Abstract
Introduction
Solanezumab, a humanized monoclonal antibody that binds soluble amyloid beta peptide, is being developed for treatment of Alzheimer's disease (AD).
Methods
Patients (n = 2042) with mild and moderate AD were randomized 1:1 to 400-mg solanezumab or placebo infusion every 4 weeks for 80 weeks and 1457 patients entered an open-label extension. Magnetic resonance imaging scans monitored for amyloid-related imaging abnormalities-edema/effusion (ARIA-E) and amyloid-related imaging abnormalities-hemorrhage/hemosiderin deposition.
Results
Sixteen patients (solanezumab, n = 11; placebo, n = 5) developed ARIA-E during the double-blind phase, and 7 patients developed ARIA-E during the open-label extension as of July 31, 2014. Unique cases are discussed including solanezumab patients who were given solanezumab, while ARIA-E was present and a patient who developed ARIA-E during placebo treatment and again during solanezumab treatment.
Discussion
Asymptomatic ARIA-E was detected in solanezumab-treated and placebo-treated AD patients. ARIA-E occurs infrequently during solanezumab and placebo treatments but may occur repeatedly in some patients.
Keywords: Amyloid-related imaging abnormalities, Alzheimer's disease, Clinical trials
1. Introduction
Magnetic resonance imaging (MRI) scans obtained during clinical trials in Alzheimer's disease (AD) patients have identified two imaging abnormalities, amyloid-related imaging abnormality edema/effusion (ARIA-E) and amyloid-related imaging abnormality hemorrhage/hemosiderin deposition (ARIA-H) [1]. ARIA-E appears as a hyperintensity in fluid-attenuated inversion recovery imaging (FLAIR) sequences and is due to parenchymal fluid accumulation (edema) or sulcal fluid effusion. ARIA-H appears as focal, round, or linear signal voids in T2*-weighted gradient recalled echo (GRE) sequences due to the iron content of residual hemosiderin deposition [1]. ARIA was initially thought to result from amyloid removal from the brain after treatment with anti-amyloid agents [1]. Subsequently, ARIA-E was found at baseline in 0.1% of untreated AD patients in two phase 3 studies of solanezumab and two phase 3 studies of semagacestat [2] and in 0.8% of patients in a retrospective review of baseline scans from three bapineuzumab studies [3]. Microhemorrhage was observed in a natural history study of memory clinic patients with a prevalence of 19%–24% and a 2-year incidence of 12% [4]. ARIA-E [3], [5], [6], [7], [8] and both ARIA-E and ARIA-H [3], [7], [8] abnormalities have been reported during treatment of AD patients with anti-amyloid antibodies, but ARIA-E has also occurred during placebo treatment [3], [6], [8]. ARIA-E is generally transient, reversible, and in milder cases, it may be clinically asymptomatic [3], [5], [6], [7], [8]. The clinical impact of ARIA is a variable depending on severity and location; ARIA-E may have concurrent symptoms such as headache, mental state changes, confusion, gait disturbances, tremor, vomiting, and/or nausea [3], [5], [7] and may require intervention beyond withholding treatment to address concomitant symptoms [5].
This article focuses on ARIA-E cases from two completed, double-blind, randomized studies [8] and one ongoing, open-label study of solanezumab in patients with mild-to-moderate AD. The limited number of cases highlights the variable and dynamic aspects of ARIA-E with both static and changing areas of edema and a wide range for time to onset and resolution in both placebo (PBO) and solanezumab (SLZ) treatment groups. Case studies were selected based on their ability to address several important clinical aspects of ARIA-E and its implications on treatment. All cases in these trials were asymptomatic.
2. Methods
Data were obtained from two completed double-blind trials (EXPEDITION and EXPEDITION 2) and their ongoing open-label extension trial (EXPEDITION-EXT, data up to July 31, 2014) of solanezumab for the treatment of mild-to-moderate AD. The specific details of the double-blind trials and baseline demographics have been described previously [8]. MRIs were obtained at baseline, endpoint and at scheduled visits. Unscheduled MRIs were obtained any time at the discretion of the investigator.
MRIs were assessed centrally by trained and validated neurologists. The neurologists are board certified and licensed physicians with subspecialty board certification in Radiology by the American Board of Radiology and specialty training in Neuroradiology with Certificates of Added Qualification in Neuroradiology, granted by the American Board of Radiology. The readers have been the central readers for the evaluation of ARIA on an average of 30 Alzheimer's trials each.
FLAIR sequences were evaluated for the presence of ARIA-E presenting either within the brain parenchyma or as sulcal FLAIR hyperintensity. ARIA-E severity was characterized as mild (confined to focal area), moderate (nonfocal, involving more than one site), and severe (multi-site, with extensive areas of involvement). When ARIA-E was detected, infusions were held unless otherwise noted, and follow-up MRIs were suggested at 4–6-week intervals. GRE sequences were obtained and evaluated for the presence of ARIA-H. Patients were not excluded due to the presence of ARIA-H in most countries except for the United Kingdom and France where patients with >2 ARIA-H were excluded. ARIA-H were counted and reported as categories 0, 1, 2 to 5, 6 to 10 and >10, according to the number of ARIA-H seen. Co-localization of ARIA-E and ARIA-H was defined as occurrence or development of both in similar locations (within approximately 1 cm) in relatively similar time frames, although development may be separated temporally.
MRI was obtained at 221 imaging centers across 16 countries for which MRI standardization training was performed. Imaging was conducted on platforms including three MRI vendors (Siemens, General Electric, and Philips) and MRI field strengths of 1.5 or 3.0 Tesla [2]. 2D FLAIR protocol was harmonized across manufacturers and field strength with range of values: Echo Time = 94–140 ms; inversion time = 2200–2800 ms; repetition time = 9000–10000 ms; slice thickness = 5 mm with 1 mm gap [2].
The percentages of patients with ARIA-E and ARIA-H were compared between treatment groups in EXPEDITION and EXPEDITION 2 using a Cochran-Mantel Haenszel test for general association controlling for study. For patients originally assigned to placebo during the double-blind studies, the entire observation period was reported; however, when they developed ARIA-E during solanezumab treatment in the open-label study, they were considered as solanezumab-treated patients. Final MRI outcome was determined using the last available MRI obtained. Some patients may have continued infusions without scheduled or unscheduled MRIs. Therefore, if ARIA-E did occur, it was not associated with any symptoms. As some analyses were conducted on data from patients in the ongoing study EXPEDITION-EXT, changes in some data may occur on subsequent final data lock.
3. Results
Two cases of ARIA-E were detected during screening of patients for the double-blind studies EXPEDITION and EXPEDITION 2, and these patients were excluded from the studies [2]. During the double-blind treatment period, ARIA-E occurred in both placebo- (PBO, n = 5) and solanezumab-treated patients (SLZ, n = 11; P = .131; Table 1). Seven cases occurred during the open-label study EXPEDITION-EXT, with one patient, Patient B, in both groups (Table 1). There was a mix of sulcal (nine instances) and parenchymal (13 instances) and mixed sulcal and parenchymal (one instance) ARIA-E (Table 1). Adverse events were reviewed for events preceding detection of and during ARIA-E, and all cases were asymptomatic. One patient (C) reported a headache and nausea approximately 1 month before the scheduled MRI and again 2 days after the scheduled MRI showed ARIA-E. However, the headache and nausea resolved each time within 48 hours after treatment with paracetamol, whereas the ARIA-E was only partially resolved at the last MRI obtained 149 days after the initial observation.
Table 1.
ARIA E summary table
| Patient ID | Original treatment assignment in EXP 1/2 | Baseline severity (Visit 1 MMSE) | Age at consent into feeder study (y) | APOE genotype | Relative onset (days)∗ | ARIA-E Sulcal and/or Parenchymal | Maximum ARIA-E severity | ARIA-H at baseline | ARIA-H at time of ARIA-E | MRI Outcome/Last observation |
|---|---|---|---|---|---|---|---|---|---|---|
| EXPEDITION | ||||||||||
| A | PBO | Mild | 79 | ε2/ε4 | 562 | Sulcal | Moderate | 1 | 6 to 10† | Complete resolution, 75 days |
| B | PBO | Mild | 80 | ε3/ε4 | 86 | Sulcal | Mild | 0 | 0 | Complete resolution, 36 days |
| C | SLZ | Mild | 81 | N/A | 365 | Parenchymal | Moderate | 6 to 10 | >10 | Partial resolution, 149 days (ED, ARIA-E) |
| D | SLZ | Mild | 73 | ε3/ε4 | 366 | Sulcal | Mild | 0 | 2 to 5† | Complete resolution, 189 days |
| E | SLZ | Mild | 80 | ε3/ε3 | 428 | Parenchymal | Mild | 6 to 10 | >10 | Complete resolution, 78 days |
| F | SLZ | Mild | 67 | ε4/ε4 | 561 | Sulcal | Severe | 1 | 2 to 5† | Partial resolution, 300 days (increased ARIA-H to >10 at discontinuation) |
| EXPEDITION 2 | ||||||||||
| G | PBO | Moderate | 69 | ε4/ε4 | 361 | Sulcal | Moderate | 2 to 5 | >10† | Partial resolution, 22 days (ED, ARIA-H) |
| H | PBO | Mild | 79 | ε2/ε3 | 566 | Parenchymal | Mild | 2 to 5 | >10 | Complete resolution, 661 days‡ |
| I | PBO | Mild | 70 | ε4/ε4 | 73 | Sulcal and Parenchymal | Mild | >10 | >10 | Partial resolution, 316 days (ED, ARIA-E) |
| J | SLZ | Mild | 93 | ε2/ε3 | 370 | Parenchymal | Severe | 0 | N/A | No change ARIA-E, 70 days (ED, ARIA-E; Increased ARIA-H to >10 at discontinuation) |
| K | SLZ | Mild | 72 | N/A | 22 | Sulcal | Moderate | 1 | 2 to 5† | Complete resolution, 227 days (ED, ARIA-H increased to 6–10) |
| L | SLZ | Mild | 69 | N/A | 83 | Sulcal | Mild | 2 to 5 | 2 to 5† | Complete resolution, 73 days |
| M | SLZ | Mild | 75 | ε3/ε3 | 197 | Parenchymal | Mild | >10 | >10 (increased) | Complete resolution, 54 days (ED) |
| N | SLZ | Mild | 74 | ε4/ε4 | 525 | Parenchymal | Moderate | 0 | 1† | Partial resolution, 51 days |
| O | SLZ | Moderate | 66 | ε3/ε4 | 361 | Parenchymal | Severe | 0 | 6 to 10† | Increased ARIA-E, 127 days (ED, ARIA-E) |
| P | SLZ | Moderate§ | 85 | ε3/ε3 | 79 | Parenchymal | Mild | 2 to 5 | N/A | Increased ARIA-E and ARIA-H to >10, 477 days |
| EXPEDITION-EXT‖ (All patients received open-label SLZ) | ||||||||||
| B | PBO | Mild | 80 | ε3/ε4 | 913 | Sulcal | Moderate | 0 | 0 | Complete resolution, 50 days |
| Q | PBO | Mild | 84 | ε3/ε4 | 915 | Parenchymal | Mild | 0 | >10 | No change, 33 days |
| R | PBO | Mild | 70 | ε4/ε4 | 730 | Sulcal | Mild | 0 | 2 to 5† | Complete resolution, 29 days |
| S | PBO | Mild | 72 | ε4/ε4 | 1295 | Parenchymal | Mild | 6 to 10 | >10 | Increased ARIA-E, 36 days |
| T | SLZ | Mild | 84 | ε3/ε3 | 1292 | Parenchymal | Moderate | 0 | 0 | No change, 32 days |
| U | SLZ | Moderate | 78 | ε2/ε3 | 1285 | Parenchymal | Mild | 0 | >10 | No change, 22 days (ED, ARIA-H) |
| V | SLZ | Mild | 79 | ε3/ε3 | 756 | Parenchymal | Mild | 2 to 5 | >10† | Increased ARIA-E, 243 days (ED, ARIA-E) |
Abbreviations: ED, early discontinuation; MMSE, Mini-Mental State Examination; N/A, not available; PBO, placebo; SLZ, solanezumab.
Relative onset (days) is calculated from date of first infusion in EXPEDITION or EXPEDITION 2.
Colocalization of new ARIA-H and ARIA-E.
Patient H developed ARIA-E during PBO treatment in EXPEDITION 2 then initiated treatment with SLZ in EXPEDITION-EXT and ARIA-E resolved during SLZ treatment during EXPEDITION-EXT.
Patient P had an MMSE score of 13 at visit 1, which was outside the inclusion and/or exclusion criteria for the study. At visit 2, Patient P had an MMSE score of 17 so is considered of moderate disease severity for this analysis.
Treatment during double-blind trial (all received SLZ in open-label trial). Relative onset of ARIA-E for patients treated with PBO in EXPEDITION and EXPEDITION 2 includes approximately 560 days of observation in those studies.
In placebo-treated and solanezumab-treated patients, ARIA-E occurred throughout the observation period with a range of onset between 73–566 and 22–1295 days, respectively (Table 1). The range for time to resolution and/or partial resolution was also variable for placebo-treated and solanezumab-treated patients, ranging from 22–316 and 51–300 days, respectively (Table 1). One patient (H) assigned to placebo in the feeder studies developed ARIA-E, switched to open-label solanezumab for 14 infusions and then stopped dosing; the patient then had complete resolution after an additional 303 days (661 days from onset). Complete resolution was observed in 10 patients (three PBO and seven SLZ) and partial resolution in 5 patients (two PBO and three SLZ; Table 1). Some solanezumab-treated patients had limited follow-up; four had no change, and four had increased ARIA-E at the last MRI obtained (Table 1).
In 19 of 22 of the ARIA-E patients, APOE genotype was available. Eleven of 19 (58%) ARIA-E patients with APOE genotyping were APOE ε4 carriers compared with 1098 of 1866 (59%) of the overall study population (Table 1). This suggests that the APOE ε4 allele is not over represented in the cohort of patients with ARIA-E. However, six of the 19 ARIA-E patients (32%) were APOE ε4 homozygotes (Table 1), compared with 242 of 1866 (13%) of the overall study population. This suggests that patients with two APOE ε4 alleles are at increased risk of developing ARIA-E.
In the pooled trial population at baseline, 1639 of 2019 (approximately 80%) patients had no ARIA-H at baseline (Fig. 1A). In addition, there were no significant differences in the categorical distribution of ARIA-H between treatment groups at baseline (Fig. 1). During the double-blind treatment period, 7.3% and 9.1% of placebo and solanezumab-treated patients had changes in ARIA-H, respectively (P = .14). In addition, there was no significant difference in the degree (number) of increases in ARIA-H between the placebo and solanezumab treatment groups (P > .08; Fig. 1B). At the time of ARIA-E detection, 3 of 23 patients (one patient was counted twice) had no ARIA-H and in two patients, ARIA-H status was not analyzable (Table 1). Sixteen of 21 patients (76%) with ARIA-E and scans assessable for ARIA-H experienced increases in ARIA-H at the time of ARIA-E (PBO, 3/5: 60% and SLZ, 13/16; 81%; Table 1). In those 16 patients, 10 had co-localization of ARIA-H changes with ARIA-E (Table 1).
Fig. 1.
(A) Baseline distribution of ARIA-H by treatment. ARIA-H was categorized as 0, 1, 2 to 5, 6 to 10, and >10, (B) Categorical increases in ARIA-H. Categorical increases were determined by the number of increases between categories 1, 2 to 5, 6 to 10, >10, and > 10 with further increases.
3.1. Summary of patients treated with solanezumab during ARIA-E
Eight patients continued to be infused with solanezumab during ARIA-E: Patient D, seven infusions, complete resolution; Patient E, three infusions, complete resolution; Patient H, 14 infusions during EXPEDITION-EXT, partial resolution at early discontinuation (ED) followed by complete resolution 10 months after infusions were stopped; Patient K, two infusions followed by increased ARIA-E, two additional infusions followed by partial resolution and cessation of infusions at which point increased ARIA-E was followed by complete resolution; Patient O, three infusions, increased ARIA-E at ED; Patient P, 17 infusions, increased ARIA-E at study end; Patient S, two infusions during EXPEDITION-EXT, increased ARIA-E at ED; Patient T, one infusion during EXPEDITION-EXT, no change at last available MRI. The infusions continued in some patients because ARIA-E was identified at a later time point and retrospective review of prior scans showed ARIA-E was initially not recognized and occurred earlier than originally thought. Other patients continued to be infused at the investigator's discretion. All patients remained asymptomatic during this time.
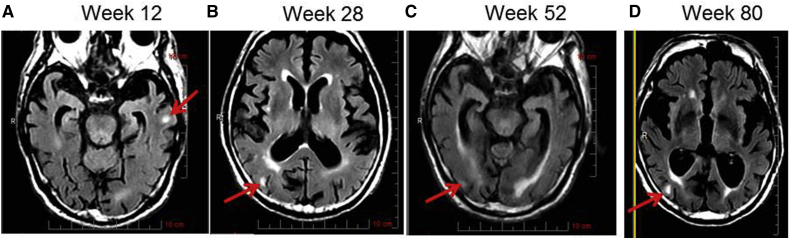
3.2. Case 1—patient treated with solanezumab during ARIA-E
Patient P, an 85-year-old male with APOE ε3/ε3 genotype, was treated with solanezumab.
ARIA-E was first recognized at the Week 80 MRI (endpoint), and prior MRIs were then retrospectively reviewed. The patient continued to be infused throughout the study. Retrospective review of Week 12 MRI, 79 days after randomization to solanezumab, identified mild left temporal ARIA-E (Fig. 2A). ARIA-H was not assessable on that MRI due to motion artifact during the GRE sequence.
Fig. 2.
FLAIR images from patient P. (A) 12 weeks, (B) 28 weeks, (C) 52 weeks, (D) 80 weeks. Red arrows indicate areas of ARIA-E.
Retrospective review of the Week 28 MRI, 198 days after randomization and 120 days after the prior MRI, showed an increase in ARIA-E evidenced by peripheral foci of T2 hyperintensity in the right parietal lobe (Fig. 2B). The left temporal ARIA-E had resolved. ARIA-H had increased (from category 2 to 5 to category 6 to 10) as a new punctate microheme in the right occipital lobe.
Retrospective review of the Week 52 MRI, 359 days after randomization and 281 days after the first appearance of ARIA-E, showed that right parietal ARIA-E had resolved. There was a very subtle new focus of FLAIR hyperintensity in the right occipital lobe, which was most compatible with mild ARIA-E (Fig. 2C). ARIA-H had increased to >10, as several of the peripheral foci of T2 shortening, previously felt to indicate normal vasculature, were now noted to indicate microhemes.
The Week 80 (endpoint) MRI, 555 days after randomization and 477 days after the first appearance of ARIA-E showed increased mild ARIA-E in the right temporal and occipital lobes and increased size and number of ARIA-H (category remained > 10; Fig. 2D). The patient completed the study while remaining asymptomatic the entire time. No further MRI follow-up was available.
3.3. Summary of patients treated or rechallenged with solanezumab after ARIA-E resolution
Four patients who developed ARIA-E, had infusions stopped, and had complete resolution were then re-challenged with solanezumab or began solanezumab treatment after placebo treatment. Three of 4 patients had no further occurrence over ∼1.5 to 2 years. After complete resolution of moderate ARIA-E while on placebo during EXPEDITION, Patient A enrolled in EXPEDITION-EXT and had no additional ARIA-E during 2 years of solanezumab infusions. After complete resolution of mild ARIA-E while on placebo during EXPEDITION, Patient B enrolled in EXPEDITION-EXT and moderate ARIA-E was detected after 13 solanezumab infusions. After complete resolution of this occurrence of ARIA, the patient was re-challenged, remained in the study for an additional year and had no further ARIA-E. Patient L was originally treated with solanezumab during EXPEDITION 2 and had ARIA-E at Week 12 (four infusions). After complete resolution of mild right and left-frontal ARIA-E, the patient received an additional 14 solanezumab infusions in EXPEDITION 2 with no recurrence of ARIA-E. Patient L then enrolled in EXPEDITION-EXT and discontinued after approximately 2 years and 8 months due to patient decision regarding inability to tolerate further MRI without an another MRI or incidence of symptomatic ARIA-E. Patient R enrolled in EXPEDITION-EXT and developed ARIA-E after seven infusions. Patient R restarted solanezumab after resolution of ARIA-E and continued for another 18 infusions with no additional incidence of ARIA-E. Infusions were stopped after ARIA-E was detected in the remaining two PBO and eight solanezumab patients with ARIA-E (patients C, F, G, I, J, M, N, Q, U, and V). These patients were not re-challenged.
3.4. Case 2—patient with more than one occurrence
Patient B, an 80-year-old female with APOE ε3/ε4 genotype, was randomized to placebo.
The Week 12 MRI, 86 days after randomization, showed mild cerebellar, left temporal, and left occipital lobe ARIA-E with a focus of abnormal sulcal FLAIR hyperintensity (Fig. 3A). No ARIA-H was detected at baseline or at the time of ARIA-E. An unscheduled MRI after 36 days showed complete resolution of ARIA-E (Fig. 3B) and study infusions restarted. An unscheduled follow-up MRI, 21 days after resumption of study infusions, showed no reoccurrence of ARIA-E. The patient completed the study without further incidence of ARIA-E and enrolled into the open-label study EXPEDITION-EXT.
Fig. 3.
FLAIR images from patient B. EXPEDITION (A) 12 weeks, and Unscheduled 1, 36 days; EXPEDITION-EXT (B) 52 weeks, and Unscheduled 1, 50 day. Arrows indicate areas of ARIA-E.
During EXPEDITION-EXT, the Week 52 MRI, 359 days after beginning solanezumab treatment, showed moderate sulcal right temporal and occipital ARIA-E (Fig. 3C). Again, no ARIA-H was detected. An unscheduled MRI, after 50 days, showed ARIA-E had again completely resolved (Fig. 3D). Patient was re-challenged with study drug and remained in the study with no further ARIA-E.
3.5. Case 3—patient with increased ARIA-E on follow-up MRIs after infusions held
Patient F, a 67 year old female with APOE ε4/ε4 genotype was randomized to solanezumab.
The Week 80 MRI (endpoint) showed moderate sulcal left temporal, left parietal, and bilateral occipital ARIA-E (Fig. 4A) and increased ARIA-H from category 1 at baseline to category 2 to 5. A thin linear macroheme (>1 cm in overall length) had also developed adjacent to the previously seen left occipital microheme. The patient completed the study; she did not enroll in EXPEDITION-EXT but continued to be followed.
Fig. 4.
FLAIR images from patient F. (A) 80 weeks, (B) unscheduled 1, (C) unscheduled 2, (D) unscheduled 3, (E) unscheduled 4, (F) unscheduled 5. Red arrows indicate areas of ARIA-E.
Unscheduled MRIs, after 85 days and 118 days, showed increased ARIA-E (severe) and ARIA-H (to 6 to 10 and to >10, respectively) (Fig. 4B and C). The next unscheduled MRI, after 195 days, showed slight worsening of ARIA-E in areas of left parietal subcortical white matter but improvement in other areas; overall, ARIA-E had resolved to moderate severity (Fig. 4D). The number of ARIA-H was unchanged; however, a linear area of hemosiderin in the right occipital lobe had slightly increased in size. The fourth unscheduled MRI, after 258 days, showed further partial resolution of ARIA-E (Fig. 4E). There was residual ARIA-E primarily in the left parietal lobe with mild involvement of the superior left occipital lobe, and subtle residual left temporal lobe ARIA-E. ARIA-H were unchanged. The final unscheduled MRI, after 300 days, showed further partial resolution of ARIA-E to mild severity (Fig. 4F) and an increase in the number and increased size of previously identified ARIA-H (category remained > 10).
3.6. Case 4—placebo-treated patient with long follow-up and time to partial resolution
Patient I was a 70-year-old female with APOE ε4/ε4 genotype, treated with placebo.
The Week 12 MRI, 73 days after randomization, showed mild sulcal and parenchymal right occipital ARIA-E (Fig. 5A). Microhemes noted in this area at screening remained unchanged. Infusions were stopped. An unscheduled MRI, after 54 days, showed no significant change in the ARIA-E (Fig. 5B) or ARIA-H. The next unscheduled MRI, after 93 days, showed additional ARIA-E (Fig. 5C) and no change in the ARIA-H. The ED (due to ARIA-E) visit MRI, after 168 days, showed no change in ARIA-E (Fig. 5D) or ARIA-H. The final MRI, after 316 days, showed partial resolution of the ARIA-E (Fig. 5E) and an increase in the number of ARIA-H to category >10 with further increase. No further follow-up was performed.
Fig. 5.
FLAIR images from patient I. (A) 12 weeks, (B) unscheduled 1, (C) unscheduled 2, (D) unscheduled 3 (ED), (E) unscheduled 4. Red arrows indicate areas of ARIA-E.
4. Discussion
In an earlier review of baseline scans from clinical trials of patients with mild to moderate AD, ARIA-E was found to occur at a very low prevalence of <0.1%–0.8% in untreated patients [2], [3]. The incidence of ARIA-E is significantly greater during treatment with some agents that target amyloid compared with placebo-treated patients [3], [5], [6], [7]. It is thought that more ARIA is associated with treatment with anti-amyloid antibodies such as bapineuzumab and gantenerumab that target the N-terminal region of Aβ because those epitopes are exposed in amyloid plaques [9] and are available for antibody binding. It has been suggested that during bapineuzumab treatment, parenchymal plaque is transiently relocated to the cerebral vasculature [10], [11] and either the increased cerebrovascular amyloid or removal of amyloid in weakened vasculature leads to ARIA-E and ARIA-H [1]. Solanezumab binds to the mid-domain of Aβ and does not bind deposited amyloid plaque, presumably because this domain is unavailable. Because solanezumab binds only soluble Aβ with very high affinity, it should not be associated with translocation of amyloid to or direct removal from the cerebral vasculature and therefore be associated with less ARIA than N-terminally directed antibodies that target deposited plaque. ARIA in placebo-treated subjects may occur from other mechanisms such as disease-mediated changes in amyloid production and/or clearance or local inflammatory processes [1].
In double-blind phase 3 trials of solanezumab, asymptomatic ARIA-E occurred in placebo-treated patients (0.5%) and solanezumab-treated patients (1.1%, P = .131; Table 1). In both treatment groups, ARIA-E was a variable dynamic process with a range of severity, sometimes occurring in one or more brain regions. In placebo-treated and solanezumab-treated patients, ARIA-E occurred with a wide range of time to onset and time to resolution with some patients having no change or increased ARIA-E at their final study MRI. No cases were clearly related to concomitant symptoms.
Phase 2 studies of bapineuzumab have reported an incidence of ARIA-E in treated patients of 9.7%, with a dose-dependent increase in incidence of up to 26.7%, whereas the incidence in placebo-treated patients was 0.8% [5]. In a combined retrospective reanalysis of MRIs from the two phase 2 studies and an open-label extension study of bapineuzumab, 17% of bapineuzumab-treated patients had ARIA-E with a dose-dependent increase in risk [3]. The mean number of infusions (every 13 weeks) before detection of ARIA-E was 2.4 (SD 1.7; range 1–7). Most ARIA-E cases (25 of 36; 69%) were identified after the first or second infusion. ARIA-E was not observed >2 years after the first exposure to bapineuzumab. The median duration of ARIA-E for the 31 of 36 patients whose ARIA-E was resolved on follow-up scans was 113 days (IQR, 61–182) [3]. Phase 3 studies of bapineuzumab have reported an incidence of ARIA-E in placebo-treated patients of 0.2% compared with a dose-dependent increase in incidence of 14.2% in the 2.0-mg/kg APOE ε4 noncarrier group and 15.3% in the 0.5-mg/kg APOE ε4 carrier group [6]. Similar to the phase 2 studies, most ARIA-E cases in the phase 3 studies occurred within the first 3 doses (∼39 weeks or 273 days) [6]. In a phase 1 study, two of eight patients treated with 200-mg gantenerumab (every 4 weeks) developed ARIA-E after 2 or 4 doses (every 4 weeks) [7].
A lower percentage of patients in the reanalysis of the bapineuzumab phase 2 studies [3] had ARIA-H at baseline (9.2%, 19 of 207) compared with our studies (18.8%). In these studies, incident ARIA-H occurred in 24 of 294 patients (8.1%) patients; however, treatment effect on incident ARIA-H was not reported in this analysis. In the phase 3 bapineuzumab studies, incident cerebral microhemorrhage and cerebral hemosiderin deposition were only reported as serious treatment-emergent adverse events at a much lower rate of 0.4% of APOE ε4 noncarriers and 0.7% of APOE ε4 carriers treated with bapineuzumab (0.5% combined) compared with 0% of placebo-treated patients ([6], supplemental material). Incident ARIA-H was similar between our studies (PBO, 7.3% and 9.1% SLZ) and the reanalyzed bapineuzumab phase 2 studies (8.1%) [3]. Although statistical analysis of treatment differences between placebo and bapineuzumab [3] and placebo and gantenerumab [7] were not reported, the treatment difference between placebo and solanezumab for incident ARIA-H was not statistically significant.
A similar percentage of APOE ε4 allele carriers was seen in solanezumab-treated patients with ARIA-E compared with the overall trial population (58% vs 59%), suggesting that the APOE ε4 allele is not overrepresented in the cohort with ARIA-E. However, the percentage of homozygous APOE ε4 patients with ARIA-E (32%) is higher than in the overall study population (13%) suggesting that APOE ε4 homozygotes have an increased risk of developing ARIA-E. In patients with ARIA-E who had APOE genotyping in the retrospective review of the phase 2 bapineuzumab trials, the APOE ε4 allele was present in 86% compared with 65% of the overall cohort [3]. Similar to the EXPEDITION studies, a greater percentage of patients who developed ARIA were APOE ε4 homozygotes compared with the percentage of APOE ε4 homozygotes in the overall trial population (36% vs 16%, respectively) [3]. Using Cox proportional hazards models, an increased risk of ARIA-E was determined of approximately 3-4-fold and 7-fold in APOE ε4 heterozygotes and homozygotes, respectively [3]. In the phase 3 bapineuzumab studies after central read of the MRIs, ARIA-E was reported in APOE ε4 carriers at 1.1% in placebo-treated patients and 16.9%–34.5% in bapineuzumab-treated patients and in noncarriers at 0.6% and 5.6%–19.9% in placebo-treated and bapineuzumab-treated patients, respectively [6]. In addition, the two patients treated with 200-mg gantenerumab in the phase 1 study who developed ARIA-E were homozygous for APOE ε4 [7]. Other data have suggested that APOE ε4 carriers have a higher vascular amyloid burden [12], [13] which could lead to increased ARIA-H and/or vascular permeability [14].
Other data also suggest an interrelationship between ARIA-H and ARIA-E as in many cases, new ARIA-H was seen in the location of ARIA-E before it occurs or after it has resolved [1], [3]. In our studies, 71% (15 of 21) of patients with ARIA-E and scans assessable for ARIA-H, experienced increases in ARIA-H at the time of ARIA-E; 48% (10 of 21) had colocalization of ARIA-H changes with ARIA-E. In the retrospective review of the phase 2 bapineuzumab studies, 82% (14 of 17) of bapineuzumab-treated patients had co-incident ARIA-H and ARIA-E, and 57% (8 of 14) of patients had co-localization [3]. Furthermore, the two gantenerumab-treated patients who developed ARIA-E also had coincident ARIA-H [7]. Taken together, these data support a relationship between incident ARIA-H and ARIA-E. However, interestingly, the one patient in the EXPEDITION studies that had two instances of ARIA-E (Patient B, one during PBO treatment and one during SLZ treatment) had no detectable ARIA-H at baseline and no detectable changes in ARIA-H before ARIA-E or after resolution.
Important clinical issues of ARIA-E include management of associated symptoms and withdrawal or continuation of treatment during ARIA-E. In our trials, when ARIA-E was detected, no intervention was performed beyond holding solanezumab infusion, and although some patients had very long time to resolution or the ARIA-E was ongoing at their last study MRI, all patients remained asymptomatic. In the phase 2 study, 6 of 12 of the bapineuzumab-treated patients with ARIA-E had symptoms of headache, confusion, vomiting, and gait disturbance, with one patient requiring treatment with steroids [5]. Some answers to whether patients can be treated through asymptomatic ARIA-E may be obtained from patients that continued treatment during very subtle asymptomatic ARIA-E that was not initially recognized and discovered only after retrospective review of MRIs. In our trials, eight patients were dosed though ARIA-E (range 3 to 17 infusions) with no associated symptoms and a variable outcome from complete resolution to increased severity at the final MRI. In the bapineuzumab phase 2 and 3 trials, an increased incidence of ARIA-E was discovered after rereview by central readers [3], [5]; these additionally identified ARIA-E patients continued treatment while mild, asymptomatic ARIA-E was present. Taken together, these data suggest that the treatment may continue during the presence of mild asymptomatic ARIA-E.
The ability to restart therapy after resolution of ARIA-E is another important aspect of clinical management. Four patients with ARIA-E were treated with or re-challenged with solanezumab after complete resolution of ARIA-E (range, 50–75 days) and 3 of 4 had no additional reoccurrence over approximately 1–2 years additional exposure. In the phase 2 study, after resolution of ARIA-E, 6 bapineuzumab-treated patients restarted treatment with 0.15 mg/kg and were titrated up to 50% of the original dose without reoccurrence of ARIA-E [5]. However, two patients with ARIA-E detected after the central reread in the bapineuzumab phase 2 studies developed ARIA-E again when re-challenged [3]. Preclinical animal models suggest that repair and remodeling of cerebral vasculature occurs [15] after ARIA [16] that may prevent further occurrence [17]. If similar mechanisms are functioning after ARIA in humans, it would explain the ability to rechallenge patients without further occurrence of ARIA-E with solanezumab or bapineuzumab in some patients [3], [5] and the differential location of 2 instances of ARIA-E in one patient in our trials.
One limitation of our data is the extent of follow-up after ARIA-E due to the patients' willingness or ability to return for unscheduled MRIs. Another limitation is that testing for evidence of amyloid pathology was not a required inclusion criterion for these studies. However, as ARIA is associated with the presence of amyloid pathology, all patients who developed ARIA are presumed to be amyloid positive. In addition, only three patients in the ARIA-E cohort participated in the optional amyloid-PET addendum and only one placebo patient had an endpoint PET scan, limiting the assessment of correlation between location and amount of amyloid plaque change and ARIA-E.
Although the presentation of ARIA-E is variable among patients participating in clinical trials, when it occurs, it appears to be manageable with close monitoring, withholding investigational therapy, treatment of any potential associated symptoms and re-initiation of treatment. Additional data and analyses will help to further guide clinicians in the management of ARIA in their patients with AD.
Research in context.
-
1.
Systematic review: The authors searched available literature using PubMed and OVID search engines to identify articles. Meeting abstracts and presentations were also reviewed. The relationship between immunotherapy and ARIA is not clear and may depend on the class and Aβ binding site of the antibody. The clinical aspects of ARIA have been described and published or presented, and the relevant references have been cited.
-
2.
Interpretation: Our findings support others' findings on ARIA in the clinical trial setting; although it is difficult to draw direct comparisons to other trials because this level of individual patient detail is not available.
-
3.
Future directions: The article may help guide clinicians' treatment decisions for individual patients with AD with ARIA in clinical trials and in general practice. Descriptions of individual cases highlight the variable presentation and resolution of ARIA, whereas summary information may guide a general approach to management of this phenomenon.
Acknowledgments
The authors would like to thank Karen Sundell, B.S., Gopalan Sethuraman, Ph.D., and Roy Yaari, M.D. for their assistance in preparation of this article. The studies detailed in this article were funded by Eli Lilly and Company.
Dedication: The authors dedicate this article in loving memory of Dr. Roza Hayduk. We will greatly miss her friendship and passion and dedication to Alzheimer's research.
Footnotes
R.H. completed work on these studies and this article before her passing and is included posthumously as a co-author.
C.C., E.S., A.H., and M.C. are employees and hold stock with Eli Lilly and Company. There are no other conflicts of interest.
References
- 1.Sperling R.A., Jack C.R., Black S.E., Frosch M.P., Greenberg S.M., Hyman B.T. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: Recommendations from the Alzheimer's Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7:367–385. doi: 10.1016/j.jalz.2011.05.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlson C., Estergard W., Oh J., Suhy J., Jack C.R., Siemers E. Prevalence of asymptomatic vasogenic edema in pretreatment Alzheimer's disease study cohorts from phase 3 trials of semagacestat and solanezumab. Alzheimers Dement. 2011;7:396–401. doi: 10.1016/j.jalz.2011.05.2353. [DOI] [PubMed] [Google Scholar]
- 3.Sperling R.A., Salloway S., Brooks D.J., Tampieri D., Barakos J., Fox N.C. Amyloid-related imaging abnormalities in patients with Alzheimer's disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol. 2012;11:241–249. doi: 10.1016/S1474-4422(12)70015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goos J.D., Henneman W.J., Sluimer J.D., Vrenken H., Sluimer I.C., Barkhof F. Incidence of cerebral microbleeds: A longitudinal study in a memory clinic population. Neurology. 2010;74:1954–1960. doi: 10.1212/WNL.0b013e3181e396ea. [DOI] [PubMed] [Google Scholar]
- 5.Salloway S., Sperling R., Gilman S., Fox N.C., Blennow K., Raskind M., Bapineuzumab 201 Clinical Trial Investigators A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73:2061–2070. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salloway S., Sperling R., Fox N.C., Blennow K., Klunk W., Raskind M. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostrowitzki S., Deptula D., Thurfjell L., Barkhof F., Bohrmann B., Brooks D.J. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch Neurol. 2012;69:198–207. doi: 10.1001/archneurol.2011.1538. [DOI] [PubMed] [Google Scholar]
- 8.Doody R.S., Thomas R.G., Farlow M., Iwatsubo T., Vellas B., Joffe S. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 9.Orgogozo J.M., Gilman S., Dartigues J.F., Laurent B., Puel M., Kirby L.C. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 10.Boche D., Zotova E., Weller R.O., Love S., Neal J.W., Pickering R.M. Consequence of Aβ immunization on the vasculature of human Alzheimer's disease brain. Brain. 2008;131:3299–3310. doi: 10.1093/brain/awn261. [DOI] [PubMed] [Google Scholar]
- 11.Weller R.O., Subash M., Preston S.D., Mazanti I., Cararero R.O. Perivascular drainage of amyloid-β peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer's disease. Brain Pathol. 2008;18:253–266. doi: 10.1111/j.1750-3639.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chalmers K., Wilcock G.K., Love S. APOE epsilon 4 influences the pathological phenotype of Alzheimer's disease by favouring cerebrovascular over parenchymal accumulation of Abeta protein. Neuropathol Appl Neurobiol. 2003;29:231–238. doi: 10.1046/j.1365-2990.2003.00457.x. [DOI] [PubMed] [Google Scholar]
- 13.Caselli R.J., Walker D., Sue L., Sabbagh M., Beach T. Amyloid load in nondemented brains correlates with APOE e4. Neurosci Lett. 2010;473:168–171. doi: 10.1016/j.neulet.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberg S.M., Rebeck G.W., Vonsattel J.P.G., Gomez-Isla T., Hyman B.T. Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol. 1995;38:254–259. doi: 10.1002/ana.410380219. [DOI] [PubMed] [Google Scholar]
- 15.Christie R., Yamada M., Moskowitz M., Hyman B. Structural and functional disruption of vascular smooth muscle cells in a transgenic mouse model of amyloid angiopathy. Am J Pathol. 2001;158:1065–1071. doi: 10.1016/S0002-9440(10)64053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schroeter S., Khan K., Barbour R., Doan M., Guido T. Immunotherapy reduces vascular amyloid-beta in PDAPP mice. J Neurosci. 2008;28:6787–6793. doi: 10.1523/JNEUROSCI.2377-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zago W., Schroeter S., Guido T., Khan K., Seubert P., Yednock T. Vascular alterations in PDAPP mice after anti-Aβ immunotherapy: Implications for amyloid-related imaging abnormalities. Alzheimers Dement. 2013;9:S105–S115. doi: 10.1016/j.jalz.2012.11.010. [DOI] [PubMed] [Google Scholar]