Abstract
Background
Prior studies have shown the association of low serum magnesium with adverse health outcomes in patients undergoing hemodialysis. There is a paucity of such studies in patients undergoing peritoneal dialysis (PD).
Study Design
Cohort Study.
Setting & Participants
10,692 patients treated with PD January 1, 2007–December 31, 2011 in facilities operated by a single large dialysis organization in the United States.
Predictor
Baseline serum magnesium levels, examined as five categories (<1.8, 1.8–<2.0, 2.0–<2.2 [reference], 2.2–<2.4, and ≥2.4 mg/dL).
Outcomes
Time to first hospitalization and time to death using competing risks regression models.
Results
The distribution of baseline serum magnesium levels in the cohort were < 1.8 mg/dl: 1928 (18%); 1.8–<2.0 mg/dl: 2204 (21%); 2.0–<2.2 mg/dl: 2765 (26%); 2.2–<2.4 mg/dl: 1765 (16%); and ≥ 2.4 mg/dl: 2030 (19%). Of the 10,692 patients, 6465 (60%) were hospitalized at least once and 1392 (13%) died during follow-up (median, 13; IQR, 7–23 months). Baseline serum magnesium <1.8 mg/dL was associated with higher risk for hospitalization and all-cause mortality after adjustment for demographic and clinical characteristics (adjusted HRs of 1.23 [95% CI, 1.14–1.33] and 1.21 [95% CI, 1.03–1.42], respectively). The higher risk for hospitalization persisted upon adjustment for laboratory variables while that for all-cause mortality was attenuated to a non-significant level. The greatest risk for hospitalization was in patients with low serum albumin (< 3.5 g/dl; p for interaction < 0.001).
Limitations
Possibility of residual confounding by unmeasured variables cannot be excluded.
Conclusions
Lower serum magnesium levels may be associated with higher risk of hospitalization in incident PD patients, particularly among those with hypoalbuminemia. Additional studies are needed to confirm these findings and investigate whether correction of hypomagnesemia reduces these risks.
INDEX WORDS: Magnesium, hypomagnesemia, end-stage renal disease (ESRD), peritoneal dialysis (PD), incident PD patients, hospitalization, all-cause mortality
Magnesium, the fourth most abundant cation in the body, is the second most plentiful intracellular cation (after potassium). As much as 70% of serum magnesium is in the free, ionized form, which has a crucial function in maintaining internal homeostasis via actions in the endocrine, musculoskeletal, nervous, and cellular messenger systems (1). The reference range for total serum magnesium in adults is 1.7–2.4 mg/dl (0.7–1.0 mmol/L or 1.4–2.0 mEq/L) (2, 3). In patients undergoing maintenance dialysis, magnesium homeostasis depends upon dietary intake, intestinal absorption, and removal by kidneys and with dialysis. In turn, serum magnesium levels can be influenced by significant changes in dietary intake; residual kidney function; dialysis dose; losses through the gastrointestinal tract, kidneys, or with dialysis; or re-distribution from the extracellular to intracellular space.
The relatively high prevalence of abnormal serum magnesium levels in patients with kidney disease has long been known but, until recently, little has been reported regarding adverse effects on health outcomes (3). Studies have shown a strong inverse association of serum magnesium levels with insulin resistance, new-onset diabetes mellitus, oxidative stress, endothelial dysfunction, hypertension, atherosclerosis, and systemic inflammation (4). Observational studies have also shown a significant association between hypomagnesemia and higher all-cause mortality in patients with chronic kidney disease not undergoing dialysis, or in those with end-stage renal disease (ESRD) treated with maintenance hemodialysis (5–7).
Commercially available peritoneal dialysate contains 0.25 mmol/L magnesium, resulting in daily net magnesium removal, and higher ultrafiltration with hypertonic solutions further increases magnesium losses into the dialysate (8–10). However, to date the data on effect of serum magnesium levels on health outcomes of patients undergoing peritoneal dialysis (PD) are sparse. We performed this study to test the hypothesis that in patients with ESRD undergoing PD, low serum magnesium levels are associated with higher risk for hospitalization and all-cause mortality.
METHODS
Study Population and Data Source
The study cohort comprised patients with ESRD who initiated treatment with PD from January 1, 2007 through December 31, 2011 and received care in one of the facilities operated by DaVita Inc., a large dialysis organization in the United States. Included patients were aged18 years or older, underwent PD for at least 60 days, and had at least one measurement of serum magnesium during the first 91-day period of treatment with PD. Patients were followed up for up to five years through December 2011 (median follow-up, 13 [IQR, 7–23] months). The Institutional Review Boards at the University of Washington and University of California Irvine approved the study and waived the requirement for informed consent since only de-identified data were received.
All data were obtained from electronic medical records of the dialysis facilities. Age was estimated by using date of birth and date of first dialysis treatment and five race/ethnicity categories were used for analyses (Whites, Blacks, Hispanics, Asians, and Others). The data on comorbid conditions were obtained from the electronic medical records of the dialysis organization as provided by the treating healthcare providers and included diabetes mellitus, hypertension, congestive heart failure, atherosclerotic heart disease, and other cardiovascular diseases. Blood samples were drawn in dialysis facilities and shipped to a central laboratory (DaVita Laboratory, Deland, FL) within 24 hours, where measurements were made using automated and standardized methods. All clinical and laboratory data, and doses of parenteral medications, were summarized for the first 91-day period of PD.
The exposure of interest was baseline serum magnesium level, defined as an average of all measurements in the first 91-days of PD. Patients were grouped into five magnesium categories (<1.8, 1.8–<2.0, 2.0–<2.2 [reference], 2.2–<2.4, and ≥2.4 mg/dL), chosen with recognition of a normal reference range of 1.8–2.4 mg/dL and a 0.2-mg/dL incremental change within this range (6).
Outcomes
The two co-primary outcomes were time to (1) first hospitalization, and (2) death from any cause. The data on each of these two outcomes was obtained from the electronic medical records of the dialysis organization. The follow-up period comprised the interval from the date of first PD treatment through the occurrence of one of the following events: a primary outcome (death or hospitalization for the respective analyses), kidney transplantation, transfer to another dialysis modality or to a facility operated by another dialysis provider, or end of administrative follow-up. Patients were considered to have transferred to another dialysis modality only if they were treated with an alternative therapy for 60 continuous days; as such, all events (death or hospitalization) within 60 days of transfer were attributed to the PD.
Statistical Analyses
Data are presented as mean ± standard deviation, median with IQR, or proportions, where appropriate. Data on gender, race/ethnicity, geographic location, serum albumin, phosphorus, calcium, potassium, and bicarbonate were missing for < 1%; serum iron saturation, ferritin and parathyroid hormone, for 1%–5%; weekly Kt/V and residual kidney function, for 12%; and 4-hour dialysate–plasma creatinine ratio, for 27%. Five sets of imputations were done using a joint multivariable multiple imputation model to account for missing laboratory variables including the exposure, covariates (age, gender, race/ethnicity, primary medical insurance, cause of ESRD, history of prior transplantation, interval from initiation of dialysis to initiation of PD, diabetes, hypertension, atherosclerotic heart disease, congestive heart failure, other cardiovascular disease, blood hemoglobin, serum albumin, calcium, phosphorus, parathyroid hormone, iron saturation, ferritin, bicarbonate, magnesium, total weekly Kt/V, residual kidney function, and 4-hour dialysate–plasma creatinine ratio), four interaction terms selected a priori (serum albumin, phosphorus, and parathyroid hormone and residual kidney function with outcome), an indicator for the outcome of interest (present or not), and the Nelson-Aalen estimator.
Two separate time-to-event analyses were undertaken using competing risks regression models to determine the association of baseline serum magnesium level with first hospitalization and all-cause mortality (11). For the analysis of all-cause mortality, competing events were kidney transplantation and transfer to in-center hemodialysis. For analysis of hospitalization, competing events were all-cause mortality, kidney transplantation, and transfer to in-center hemodialysis. Sub-hazard ratios (HRs) were obtained for the outcome of interest and combined using Rubin’s rules.
Data were analyzed using the following levels of adjustment: (1) unadjusted; (2) case mix-adjusted for demographic and clinical characteristics, including age, gender, race/ethnicity, primary insurance, geographic location of dialysis facility (Northeast, South, Midwest, or West), year of incidence, cause of ESRD, prior kidney transplantation, comorbid conditions (diabetes mellitus, hypertension, congestive heart failure, atherosclerotic heart disease, other cardiovascular diseases), and interval from initiation of dialysis to initiation of PD; (3) case-mix plus laboratory variables that included all of the aforementioned covariates and hemoglobin, serum albumin, uncorrected calcium, phosphorus, intact parathyroid hormone, ferritin, iron saturation, bicarbonate, potassium, total weekly Kt/V, residual kidney function, 4-hour dialysate–plasma creatinine ratio from the peritoneal equilibration test, and treatment with automated PD within the first 91-day period of PD.
Two different sensitivity analyses were done. First, the associations of baseline serum magnesium level as a continuous variable were examined with HRs expressed for risk associated with every 0.2-mg/dl lower serum levels. Second, the analyses were repeated by imputing the value for serum magnesium in 7152 patients with missing values for the first 91 days of initiation of PD.
Effect modification of the association of serum magnesium (< 1.8 mg/dl, compared to ≥ 1.8 mg/dl) with time to first hospitalization and all-cause mortality was examined for four a priori covariates (serum albumin < and ≥ 3.5 g/dl, parathyroid hormone < and ≥ 300 pg/ml, phosphorus < and ≥ 5 mg/dl, and residual kidney function < and ≥ 55 L/week/1.73 m2). Sub-group analyses were performed for covariates with p-value for the interaction term < 0.05. The same demographic, clinical, and laboratory covariates were used for these sub-group analyses as already mentioned, except the variable used to stratify the population for the particular analyses.
All analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC) and Stata, version 13.1 (StataCorp LP, College Station, TX).
RESULTS
Study Cohort Description
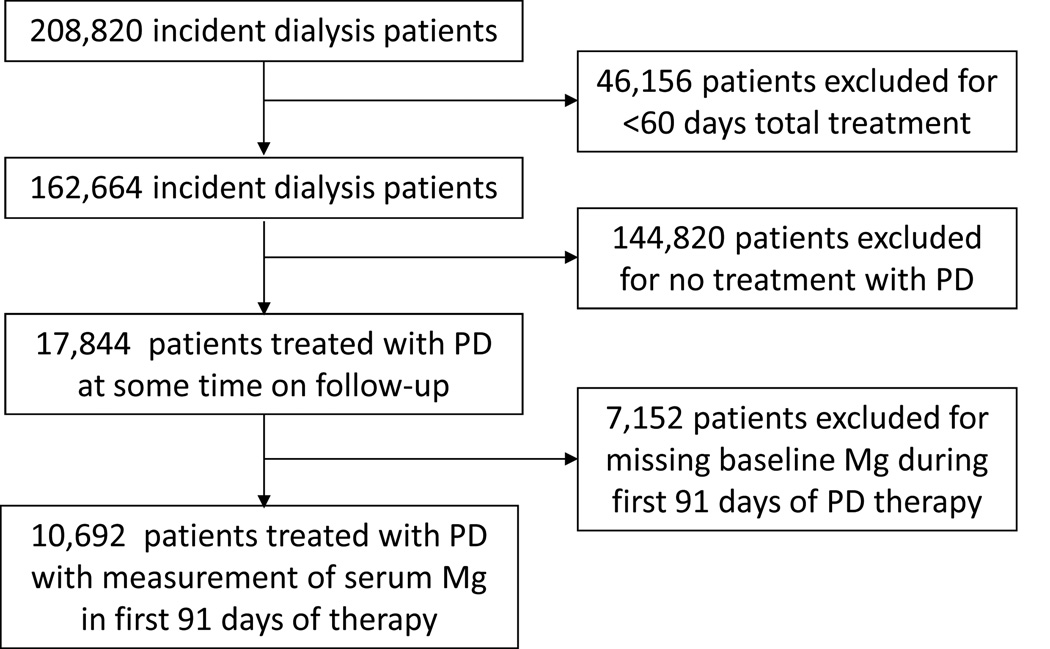
A total of 208,820 patients with ESRD who initiated dialysis therapy in participating facilities were identified. Of these, 17,844 patients received PD for at least 60 days (Figure 1). The study cohort comprised 10,692 patients who had serum magnesium measured during the first 91-days of PD (Figure 1). Characteristics of patients included in the cohort compared with those excluded are reported in Table S1 (provided as online supplementary material).
Figure 1. Flow chart summarizing how the cohort was assembled.
Abbreviations: PD, peritoneal dialysis; Mg, magnesium.
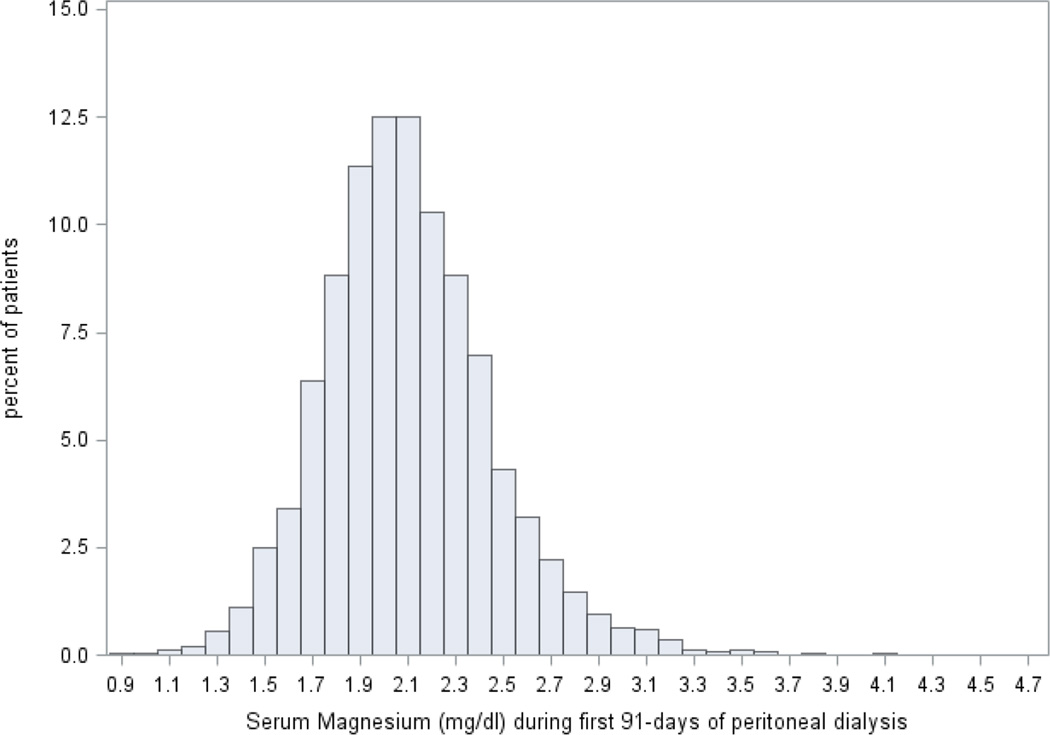
Mean serum magnesium level of the cohort was 2.1 ± 0.4 (standard deviation) mg/dL (median, 2.1 [IQR, 1.9–2.3] mg/dL; Figure 2). Baseline clinical characteristics of these patients, stratified by category of baseline serum magnesium, are presented in Table 1. Of the cohort, 1928 (18%) had hypomagnesemia (serum magnesium < 1.8 mg/dl) and these patients were older and more likely to be Black and had a longer interval from initiation of dialysis to treatment with PD. Additionally, patients with hypomagnesemia had lower hemoglobin, serum albumin, creatinine, urea nitrogen, potassium, uncorrected calcium, phosphorus, total iron-binding capacity, and higher ferritin, parathyroid hormone, and alkaline phosphatase levels. Patients with lower serum magnesium levels also were more likely to have higher 4-hour dialysate–plasma creatinine.
Figure 2. The distribution of average serum Mg over the first 3 months of peritoneal dialysis (n=10,692).
The data reflect the averaged measurements made during the first 3 months of PD treatment.
Table 1.
Selected characteristics of patients according to serum magnesium level
| Characteristics | Total (N=10,692) |
< 1.8 mg/dL (n=1928) |
1.8– < 2.0 mg/dL (n=2204) |
2.0 – < 2.2 mg/dL (n=2765) |
2.2 – < 2.4 mg/dL (n=1765) |
≥ 2.4 mg/dL (n=2030) |
|---|---|---|---|---|---|---|
| Serum Magnesium, mg/dl | 2.1 ± 0.4 | 1.6 ± 0.2 | 1.9 ± 0.1 | 2.1 ± 0.1 | 2.3 ± 0.1 | 2.6 ± 0.3 |
| Age (y) | 56 ± 16 | 58 ± 15 | 57 ± 15 | 56 ± 16 | 55 ± 16 | 54 ± 16 |
| Age ≥65 y | 3380 (32) | 723 (38) | 717 (33) | 890 (32) | 512 (29) | 538 (27) |
| Female sex* | 4772(45) | 876 (45) | 966 (44) | 1205 (44) | 793 (45) | 932 (46) |
| Race /Ethnicity | ||||||
| Whites | 6275 (59) | 1099 (57) | 1285 (59) | 1623 (59) | 1046 (59) | 1222 (60) |
| Blacks | 2461 (23) | 572 (30) | 572 (26) | 598 (22) | 361 (20) | 358 (18) |
| Hispanics | 1199 (11) | 155 (8) | 205 (9) | 334 (12) | 225 (13) | 280 (14) |
| Asian | 397 (4) | 39 (2) | 76 (3) | 110 (4) | 70 (4) | 102 (5) |
| Others | 332 (3) | 59 (3) | 56 (3) | 90 (3) | 60 (3) | 67 (3) |
| Primary insurance | ||||||
| Medicare | 4924 (46) | 950 (49) | 1010 (46) | 1280 (46) | 816 (46) | 868 (43) |
| Medicaid | 488 (5) | 77 (4) | 95 (4) | 122 (4) | 69 (4) | 125 (6) |
| Other | 5280 (49) | 901 (47) | 1099 (50) | 1363 (49) | 880 (50) | 1037 (51) |
| Cause of ESRD* | ||||||
| Diabetes | 4299 (40) | 812 (42) | 897 (41) | 1101 (40) | 679 (38) | 810 (40) |
| Hypertension | 2834 (27) | 505 (26) | 581 (26) | 750 (27) | 452 (26) | 546 (27) |
| Glomerular disease | 1703 (16) | 274 (14) | 348 (16) | 445 (16) | 302 (17) | 334 (16) |
| Other | 1856 (17) | 337 (18) | 378 (17) | 469 (17) | 332 (19) | 340 (17) |
| Comorbidities | ||||||
| Diabetes | 6778 (63) | 1281 (66) | 1405 (64) | 1726 (62) | 1095 (62) | 1271 (63) |
| Hypertension* | 5722 (54) | 1017 (53) | 1179 (53) | 1488 (54) | 923 (52) | 1115 (55) |
| Congestive Heart Failure* | 2091 (20) | 383 (20) | 416 (19) | 515 (19) | 336 (19) | 441 (22) |
| Atherosclerotic Heart Disease | 1854 (17) | 312 (16) | 341 (15) | 486 (18) | 310 (18) | 405 (20) |
| Other Cardiovascular Diseases* | 1637 (15) | 302 (16) | 329 (15) | 429 (16) | 247 (14) | 330 (16) |
| Dyslipidemia | 5146 (48) | 865 (45) | 1093 (50) | 1335 (48) | 840 (48) | 1013 (50) |
| Interval from dialysis initiation to PD initiation (d) |
28 [9–142] | 33 [12–172] | 27 [10–137] | 26 [9–130] | 24 [9–125] | 29 [8–149] |
| Ever treated with hemodialysis | 4392 (41) | 804 (42) | 861 (39) | 1109 (40) | 709 (40) | 909 (45) |
| History of previous transplant* | 272 (3) | 60 (3) | 57 (3) | 68 (2) | 45 (3) | 42 (2) |
| Geographic Location | ||||||
| Northeast | 1510 (14) | 249 (13) | 304 (14) | 398 (14) | 251 (14) | 308 (15) |
| Midwest | 2206 (21) | 431 (22) | 462 (21) | 560 (20) | 362 (21) | 391 (19) |
| South | 4302 (40) | 878 (46) | 930 (42) | 1089 (40) | 669 (38) | 736 (36) |
| West | 2646 (25) | 364 (19) | 505 (23) | 710 (26) | 478 (27) | 589 (29) |
| Serum albumin (g/dl) | 3.6 ± 0.5 | 3.4 ± 0.5 | 3.5 ± 0.5 | 3.6 ± 0.4 | 3.7 ± 0.4 | 3.7 ± 0.5 |
| Albumin <3.5 g/dL | 3913 (36) | 1001 (52) | 912 (41) | 976 (35) | 518 (29) | 506 (25) |
| Serum creatinine (mg/dl) | 6.9 ± 3.4 | 6.1 ± 3.0 | 6.4 ± 3.2 | 6.7 ± 3.2 | 7.2 ± 3.4 | 8.3 ± 4.0 |
| Serum urea nitrogen (mg/dl) | 50 ± 16 | 45 ± 15 | 47 ± 15 | 49 ± 15 | 52 ± 15 | 59 ± 17 |
| Blood hemoglobin (g/dl) | 11.7 ± 1.3 | 11.3 ± 1.3 | 11.6 ± 1.3 | 11.8 ± 1.3 | 11.8 ± 1.3 | 11.8 ± 1.4 |
| Hemoglobin <10 g/dL | 990 (9) | 269 (14) | 226 (10) | 201 (7) | 128 (7) | 166 (8) |
| Serum ferritin (ng/ml) | 307 [152–559] | 385 [198–664] | 320 [165–591] | 298 [146–534] | 271 [138–506] | 290 [131–509] |
| Serum TIBC (mg/dl) | 246 ± 47 | 233 ± 48 | 244 ± 48 | 249 ± 45 | 250 ± 44 | 255 ± 47 |
| Iron Saturation, % | 31 ± 12 | 31 ± 13 | 30 ± 12 | 30 ± 12 | 30 ± 11 | 32 ± 14 |
| Serum calcium^(mg/dl) | 8.8 ± 0.7 | 8.4 ± 0.7 | 8.7 ± 0.6 | 8.8 ± 0.6 | 8.9 ± 0.6 | 8.9 ± 0.6 |
| Serum phosphorus (mg/dl) | 4.9 ± 1.3 | 4.4 ± 1.1 | 4.7 ± 1.1 | 4.8 ± 1.2 | 5.1 ± 1.2 | 5.6 ± 1.4 |
| Serum PTH (pg/ml) | 302 [189–479] | 325 [212–510] | 310 [188–489] | 291 [182–460] | 290 [185–469] | 294 [182–478] |
| PTH <150 pg/mL | 1753 (17) | 242 (13) | 383 (18) | 489 (18) | 281 (16) | 358 (18) |
| Serum alkaline phosphatase (U/L) | 82 [65–107] | 86 [67–113] | 83 [65–106] | 81 [64–105] | 81 [65–104] | 81 [65–105] |
| Serum potassium, mEq/L | 4.1 ± 0.6 | 3.9 ± 0.5 | 4.1 ± 0.5 | 4.1 ± 0.5 | 4.2 ± 0.5 | 4.3 ± 0.6 |
| Serum sodium, mEq/L | 139 ± 3 | 139 ± 3 | 139 ± 3 | 139 ± 3 | 139 ± 3 | 139 ± 3 |
| Bicarbonate(mEq/L) | 25 ± 3 | 26 ± 3 | 26 ± 3 | 26 ± 3 | 25 ± 3 | 25 ± 3 |
| Weekly total Kt/V | 2.5 ± 0.7 | 2.5 ± 0.7 | 2.6 ± 0.7 | 2.5 ± 0.7 | 2.5 ± 0.7 | 2.3 ± 0.6 |
| Peritoneal Kt/V | 1.5 ± 0.4 | 1.5 ± 0.4 | 1.5 ± 0.4 | 1.5 ± 0.4 | 1.5 ± 0.4 | 1.5 ± 0.4 |
| Residual kidney function (L/wk/ 1.73 m2) | 56 [27–92] | 57 [29–94] | 62 [32–100] | 61 [30–98] | 55 [27–89] | 42 [17–73] |
| 4-hour D/P creatinine ratio | 0.65 ± 0.12 | 0.67 ± 0.12 | 0.67 ± 0.12 | 0.65 ± 0.12 | 0.65 ± 0.13 | 0.63 ± 0.12 |
| Use of automated PD | ||||||
| Within first 91-days | 5142 (48) | 880 (46) | 1025 (47) | 1310 (47) | 851 (48) | 1076 (53) |
| At any time in follow-up* | 8542 (80) | 1502 (78) | 1757 (80) | 2218 (80) | 1421 (81) | 1644 (81) |
| Year of PD incidence | ||||||
| 2007 | 2098 (20) | 215 (11) | 316 (14) | 468 (17) | 427 (24) | 672 (33) |
| 2008 | 2170 (20) | 338 (18) | 417 (19) | 606 (22) | 372 (21) | 437 (21) |
| 2009 | 2326 (22) | 468 (24) | 535 (24) | 632 (23) | 351 (20) | 340 (17) |
| 2010 | 2442 (23) | 527 (27) | 564 (26) | 623 (22) | 367 (21) | 361 (18) |
| 2011 | 1656 (15) | 380 (20) | 372 (17) | 436 (16) | 248 (14) | 220 (11) |
p-value for differences between groups > 0.05
Uncorrected
Note: Unless otherwise indicated, values for categorical variables are given as number (percentage); values for continuous variables, as mean ± standard deviation or median [interquartile range]. Conversion factors for units: calcium in mg/dL to mmol/L, ×0.2495; creatinine in mg/dL to µmol/L, ×88.4; serum urea nitrogen in mg/dL to mmol/L, ×0.357.
Abbreviations and definitions: TIBC, total iron-binding capacity; PTH, parathyroid hormone; iPTH, intact parathyroid hormone; D/P creatinine ratio, ratio of creatinine concentration in the dialysate vs in plasma; Residual kidney function, mean of 24-hour urinary urea and creatinine clearance
Association of Serum Magnesium Level With Hospitalization
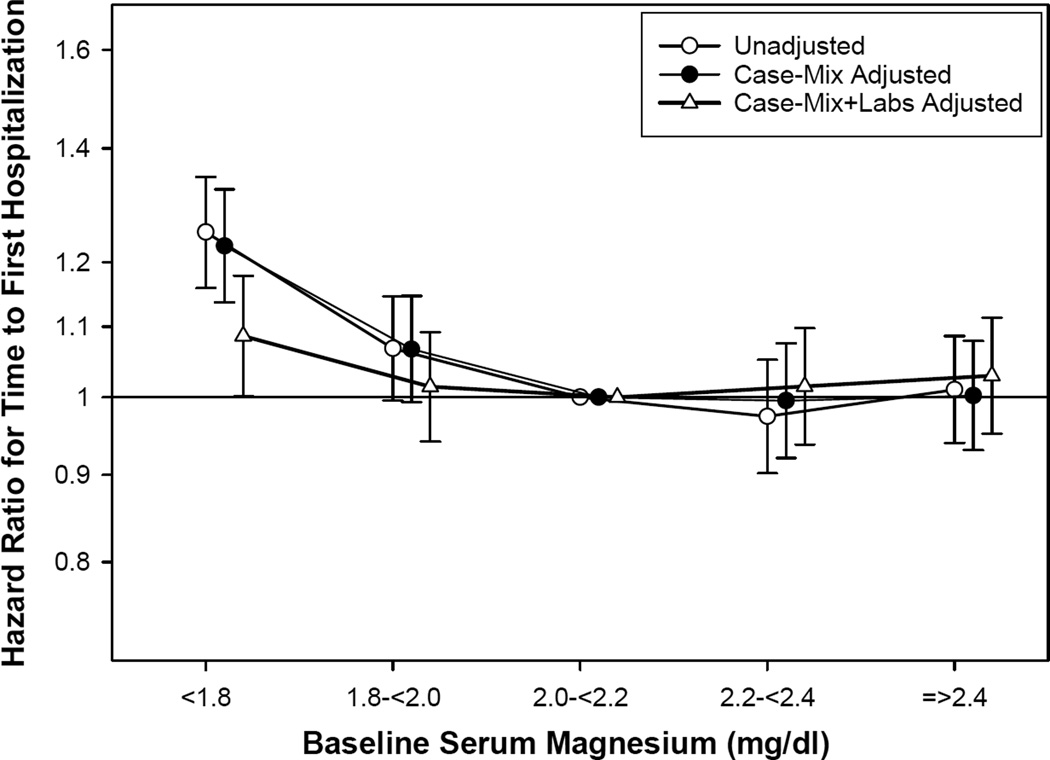
Of the 10,692 patients, 6465 (60%) were hospitalized at least once during the follow-up period. As shown in Table 2 and Figure 3, baseline magnesium level <1.8 mg/dL was associated with a significantly higher risk for hospitalization in unadjusted, case-mix adjusted, and case-mix and laboratory data-adjusted models (reference, magnesium 2.0–<2.2 mg/dl). Using baseline serum magnesium as a continuous variable (per 0.2-mg/dl decrease; Table S2) or dividing the cohort into two groups (<1.8 vs. ≥1.8 mg/dL), similar trends were identified.
Table 2.
Association of categories of baseline serum magnesium with hospitalization and all-cause mortality in incident PD patients (n=10,692)
| Hospitalization | All-Cause Mortality | |||||
|---|---|---|---|---|---|---|
| Serum Magnesium Category |
Unadjusted | Case-Mix Adjusteda | Case-Mix + Laboratory Adjustedb |
Unadjusted | Case-Mix Adjusteda |
Case-Mix + Laboratory Adjustedb |
| < 1.8 mg/dL | 1.25 (1.16, 1.35); < 0.001 |
1.23 (1.14, 1.33); < 0.001 |
1.09 (1.00, 1.18); 0.05 | 1.28 (1.09, 1.50); 0.003 |
1.21 (1.03, 1.42); 0.02 |
0.97 (0.81, 1.15); 0.7 |
| 1.8–<2.0 mg/dL | 1.07 (1.00, 1.15); 0.07 | 1.07 (0.99, 1.15); 0.08 |
1.01 (0.94, 1.09); 0.7 | 1.01 (0.86, 1.18); 0.9 | 0.99 (0.85, 1.16); 0.9 | 0.91 (0.77, 1.06); 0.2 |
| 2.0–<2.2 mg/dL | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2.2–<2.4 mg/dL | 0.97 (0.90, 1.05); 0.5 | 0.99 (0.92, 1.07); 0.9 | 1.02 (0.94, 1.10); 0.7 | 0.93 (0.79, 1.09); 0.4 | 0.99 (0.84, 1.17); 0.9 | 1.03 (0.87, 1.22); 0.7 |
| ≥ 2.4 mg/dL | 1.01 (0.94, 1.09); 0.8 | 1.00 (0.93, 1.08); 0.9 | 1.03 (0.95, 1.11); 0.5 | 0.89 (0.76, 1.05); 0.2 | 0.93 (0.80, 1.09); 0.4 | 0.96 (0.81, 1.13); 0.6 |
Note: Associations are given as adjusted hazard ratio (95% confidence interval); P.
PD, peritoneal dialysis
Data adjusted for age, gender, diabetes, race/ethnicity, primary insurance, geographic location, year of Incidence, ESRD reason, prior transplant, comorbid conditions(hypertension, congestive heart failure, atherosclerotic heart disease, other cardiovascular diseases), and interval from initiation of dialysis to initiation of PD
Data adjusted for the demographic characteristics above plus hemoglobin, serum albumin, uncorrected calcium, phosphorus, intact parathyroid hormone, ferritin, iron saturation, bicarbonate, potassium, total weekly Kt/V urea, residual kidney function, D/P creatinine, use of automated PD
Figure 3. Hazard ratios for hospitalization by categories of baseline serum magnesium (reference: serum magnesium, 2.0 – < 2.2 mg/dl).
Competing risks regression models with 3 levels of adjustments:(1) unadjusted; (2) case-mix adjusted for age, gender, diabetes, race/ethnicity, primary insurance, geographic location, year of incidence, cause of end-stage renal disease, prior kidney transplant, comorbid conditions (hypertension, congestive heart failure, atherosclerotic heart disease, other cardiovascular diseases), and interval from initiation of dialysis to initiation of peritoneal dialysis; and (3) case-mix and laboratory data (hemoglobin, albumin, uncorrected calcium, phosphorus, intact parathyroid hormone, ferritin, iron saturation, bicarbonate, potassium, total weekly Kt/V urea, residual kidney function, D/P creatinine, use of automated PD).
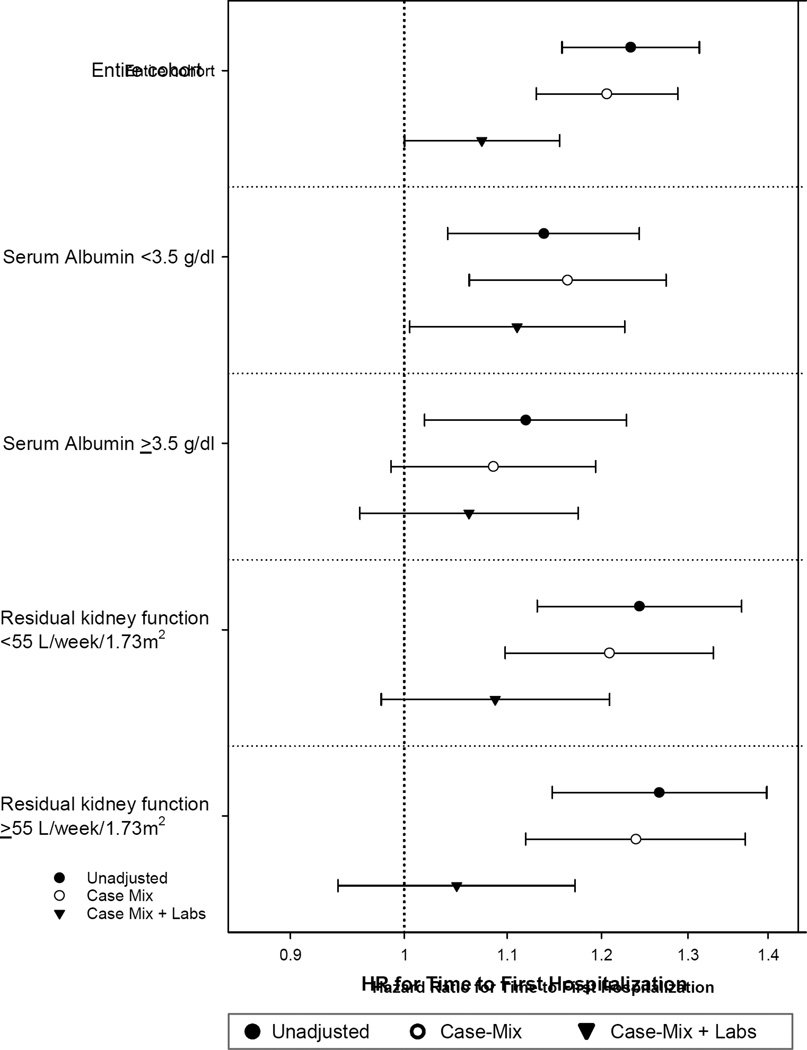
There was a significant effect modification for the association of serum magnesium with time to first hospitalization by serum albumin and residual kidney function (p for interaction terms in adjusted models, <0.001 and 0.005, respectively); there was no significant effect modification with serum parathyroid hormone or phosphorus. The risk for hospitalization with lower serum magnesium levels was higher in patients with serum albumin < 3.5 g/dl or with residual kidney function < 55 L/week/1.73 m2 (Table S3 and Figure 4).
Figure 4. Association of serum magnesium with time to first hospitalization stratified by serum albumin and residual kidney function among patients treated with peritoneal dialysis.
The lines represent hazards ratio for hospitalization for patients with serum magnesium level < 1.8 mg/dl (reference: ≥ 1.8 mg/dl) in the study population stratified by serum albumin (< and ≥ 3.5 g/dl) and residual kidney function (< and ≥ 55 L/week/1.73 m2). Competing risks regression models with 3 levels of adjustments:(1) unadjusted; (2) case-mix adjusted for adjusted for age, gender, diabetes, race/ethnicity, primary insurance, geographic location, year of incidence, cause of end-stage renal disease, prior kidney transplant, comorbid conditions (hypertension, congestive heart failure, atherosclerotic heart disease, other cardiovascular diseases), and interval from initiation of dialysis to initiation of peritoneal dialysis; and (3) case-mix and laboratory data adjusted for all covariates above plus hemoglobin, uncorrected calcium, phosphorus, intact parathyroid hormone, ferritin, iron saturation, bicarbonate, potassium, total weekly Kt/V urea, D/P creatinine, and use of automated PD. The covariate used to stratify the population was not used for additional adjustment – residual kidney function was an additional covariate in analyses stratified by serum albumin and serum albumin in analyses stratified by residual kidney function.
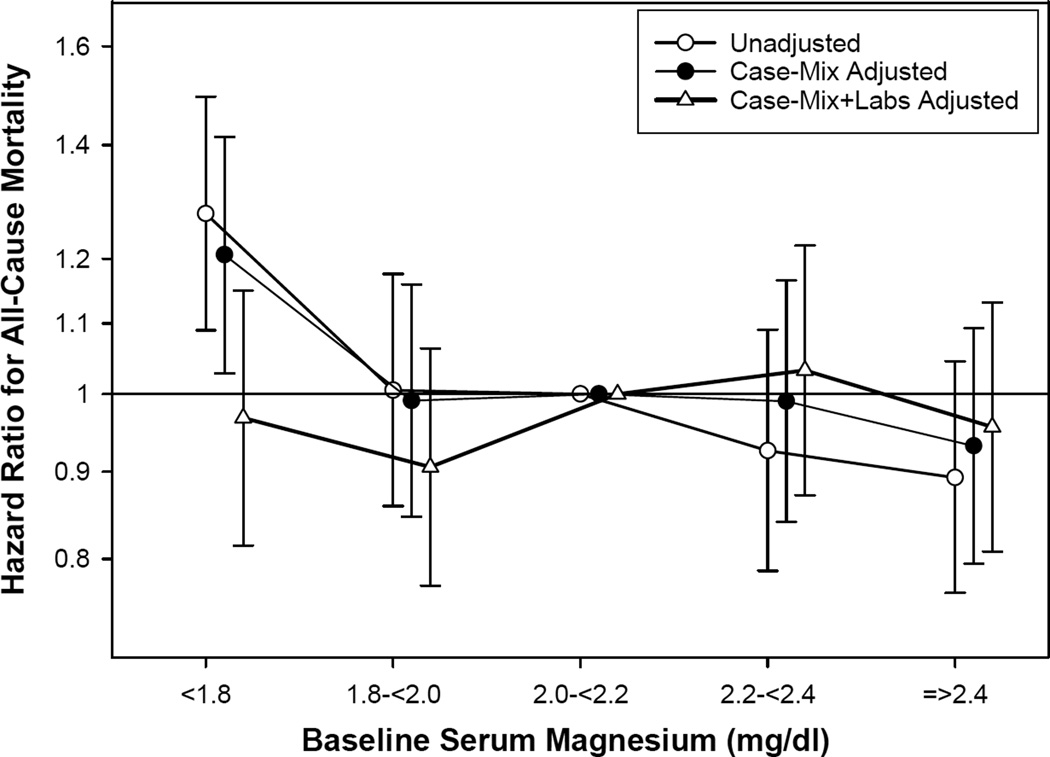
Association of Serum Magnesium Level With All-Cause Mortality
During follow-up, 1392 (13%) patients died (mortality rate, 93 [95% confidence interval, 89–98] per 1000 patient-years). Individuals with serum magnesium < 1.8 mg/dl had a significantly higher risk for death in unadjusted models and after adjustment for differences in case-mix, compared to patients with magnesium levels of 2.0–< 2.2 mg/dL (Table 2 and Figure 5). However, the association was attenuated to a non-significant level upon further adjustment for laboratory variables. Using serum magnesium as a continuous variable (per 0.2-mg/dl decrease; Table S2), or dividing the cohort into two groups (< 1.8 vs ≥ 1.8 mg/dL), the same trends were observed.
Figure 5. Hazard ratios for all-cause mortality by categories of baseline serum magnesium (reference: serum magnesium, 2.0 – < 2.2 mg/dl).
Competing risks regression models with 3 levels of adjustments:(1) unadjusted; (2) case-mix adjusted for adjusted for age, gender, diabetes, race/ethnicity, primary insurance, geographic location, year of incidence, cause of end-stage renal disease, prior kidney transplant, comorbid conditions (hypertension, congestive heart failure, atherosclerotic heart disease, other cardiovascular diseases), and interval from initiation of dialysis to initiation of peritoneal dialysis; and (3) case-mix and laboratory data adjusted for all covariates above plus hemoglobin, albumin, uncorrected calcium, phosphorus, intact parathyroid hormone, ferritin, iron saturation, bicarbonate, potassium, total weekly Kt/V urea, residual kidney function, D/P creatinine, use of automated PD.
There was significant effect modification of the association of serum magnesium with all-cause mortality by serum albumin and parathyroid hormone levels (p for interaction term <0.001, and 0.007, respectively); there was no significant effect modification by residual kidney function, or serum phosphorus. However, none of the associations in sub-group analyses reached statistical significance (Table S3).
DISCUSSION
To our knowledge, this is the first report from a large nationally representative cohort of patients undergoing PD of the association between serum magnesium levels and hospitalization and all-cause mortality. We demonstrate that baseline serum magnesium <1.8 mg/dL is associated with a significantly higher risk for hospitalization, particularly in individuals with serum albumin < 3.5 g/dl. The association of low serum magnesium levels with all-cause mortality was attenuated to a non-significant level after adjustment for laboratory variables.
Hospitalizations are an important contributor to patient morbidity and reflect the high burden of disease in patients undergoing dialysis. It also results in significant societal and financial burden. In the United States, there has been a gradual decline in hospitalizations for patients undergoing maintenance dialysis (12). In 2013, patients undergoing PD had an adjusted rate of hospitalization of 1.7 per patient-year as compared to 2.0 per patient-year in 2005, a decline of 15% (12). Identifying modifiable risk factors for hospitalizations is important to develop and test potentially effective interventions. To our knowledge, no study thus far has examined the association of serum magnesium with hospitalization in patients with kidney disease. Our study suggests that serum magnesium levels < 1.8 mg/dL is one such factor, as the association remained statistically significant despite adjustment for potential confounders. Hypomagnesemia may be associated with higher rate of hospitalization by several mechanistic pathways. First, cardiovascular disease is a common cause of hospitalization in PD patients, with fluid overload and metabolic abnormalities as important contributors (12–14). Hypomagnesemia is a recognized risk for cardiac rhythm disturbances and is associated with a higher risk for cardiovascular events (3). There is also growing evidence supporting an association between lower serum magnesium levels and dyslipidemia, metabolic syndrome, endothelial dysfunction, atherosclerosis, and vascular calcification, all of which could potentially be mechanisms to explain the observations in our study (15–19). Second, infection is an ongoing threat to the successful execution of PD. Limited evidence suggests that low serum magnesium levels in PD patients are associated with poorer nutritional status, deteriorating cellular health, and increased inflammation (10, 20). In the present study, lower serum magnesium levels were associated with lower serum urea nitrogen, albumin, phosphorus, and potassium, which are consistent with a higher prevalence of protein-energy wasting, an important risk factor for infectious complications in PD patients (21, 22). However, none of these mechanistic pathways were evaluated in this study and thus, remain speculative.
Several previous studies have shown an association between low serum magnesium levels and higher all-cause and cardiovascular mortality and sudden cardiac death in patients undergoing maintenance hemodialysis (6, 7, 23, 24). In contrast, the data for patients undergoing PD is substantially more limited. To our knowledge, only one previous single-center study has examined the association of serum magnesium with all-cause mortality. It demonstrated a significantly higher risk for death in patients with serum magnesium < 1.6 mg/dl despite adjustment for age, race, sex, diabetes, and dialysis vintage (25). Consistent with this prior study, patients with serum magnesium < 1.8 mg/dl had a significantly higher risk for death in models adjusted for differences in demographics and case-mix. However, the higher risk was attenuated to a non-significant level upon adjustment for laboratory variables. This finding of the association of low serum magnesium level with all-cause mortality attenuated to non-significant levels with adjustment for laboratory variables is consistent with the two recent studies from the United States of patients undergoing maintenance hemodialysis (6, 7). This raises the possibility that low serum magnesium may be in the causal pathway of the higher risk of death observed with some laboratory parameters and needs to be examined in future studies.
In the present study, not surprisingly, serum albumin level was an important effect modifier for the association of low serum magnesium with hospitalization and all-cause mortality. Serum albumin acts as a transport protein for numerous substances, including magnesium, calcium, and zinc (26). Approximately 30% of serum magnesium is bound to protein, primarily albumin, and thus total measured magnesium levels may be affected by hypoalbuminemia. Furthermore, hypoalbuminemia will decrease the Gibbs-Donnan effect and thus tend to increase diffusion of magnesium into dialysate (3). In our prior study of patients undergoing maintenance hemodialysis, the greatest risk of all-cause mortality with low serum magnesium levels was in patients with low serum albumin level (6). In the present study of patients undergoing PD, the highest risk for hospitalization with low magnesium levels was seen in patients with low serum albumin levels.
Strengths of our study include a large contemporary and nationally representative cohort of patients undergoing PD with follow-up of up to five years. However, there are several limitations that should be considered when interpreting our findings. First, a significant proportion of patients were excluded due to lack of serum magnesium measurements. However, it is reassuring to note that there was no clinically meaningful difference in characteristics of patients included in the study to those who were excluded (Table S1). Furthermore, repeating the analyses after imputing the exposure in those with missing values yielded the same results. Second, because there was no information for specific cause of hospitalization or death in this cohort, we could not investigate the association between serum magnesium level and non-fatal or fatal cardiovascular events. Third, given the observational nature of the study, we cannot be certain that the associations described in this report are causal. Even though there was adjustment for a large number of covariates, the risk of residual confounding remains. Finally, the current study only provided associations with baseline serum magnesium levels. A study examining the trends of serum magnesium over time in patients undergoing hemodialysis has shown a small decline over time (Δ −0.011 [95% confidence interval, −0.017 to −0.009] mmol/L/year) (24). Our ability to examine the effect of change in magnesium levels over time to outcomes was limited by the number of patients with repeat measurements and should be examined in the future.
In conclusion, to our knowledge, this is the first study to examine the associations between serum magnesium levels and hospitalization and all-cause mortality in a large nationally representative cohort of patients undergoing PD. We observed that lower serum magnesium level is associated with a higher risk for hospitalization, particularly in patients with hypoalbuminemia. Future studies should confirm these findings, and focus on the mechanisms underlying the association of hypomagnesemia with poor outcomes and the potential benefit with magnesium supplementation on clinical events and survival among hypomagnesemic PD patients.
Supplementary Material
Acknowledgments
We thank DaVita Clinical Research for providing the clinical data for this study.
Support: This work was supported by grants from the National Institutes of Health: R01DK95668 (Drs Mehrotra and Kalantar-Zadeh), Dr Yang was supported by Natural Science Foundation of China (grant 81570614) and the grant from the First Affiliated Hospital of Sun Yat-sen University, China. Dr Obi has been supported by the Shinya Foundation for International Exchange of Osaka University Graduate School of Medicine Grant, Japan. The funders of this study had no role in study design, collection, analysis, and interpretation of data, writing the report, and the decision to submit the report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Research area and study design: XY, MBR, KK-Z, RM; data acquisition: MS, ES; data analysis/interpretation: XY, MS, ES, MBR, YO, SVA, KK-Z, RM; statistical analysis: MS, ES; supervision or mentorship: XY, MBR, YO, SVA, KK-Z, RM. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. RM takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Supplementary Material
Table S1: Comparison of baseline characteristics of patients included in final cohort vs those excluded.
Table S2: Association of serum Mg as continuous variable with hospitalization and all-cause mortality in incident PD patients.
Table S3: Association of serum Mg < 1.8 mg/dl with time to first hospitalization and mortality across prespecified subgroups.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Supplementary Material Descriptive Text for Online Delivery
Supplementary Table S1 (PDF). Comparison of baseline characteristics of patients included in final cohort vs those excluded.
Supplementary Table S2 (PDF). Association of serum Mg as continuous variable with hospitalization and all-cause mortality in incident PD patients.
Supplementary Table S3 (PDF). Association of serum Mg < 1.8 mg/dl with time to first hospitalization and mortality across prespecified subgroups.
REFERENCES
- 1.Ayuk J, Gittoes NJ. Contemporary view of the clinical relevance of magnesium homeostasis. Ann Clin Biochem. 2014;51:179–188. doi: 10.1177/0004563213517628. [DOI] [PubMed] [Google Scholar]
- 2.Lowenstein FW, Stanton MF. Serum magnesium levels in the United States, 1971–1974. Journal of the American College of Nutrition. 1986;5:399–414. doi: 10.1080/07315724.1986.10720143. [DOI] [PubMed] [Google Scholar]
- 3.Alhosaini M, Leehey DJ. Magnesium and Dialysis: The Neglected Cation. Am J Kidney Dis. 2015;66:523–531. doi: 10.1053/j.ajkd.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 4.Kanbay M, Yilmaz MI, Apetrii M, et al. Relationship between serum magnesium levels and cardiovascular events in chronic kidney disease patients. Am J Nephrol. 2012;36:228–237. doi: 10.1159/000341868. [DOI] [PubMed] [Google Scholar]
- 5.Van Laecke S, Nagler EV, Verbeke F, Van Biesen W, Vanholder R. Hypomagnesemia and the risk of death and GFR decline in chronic kidney disease. Am J Med. 2013;126:825–831. doi: 10.1016/j.amjmed.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 6.Li L, Streja E, Rhee CM, et al. Hypomagnesemia and Mortality in Incident Hemodialysis Patients. Am J KIdney Dis. 2015;66:1047–1055. doi: 10.1053/j.ajkd.2015.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lacson E, Jr, Wang W, Ma L, Passlick-Deetjen J. Serum Magnesium and Mortality in Hemodialysis Patients in the United States: A Cohort Study. Am J KIdney Dis. 2015;66:1056–1066. doi: 10.1053/j.ajkd.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Ejaz AA, McShane AP, Gandhi VC, Leehey DJ, Ing TS. Hypomagnesemia in continuous ambulatory peritoneal dialysis patients dialyzed with a low-magnesium peritoneal dialysis solution. Perit Dial Int. 1995;15:61–64. [PubMed] [Google Scholar]
- 9.Rippe B, Venturoli D, Simonsen O, de Arteaga J. Fluid and electrolyte transport across the peritoneal membrane during CAPD according to the three-pore model. Perit Dial Int. 2004;24:10–27. [PubMed] [Google Scholar]
- 10.Ye H, Zhang X, Guo Q, et al. Prevalence and factors associated with hypomagnesemia in Southern Chinese continuous ambulatory peritoneal dialysis patients. Perit Dial Int. 2013;33:450–454. doi: 10.3747/pdi.2012.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fine JP, Grey RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 12.U.S. Renal Data System. USRDS 2015 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2015. 2015. [Google Scholar]
- 13.Wang AY, Brimble KS, Brunier G, et al. ISPD Cardiovascular and Metabolic Guidelines in Adult Peritoneal Dialysis Patients Part II - Management of Various Cardiovascular Complications. Perit Dial Int. 2015;35:388–396. doi: 10.3747/pdi.2014.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang AY, Brimble KS, Brunier G, et al. ISPD Cardiovascular and Metabolic Guidelines in Adult Peritoneal Dialysis Patients Part I - Assessment and Management of Various Cardiovascular Risk Factors. Perit Dial Int. 2015;35:379–387. doi: 10.3747/pdi.2014.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Averbukh Z, Rosenberg R, Galperin E, et al. Cell-associated magnesium and QT dispersion in hemodialysis patients. Am J Kidney DIs. 2003;41:196–202. doi: 10.1053/ajkd.2003.50004. [DOI] [PubMed] [Google Scholar]
- 16.Maier JA, Malpuech-Brugere C, Zimowska W, Rayssiguier Y, Mazur A. Low magnesium promotes endothelial cell dysfunction: implications for atherosclerosis, inflammation and thrombosis. Biochimica et biophysica acta. 2004;1689:13–21. doi: 10.1016/j.bbadis.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 17.He K, Liu K, Daviglus ML, et al. Magnesium intake and incidence of metabolic syndrome among young adults. Circulation. 2006;113:1675–1682. doi: 10.1161/CIRCULATIONAHA.105.588327. [DOI] [PubMed] [Google Scholar]
- 18.Louvet L, Buchel J, Steppan S, Passlick-Deetjen J, Massy ZA. Magnesium prevents phosphate-induced calcification in human aortic vascular smooth muscle cells. Nephrol Dial Transplant. 2013;28:869–878. doi: 10.1093/ndt/gfs520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dey R, Rajappa M, Parameswaran S, Revathy G. Hypomagnesemia and atherogenic dyslipidemia in chronic kidney disease: surrogate markers for increased cardiovascular risk. Clin Exp Nephrol. 2015;19:1054–1061. doi: 10.1007/s10157-015-1097-z. [DOI] [PubMed] [Google Scholar]
- 20.Fein P, Suda V, Borawsky C, et al. Relationship of serum magnesium to body composition and inflammation in peritoneal dialysis patients. Adv Perit DIal. 2010;26:112–115. [PubMed] [Google Scholar]
- 21.Wang Q, Bernardini J, Piraino B, Fried L. Albumin at the start of peritoneal dialysis predicts the development of peritonitis. Am J Kidney Dis. 2003;41:664–669. doi: 10.1053/ajkd.2003.50128. [DOI] [PubMed] [Google Scholar]
- 22.Fan X, Huang R, Wang J, et al. Risk factors for the first episode of peritonitis in Southern Chinese continuous ambulatory peritoneal dialysis patients. PloS One. 2014;9:e107485. doi: 10.1371/journal.pone.0107485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakaguchi Y, Fujii N, Shoji T, Hayashi T, Rakugi H, Isaka Y. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int. 2014;85:174–181. doi: 10.1038/ki.2013.327. [DOI] [PubMed] [Google Scholar]
- 24.de Roij van Zuijdewijn CL, Grooteman MP, Bots ML, et al. Serum Magnesium and Sudden Death in European Hemodialysis Patients. PloS One. 2015;10:e0143104. doi: 10.1371/journal.pone.0143104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fein P, Weiss S, Ramos F, Singh P, Chattopadhyay J, Avram MM. Serum magnesium concentration is a significant predictor of mortality in peritoneal dialysis patients. Adv Perit Dial. 2014;30:90–93. [PubMed] [Google Scholar]
- 26.Doweiko JP, Nompleggi DJ. The role of albumin in human physiology and pathophysiology, Part III: Albumin and disease states. J Parenteral Enter Nutr. 1991;15:476–483. doi: 10.1177/0148607191015004476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.