ABSTRACT
We recently described that all three 6-phosphogluconate dehydrogenase (6PGDH) isoforms of Arabidopsis (PGD) with similar length show dual localization: PGD1 and PGD3 in the cytosol and in plastids, and PGD2 in the cytosol and in peroxisomes. We set out to investigate heterodimer formation, however due to only weak homodimerization of all Arabidopsis PGD isoforms in yeast cells, we conducted further protein-protein interaction studies in planta to investigate homomer versus heteromer formation and their sub-cellular localization. Bimolecular fluorescence complementation (BiFC) analyses in co-transfected Arabidopsis protoplasts demonstrated that all PGD isoforms may form homo- and heterodimers. Notably, with free N-terminal ends, PGD1-PGD3 heterodimers were detected both in the cytosol and in the plastid stroma, but heterodimers with PGD2 only in the cytosol. This independently confirmed that PGD2 cannot enter plastids. On the other hand, with free C-terminal ends, PGD1-PGD2 and PGD3-PGD2 heterodimers were confined to the cytosol, indicating that only PGD2 homodimers are imported by peroxisomes. Together these findings suggest functional redundancy of PGD1 and PGD3 inside plastids, and relevance of PGD1-PGD2 or PGD3-PGD2 heterodimer formation in the cytosol: this could retain sufficient 6PGDH activity needed for NADPH provision, especially during stress defense and initiation of developmental responses.
KEYWORDS: A. thaliana, dimerization, OPPP, PGD isoforms, sub-cellular targeting
Abbreviations
- BiFC
bimolecular fluorescence complementation
- CaMV
Cauliflower mosaic virus
- G6PDH/G6PD
glucose-6-phosphate dehydrogenase activity/isoform
- OPPP
oxidative pentose-phosphate pathway
- 6PGDH/PGD
6-phosphogluconate dehydrogenase activity/isoform
- 6PGL/PGL
6-phosphogluconolactonase activity/isoform
- Rboh
respiratory burst oxidase homologs
- roGFP
redox-sensitive green fluorescent protein
- ROS
reactive oxygen species
- Trx
thioredoxin
- YFP
yellow fluorescent protein
- Y2H
yeast-2 hybrid
In the night, the oxidative pentose-phosphate pathway (OPPP) operates in the plastid stroma of plant cells as formal reversion of the Calvin-Benson cycle.1 The first three steps, known as the oxidative part of the OPPP, have recently been shown to play important roles in the cytosol and also in peroxisomes.2,3 Mainly because the first and third OPPP steps provide NADPH, which is not only essential for anabolic biosynthesis, but also for protecting the different cellular compartments against oxidative stress.4 Besides, NADPH is the substrate of respiratory burst oxidase homologs (Rbohs) that are specifically activated during stress and developmental responses.5,6,7 Moreover, NADPH is also required for the biosynthesis of nitric oxide (NO) and of jasmonic acid (JA) in peroxisomes.8
During the past years we have demonstrated that certain plastid-annotated Arabidopsis OPPP isoforms may alternatively target peroxisomes.2,3 This re-direction of plastid-destined precursors (default localization) to peroxisomes (transient localization) occurs under conditions favoring changes in the NADPH/NADP ratio and the linked cytosolic glutathione pool. Adaptations in target enzymes are initiated by redox transmitters, like cognate thioredoxins (e.g. Trx m2).2,3 In case of the first OPPP step, catalyzed by glucose-6-phosphate dehydrogenase (G6PDH), the plastidial Arabidopsis isoform G6PD1 is retained in the cytosol through interaction with G6PD4 (another redox-responsive but catalytically inactive plastidial isoform), followed by folding and peroxisomal import of G6PD1 upon exposure of a cryptic C-terminal targeting signal.2 For the second OPPP step, catalyzed by 6-phosphogluconolactonase (6PGL), we could show that the plastidial isoform PGL31,9,10 may be re-directed from plastids to peroxisomes, here involving a redox-dependent chaperon switch of thioredoxin from holdase to foldase function in the cytosol.11,12 Notably, this thioredoxin co-chaperon switch is influenced by the trophic state and developmental program of the investigated tissue, i.e. soil-grown vs. tissue culture plants (raised on 1% sucrose), or leaves versus roots.3
In this work we investigated whether dual targeting of the three Arabidopsis 6-phosphogluconate dehydrogenase isoforms PGD1 (At1g64190), PGD2 (At3g02360), and PGD3 (At5g41670), that catalyze the last step of the oxidative part of the OPPP in different plant cell compartments,8 may serve a particular function. Especially we wondered (besides homodimer formation that is essential for catalytic activity),13 whether the dual cytosolic/plastidial isoforms PGD1 and PGD3 may form heterodimers with the dual cytosolic/peroxisomal isoform PGD2; and if this should be the case, what might be the possible benefit for the cell? Potential for heteromerization is implied by mutual, cyanobacterial origin of all plant 6PGDH enzymes,14 but to this end has only been found to occur among the cytosolic Pgd1 and Pgd2 enzymes of maize.15
First we studied Arabidopsis PGD homodimerization in yeast, essentially as described in Meyer et al.2 (for oligonucleotide primers, see Table 1). However, the yeast-2 hybrid-based approach was impeded by surprisingly weak growth of the analyzed binding domain (BD) - activation domain (AD) combinations, which was barely detectable above background (Fig. 1A; compare to the empty vector controls). This result indicated problems of either formation or stability of the different PGD homodimers in the yeast cell nucleus.
Table 1.
Additional oligonucleotide primers (to Hölscher et al.)8 used in this study.
| Oligonucleotide | Sequence | Vector |
|---|---|---|
| PGD1_NdeI_s | NNNCATATGATGGAGTCCGCCGCACTATC | pGBKT7, pGADT7 |
| PGD2_NdeI_s | NNNCATATGGCTGTTCAACCTACAAGAATAG | pGBKT7, pGADT7 |
| PGD3_NcoI_s | NNNCCATGGAGTCCGTCGCTCTATC | pGBKT7, pGADT7 |
| PGD2_BamHI_as | NNNGGATCCTCAGATCTTAGATTGTCTTGCAATC | pGBKT7, pGADT7 |
| PGD1_BamHI_as | NNNGGATCCTCAATGGTTCTTCCTGGCAA | pGBKT7, pGADT7 |
| pSPYNE(R), pSPYCE(MR) | ||
| PGD3_BamHI_as | NNNGGATCCTTACTGACTCTTCCTTGCAAG | pGBKT7, pGADT7 |
| pSPYNE(R), pSPYCE(MR) | ||
| PGD1_SpeI_s | NNACTAGTATGGAGTCCGCCGCACTATC | pSPYNE, pSPYCE(M), pSPYNE(R), pSPYCE(MR) |
| PGD3_SpeI_s | NNACTAGTATGGAGTCCGTCGCTCTATC | pSPYNE, pSPYCE(M), pSPYNE(R), pSPYCE(MR) |
| matPGD1/3_SpeI_s | NNACTAGTATGGGCCAAAACCTCGCC | pSPYNE, pSPYCE(M), pSPYNE(R), pSPYCE(MR) |
| PGD1(-STOP)_BamHI_as | NNNGGATCCATGGTTCTTCCTGGCAA | pSPYNE, pSPYCE(M) |
| PGD3(-STOP)_BamHI_as | NNNGGATCCCTGACTCTTCCTTGCAAG | pSPYNE, pSPYCE(M) |
Abbreviations: as, antisense; s, sense. Restrictions sites in oligonucleotides underlined, stop codons in bold.
Figure 1.
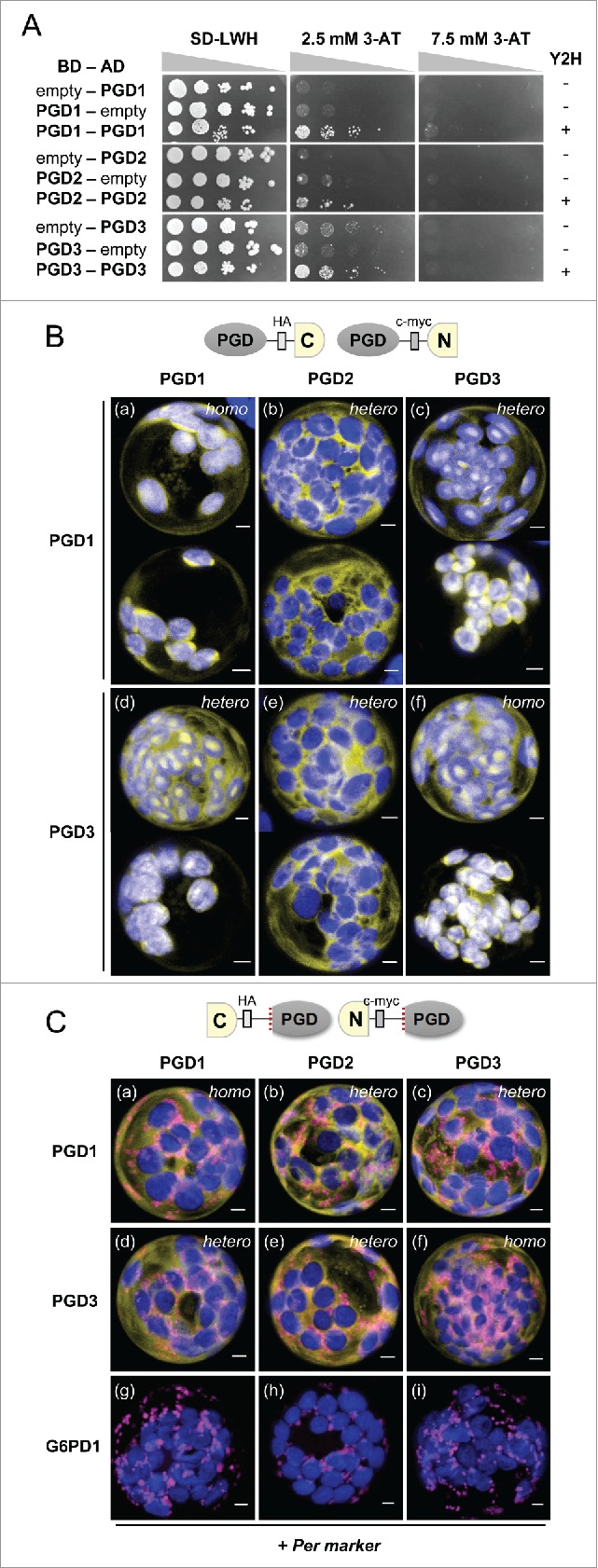
Extent of dimer formation among the 3 Arabidopsis PGD isoforms. A, Dimerization analyses of the indicated binding (BD) and activation domain (AD) construct combinations in yeast strain SMY3.7 With the empty vector, none of the PGD isoforms showed significant growth (Y2H, - signs) on selective drop-out medium (SD-LWH) in the presence of 3-Amino-1,2,4-triazole (3-AT, top; successive 1:10 dilutions are depicted as triangles). Weak growth of PGD1-PGD1, PGD2-PGD2, and PGD3-PGD3 combinations detected on 2.5 mM 3-AT plates indicates homo-dimerization (Y2H, + signs). B-C, Bimolecular fluorescence complementation (BiFC) analyses upon co-expression of the schematically indicated split YFP combinations (top, driven by the CaMV 35S promoter) in Arabidopsis protoplasts isolated from tissue-culture plants (grown on solidified half-strength Murashige & Skoog medium, pH 5.7 with 1% sucrose). PGD isoforms listed on the left were fused to the C-terminal half and those listed above the columns to the N-terminal half of split YFP. B, With free N-terminal ends, PGD1 and PGD3 formed homodimers (a and f) as well as heterodimers (c and d) in the cytosol and inside plastids, but with PGD2 only heterodimers in the cytosol (b and e). Two representative cells are shown for each combination; the images were recorded 24-48 h after transfection. C, With free C-terminal ends, all full-length split YFP-PGD combinations labeled the cytosol (data not shown). For the mature versions (that allow peroxisome import of PGD2)8 heterodimers were formed in the cytosol, but none of them co-localized with the peroxisome (Per) marker (PGD2-PGD1, panel b; PGD2-PGD3, panel e), by contrast to PGD2 homodimers8 used as positive control (not shown) or in combination with G6PD12 used as negative control (panels g-i). The images were recorded 48-72 h after transfection. Only merged channels are shown (maximal projections of approximately 35 single sections): reconstituted YFP signals in yellow, peroxisome marker (PGL3_short-OFP)2 in magenta, and chlorophyll auto-fluorescence in blue. Co-localization or very close signals (less than 200 nm) appear whitish. Homo, homo-dimers; hetero, hetero-dimers. Bars = 3µm.
To simultaneously monitor dual targeting of the PGD combinations, which is decided in the plant cell cytosol (∼75% reduced NADPH/NADP pool, according to roGFP - a redox-active reporter of the glutathione status),16 we focused on split YFP-based bimolecular fluorescence complementation (BiFC) of CaMV 35S promoter-driven constructs in Arabidopsis protoplasts, as done in our most recent publication.8 These protein-protein interaction studies were conducted with fusion constructs, leaving either the N-terminal or C-terminal ends of the interaction partners accessible, to enable plastidial or peroxisomal import, respectively.
With free N-terminus, the two plastidial isoforms PGD1 and PGD3 showed homo- (Fig. 1B; panels a and f) as well as hetero-dimerization (panels c and d) in both the cytosol and in the plastid stroma to various extents. By contrast, hetero-dimerization with the peroxisomal isoform PGD2 was confined to the cytosol (Fig. 1B; panels b and e), which independently confirmed that PGD2 does not enter plastids.7
To enable peroxisome targeting analyses of folded fusion proteins with free C-terminal ends,8 mature versions of split YFP-PGD1 and split YFP-PGD3 fusions had to be cloned (for oligonucleotide primers, see Table 1). By contrast to the corresponding full-length versions that all interacted in the cytosol (data not shown), the results obtained for the new mature PGD combinations shown in Fig. 1C demonstrate that neither PGD1-PGD2 nor PGD3-PGD2 heterodimers co-localize with the peroxisome (Per) marker (OFP-PGL3_C-short).2 This is an important result, since also mature PGD2-PGD2 homodimers only localized to peroxisomes when both PTS1 targeting signals (C-terminal SKI motifs) were freely accessible.8 This combination served as positive control (not shown) and co-expression with split YFPC-G6PD1 (showing peroxisomal localization only with G6PD4)2 as negative control (Fig. 1C, panels g–i).
Finally, we may draw the following conclusions: In case of the two dual cytosolic/plastidial Arabidopsis isoforms PGD1 and PGD3, heterodimerization seems to reflect functional redundancy, because the corresponding mRNA expression patterns largely overlap.8 By contrast, heterodimers of the dual cytosolic/plastidial isoforms PGD1 or PGD3 formed with PGD2 were not imported by peroxisomes. Thus, PGD2 heterodimers with PGD1 or PGD3 may serve the specific function of enzyme retention, needed to ensure flux through the cytosolic part of the OPPP. This becomes especially important during stress signaling, when the first OPPP enzyme, cytosolic G6PD6 (and possibly also G6PD5) is activated by phosphorylation via protein kinase ASKα (GSK3-type).6,7 This fast posttranslational activation of the oxidative OPPP part in the Arabidopsis cytosol was shown to occur under conditions of both abiotic and biotic stress, i.e., either induced by salt or drought,6 and recently also by pathogen attack.7 Similar mechanisms may as well lead to fast activation of cytosolic G6PDH enzymes during stress and developmental change in tobacco and tomato.5,17
We assume that due to the high degree of amino-acid conservation,8,14 the different PGD heterodimers should be active. But because dimerization does not necessarily reflect catalytic activity, as indicated by C-terminally truncated PGD versions in yeast,18 this needs to be clarified by future studies. In any case, our finding of extensive hetero-dimerization potential among the three Arabidopsis PGD isoforms calls for a specific surveillance mechanism in the cytosol, like for the first two OPPP steps.2,3 A similar redox-sensitive mechanism would respond to stress and/or developmental change, and thus contribute to fast coordinated adjustment of the NADPH/NADP redox state, in both the cytosol and in peroxisomes.
Disclosure of potential confllicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Anna Xenia Bestehorn for experimental support and Hannes Lansing for editorial contributions. This work was supported by the Deutsche Forschungsgemeinschaft (DFG grant SCHA 541/12-1 to A.v.S.).
References
- 1.Kruger NJ, von Schaewen A. The oxidative pentose phosphate pathway: structure and organisation. Curr Opin Plant Biol 2003; 6:236-46; PMID:12753973; http://dx.doi.org/ 10.1016/S1369-5266(03)00039-6 [DOI] [PubMed] [Google Scholar]
- 2.Meyer T, Hölscher C, Schwöppe C, von Schaewen A. Alternative targeting of Arabidopsis plastidic glucose-6-phosphate dehydrogenase G6PD1 involves cysteine-dependent interaction with G6PD4 in the cytosol. Plant J 2011; 66:745-58; PMID:21309870; http://dx.doi.org/ 10.1111/j.1365-313X.2011.04535.x [DOI] [PubMed] [Google Scholar]
- 3.Hölscher C, Meyer T, von Schaewen A. Dual-targeting of Arabidopsis 6-Phosphogluconolactonase 3 (PGL3) to chloroplasts and peroxisomes involves interaction with Trx m2 in the Cytosol. Mol Plant 2014; 7:252-5; PMID:24008768; http://dx.doi.org/ 10.1093/mp/sst126 [DOI] [PubMed] [Google Scholar]
- 4.Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 2005; 17:1866-75; PMID:15987996; http://dx.doi.org/ 10.1105/tpc.105.033589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scharte J, Schön H, Tjaden Z, Weis E, von Schaewen A. Isoenzyme replacement of glucose-6-phosphate dehydrogenase in the cytosol improves stress tolerance in plants. Proc Natl Acad Sci USA 2009; 106:8061-6; PMID:19416911; http://dx.doi.org/ 10.1073/pnas.0812902106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dal Santo S, Stampfl H, Krasensky J, Kempa S, Gibon Y, Petutschnig E, Rozhon W, Heuck A, Clausen T, Jonak C. Stress-induced GSK3 regulates the redox stress response by phosphorylating glucose-6-phosphate dehydrogenase in Arabidopsis. Plant Cell 2012; 24:3380-92; PMID:22885737; http://dx.doi.org/ 10.1105/tpc.112.101279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stampfl H, Fritz M, Dal Santo S, Jonak C. The GSK3/Shaggy-like kinase ASKα contributes to pattern-triggered immunity. Plant Physiol 2016; 171:1366-77; PMID:27208232; http://dx.doi.org/ 10.1104/pp.15.01741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hölscher C, Lutterbey MC, Lansing H, Meyer T, von Schaewen A. Defects in peroxisomal 6-phosphogluconate dehydrogenase isoform PGD2 prevent gametophytic interaction in Arabidopsis thaliana. Plant Physiol 2016; 171:192-205; PMID:26941195; http://dx.doi.org/ 10.1104/pp.15.01301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong Y, DeFraia C, Williams D, Zhang X, Mou Z. Characterization of Arabidopsis 6-phosphogluconolactonase T-DNA insertion mutants reveals an essential role for the oxidative section of the plastidic pentose phosphate pathway in plant growth and development. Plant Cell Physiol 2009; 50:1277-91; PMID:19457984; http://dx.doi.org/ 10.1093/pcp/pcp070 [DOI] [PubMed] [Google Scholar]
- 10.Bussell JD, Keech O, Fenske R, Smith SM. Requirement for the plastidial oxidative pentose phosphate pathway for nitrate assimilation in Arabidopsis. Plant J 2013; 75:578-91; PMID:23621281; http://dx.doi.org/ 10.1111/tpj.12222 [DOI] [PubMed] [Google Scholar]
- 11.Berndt C, Lillig CH, Holmgren A. Thioredoxins and glutaredoxins as facilitators of protein folding. Biochim Biophys Acta 2008; 1783:641-50; PMID:18331844; http://dx.doi.org/ 10.1016/j.bbamcr.2008.02.003 [DOI] [PubMed] [Google Scholar]
- 12.Sanz-Barrio R, Fernández-San Millán A, Carballeda J, Corral-Martínez P, Seguí-Simarro JM, Farran I. Chaperone-like properties of tobacco plastid thioredoxins f and m. J Exp Bot 2012; 63:365-79; PMID:21948853; http://dx.doi.org/ 10.1093/jxb/err282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams MJ, Ellis GH, Gover S, Naylor CE, Phillips C. Crystallographic study of coenzyme, coenzyme analogue and substrate binding in 6-phosphogluconate dehydrogenase: implications for NADP specificity and the enzyme mechanism. Structure 1994; 2:651-68; PMID:7922042; http://dx.doi.org/ 10.1016/S0969-2126(00)00066-6 [DOI] [PubMed] [Google Scholar]
- 14.Krepinsky K, Plaumann M, Martin W, Schnarrenberger C. Purification and cloning of chloroplast 6-phosphogluconate dehydrogenase from spinach. Cyanobacterial genes for chloroplast and cytosolic isoenzymes encoded in eukaryotic chromosomes. Eur J Biochem 2001; 268:2678-86; PMID:11322889; http://dx.doi.org/ 10.1046/j.1432-1327.2001.02154.x [DOI] [PubMed] [Google Scholar]
- 15.Bailey-Serres J, Nguyen MT. Purification and characterization of cytosolic 6-phosphogluconate dehydrogenase isozymes from maize. Plant Physiol 1992; 100:1580-3; PMID:16653162; http://dx.doi.org/ 10.1104/pp.100.3.1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bratt A, Rosenwasser S, Meyer A, Fluhr R. Organelle redox autonomy during environmental stress. Plant Cell Environ 2016; PMID:27037976; http://dx.doi.org/ 10.1111/pce.12746. [DOI] [PubMed] [Google Scholar]
- 17.Landi S, Nurcato R, De Lillo A, Lentini M, Grillo S, Esposito S. Glucose-6-phosphate dehydrogenase plays a central role in the response of tomato (Solanum lycopersicum) plants to short and long-term drought. Plant Physiol Biochem 2016; 105:79-89; PMID:27085599; http://dx.doi.org/ 10.1016/j.plaphy.2016.04.013 [DOI] [PubMed] [Google Scholar]
- 18.He W, Wang Y, Liu W, Zhou CZ. Crystal structure of Saccharomyces cerevisiae 6-phosphogluconate dehydrogenase Gnd1. BMC Struct Biol 2007; 7:38; PMID:17570834; http://dx.doi.org/ 10.1186/1472-6807-7-38 [DOI] [PMC free article] [PubMed] [Google Scholar]


