Abstract
Objectives
Overexpression of ATP-binding cassette (ABC) transporters is a frequent cause of multidrug resistance in cancer cells and pathogenic microorganisms. One example is the Cdr1p transporter from the human fungal pathogen Candida albicans that belongs to the pleiotropic drug resistance (PDR) subfamily of ABC transporters found in fungi and plants. Cdr1p is overexpressed in several azole-resistant clinical isolates, causing azole efflux and treatment failure. Cdr1p appears as a doublet band in western blot analyses, suggesting that the protein is post-translationally modified. We investigated whether Cdr1p is phosphorylated and the function of this modification.
Methods
Phosphorylated residues were identified by MS. Their function was investigated by site-directed mutagenesis and expression of the mutants in a C. albicans endogenous system that exploits a hyperactive allele of the Tac1p transcription factor to drive high levels of Cdr1p expression. Fluconazole resistance was measured by microtitre plate and spot assays and transport activity by Nile red accumulation.
Results
We identified a cluster of seven phosphorylated amino acids in the N-terminal extension (NTE) of Cdr1p. Mutating all seven sites to alanine dramatically diminished the ability of Cdr1p to confer fluconazole resistance and transport Nile red, without affecting Cdr1p localization. Conversely, a Cdr1p mutant in which the seven amino acids were replaced by glutamate was able to confer high levels of fluconazole resistance and to export Nile red.
Conclusions
Our results demonstrate that the NTE of Cdr1p is phosphorylated and that NTE phosphorylation plays a major role in regulating Cdr1p and possibly other PDR transporter function.
Introduction
Transporters of the ATP-binding cassette (ABC) superfamily are found in all living organisms, from microorganisms to human. They are located at the plasma membrane or the membranes of intracellular organelles and are involved in transporting various substrates, such as metabolic products, lipids, xenobiotics and chemotherapeutic drugs.1,2 They typically contain at least one nucleotide-binding domain (NBD) with highly conserved sequence motifs that participate in ATP binding and hydrolysis, providing the energy necessary to translocate their substrates across the channel formed by their transmembrane domains (TMDs). ABC transporters containing one set of TMDs and one NBD, with either a forward (TMD-NBD) or reverse (NBD-TMD) topological arrangement, are referred to as half transporters and require dimerization to be functional. Full transporters, on the other hand, correspond to the duplication of a half transporter unit spaced by a linker domain, which can also adopt a forward (TMD–NBD)2 or reverse (NBD–TMD)2 topology. The (TMD–NBD) and (TMD–NBD)2 topology is typical of many ABC transporters in humans, including the TAP1/2 antigenic peptide transporters, the multidrug transporter P-glycoprotein and the cystic fibrosis transmembrane conductance regulator CFTR.3,4 Transporters with the reverse NBD–TMD configuration are also found in humans and are exemplified by the multidrug transporter ABCG2 (also known as breast cancer resistance protein, BCRP).5 A standard nomenclature has been adopted for human and mouse ABC transporters, based on their topological arrangement (subfamily A-G) (http://www.genenames.org/genefamilies/ABC).
Fungi and plants possess a unique subfamily of ABC transporters, not found in humans, comprised of full transporters with an (NBD–TMD)2 configuration as seen in the pleiotropic drug resistance (PDR) subfamily.6,7 These transporters display unusual structural features such as non-canonical nucleotide binding sequences and a cytosolic N-terminal extension (NTE) of 100–200 amino acids that is rich in polar and charged amino acids and of still unknown function.6,8 The best-characterized member of this subfamily is Saccharomyces cerevisiae Pdr5p, which exports a wide spectrum of xenobiotics out of the cell and contributes to a PDR phenotype.9 Pdr5p has also been shown to transport phospholipids as well as structurally unrelated chemical compounds.10,11 Other members of this subfamily in S. cerevisiae, such as Snq2p, Pdr10p and Pdr15p, have common but also distinct substrate specificities and appear to be less active at exporting growth inhibitory compounds as compared with Pdr5p.12–14 These transporters are controlled by the Pdr1p and Pdr3p transcription factors and they are constitutively overexpressed in strains carrying hyperactive mutations in these regulators.10 Finally, it has been shown that Pdr5p homodimerizes15 and also physically interacts with other full transporters such as Snq2p, although the role of these interactions remains poorly understood.16,17
An important mechanism of clinical azole drug resistance in the human fungal pathogen Candida albicans is the selection of gain-of-function (gof) mutations in the Tac1p transcription factor, leading to the constitutive overexpression of the CDR1 and CDR2 genes encoding transporters of the PDR subfamily highly homologous to S. cerevisiae Pdr5p.18 We and others have shown that Cdr1p plays a more important role than Cdr2p in conferring clinical azole resistance, despite their high level of sequence homology.19,20 Research efforts have focused recently on identifying Cdr1p inhibitors, such as chemical modulators and peptide mimics, as a strategy to overcome clinical azole resistance.21 Overexpression of genes homologous to C. albicans CDR1 and CDR2 is also frequent in azole-resistant Candida species of medical importance such as Candida glabrata, Candida dubliniensis, Candida tropicalis and Candida parapsilosis, as well as in Aspergillus fumigatus.22,23 Given the apparently widespread role played by this family of transporters in clinical azole resistance, it is of paramount importance to obtain a detailed understanding of the molecular mechanisms regulating their function, as a prerequisite to the development of improved therapeutic strategies.
Phosphorylation has been shown to regulate ABC transporter function across subfamilies. For example, the serine/threonine kinase Pim1 phosphorylates mammalian P-glycoprotein, regulating its stability and cell surface expression.24 Pim1 can also phosphorylate ABCG2, a modification required for its dimerization as well as optimal transport activity.25 In fungi, phosphorylation of the linker domain of S. cerevisiae Ste6p, a full transporter homologous to P-glycoprotein, has been shown to control its ubiquitination and protein turnover.26,27 Other examples include Ycf1p, the CFTR homologue in yeast, in which phosphorylation of the linker domain enhances transporter activity28 whereas N-terminal phosphorylation diminishes its activity.7,29 ABC transporters of the PDR family are also regulated by phosphorylation.30–32 Phosphorylation of Pdr5p is important for its stability as deletion of genes encoding casein kinase I abolished Pdr5p phosphorylation and caused Pdr5p vacuolar degradation.30 Using a heterologous expression system in S. cerevisiae, it was shown that PKA-dependent phosphorylation of C. glabrata Pdh1p is important for drug export mediated by this transporter.31 In the case of C. glabrata Cdr1p, Akt-dependent phosphorylation was demonstrated by using an antibody that recognizes Akt phosphorylated substrate peptides. Mutation of the putative Akt recognition sites located in NBD1 of C. glabrata Cdr1p reduced its ATPase activity, suggesting that phosphorylation plays a direct role in modulating transporter function.32 Despite their important role in clinical azole resistance, nothing is known about the phosphorylation of C. albicans Cdr1p and Cdr2p. In the present study, we investigated whether C. albicans Cdr1p is phosphorylated and whether phosphorylation regulates its function.
Materials and methods
Strains, growth media and chemicals
The C. albicans strains used in this study are listed in Table 1. Strains were routinely grown at 30°C in YPD medium containing 1% yeast extract (BD Biosciences, Sparks, MD, USA), 2% Bacto Peptone (BD Biosciences) and 2% glucose (Sigma, St Louis, MO, USA). For solid media, 2% agar (Difco, BD Biosciences) was added. Escherichia coli DH10B cells were used for all DNA cloning procedures and maintenance of plasmid constructs. E. coli cells were grown in LB medium to which chloramphenicol (34 mg/L) was added as required. DNA primers were purchased from Integrated DNA Technologies (Coralville, IA, USA). Acid-washed glass beads (425–600 μm), chemicals and antifungal compounds were purchased from Sigma, unless otherwise stated. Stock solutions of fluconazole were prepared in water at a concentration of 5 mg/mL.
Table 1.
C. albicans strains used in this study
| Strain | Parent | Genotype | Reference |
|---|---|---|---|
| 5674 | 5457 | azole-resistant clinical isolate | 37 |
| STY7 | 5674 | cdr2AΔ::FRT/cdr2BΔ::FRT | 20 |
| STY31 | 5674 | cdr1AΔ::FRT/cdr1BΔ::FRT cdr2AΔ::FRT/cdr2BΔ::FRT | 20 |
| STY31 + CDR1WT | STY31 | CDR1-SAT1/cdr1BΔ::FRT cdr2AΔ::FRT/cdr2BΔ::FRT | this study |
| STY31 + CDR17A | STY31 | CDR17A-SAT1/cdr1BΔ::FRT cdr2AΔ::FRT/cdr2BΔ::FRT | this study |
| STY31 + CDR17E | STY31 | CDR17E-SAT1/cdr1BΔ::FRT cdr2AΔ::FRT/cdr2BΔ::FRT | this study |
Construction of plasmids and strains used in this study
The primers used for strain construction are listed in Table S1 (available as Supplementary data at JAC Online). For reintroducing the CDR1 gene in strain STY31, a smaller version of plasmid pSFS2A33 was constructed to facilitate CDR1 cloning. This was achieved by digesting pSFS2A with BamHI and HindIII, yielding a fragment of 1.4 kb containing ∼70% of the FLP recombinase sequence and a 6.1 kb fragment consisting of the rest of the plasmid with the SAT1 gene. The 6.1 kb fragment was gel-purified, blunt-ended, religated and used to transform DH10B bacteria. One plasmid (pSFS2Ashort), displaying the correct restriction pattern, was selected for constructing the CDR1-complementing plasmid pCDR1, as follows. First, the CDR1down DNA fragment (corresponding to positions +4826 to +5492 of the CDR1 gene relative to the ATG translation start codon) was PCR-amplified from C. albicans SC5314 genomic DNA with KOD HotStart DNA polymerase (Novagen, La Jolla, CA, USA) using primers MR1836 and MR1305, digested with XhoI and ApaI, and subsequently cloned between the XhoI and ApaI sites of plasmid pSFS2Ashort, generating plasmid pSFshortCDR1down. Next, primers MR1512 and MR1835 were used to amplify the CDR1 promoter, ORF and terminator (corresponding to positions −472 to +4826 relative to the ATG); the resulting fragment was digested with SacII and NotI and cloned between the SacII and NotI sites of pSFshortCDR1down to yield plasmid pCDR1. Primer MR1512 also introduced an ApaI site at the 5′-end of the fragment to allow the release of the cassette by digestion with ApaI before C. albicans transformation. Sequencing of the entire CDR1 ORF in pCDR1 confirmed that the PCR-amplified sequence was correct.
Site-directed mutagenesis of the phosphosites was performed using the QuikChange mutagenesis kit (Agilent Technologies). The CDR1-7A mutation (S8A, S12A, T49A, T51A, S54A, S59A and T68A) was generated in successive steps, as follows. Initially, the T49A, T51A and S54A mutations were introduced by PCR using plasmid pCDR1 as a template, the mutagenic primer MR2023 and KOD Hot Start DNA polymerase (Novagen). Subsequently, the S59A mutation was introduced into the T49A, T51A and S54A mutant plasmid as described above, using primer pairs MR2566 and MR2568. In addition to the S59A mutation, these primers also introduced a silent mutation of A to T at position +180 relative to the ATG, creating a PstI site to facilitate the identification of positive mutant clones. A 1.4 kb HindIII–SacII fragment containing the T49A, T51A, S54A and S59A mutations was subcloned into pBluescript for subsequent introduction of the S8A and S12A mutations, using primers MR2569 and MR2570. Finally, the T68A mutation was introduced into the S8A, S12A, T49A, T51A, S54A and S59A mutant plasmid using primers MR2571 and MR2572, generating the pBluescript-7A mutant plasmid. This 1.4 kb HindIII–SacII mutated fragment was subcloned back into the HindIII–SacII digested pCDR1 to yield the pCDR1-7A mutant plasmid.
The 7E mutation (S8E, S12E, T49E, T51E, S54E, S59E and T68E) was created as follows. The T49E, T51E and S54E mutations were generated using pCDR1 as a template and the mutagenic primer MR2330. A 1.4 kb HindIII–SacII fragment containing the T49E, T51E and S54E mutations was subcloned into pBluescript. The S8E and S12E mutations were created using the mutagenic primers MR2588 and MR2589 and the S59E mutation was subsequently generated using primers MR2618 and MR2619. Finally, the T68E mutation was introduced using primers MR2612 and MR2613 to yield the pBluescript-7E mutant plasmid. This HindIII–SacII mutated fragment was subcloned back into the HindIII–SacII digested pCDR1 to yield the pCDR1-7E mutant plasmid. The CDR1 fragments subjected to mutagenesis were sequenced in their entirety to confirm the desired mutations and to verify that no additional mutations had been introduced during the mutagenesis.
C. albicans strains expressing the Cdr1p WT or Cdr1p 7A and 7E mutants were constructed by introducing one copy of the CDR1 WT or mutant alleles at the CDR1 chromosomal locus in strain STY31 (cdr1Δ/cdr1Δ cdr2Δ/cdr2Δ).20 To allow integration by homologous recombination, the CDR1 WT, 7A and 7E transformation cassettes were released from their respective plasmids by ApaI digestion (1–5 μg) and used to transform strain STY31. C. albicans transformation was performed by electroporation or by the lithium acetate procedure as described previously.20 Nourseothricin-resistant transformants were selected on YPD agar plates supplemented with 200 mg/L nourseothricin (Werner Bioagents, Jena, Germany). Nourseothricin-resistant transformants containing the correctly integrated cassette were identified by Southern blotting or PCR. Strains containing the cassette integrated at the CDR1-A allele (as determined by PCR analysis using primers MR1613 and MR1614) were selected to ensure similar levels of expression.20 At least two independent strains per construct were functionally analysed to verify that they yielded similar results.
Total protein extractions, membrane protein preparation and western blotting
Total protein extracts were prepared using a glass beads protocol. Briefly, cells in the exponential phase were harvested and resuspended in 100 μL of TE buffer (50 mM Tris–HCl, pH 7.5/1.5 mM EDTA) containing protease inhibitors (1 mM PMSF and 5 μg/mL each of leupeptin, pepstatin and aprotinin). Cells were disrupted by vortexing in the presence of glass beads for 5 cycles of 1 min of vortexing followed by 1 min of cooling on ice. Total proteins were obtained following centrifugation at 3000 rpm (1865 g) for 1 min at 4°C to remove cell debris. Total membrane extracts were prepared from cultures of logarithmically growing cells using a Freezer Mill (SPEX CetriPrep, Metuchen, NJ, USA) as described previously.20 Protein concentrations were determined using a micro-BCA protein assay kit (Pierce, Rockford, IL, USA) and samples (25 μg) were separated by low-bis SDS–PAGE (6% or 8% acrylamide, 37.5:0.25 acrylamide:bis-acrylamide). The gels were either stained with Coomassie blue or transferred to a nitrocellulose membrane with a Trans Blot SD Semi-Dry transfer apparatus (Bio-Rad). The membrane was stained with Ponceau S reagent (0.1% Ponceau S in 5% acetic acid) prior to immunodetection. Immunodetection of Cdr1p was performed with either a generic anti-Cdrp antibody that recognizes both Cdr1p and Cdr2p (1:1000 dilution)34 or a specific anti-Cdr1p antibody (1:4000 dilution)35 using the Western Lightning Plus-ECL kit (PerkinElmer, Woodbridge, ON, Canada). Dephosphorylation assays were performed by incubating total membrane extracts (25 μg) at 37°C for 30 min in the presence of 200 U of λ-phosphatase (New England Biolabs) with or without phosphatase inhibitors (2 mM imidazole, 1.2 mM sodium molybdate, 1 mM sodium orthovanadate and 4 mM sodium tartrate dehydrate).
MS
Total membrane extracts from the C. albicans STY7 strain (CDR1/CDR1 cdr2Δ/cdr2Δ) were separated by 6% SDS–PAGE, stained with colloidal Coomassie (10% ammonium sulphate/0.1% Coomassie G250/3% orthophosphoric acid/20% ethanol) and the gel band corresponding to Cdr1p was excised and digested with trypsin. The tryptic peptides were analysed by nanoLC-MS using an Eksigent system (Dublin, CA, USA) interfaced to an LTQ-Orbitrap mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) via a nanoelectrospray ionization source. LC separations were performed using custom-made C18 columns. The sample injection volume was 5 μL, and tryptic digests were first loaded on the pre-column at a flow rate of 4 μL/min and subsequently eluted onto the analytical column using a gradient from 10% to 60% aqueous acetonitrile (0.2% formic acid) over 56 min with a flow rate of 0.6 μL/min. The data-dependent acquisition mode was enabled and each Orbitrap survey scan (resolution: 60 000) was followed by three MS/MS scans with dynamic exclusion for a duration of 30 s on the LTQ linear ion trap mass spectrometer. Multiply charged ions with intensity values >10 000 counts were selected for MS/MS sequencing. The normalized collision energy was set to 25%. Each sample was analysed with at least three replicates. Mass calibration used an internal lock mass (protonated (Si(CH3)2O)6; m/z 445.12057) and provided mass accuracy within 5 ppm for all nanoLC-MS experiments. Raw MS/MS spectra acquired from replicate liquid chromatography tandem MS (LC-MS/MS) analyses (n = 3) were combined into a single file using Mascot Distiller (version 2.1.1.0, Matrix Science) to reduce spectral redundancy and to correctly identify precursor m/z from survey scans. Peptides of interest were confirmed by back-blasting against the Candida genome database (http://www.candidagenome.org/). Assignment of phosphorylation sites was validated through manual inspection of relevant MS/MS spectra.
Drug susceptibility testing
Liquid microtitre plate assays were performed as described previously.20 Cell growth was measured spectrophotometrically at OD620 after 24 and 48 h of incubation at 30°C in YPD. During the course of these experiments, fluconazole-susceptible STY31-derived strains were found to give rise to fluconazole-resistant cells randomly, independently of the fluconazole concentration. These outliers were removed by performing 6 or 12 replicates for fluconazole-resistant or fluconazole-susceptible strains, respectively, calculating the median OD and excluding OD values that fell outside ±80% of the median. A minimum of three independent replicates for each strain at each fluconazole concentration after removal of outliers was included in the final calculations. The relative growth was calculated as the percentage of cell growth in drug-containing medium relative to the cell growth in the absence of drugs; the result was plotted as percent inhibition versus drug concentration. The IC50 values (fluconazole concentration at which 50% of the growth is inhibited) were determined by intrapolation from the growth inhibition curves, using GraphPad Prism version 6.00 for Mac (GraphPad Software, San Diego, CA, USA). Spot assays were performed as previously described.20 Briefly, cells were resuspended in YPD to an OD600 of 0.1. Ten-fold serial dilutions of each strain were spotted on YPD plates containing different concentrations of fluconazole and grown for 48 h at 30°C.
Indirect immunofluorescence
C. albicans strains that express WT or phosphomutant Cdr1p were grown to early logarithmic phase (OD600 of 0.5) and approximately 1 OD600 unit of cells was fixed in fixation solution (0.1 M KPO4, pH 6.4/3.7% formaldehyde) for 1 h at room temperature. Cells were then washed extensively with 0.1 M KPO4, pH 6.4 and resuspended in 225 μL of freshly prepared spheroplasting solution [1 M K2HPO4/37 mM citric acid/1.2 M sorbitol/20 μL of glusulase (DuPont, Boston, MA, USA)/2 μL of 10 mg/mL zymolase 100 T (MP Biomedicals, Solon, OH, USA)] and incubated at 30°C for 30 min. The generic anti-Cdrp antibody was pre-adsorbed in the presence of STY31 cells to reduce non-specific staining and was used at a 1:250 dilution while the secondary Alexa Fluor® 488-labelled goat anti-rabbit IgG antibody was used at a 1:500 dilution (Molecular Probes, Eugene, OR, USA). Epifluorescence microscopy was performed with a Zeiss LSM700 laser scanning confocal microscope. Image analysis was carried out using Zeiss AxioVision 4.8 software.
Nile red accumulation assay
C. albicans strains expressing WT or Cdr1p phosphomutants were grown to early logarithmic phase (OD600 of 0.5). The equivalent of 15 OD600 units of cells was harvested by centrifugation and washed twice with PBS. The cell pellet was then resuspended in 1.5 mL of PBS + 2% glucose and incubated for 1 h at 30°C in a shaking incubator. After the incubation, Nile red (Sigma) was added to the cells at a final concentration of 6 μM from a 1 mM stock in DMSO and incubated with agitation for 30 min at 30°C. Following this incubation, 200 μL of the cell suspension was transferred to each well of a black 96-well plate (PerkinElmer) in triplicate and the fluorescence intensity was read in a FlexStation II microplate reader (Molecular Devices, Sunnyvale, CA, USA) using an excitation wavelength of 553 nm and an emission wavelength of 636 nm.
Results
Investigation of Cdr1p phosphorylation
Cdr1p migrates as a broad band or a doublet in western blots,20,36 suggesting that the protein may be post-translationally modified. To test whether this pattern was due to phosphorylation, we performed a dephosphorylation assay using total cell membranes containing high levels of Cdr1p and Cdr2p prepared from the well-characterized clinical isolate 5674 (Figure 1a, top panel). This isolate is resistant to several azole derivatives and overexpresses CDR1, CDR2 and many other genes due to a gof mutation in Tac1p.20,37,38 Treatment with λ-phosphatase slightly increased the mobility of Cdr1p as detected by the anti-Cdr1p antibody that recognizes the N-terminal domain of Cdr1p20,35 (Figure 1a, top panel, compare lanes 1 and 2). This mobility shift was inhibited by the addition of phosphatase inhibitors (Figure 1a, lane 3). A similar Cdr1p migration pattern was detected in strain STY7, a cdr2Δ/cdr2Δ mutant isogenic to strain 5674,20 using the anti-Cdr1p antibody as well as the generic anti-Cdrp antibody that recognizes the NBD2 of Cdr1p (Figure 1a, bottom panel). These results indicated that Cdr1p is phosphorylated.
Figure 1.
Cdr1p phosphorylation. (a) Western blot analysis. Total membrane extracts prepared from strains 5674 (top panel) and STY7 (bottom panel) were treated or not with λ-phosphatase in the presence or absence of phosphatase inhibitors and separated by SDS–PAGE. Cdr1p was detected using either a specific anti-Cdr1p or a generic anti-Cdrp antibody. (b) Schematic representation of Cdr1p depicting the NTE, the two ABC core regions containing the NBDs and the transmembrane regions containing 12 TMDs organized in an NBD1–TMD1–NBD2–TMD2 topology. Cdr1p phosphorylated residues identified by MS are shown as filled circles. (c) Sequence alignment of the NTE regions of Cdr1p, Cdr2p and their orthologues in different Candida species, C. tropicalis (Ct), C. dubliniensis (Cd), C. glabrata (Cg) (www.candidagenome.org) and S. cerevisiae Pdr5p using the COBALT multiple alignment tool (www.ncbi.nlm.nih.gov/tools/cobalt/recobalt.cgi). Negatively charged residues are in bold. Phosphorylated residues identified by MS in CaCdr1p (this study) and ScPdr5p39 are circled. PPase, phosphatase. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
MS was used to identify phosphorylated sites in Cdr1p. For this purpose, we used strain STY7 (cdr2Δ/cdr2Δ) to avoid possible peptide sequence misassignment due to the presence of Cdr2p. Total membranes were prepared from STY7 and separated by SDS–PAGE. The gel band corresponding to Cdr1p was excised, trypsin-digested and submitted to LC-MS/MS analysis. We obtained ∼66% sequence coverage for full-length Cdr1p (Figure S1). Most TMDs were not detected by MS due to the low frequency of trypsin cleavage sites in these domains. Sequence coverage of ∼92% was obtained for the cytosolic NTE and NBD1 domains and ∼83% for the cytosolic linker and NBD2 domains (Figure S1). Twelve unique phosphosites were identified. Interestingly, seven of these sites were found to cluster in the NTE region (pSer8, pSer12, pThr49, pThr51, pSer54, pSer59 and pThr68) while the other five were located in the linker (pSer846, pThr847 and pSer849) and NBD2 domains (pThr1057 and pSer1062) (Figure 1b). Phosphorylation of S. cerevisiae Pdr5p has been reported in various high-throughput phosphoproteomic studies from cells grown under different conditions, these data being curated in the PhosphoGRID database (www.PhosphoGRID.org).39 Strikingly, we found that, of the 21 Pdr5p phosphosites recorded in PhosphoGRID, 11 are located in the NTE region and 4 are conserved with the phosphosites we identified in Cdr1p (pThr49, pThr51, pSer54, pSer59) (Figure 1c). Taken together, these results suggested that NTE phosphorylation may be important for PDR transporter function. We therefore decided to investigate the contribution of NTE phosphorylation to Cdr1p transport activity, using site-directed mutagenesis.
C. albicans Cdr1p expression systems
To test the function of different Cdr1p mutants, we first engineered a C. albicans homologous expression system that exploits results we obtained while characterizing strain 5674, namely the presence in that strain of a strong hyperactive allele of the Tac1p transcription factor (Tac1p N972D) that drives high levels of CDR1 and CDR2 expression38 coupled to a strong azole hypersusceptibility phenotype conferred by the synergistic effect of deleting both CDR1 and CDR2.20 This C. albicans protein expression system is similar to that of the S. cerevisiae system consisting of a multidrug hypersusceptible strain (AD1-8u-) lacking seven major ABC transporters and containing a hyperactive allele of the PDR1 gene (pdr1-3) that drives high levels of expression of a gene integrated at the chromosomal PDR5 locus.10 This strategy has been successfully used for expressing various fungal and human transporter proteins and for performing structure–function studies.40–42
We constructed a plasmid carrying the CDR1 open reading frame plus ∼0.5 kb of upstream sequences, the dominant selectable marker SAT1 followed by ∼0.7 kb of CDR1 downstream sequences (Figure 2a) and, after release of the integration cassette by restriction enzyme digestion, used this cassette to transform the 5674-derived cdr1Δ/cdr1Δ cdr2Δ/cdr2Δ strain STY31.20 Upon integration at the CDR1 endogenous locus by homologous recombination, the expression of CDR1 in the resulting strain is under the control of its own promoter, driven by the Tac1p N972D gof mutation (Figure 2b). We verified the functional expression of CDR1 in this system using a liquid microtitre plate assay (Figure 2c). As previously reported, strain STY31 was extremely susceptible to fluconazole, with an IC50 of 0.33 mg/L (Figure 2c). Reintroducing one allele of CDR1 in STY31 resulted in a marked increase in resistance to fluconazole (108-fold), with an IC50 of 35.7 mg/L (Figure 2c). Strain STY7, the 5674-derived cdr2Δ/cdr2Δ mutant that carries two copies of the CDR1 gene, was more resistant to fluconazole than the CDR1-complemented STY31 strain, with an IC50 of 92.6 mg/L. Taken together, these results confirmed the functional expression of CDR1 in strain STY31 and showed that this system provides a sensitive read-out and large range (>100-fold) for structure–function studies of Cdr1p or other genes integrated at the CDR1 locus.
Figure 2.

Expression of CDR1 in strain STY31 (Tac1pN972D cdr1Δ/cdr1Δ cdr2Δ/cdr2Δ). (a) Schematic representation of plasmid pCDR1 containing the CDR1 integration cassette. The cassette consists of, from left to right: CDR1up, CDR1 upstream region; the entire ORF of the CDR1 allele; SAT1 cassette (derived from pSFS2Ashort); and CDR1down, CDR1 downstream region. Abbreviations for restriction sites are: S, SacII; A, ApaI; N, NotI; and X, XhoI. The ApaI site shown in brackets was introduced by PCR. (b) CDR1 expression system. High-level expression of CDR1 is driven by the transcription factor Tac1p carrying an activating mutation (Tac1pN972D) in the STY31 strain lacking the endogenous CDR1 and CDR2 genes. (c) Fluconazole resistance profiles of the STY31, STY7 and STY31 + CDR1 strains. Cells were incubated for 48 h at 30°C in liquid YPD medium with the indicated concentrations of fluconazole. The data are presented as the relative growth of cells in fluconazole-containing medium compared with the growth of the same strain in fluconazole-free medium, which was set at 100%. The data presented come from one representative experiment performed in duplicate. FLC, fluconazole.
NTE phosphorylation is important for Cdr1p-mediated azole resistance and Nile red export
To assess the functional importance of the clustered NTE phosphosites, we constructed two Cdr1p NTE mutants in the pCDR1 plasmid, with all seven phosphorylated serines or threonines mutated to alanine (CDR1-7A: S8A/S12A/T49A/T51A/S54A/S59A/T68A; phosphonull mutant) or to glutamic acid (CDR1-7E: S8E/S12E/T49E/T51E/S54E/S59E/T68E; phosphomimetic mutant). Upon integration in the STY31 strain, Cdr1p expression was analysed by western blotting using total protein extracts and the generic anti-Cdrp antibody whose epitope is outside the region targeted for mutagenesis (Figure 3a). Interestingly, immunodetection of CDR1-7A revealed a slight increase in gel mobility when compared with the CDR1-WT or the CDR1-7E mutant, similar to the dephosphorylated form of Cdr1p (Figure 1a). In contrast, the mobility of the CDR1-7E mutant appeared slightly retarded as compared with that of the CDR1-WT. We also observed a small but consistent variation in the signal intensity of the CDR1-7A mutant, which was always expressed at a slightly higher level than CDR1-WT or the CDR1-7E mutant (Figure 3a), suggesting that the phosphorylation status of the Cdr1p NTE could play a role in protein stability. We also examined whether NTE phosphorylation affects Cdr1p plasma membrane localization, using indirect immunofluorescence microscopy (Figure 3b). In the strain expressing Cdr1p WT, a fluorescence signal was detected at the cell periphery, consistent with the previously described plasma membrane localization of Cdr1p.43 Cdr1p displayed a punctate distribution pattern, consistent with its association with lipid rafts.44 Very similar patterns and levels of fluorescence were detected in the strains expressing the CDR1-7A or CDR1-7E mutants, demonstrating that these mutations do not affect the plasma membrane localization of Cdr1p.
Figure 3.

Expression analyses of the Cdr1p NTE phosphomutants. (a) Total membranes extracted from STY31 and strains expressing WT Cdr1p or the Cdr1p NTE phosphorylation mutants 7A and 7E were analysed by western blotting with the generic anti-Cdrp antibody (top panel). Ponceau staining of the membrane before incubation with the antibody is shown as a control for equal protein loading and transfer (bottom panel). (b) Indirect immunofluorescence detection of the indicated strains was performed using the generic anti-Cdrp antibody followed by incubation with a fluorescent secondary antibody and visualization by epifluorescence microscopy. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
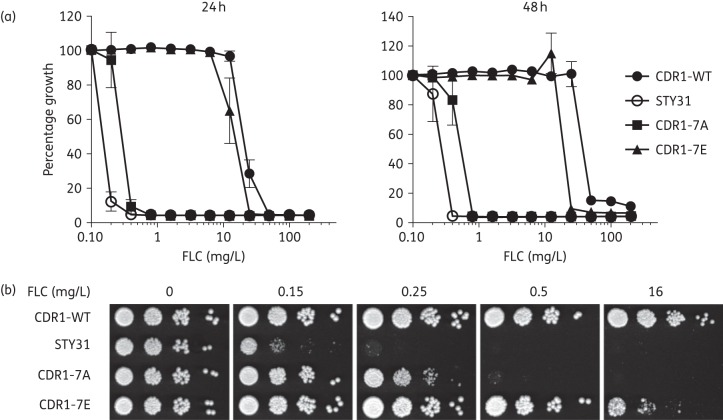
We next performed drug resistance assays with fluconazole to evaluate the functional importance of NTE phosphorylation on Cdr1p-mediated azole resistance (Figure 4 and Table 2). Our results showed that abolishing NTE phosphorylation strongly diminished Cdr1p-mediated fluconazole resistance since the strain expressing CDR1-7A was highly susceptible to fluconazole (Figure 4a). This result clearly showed that phosphorylation of the NTE is critical for Cdr1p function. In contrast, the phosphomimetic mutant CDR1-7E displayed high levels of fluconazole resistance, exhibiting between a 98- or 67-fold increase in fluconazole resistance as compared with STY31 after 24 and 48 h of growth, respectively (Table 2). However, the CDR1-7E mutant did not reach the same levels of resistance as that of the CDR1-WT. This was more evident after 48 h of growth, at which timepoint the level of CDR1-7E resistance was ∼50% of that of CDR1-WT (Table 2) (see the Discussion section). A fluconazole resistance assay was also carried out on solid media, and yielded very similar results (Figure 4b).
Figure 4.
Fluconazole resistance profiles of the Cdr1p NTE phosphomutants. (a) The indicated strains were analysed by MIC assay. Cells were incubated for 24 and 48 h at 30°C in liquid YPD medium with the indicated concentrations of fluconazole. The data are the mean of at least three independent experiments performed in duplicate. (b) Spot assay. Serial 10-fold dilutions of the cells, starting at an OD600 of 0.1, were spotted onto YPD plates containing fluconazole at the indicated concentrations or no drug. The plates were incubated for 48 h at 30°C. FLC, fluconazole.
Table 2.
Fluconazole IC50 (mg/L)
| IC50 |
Fold increase relative to STY31 |
Percentage fluconazole resistance relative to CDR1-WT |
||||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| STY31 | 0.16 | 0.30 | 1 | 1 | 0.8 | 0.8 |
| CDR1WT | 21.1 | 40.0 | 132 | 133 | 100 | 100 |
| CDR17A | 0.30 | 0.60 | 2 | 2 | 1.4 | 1.5 |
| CDR17E | 15.6 | 20.0 | 98 | 67 | 73.9 | 50 |
We next evaluated transporter function of these mutants by Nile red accumulation assay. Nile red is a lipophilic dye that strongly fluoresces in a hydrophobic environment such as membrane bilayers. It has been shown that Nile red is actively extruded from S. cerevisiae cells expressing Cdr1p, resulting in reduced intracellular accumulation of this compound.45 Equal numbers of cells from the different mutant strains were incubated with Nile red for 30 min at 30°C, prior to fluorescence quantification. This experiment showed that the strain expressing CDR1-WT accumulated 25-fold less Nile red than the STY31 strain, confirming that Cdr1p is active at exporting Nile red in our expression system (Figure 5). CDR-7A cells accumulated high levels of fluorescence, although to a lesser extent than STY31, while CDR1-7E cells accumulated very little Nile red, like CDR1-WT. Thus the results of the Nile red accumulation assay paralleled those of the fluconazole resistance assay. Taken together, these results demonstrate that NTE phosphorylation is crucial for Cdr1p transport function.
Figure 5.

Nile red accumulation assay. The equivalent of 15 OD600 units of log phase cells from the indicated strains were incubated with Nile red for 30 min at 30°C. After incubation, intracellular fluorescence was measured. Fluorescence accumulation is presented as relative fluorescence units (RFU) in which the basal levels of fluorescence in the absence of cells (PBS + Nile red) has been subtracted from the total fluorescence values. The data presented are average values from three independent experiments.
Discussion
Yeast ABC transporters of the PDR subfamily appear to be regulated by phosphorylation. Previous studies have shown that the level of phosphorylation could directly influence the stability and activity of S. cerevisiae Pdr5p and C. glabrata Cdr1p, respectively.30,32 Despite these findings, our current knowledge regarding phosphorylation-dependent regulation of yeast PDR transporters is far from complete and only a few kinases involved in ABC transporter phosphorylation have been identified. In the present study, we provided direct evidence that C. albicans Cdr1p is phosphorylated. Furthermore, using LC-MS/MS analyses, we identified a cluster of seven phosphosites located in the NTE region and showed that mutating these phosphosites to alanine almost abolished Cdr1p function. At this point, we do not know whether the seven sites are phosphorylated simultaneously in a single Cdr1p molecule and whether phosphorylation of each of the seven sites is required for optimal Cdr1p function.
We investigated the importance of NTE phosphorylation by mutating all seven phosphosites to non-phosphorylatable alanine (CDR1-7A) as well as to phosphomimetic glutamic acid (CDR1-7E). CDR1-7A was strongly defective in its transport function as measured by the fluconazole resistance and Nile red accumulation assays, demonstrating that phosphorylation of the NTE is crucial for Cdr1p function (Figure 4). This, however, does not exclude the possibility that phosphorylation of residues in other regions of Cdr1p could be equally important. Finally, it is possible that the residual activity of CDR1-7A is due to other phosphosites elsewhere in the protein, including some not identified in this study.
On the other hand, the function of the CDR1-7E mutant roughly mimicked that of CDR1-WT, as demonstrated by two independent functional assays (Figures 4 and 5), supporting the proposition that a negatively charged NTE is important for Cdr1p function. The CDR1-7E, however, was less resistant to fluconazole as compared with the CDR1-WT, a phenotype that was more obvious after a prolonged incubation period (Figure 4 and Table 2, 48 h incubation), suggesting that the unregulated, constitutive simultaneous phosphorylation of all residues mimicked by the 7E mutations is not optimal for Cdr1p function.
During the course of our study, new phosphorylation sites in S. cerevisiae Pdr5p, of uncharacterized function, have been reported independently from several large-scale MS studies and these data have been compiled in the PhosphoGRID database (http://www.phosphogrid.org/).39 Currently, 11 phosphosites have been identified in the NTE of Pdr5p. A sequence alignment showed that residues corresponding to pThr49, pThr51, pSer54 and pSer59 in Cdr1p are also phosphorylated in Pdr5p and are highly conserved among PDR transporters (Figure 1c). Notably, we also observed that some Ser/Thr phosphosites in either Cdr1p or Pdr5p NTE have corresponding negatively charged residues in other PDR transporters, consistent with a positional importance of clustered negatively charged residues within the NTE, creating a so-called acidic patch.6 Phosphosites identified outside the NTE region are less conserved between Cdr1p and Pdr5p; however, it cannot be excluded that additional phosphorylation sites, more labile in nature, were not identified in our study or in large-scale proteomic studies. Taken together, these results demonstrate the presence of an evolutionarily conserved acidic patch within the PDR transporter NTE, formed by negatively charged and phosphorylated residues, that seems to play an important role in transporter function.
How does NTE phosphorylation regulate Cdr1p function? Due to the lack of knowledge about the three-dimensional structure of transporters of the PDR subfamily, the position of the NTE relative to the core ABC transporter is unknown. However, a recent computational modelling study of Pdr5p placed the NTE beneath both NBDs, similar to the location of the regulatory domains of the ABC metal-chelate molybdate/tungstate transporter.8,46 This implies that NTE phosphorylation could impact on NBD function. Interestingly, recent NMR and three-dimensional electron cryomicroscopy studies showed that phosphorylation of the R-domain (linker domain) of CFTR plays a positive regulatory role in its activity by pushing the R-domain away from the NBD core, thereby allowing entry of ions for transportation.47–49 Alternatively, given the well-documented role of acidic patches in mediating protein–protein interaction,50,51 it is possible that the NTE region of Cdr1p could serve as a platform for interacting with a regulatory protein and that such interaction would be mediated by phosphorylation.
What are the kinases responsible for Cdr1p NTE phosphorylation? In general, spatial proximity is a key factor that influences substrate selection by a protein kinase.52 Yeast casein kinase I and II (CKI and CKII) are both peripheral plasma membrane protein kinases, located in the same subcellular compartment as Cdr1p. CKI has been shown to be involved in the phosphorylation of several membrane proteins in S. cerevisiae, including Pdr5p, the uracil permease Fur4p as well as the glucose transporter Rgt2p.30,53,54 CKII, on the other hand, has been shown to phosphorylate S. cerevisiae Ycf1p.29,55 Analysing the Cdr1p NTE phosphosites and their surrounding residues did not predict the canonical consensus recognition motif for CKI but identified Ser54 as a possible substrate for CKII.56,57 This is interesting since CKII has been shown to regulate azole susceptibility in C. albicans,58 qualifying it as a likely candidate. Finally, the kinase-predicting engine NetPhosK (http://www.cbs.dtu.dk/services/NetPhosK/) predicted, among different possibilities, that Ser8, Thr51 and Ser54 could be substrates for protein kinase C (PKC). Taken together, it is likely that at least two different kinases are involved in phosphorylating the Cdr1p NTE, a possibility currently being investigated.
Funding
This work was supported by a research grant to M. R. from the Canadian Institutes of Health Research (MT-15679).
Transparency declarations
None to declare.
Supplementary data
Acknowledgements
We would like to thank Joachim Morschhäuser for the gift of pSFS2A, Dominique Sanglard for the anti-Cdr1p antibody, the IRIC Genomic Platform for DNA sequencing and the IRIC Proteomic Platform for MS analysis.
References
- 1.Dean M, Annilo T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu Rev Genomics Hum Genet 2005; 6: 123–42. [DOI] [PubMed] [Google Scholar]
- 2.Jones PM, O'Mara ML, George AM. ABC transporters: a riddle wrapped in a mystery inside an enigma. Trends Biochem Sci 2009; 34: 520–31. [DOI] [PubMed] [Google Scholar]
- 3.Theodoulou FL, Kerr ID. ABC transporter research: going strong 40 years on. Biochem Soc Trans 2015; 43: 1033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dean M. The genetics of ATP-binding cassette transporters. Meth Enzymol 2005; 400: 409–29. [DOI] [PubMed] [Google Scholar]
- 5.Robey RW, To KK, Polgar O et al. ABCG2: a perspective. Adv Drug Deliv Rev 2009; 61: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamping E, Baret PV, Holmes AR et al. Fungal PDR transporters: phylogeny, topology, motifs and function. Fungal Genet Biol 2010; 47: 127–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paumi CM, Chuk M, Snider J et al. ABC transporters in Saccharomyces cerevisiae and their interactors: new technology advances the biology of the ABCC (MRP) subfamily. Microbiol Mol Biol Rev 2009; 73: 577–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutledge RM, Esser L, Ma J et al. Toward understanding the mechanism of action of the yeast multidrug resistance transporter Pdr5p: a molecular modeling study. J Struct Biol 2011; 173: 333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golin J, Ambudkar SV. The multidrug transporter Pdr5 on the 25th anniversary of its discovery: an important model for the study of asymmetric ABC transporters. Biochem J 2015; 467: 353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Decottignies A, Grant AM, Nichols JW et al. ATPase and multidrug transport activities of the overexpressed yeast ABC protein Yor1p. J Biol Chem 1998; 273: 12612–22. [DOI] [PubMed] [Google Scholar]
- 11.Golin J, Ambudkar SV, Gottesman MM et al. Studies with novel Pdr5p substrates demonstrate a strong size dependence for xenobiotic efflux. J Biol Chem 2003; 278: 5963–9. [DOI] [PubMed] [Google Scholar]
- 12.Rockwell NC, Wolfger H, Kuchler K et al. ABC transporter Pdr10 regulates the membrane microenvironment of Pdr12 in Saccharomyces cerevisiae. J Membr Biol 2009; 229: 27–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Decottignies A, Lambert L, Catty P et al. Identification and characterization of SNQ2, a new multidrug ATP binding cassette transporter of the yeast plasma membrane. J Biol Chem 1995; 270: 18150–7. [DOI] [PubMed] [Google Scholar]
- 14.Wolfger H, Mamnun YM, Kuchler K. The yeast Pdr15p ATP-binding cassette (ABC) protein is a general stress response factor implicated in cellular detoxification. J Biol Chem 2004; 279: 11593–9. [DOI] [PubMed] [Google Scholar]
- 15.Ferreira-Pereira A, Marco S, Decottignies A et al. Three-dimensional reconstruction of the Saccharomyces cerevisiae multidrug resistance protein Pdr5p. J Biol Chem 2003; 278: 11995–9. [DOI] [PubMed] [Google Scholar]
- 16.Kolaczkowska A, Kolaczkowski M, Goffeau A et al. Compensatory activation of the multidrug transporters Pdr5p, Snq2p, and Yor1p by Pdr1p in Saccharomyces cerevisiae. FEBS Lett 2008; 582: 977–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snider J, Hanif A, Lee ME et al. Mapping the functional yeast ABC transporter interactome. Nat Chem Biol 2013; 9: 565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coste AT, Karababa M, Ischer F et al. TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2. Eukaryot Cell 2004; 3: 1639–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmes AR, Lin Y-H, Niimi K et al. ABC transporter Cdr1p contributes more than Cdr2p does to fluconazole efflux in fluconazole-resistant Candida albicans clinical isolates. Antimicrob Agents Chemother 2008; 52: 3851–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsao S, Rahkhoodaee F, Raymond M. Relative contributions of the Candida albicans ABC transporters Cdr1p and Cdr2p to clinical azole resistance. Antimicrob Agents Chemother 2009; 53: 1344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prasad R, Banerjee A, Khandelwal NK et al. The ABCs of Candida albicans multidrug transporter Cdr1. Eukaryot Cell 2015; 14: 1154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paul S, Moye-Rowley WS. Multidrug resistance in fungi: regulation of transporter-encoding gene expression. Front Physiol 2014; 5: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Espinel-Ingroff A, Pfaller MA, Bustamante B et al. Multilaboratory study of epidemiological cutoff values for detection of resistance in eight Candida species to fluconazole, posaconazole, and voriconazole. Antimicrob Agents Chemother 2014; 58: 2006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie Y, Burcu M, Linn DE et al. Pim-1 kinase protects P-glycoprotein from degradation and enables its glycosylation and cell surface expression. Mol Pharmacol 2010; 78: 310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie Y, Xu K, Linn DE et al. The 44-kDa Pim-1 kinase phosphorylates BCRP/ABCG2 and thereby promotes its multimerization and drug-resistant activity in human prostate cancer cells. J Biol Chem 2008; 283: 3349–56. [DOI] [PubMed] [Google Scholar]
- 26.Kelm KB, Huyer G, Huang JC et al. The internalization of yeast Ste6p follows an ordered series of events involving phosphorylation, ubiquitination, recognition and endocytosis. Traffic 2004; 5: 165–80. [DOI] [PubMed] [Google Scholar]
- 27.Kölling R. Mutations affecting phosphorylation, ubiquitination and turnover of the ABC-transporter Ste6. FEBS Lett 2002; 531: 548–52. [DOI] [PubMed] [Google Scholar]
- 28.Eraso P, Martínez-Burgos M, Falcón-Pérez JM et al. Ycf1-dependent cadmium detoxification by yeast requires phosphorylation of residues Ser908 and Thr911. FEBS Lett 2004; 577: 322–6. [DOI] [PubMed] [Google Scholar]
- 29.Paumi CM, Chuk M, Chevelev I et al. Negative regulation of the yeast ABC transporter Ycf1p by phosphorylation within its N-terminal extension. J Biol Chem 2008; 283: 27079–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Decottignies A, Owsianik G, Ghislain M. Casein kinase I-dependent phosphorylation and stability of the yeast multidrug transporter Pdr5p. J Biol Chem 1999; 274: 37139–46. [DOI] [PubMed] [Google Scholar]
- 31.Wada S-I, Niimi M, Niimi K et al. Candida glabrata ATP-binding cassette transporters Cdr1p and Pdh1p expressed in a Saccharomyces cerevisiae strain deficient in membrane transporters show phosphorylation-dependent pumping properties. J Biol Chem 2002; 277: 46809–21. [DOI] [PubMed] [Google Scholar]
- 32.Wada S-I, Tanabe K, Yamazaki A et al. Phosphorylation of Candida glabrata ATP-binding cassette transporter Cdr1p regulates drug efflux activity and ATPase stability. J Biol Chem 2005; 280: 94–103. [DOI] [PubMed] [Google Scholar]
- 33.Reuss O, Vik A, Kolter R et al. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene 2004; 341: 119–27. [DOI] [PubMed] [Google Scholar]
- 34.Gauthier C, Weber S, Alarco A-M et al. Functional similarities and differences between Candida albicans Cdr1p and Cdr2p transporters. Antimicrob Agents Chemother 2003; 47: 1543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Micheli M, Bille J, Schueller C et al. A common drug-responsive element mediates the upregulation of the Candida albicans ABC transporters CDR1 and CDR2, two genes involved in antifungal drug resistance. Mol Microbiol 2002; 43: 1197–214. [DOI] [PubMed] [Google Scholar]
- 36.Coste A, Turner V, Ischer F et al. A mutation in Tac1p, a transcription factor regulating CDR1 and CDR2, is coupled with loss of heterozygosity at chromosome 5 to mediate antifungal resistance in Candida albicans. Genetics 2006; 172: 2139–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saidane S, Weber S, De Deken X et al. PDR16-mediated azole resistance in Candida albicans. Mol Microbiol 2006; 60: 1546–62. [DOI] [PubMed] [Google Scholar]
- 38.Znaidi S, De Deken X, Weber S et al. The zinc cluster transcription factor Tac1p regulates PDR16 expression in Candida albicans. Mol Microbiol 2007; 66: 440–52. [DOI] [PubMed] [Google Scholar]
- 39.Sadowski I, Breitkreutz B-J, Stark C et al. The PhosphoGRID Saccharomyces cerevisiae protein phosphorylation site database: version 2.0 update. Database (Oxford) 2013; 2013: bat026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furman C, Mehla J, Ananthaswamy N et al. The deviant ATP-binding site of the multidrug efflux pump Pdr5 plays an active role in the transport cycle. J Biol Chem 2013; 288: 30420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamping E, Monk BC, Niimi K et al. Characterization of three classes of membrane proteins involved in fungal azole resistance by functional hyperexpression in Saccharomyces cerevisiae. Eukaryot Cell 2007; 6: 1150–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keniya MV, Holmes AR, Niimi M et al. Drug resistance is conferred on the model yeast Saccharomyces cerevisiae by expression of full-length melanoma-associated human ATP-binding cassette transporter ABCB5. Mol Pharm 2014; 11: 3452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jha S, Dabas N, Karnani N et al. ABC multidrug transporter Cdr1p of Candida albicans has divergent nucleotide-binding domains which display functional asymmetry. FEMS Yeast Res 2004; 5: 63–72. [DOI] [PubMed] [Google Scholar]
- 44.Pasrija R, Panwar SL, Prasad R. Multidrug transporters CaCdr1p and CaMdr1p of Candida albicans display different lipid specificities: both ergosterol and sphingolipids are essential for targeting of CaCdr1p to membrane rafts. Antimicrob Agents Chemother 2008; 52: 694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ivnitski-Steele I, Holmes AR, Lamping E et al. Identification of Nile red as a fluorescent substrate of the Candida albicans ATP-binding cassette transporters Cdr1p and Cdr2p and the major facilitator superfamily transporter Mdr1p. Anal Biochem 2009; 394: 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerber S, Comellas-Bigler M, Goetz BA et al. Structural basis of trans-inhibition in a molybdate/tungstate ABC transporter. Science 2008; 321: 246–50. [DOI] [PubMed] [Google Scholar]
- 47.Kanelis V, Hudson RP, Thibodeau PH et al. NMR evidence for differential phosphorylation-dependent interactions in WT and DeltaF508 CFTR. EMBO J 2010; 29: 263–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang L, Aleksandrov LA, Zhao Z et al. Architecture of the cystic fibrosis transmembrane conductance regulator protein and structural changes associated with phosphorylation and nucleotide binding. J Struct Biol 2009; 167: 242–51. [DOI] [PubMed] [Google Scholar]
- 49.Stolarczyk EI, Reiling CJ, Paumi CM. Regulation of ABC transporter function via phosphorylation by protein kinases. Curr Pharm Biotechnol 2011; 12: 621–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leung JW, Agarwal P, Canny MD et al. Nucleosome acidic patch promotes RNF168- and RING1B/BMI1-dependent H2AX and H2A ubiquitination and DNA damage signaling. PLoS Genet 2014; 10: e1004178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mersman DP, Du H-N, Fingerman IM et al. Charge-based interaction conserved within histone H3 lysine 4 (H3K4) methyltransferase complexes is needed for protein stability, histone methylation, and gene expression. J Biol Chem 2012; 287: 2652–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ptacek J, Devgan G, Michaud G et al. Global analysis of protein phosphorylation in yeast. Nature 2005; 438: 679–84. [DOI] [PubMed] [Google Scholar]
- 53.Marchal C, Haguenauer-Tsapis R, Urban-Grimal D. Casein kinase I-dependent phosphorylation within a PEST sequence and ubiquitination at nearby lysines signal endocytosis of yeast uracil permease. J Biol Chem 2000; 275: 23608–14. [DOI] [PubMed] [Google Scholar]
- 54.Moriya H, Johnston M. Glucose sensing and signaling in Saccharomyces cerevisiae through the Rgt2 glucose sensor and casein kinase I. Proc Natl Acad Sci USA 2004; 101: 1572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pickin KA, Ezenwajiaku N, Overcash H et al. Suppression of Ycf1p function by Cka1p-dependent phosphorylation is attenuated in response to salt stress. FEMS Yeast Res 2010; 10: 839–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ubersax JA, Ferrell JE. Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol 2007; 8: 530–41. [DOI] [PubMed] [Google Scholar]
- 57.Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J 2003; 17: 349–68. [DOI] [PubMed] [Google Scholar]
- 58.Bruno VM, Mitchell AP. Regulation of azole drug susceptibility by Candida albicans protein kinase CK2. Mol Microbiol 2005; 56: 559–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.