Abstract
A boundary lubricant attaches and protects sliding bearing surfaces by preventing interlocking asperity-asperity contact. Proteoglycan-4 (PRG4) is a boundary lubricant found in the synovial fluid that provides chondroprotection to articular surfaces. Inflammation of the diarthrodial joint modulates local PRG4 concentration. Thus, we measured the effects of inflammation, with Interkeukin-1α (IL-1α) incubation, upon boundary lubrication and PRG4 expression in bovine cartilage explants. We further aimed to determine whether the addition of exogenous human recombinant PRG4 (rhPRG4) could mitigate the effects of inflammation on boundary lubrication and PRG4 expression in vitro.
Cartilage explants, following a 7-day incubation with IL-1α, were tested in a disc-on-disc configuration using either rhPRG4 or saline (PBS control) as a lubricant. Following mechanical testing, explants were studied immunohistochemically or underwent RNA extraction for RT-PCR. We found that static coefficient of friction (COF) significantly decreased to 0.14 ±0.065 from 0.21 ±0.059 (p=0.014) in IL-1α stimulated explants lubricated with rhPRG4, as compared to PBS. PRG4 expression was significantly up regulated from 30.8 ± 19 copies in control explants lubricated with PBS to 3330 ± 1760 copies in control explants lubricated with rhPRG4 (p<0.001). Explants stimulated with IL-1α displayed no increase in PRG4 expression upon lubrication with rhPRG4, but with PBS as the lubricant, IL-1α stimulation significantly increased PRG4 expression compared to the control condition from 30.8 ± 19 copies to 401 ± 340 copies (p=0.015). Overall, these data suggest that exogenous rhPRG4 may provide a therapeutic option for reducing friction in transient inflammatory conditions and increasing PRG4 expression.
Introduction
Osteoarthritis (OA) is a debilitating joint disease that affects 1 in 2 individuals by the age of 85.1 Treatment includes symptomatic relief using nonsteroidal anti-inflammatory drugs (NSAIDs), viscosupplementation, and ultimately arthroplasty. Joint replacement surgery led to $28.5 billion of hospitalization expenditures in 2009 in the United States2 and comprised 35% of total arthritis-related procedures requiring hospitalization.3 Post-traumatic osteoarthritis (PTOA) encompasses 12% of the OA case burden,4 and is suspected to involve mechanical, biological, and environmental causes which together initiate and exacerbate cartilage degeneration.5 Inflammation following joint trauma creates a nidus for the development of PTOA.6 Interleukin-1 (IL-1) is one among many cytokines involved in joint inflammation.7 The importance of IL-1 in the initiation and progression of OA is reflected by the clinical interest of soluble IL-1 antagonists.8
Proteoglycan-4 (PRG4), also referred to as lubricin, is a large, mucinous glycoprotein that coats the articular surfaces and provides articular cartilage boundary lubrication during high load and slow sliding speeds9. IL-1α and inflammation in general modulate PRG4 expression by synoviocytes as well as superficial and intermediate zone chondrocytes.10 The N-terminus of PRG4 creates a disulfide bond between PRG4 monomers, enabling PRG4 to form a multilayer molecular film along the articular surface.11 Hydration forces provided by O-linked mucin domain glycosylations are also likely involved in repulsion of molecules, since removal of distal NeuAc (sialic acid) and penultimate Gal (galactose) decreases lubricating ability in vitro.12 Under conditions of reciprocating cyclic motion, boundary lubricants must be replenished to prevent articular wear.13 Recently a link between elevated coefficient of friction (COF), increased mechanical strain, and increased chondrocyte caspase-3 activation in the absence of inflammation has been established both in vitro and in vivo in animal models lacking normal PRG4 lubrication.13; 14
These observations coupled with the well-known catabolic effects resulting from mechanical injuries due to inflammatory mediators, such as IL-1, have initiated new inquiry into the anabolic role that PRG4 plays in the protection of articular chondrocytes.15; 16 Downstream catabolic effects of IL-1 include stimulation of matrix metalloproteinase (MMP) production, increased proteoglycan release, and increased cartilage matrix degradation, which may initiate the phenomenon of PTOA.17 These same metabolic dyscrasias are also accompanied by synovitis in OA.18 Overexpression of IL-1β in rabbits results in cellular hyperplasia, articular cartilage loss, and clinical synovitis.19 Isolated bovine and rat chondrocytes treated with IL-1α showed decreased PRG4 secretion and decreased PRG4 expression.20; 21 IL-1β has also been shown to down-regulate PRG4 expression in chondrocytes.20-22 However, other studies suggest that inflammation increases PRG4 production.23-25 Whether exogenous PRG4 can attenuate the impact of IL-1 stimulation on native PRG4 expression by cartilage has not yet been reported.
PRG4 supplementation has been shown to improve the diminished lubricating ability of osteoarthritic, human synovial fluid.26 In rodent PTOA models, following either meniscectomy27 or anterior-cruciate ligament transection (ACLT),28-30 the intra-articular dosing of PRG4 has demonstrated improved histology,27-30 increased cartilage thickness,30 and preservation of glycosaminoglycan compared to untreated controls.28; 29 Rats under forced exercise, in particular, showed greater damage without PRG4 supplementation following ACLT.29 Additionally, Teeple et al. showed some radiographic improvement following PRG4 supplementation in post-ACLT rat knees.30 Either recombinant human PRG4 (rhPRG4) or purified human synovial fluid PRG4 was used in the aforementioned studies. The half-life of rhPRG4 is short, following intra-articular PRG4 administration.31 Thus, chondroprotective effects likely involve native expression of PRG4 to support these observations.7; 29; 31; 32 We aimed to determine the effects of IL-1α on boundary lubrication and its biological impact on PRG4 expression in a cartilage-on-cartilage bovine explant model.14 We posited that the posterior area of the femoral condyle, with less extractable PRG4, would be more susceptible to increases in friction due to IL-1α stimulation.33 We further aimed to determine whether the addition of exogenous rhPRG4 could mitigate the effects of inflammation on boundary lubrication and PRG4 expression in vitro. We hypothesized that IL-1α stimulation of articular cartilage explants will induce cartilage catabolism, leading to a decrease in PRG4 expression and an increase in COF. Furthermore, we hypothesized that the introduction of rhPRG4, as a boundary lubricant, will have a restorative effect on cartilage through increasing chondrocyte native PRG4 expression and decreasing COF in IL-1α treated explants.
Materials Methods
Explant Harvest and Culture
Osteochondral explant pairs, 6 and 12mm in diameter, were cored from bovine femoral condyles within 4 hours of slaughter. Explants intended for both friction and immunohistochemical staining were acquired from 99 explants (79 mechanically stimulated, 20 controls) collected from 20 bovine femoral condyles from 20 adult steers between the ages of 12-16 months. Cartilage surfaces that appeared grossly deformed or damaged were rejected. Explant pairs were harvested from the anterior, mid, and posterior regions of the medial femoral condyle (Figure 1). A separate group of explants consisting of 12 explants harvested from 3 bovine femoral condyles from 3 donors was used for PRG4 expression evaluation. Another group of explants, consisting of twenty 12mm explants and ten 6mm and 12mm explant pairs, was harvested from 5 bovine femoral condyles from 5 donors and used for dual imaging of native PRG4 and exogenous rhPRG4. All osteochondral explant groups were harvested from the medial region of the femoral condyle. Explant pairs underwent three washes with 5 ml of Dulbeccos Modified Eagles Medium containing 4.5g/L D-Glucose and L-glutamine (DMEM) from (Gibco by Life Technologies) and centrifugation at 2500 rpm for 5 minutes. Explants were then cultured for 7 days in the same DMEM containing 10% fetal bovine serum (Hyclone GE Life Sciences) and 1% penicillin streptomycin containing 5,000 units/ml penicillin and 5,000 μg/ml streptomycin (Gibco; Life Technologies)14 either with or without recombinant IL-1α at a concentration of 10 ng/ml in PBS (R&D Systems Inc., Minneapolis, MN).14; 22 Medium containing IL-1α was changed at days 3 and 5 and new medium containing IL-1α was added.
Figure 1.
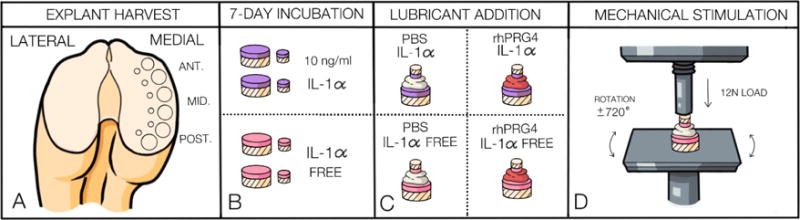
Diagram demonstrating methods of explant harvest, preparation, and mechanical stimulation of bovine cartilage explant pairs. Panel A displays the medial femoral condylar region of the bovine knee from which 12 and 6mm explant pairs were harvested from the anterior, mid, and posterior regions. Panel B displays the two groups of explant pairs incubated in culture medium supplemented with or without IL-1α for 7-days following explant pair harvest. Panel C shows the explant pairs with their intervening test lubricants. Panel D displays the mechanical stimulation set up including explant pair mounting to the machine components using ethyl cyanoacrylate glue, the magnitude and direction of the load applied to the explant pairs, and the platform rotation from which torque values were obtained for COF calculation.
Manufacture and Purification of rhPRG4
The expression of rhPRG4 by CHO-M cells has been described previously.34 CHO-M cells were transfected with the full-length human PRG4 gene resulting in a full-length PRG4 (1404 amino acids in length). The presence of O-linked glycosylations comprised of (β1,3)Gal-GalNAc was previously confirmed by mass spectrometry.35
Mechanical Stimulation Methodology
Explant pairs lubricated with 100μl of either phosphate-buffered saline (PBS) or rhPRG4 (Lubris, LLC, Framingham, MA) from a stock solution of 1mg/ml in PBS were mounted into a materials testing system (BOSE Electroforce 3200; Prairie Eden, MN). Mechanical stimulation of opposing explant pairs was preceded by 12N static compression for 8 minutes, resulting in approximately 18% compressive strain. This interval allowed the pore pressure to depressurize within the cartilage samples to approximate towards zero. After depressurization, the explant pairs underwent 12 cycles of ±720˚rotation while under constant axial strain. Static COF, a measure of the stick-slip condition, and dynamic COF (equilibrium COF) were calculated from the torque and axial load measured during the rotation sequence. The following equations were used:
Static COF= Tmax/(2/3*R*Feq)
Dynamic COF= Tave/(2/3*R*Feq)
where Tmax= torque max within the first 20˚ of rotation, Tavg=torque average for the last 720˚ of rotation, R=radius of the 6 mm explant, and Feq=equilibrium force obtained after cartilage decompression.14
Digital calipers were used to measure the initial total cartilage thickness values of the 12mm and 6mm explant bearing pairs. Four caliper values were measured and averaged for each explant in the pair and the total cartilage thickness was determined as the sum of the averaged explant thickness values forming the explant pair. Total cartilage thickness values were 1.48 ±0.398 mm for the 6mm explants, 1.28±0.274 mm for the 12 mm explants, and 2.76± 0.609 mm for total cartilage thickness of all explant pairs. The total cartilage thickness of an explant pair was compared to the change in measured displacement across these two cartilage surfaces after mechanical loading had occurred. This comparison yielded a change in cartilage thickness for each of the explant pairs. Explant pairs with apparent compressive strains of 10% or less, or 30% or more were excluded. Secondly, if the standard deviation for the equilibrium force (Feq) within explant pairs harvested from the same knee joint was 0.5 or greater, explant pairs whose Feq were less than 2N, were excluded. These two criteria were used to minimize the impact of outliers on variability in COF, attributable in our experience, to abnormal specimen variability, inadequate mounting, and confounders caused by geometry of two surfaces that are not normally apposed or parallel to one another. Of the 79 explant pairs mechanically tested, 17 were excluded. Nominal Feq for the accepted explants was 2.84±0.622 N. Data points used for the COF statistical analysis within each mechanical testing subset from the anterior, mid, and posterior region respectively for the 79 explants taken from 20 bovine femoral condyles are as follows: Control-PBS (n=5, n=6, n=3); IL-1-PBS (n=4, n=6, n=3); Control-rhPRG4 (n=6, n=9, n=4); IL-1-rhPRG4 (n=4, n=6, n=6).
Histology Preparation
After COF measurement, the 12mm explants from each testing pair were placed in formalin, decalcified with EDTA, and sectioned into 10μm thick sections. PRG4 accumulation was determined from 62 explant pairs. PRG4 accumulation was visualized in these samples and their 20 non-mechanically stimulated 12mm explant controls. Monoclonal antibody 9G3 (EMD Millipore; Darmstadt, Germany), which reacts with an epitope in the mucin domain of PRG4, was used to visualize PRG4 accumulation.36 Control explants for each treatment group were immediately placed in formalin after 7 days of culture and not exposed to lubricant supplementation or mechanical stimulation.
Real Time Polymerase Chain Reaction (RT-PCR)
The second group of 12 explants from 3 donors was cultured with or without 10ng/ml of IL-1α for 7 days. Each of 4 explant pairs harvested from each bovine femoral condyle was assigned a different experimental group (either IL-PBS, IL-rhPRG4, Control-PBS, or Control-rhPRG4). Immediately after testing, the cartilage section of the osteochondral explant was separated from the bone. Cartilage samples were placed in an RNA preservation solution (RNAlater, Thermo Scientific) and stored at −80°C until RNA isolation was performed. Three RNA samples were obtained for each of the 4 explant test groups. These three RNA samples were then pooled and evaluated in triplicate for RT-PCR.
Cartilage was minced with a #15 scalpel blade for RNA extraction. The RNA storage solution was carefully removed, and cartilage pieces were moved into 2ml lysing matrix tubes (MP Biomedical) to which 700μl QIAzol lysis buffer (QIAGEN, GmbH,D-Hilden, Germany) was pipetted. The cartilage specimens were homogenized 10 times for 15 seconds, with 2 minutes of rest in ice between subsequent 15-second homogenization cycles (MP FastPrep-24 homogenizer MP Biomedicals, LLC. Santa Ana, CA). Total RNA was extracted and purified using miRNeasy micro kit (QIAGEN, GmbH,D Hilden, Germany) according to the manufacturer’s protocol. The concentration of eluted RNA was determined via NanoDrop software (Thermo Scientific). cDNA was synthesized from 5 μg of total RNA. RT-PCR performed on a Mastercycler Realplex4 (Eppendorf North America) system using qPCR Kit Kapa Sybr fast master mix (Kapabiosystems, Boston, MA, USA) and under conditions recommended by these manufacturers. After agarose gel analysis and purification of a 135 bp PCR product (QIAquick gel extraction kit. Qiagen Sciences,MD 20874,USA), the DNA concentration was measured and a standard curve was created based on dilution. The same was also accomplished for glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Primer sequences for bovine PRG4 are as follows: Forward: 5- ACTCCACCACCTCCACCTCGG -3, Reverse: 5- GCTGAACGCTGCCACCTCTC -3. GAPDH was used as the internal control: Forward: 5-AGGTCATCCATGACCACTTTGG -3; Reverse: 5-GCAGGTCAGATCCACAACAG-3. The standard or sample volumes were 20 μl per reaction. qPCR program for PRG4 was: 95° C for 15 min, then 95°C 15 sec, 65°C 15 sec, 72°C 20 sec for 45 cycles. qPCR program for GAPDH was: 95°C for 15 min, then 95°C 15 sec, 55°C 15 sec, 72°C 20 sec for 45 cycles. The expression levels (number of copies) were normalized to GAPDH levels. PRG4 copy number was obtained from a standard curve with 100% efficiency. Sample copy number was expressed as number of copies of PRG4 per 100 copies of GAPDH.
Dual Imaging of rhPRG4 and Native PRG4
The third group of explants, described above, was harvested for PRG4 dual imaging of rhPRG4 and resident bovine PRG4. This group of explants consisted of twenty 12mm explants that were not mechanically stimulated and ten 6mm and 12mm explant pairs that were mechanically stimulated. These explants were harvested from 5 bovine femoral condyles from 5 donors and cultured with or without 10ng/ml of IL-1α for 7 days. Each of the four 12 mm explants and two 6mm and 12mm explant pairs harvested from each bovine femoral condyle were assigned to a different non-mechanical (IL-rhPRG4, IL, Control-rhPRG4, or Control) or mechanical (IL-rhPRG4 or Control-rhPRG4) testing group. rhPRG4 was labeled using a Pierce NHS-rhodamine antibody labeling kit (Thermo Fisher Scientific; Grand Island, NY) according to manufacturer instructions using a 2mg/ml concentration of rhPRG4. Explants were then exposed to 100μl of PBS or rhPRG4-rhodamine as a lubricant for 15 minutes with and without mechanical stimulation using the mechanical stimulation protocol described above. Cartilage was removed from bone immediately after experimentation and frozen at −80°C. Sections of frozen cartilage were then cut and stained with 9G3 and DAPI before being imaged. Two sections of cartilage were stained and imaged per sample with 5 samples evaluated per test group.
Statistical Analysis
Three-way analyses of variance (ANOVA) were used to determine the effects of lubricant (PBS vs. rhPRG4), IL-1α incubation condition (IL-1α treated vs. control), and cartilage harvest region (anterior, mid, and posterior) on static and dynamic COF values in mechanically stimulated bovine cartilage explants. Lubricant and IL-1α incubation condition were across-subject factors and harvest region was a within-subject factor in the model. If significant interactions were detected, partial F-tests were used post hoc to test simple effects (e.g. lubricant effect within IL-1α incubation condition or IL-1α effect within lubricant). Similarly, a two-way ANOVA followed by partial F-tests were used to determine the effects of the lubricant and IL-1α incubation condition on PRG4 expression assayed via RT-PCR. Because RT-PCR data was log transformed prior to analysis, geometric means and their associated standard errors are presented. All analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC). Statistical significance determined based on p < 0.05.
Results
Application of rhPRG4 to Cartilage Explants Stimulated with IL-1 Restores Low Friction
PRG4 accumulation at the articular surface (Figure 2) was qualitatively diminished in IL-1α treated, non-mechanically stimulated explants (IL) compared to their IL-1α free, non-mechanically stimulated counterparts (Control). The lack of PRG4 accumulation at the articular surface of IL-1α stimulated explants was restored upon lubrication with rhPRG4 but diminished upon lubrication with PBS (Figure 2). These qualitative observations in PRG4 surface accumulation parallel the static COF data in Figure 3a, where explants with decreased PRG4 available at the articular surface demonstrated higher static COF. Three-way analysis of variance results indicate that the effect of lubricant on static COF values was dependent upon whether or not IL-1α was present (lubricant by- IL-1α condition interaction, p=0.022). IL-1α treated explants lubricated with PBS during mechanical stimulation displayed significantly higher static COF values compared to IL-1α free explants lubricated with PBS (p=0.02,). No significant difference was observed between IL-1α conditions in rhPRG4 lubricated explants (p=0.37). Additionally, IL-1α treated, rhPRG4 lubricated explants displayed significantly lower static COF compared to IL-1α treated, PBS lubricated explants (p=0.014). The significantly lower mean static COF (0.14 ±0.065) for IL-1α treated, rhPRG4 lubricated explants compared to the mean static COF (0.21 ±0.059) for IL-1α treated, PBS lubricated explants indicates that rhPRG4 has the ability to increase boundary lubrication in IL-1α treated explants. Mean static COF (0.16 ±0.066) of IL-1α free explants lubricated with rhPRG4 and the static COF (0.15 ±0.052) for IL-1α free explants lubricated with PBS were not significantly different (p=0.44). Thus, residual PRG4 at the articular surface of IL-1α free explants was capable of compensating for the lack of lubrication provided by PBS and may account for the lack of significant difference in friction values for IL-1α free explants lubricated with rhPRG4 compared to lubrication with PBS (Figure 2).
Figure 2.
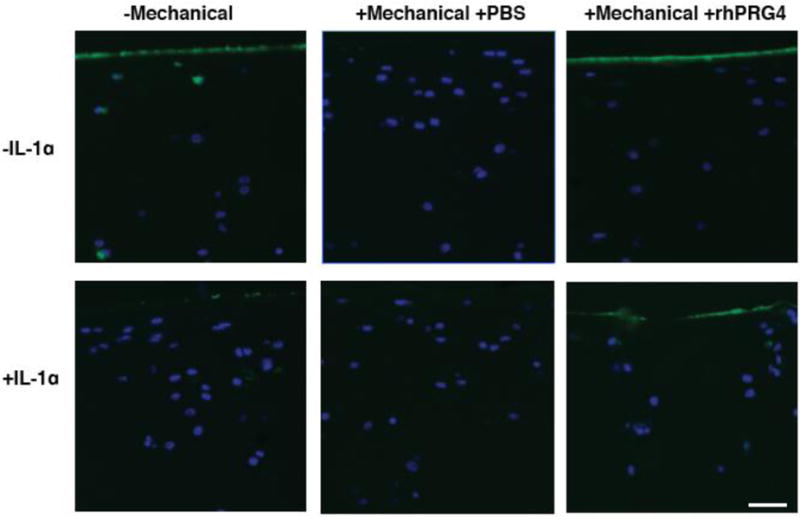
PRG4 Immunohistochemistry. Immunohistological detection of PRG4 by 9g3 (green) and cell nuclei via DAPI (blue) in non-mechanically stimulated (−Mechanical) and mechanically stimulated (+Mechanical) bovine cartilage explants cultured with IL-1α (+IL-1α, IL) or IL-1α free media (−IL-1α, Control) is shown in six representative images. Either phosphate buffered saline (PBS) or recombinant PRG4 (rhPRG4) was used as a lubricant for mechanically stimulated explants. IL-1α treated, non-mechanically stimulated explants (n=20) displayed decreased PRG4 compared to control treated, non-mechanically stimulated explants (Control, n=20). IL-PBS explants (n=13) displayed no change in PRG4 while IL-rhPRG4 explants (n=16) displayed increased PRG4 on the surface comparable to Control explants and Control-rhPRG4 explants (n=19). Control-PBS explants (n=14) displayed marked decrease in PRG4 compared to Control and Control-rhPRG4 explants. Scale bar is 50 microns.
Figure 3.
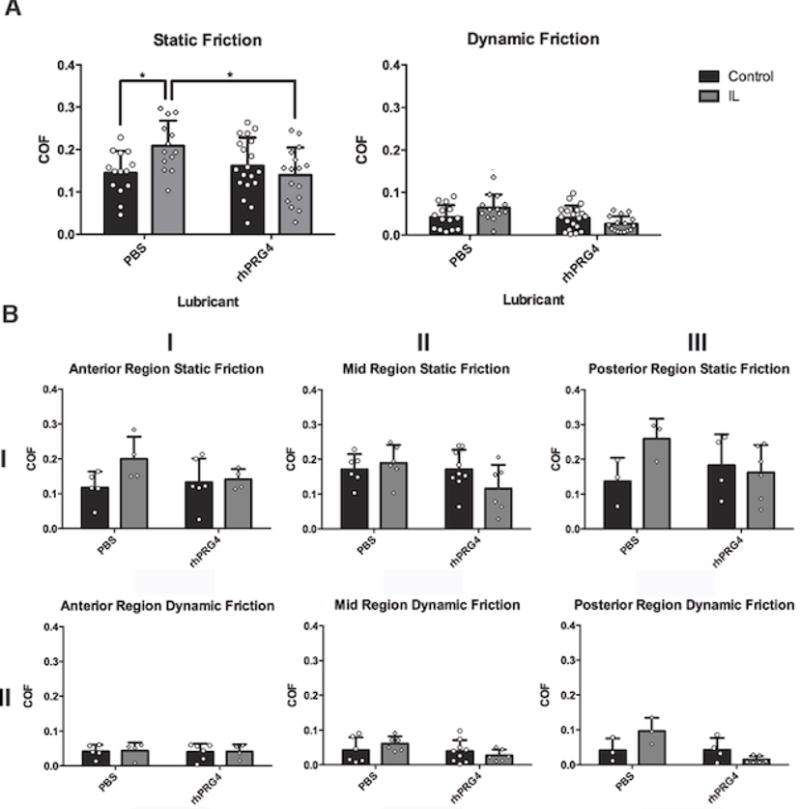
(A) Total COF Across Cartilage Explant Groups. Dynamic (right) and static (left) coefficient of friction (COF) data for mechanically stimulated, IL-1α supplemented (IL, gray) or IL-1α free (Control, black) explants lubricated with either phosphate buffered saline (PBS, left) or recombinant PRG4 (rhPRG4, right) are illustrated above. IL-PBS explants (n=13) displayed significantly higher static COF compared to Control-PBS explants (n=14, *p=0.02) IL-PBS explants also displayed significantly higher static COF compared to IL-rhPRG4 explants (n=16, *p=0.014). Values represented as mean ± standard deviation. (B) COF by Cartilage Region. Dynamic (right) and static (left) COF of the anterior, mid and posterior areas of the medial femoral condyle is shown. No significant difference was found for static and dynamic COF by cartilage region.
When dynamic COF was examined, analyses of variance results indicated no significant effect of lubricant (p=0.08), IL-1α incubation condition (p=0.90) nor any evidence of an interaction (p=0.21).
Static and dynamic COF values separated by location from the anterior, mid, and posterior regions of the femoral condyle are displayed in Figure 3B. Based on three-way analyses of variance results, there was no evidence that static or dynamic COFs differed across regions (p=0.26 and p=0.59, respectively), nor was their evidence that the effects of lubricant and/or IL-1α incubation condition were region dependent (all interactions, p’s > 0.10).
Labeled rhPRG4 Accumulates on the Articular Surface
Rhodamine labeled rhPRG4 was used to assess the extent to which rhPRG4 attached and infiltrated the cartilage in IL-1α stimulated explants lubricated with rhPRG4 and IL-1α free explants lubricated with rhPRG4. A greater amount of rhodamine labeled rhPRG4 was observed in the cartilage of IL-1α stimulated explants compared to IL-1α free controls (Figure 4). It appeared that rhPRG4 markedly accumulated on the cartilage surface.
Figure 4.
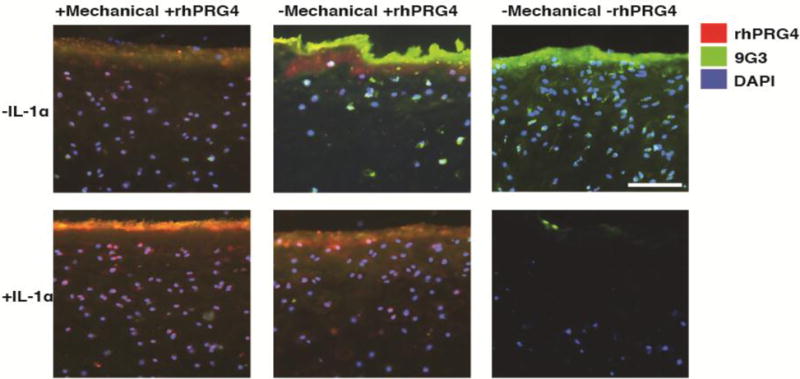
Immunohistochemistry of PRG4 in the Presence of Exogenous Rhodamine Labeled rhPRG4. Representative images showing exogenous rhPRG4 (red) and a combination of exogenous and endogenous PRG4 for IL-1α treated (+IL-1α) and control (−IL-1α) explants with (+rhPRG4) or without (−rhPRG4) rhodamine labeled rhPRG4. Green indicates detection of all PRG4 present by 9g3(whether endogenous or exogenous) and red indicates exogenous, rhodamine-labeled rhPRG4. Counter staining of cell nuclei with DAPI is blue. Samples lubricated with rhPRG4 were either mechanically stimulated (+Mechanical) or not mechanically stimulated (−Mechanical). IL-1α stimulated explants without rhPRG4 (n=5) exhibited little to no endogenous PRG4, while IL-1α stimulated samples with rhPRG4 (n=5) displayed greater amounts of exogenous rhPRG4 penetrating into the cartilage superficial zone. IL-1α stimulated samples appear to exhibit more exogenous rhPRG4 attachment than their IL-1α free counterparts (n=5). This suggests that IL-1α stimulation diminishes endogenous PRG4 while allowing for increased exogenous rhPRG4 attachment and penetration into the cartilage tissue. Scale bar is 100 microns.
Restoration of PRG4 Expression via rhPRG4 in IL-1α Stimulated Cartilage Explants
Based on the two-way analysis of variance, there was a significant interaction between lubricant and IL-1α incubation condition on PRG4 expression (p=0.006). PRG4 expression was significantly up regulated from 30.8 ± 19 copies in IL-1α free explants lubricated with PBS to 3330 ± 1760 copies in IL-1α free explants lubricated with rhPRG4 (p<0.001)(Figure 5). These data indicate that rhPRG4 significantly increases native PRG4 expression in cartilage explants undergoing mechanical stimulation when not exposed to inflammation. Explants stimulated with IL-1α did not respond in this manner. There was a significant increase in PRG4 expression from 30.8 ± 19 copies in IL-1α free explants lubricated with PBS to 401 ± 340 copies in IL-1α stimulated explants lubricated with PBS (p=0.015). Conversely, explants stimulated with IL-1α and lubricated with rhPRG4 trended toward significantly decreased PRG4 expression from 3330 ± 1760 copies in IL-1α free explants lubricated with rhPRG4 to 525 ± 41.2 copies (p=0.057).
Figure 5.
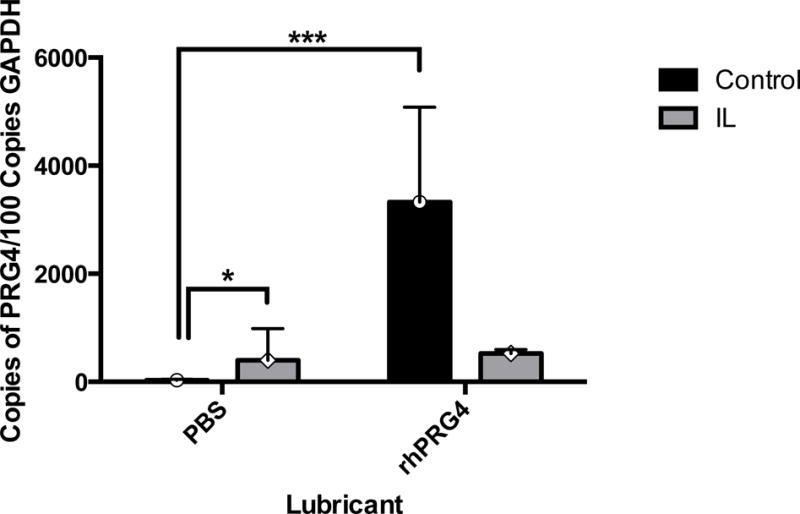
PRG4 mRNA Expression Across Cartilage Explant Groups PRG4 mRNA for mechanically stimulated bovine cartilage explants cultured with IL-1α supplemented medium (IL, gray) or IL-1α free medium (Control, black) is shown above. Either phosphate buffered saline (PBS) or recombinant PRG4 (rhPRG4) was used as a lubricant during mechanical stimulation. PRG4 expression was significantly up regulated from 30.8 ± 19 copies in control explants lubricated with PBS to 3330 ± 1760 copies in control explants lubricated with rhPRG4 (n=3, p<0.001). PRG4 expression significantly increased from 30.8 ± 19 copies in IL-1α free explants lubricated with PBS to 401 ± 340 copies in IL-1α stimulated explants lubricated with PBS (n=3, p=0.015). Explants stimulated with IL-1α and lubricated with rhPRG4 trended toward significantly decreased PRG4 expression from 3330 ± 1760 copies in IL-1α free explants lubricated with rhPRG4 to 525 ± 41.2 copies (n=3, p=0.057). Values represented as geometric mean ± geometric standard error.
Discussion
This study indicated that IL-1α stimulation of bovine explants resulted in significantly increased static COF under lubrication with PBS and that rhPRG4 was capable of significantly lowering increased COF under this inflammatory condition in vitro. Furthermore, we showed that in the IL-1α free condition PRG4 expression is significantly up regulated with use of rhPRG4 as a lubricant and that the effect of IL-1α stimulation on PRG4 expression compared to the IL-1α free condition is lubricant dependent under mechanical stimulation. However, we did not observe the same findings upon analyzing explants separately from the anterior, mid, and posterior regions of the femoral condyle. This is perhaps due to small regional sample size numbers, especially in the posterior region, which partly occurred as a result of our exclusion criteria. This observation must be reconciled with other studies that showed explants from the anterior femoral condyle contained greater extractable amounts of PRG4 compared to explants from the posterior area.33; 37
Recently, Peng et al found significant differences in equilibrium COF between the anterior-most and posterior-most cartilage regions of the femoral condyle in a pin-on-disc tribological system with cartilage harvested from calves.37 Our present study evaluated static and dynamic COF in the anterior, mid, and posterior regions of the femoral condyle using a cartilage disc-on-cartilage disc set up with explants harvested from skeletally mature adult steers. Peng et al only allowed for 2 minutes of cartilage depressurization following a 0.1 MPa load and performed studies at a 0.5 mm/s sliding speed, which is faster than commonly used in other tribological studies.37-41 Our present work used 8 minutes of depressurization following a 0.42 MPa load and a 0.3mm/s sliding speed coupled with rigorous exclusion criteria.
Immunohistochemistry confirmed that rhodamine-labeled rhPRG4 attached to the cartilage surface, possibly infiltrated the superficial zone, and provided a surface coating that recapitulates native PRG4, particularly in the IL-1α stimulated explants. This rhPRG4 coating acted as an anti-adhesive and lubricating physisorbed layer that diminished both static and dynamic friction in IL-1α stimulated, bovine cartilage explants. These in vitro inflamed explants showed little to no residual, native PRG4 at the cartilage surface. Thus, the significant reduction in dynamic and static friction between IL-1α stimulated explants lubricated with saline compared to IL-1α stimulated explants lubricated with rhPRG4 was due to the restoration of boundary lubrication by the addition of exogenous rhPRG4. Also, the lack of a statistically significant difference in friction between IL-1α stimulated explants lubricated with rhPRG4 and IL-1α free explants lubricated with saline indicates that rhPRG4 may be lubricating just as effectively as the naturally expressed residual bovine PRG4 found on IL-1α free explants. The exact mechanism by which IL-1α removes PRG4 from the surface has yet to be fully determined. However, we posit that the combination of PRG4 down regulation by IL-1α, along with increased MMP production in IL-1α exposed cartilage,17 promotes the removal of PRG4 from the cartilage surface.
Our current work indicates that exogenous rhPRG4 and, thus perhaps, synovial PRG4 have mechanobiological effects. The articular surface of IL-1α free cartilage re-lubricated by rhPRG4 resulted in more native PRG4 expression. The mechanism through which rhPRG4 up regulates native PRG4 expression has yet to be fully elucidated. However, prior reports describe that the combination of load and lubricated mechanical sliding increases PRG4 expression.42; 43 The up regulation of PRG4 expression in IL-1α stimulated explants evidently depends upon the lubricant used. PBS as the lubricant led to an increase in PRG4 expression, whereas rhPRG4 as the lubricant may have led to a decrease in PRG4 expression. Overall, the IL-1α stimulation appeared to interfere with the normal mechanobiological response of cartilage to the combination of load and lubricated mechanical sliding. These results also support PRG4’s role as the primary boundary lubricant in synovial fluid since neither HA nor surface-active phospholipids were added. Overall, HA may play a synergistic role with PRG4 in lowering COF,39 but the role phospholipids play in boundary lubrication is unclear.44-47 In regards to whether or not PRG4 has a direct anti-inflammatory effect, a recent study showed that PRG4 competes with HA in binding CD44, and initiating an anti-inflammatory response.48
Intra-articular delivery of rhPRG4 may be therapeutically applicable to other forms of joint inflammation as well. Indeed, human synovial fluid samples from patients with OA and RA patients,49 as well as menisci from OA patients,50 have been shown to contain decreased PRG4 compared to control samples. In other work, human RA tissue samples from patients with advanced disease progression displayed significantly decreased PRG4 expression compared to control samples.51
Prior studies indicate that PRG4 protein levels are decreased with inflammation,20; 52; 53 and that PRG4 mRNA expression is down regulated.21; 22; 54 However, PRG4 levels were increased at certain time-points in models of PTOA.23-25 Quantification of PRG4 using ELISA and Western blot carry the limitations inherent in the anti-PRG4 antibodies currently available. These antibodies detect either a terminal end of the PRG4 protein or the glycosylated mucin domain.36 Thus, PRG4 protein fragments, generated by increased proteolytic activity in inflammatory conditions, may have been detected and reported in lieu of intact PRG4 protein in these previous studies.
Limitations of this study include that bovine cartilage samples were obtained from specimens of varying ages and only from the medial femoral condyles of the animals. We had greater consistency in harvesting from a curved surface like the femoral condyle but this also creates the limitation of not having a paired tibial plateau cartilage surface. Only 8 minutes for cartilage depressurization was allowed in order to maintain cartilage viability during friction testing to enable subsequent immunohistology and RNA isolation. Eight minutes approximates a majority of the depressurization but is not maximal.55 However, the equilibrium loads used in this study were similar to prior tribological studies.56 Additionally, the cartilage explant bearings in this study were cultured to maintain cell viability and only mechanically stimulated once. This enabled us to determine the mechanical, cartilage-on-cartilage, frictional effects as well as the biological effects of IL-1α stimulation on protein expression in chondrocytes for the 12 mm explant from each explant pair. A gimbal system57; 58 was not in place in our test system to maintain perfect co-planarity as the two oscillating cartilage surfaces moved past one another during the friction tests. Thus variations in friction caused by surface imperfections or imprecise mounting could also cause a sporadic increase in friction, which is why criteria were established for acceptability of the data.
Conclusions
Exogenous rhPRG4 significantly reduced static friction values for IL-1α stimulated, bovine cartilage explants. Immunohistochemistry for PRG4 showed removal of PRG4 from the cartilage surface during IL-1 stimulation. This PRG4 deficiency at the surface was restored upon addition of rhPRG4 as a lubricant. Additionally, IL-1α free cartilage explants showed significantly increased PRG4 expression when lubricated with rhPRG4 compared to saline. IL-1 alters the normal mechanobiological response of cartilage to the combination of load and lubricated mechanical sliding.
Acknowledgments
Funding for the study was provided by US National Institutes of Health Grants R01-AR050180, R42AR057276, P20- GM104937 and CDMRP-PR110746. Competing Interests: Please note that Dr. Jay was granted a United States patent “Tribonectin polypeptides and uses thereof,” US6743774B1 for the therapeutic use of PRG4 in joints. He receives support from a Phase II STTR from the NIH for the clinical translation efforts related to his patent. Dr. Schmidt is a paid consultant of Lubris, LLC, who provided the recombinant human PRG4 for the study. Dr. Schmidt was granted two United States patents “Ophthalmic device, and method of use thereof, for increasing ocular boundary lubrication,” US8563028B2 and “Therapeutic Modulation of Ocular Surface Lubrication,” US9138457B2 for therapeutic use of PRG4 in the eye. The present paper does not materially or financially affect Dr. Jay’s or Dr. Schmidt’s patents relating to recombinant PRG4. The commercial relationship does not alter the authors’ adherence to all the JOR policies on sharing data and materials.
Footnotes
Author Contributions
K.L. and L.Z. have made substantial contributions to research. K.L., K.E., T.S., B.F., G.B. and G.J. have made significant contributions to research design or acquisition, analysis and interpretation of data and to drafting of paper or revising it critically. All authors have read and approved the final submitted manuscript.
All other authors have no conflicts of interest.
References
- 1.Murphy L, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis and rheumatism. 2008;59:1207–1213. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective: A population-based review of the fourth most common cause of hospitalization in U.S. adults. Orthopedic nursing. 2012;31:85–91. doi: 10.1097/NOR.0b013e31824fcd42. [DOI] [PubMed] [Google Scholar]
- 3.Gabriel SE, Crowson CS, Campion ME, et al. Direct medical costs unique to people with arthritis. The Journal of rheumatology. 1997;24:719–725. [PubMed] [Google Scholar]
- 4.Brown TD, Johnston RC, Saltzman CL, et al. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. Journal of orthopaedic trauma. 2006;20:739–744. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 5.Zhang LZ, Z H, Jiang Y, Tu YH, Jiang PH, Yang AL. Mechanical and biologic link between cartilage and subchondral bone in osteoarthritis. Arthritis Care Res. 2012;64:960–967. doi: 10.1002/acr.21640. [DOI] [PubMed] [Google Scholar]
- 6.Anderson DD, Chubinskaya S, Guilak F, et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2011;29:802–809. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine & growth factor reviews. 2002;13:323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 8.Evans CH, Gouze JN, Gouze E, et al. Osteoarthritis gene therapy. Gene therapy. 2004;11:379–389. doi: 10.1038/sj.gt.3302196. [DOI] [PubMed] [Google Scholar]
- 9.Jay GD, Waller KA. The biology of lubricin: near frictionless joint motion. Matrix biology: journal of the International Society for Matrix Biology. 2014;39:17–24. doi: 10.1016/j.matbio.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Klein TJ, Schumacher BL, Schmidt TA, et al. Tissue engineering of stratified articular cartilage from chondrocyte subpopulations. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2003;11:595–602. doi: 10.1016/s1063-4584(03)00090-6. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt TA, Plaas AH, Sandy JD. Disulfide-bonded multimers of proteoglycan 4 PRG4 are present in normal synovial fluids. Biochimica et biophysica acta. 2009;1790:375–384. doi: 10.1016/j.bbagen.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Jay GD, Harris DA, Cha CJ. Boundary lubrication by lubricin is mediated by O-linked beta(1-3)Gal-GalNAc oligosaccharides. Glycoconjugate journal. 2001;18:807–815. doi: 10.1023/a:1021159619373. [DOI] [PubMed] [Google Scholar]
- 13.Waller KA, Zhang LX, Elsaid KA, et al. Role of lubricin and boundary lubrication in the prevention of chondrocyte apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:5852–5857. doi: 10.1073/pnas.1219289110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waller KA, Zhang LX, Fleming BC, et al. Preventing friction-induced chondrocyte apoptosis: comparison of human synovial fluid and hylan G-F 20. J Rheumatol. 2012;39:1473–1480. doi: 10.3899/jrheum.111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karamchedu NP, Tofte JN, Waller KA, et al. Superficial zone cellularity is deficient in mice lacking lubricin: a stereoscopic analysis. Arthritis research & therapy. 2015;18:64. doi: 10.1186/s13075-016-0967-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruan MZC, C V, Cela R, Clarke C, Lundgren-Akerlund E, Barry MA, Lee BHL. Treatment of osteoarthritis using a helper-dependent adenoviral vector retargeted to chondrocytes. Molecular Therapy- Methods & Clinical Development. 2016:3. doi: 10.1038/mtm.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sofat N. Analyzing the role of endogenous matrix molecules in the development of osteoarthritis. International Journal of Experimental Pathology. 2009;90:463–479. doi: 10.1111/j.1365-2613.2009.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nature reviews Rheumatology. 2010;6:625–635. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 19.Ghivizzani SC, Kang R, Georgescu HI, et al. Constitutive intra-articular expression of human IL-1 beta following gene transfer to rabbit synovium produces all major pathologies of human rheumatoid arthritis. Journal of immunology. 1997;159:3604–3612. [PubMed] [Google Scholar]
- 20.Schmidt TA, Gastelum NS, Han EH, et al. Differential regulation of proteoglycan 4 metabolism in cartilage by IL-1alpha, IGF-I, and TGF-beta1. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2008;16:90–97. doi: 10.1016/j.joca.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Cheng J, Wang Y, Wang Z, et al. Differential regulation of proteoglycan-4 expression by IL-1alpha and TGF-beta1 in rat condylar chondrocytes. The Tohoku journal of experimental medicine. 2010;222:211–218. doi: 10.1620/tjem.222.211. [DOI] [PubMed] [Google Scholar]
- 22.Jones AR, Flannery CR. Bioregulation of lubricin expression by growth factors and cytokines. Eur Cell Mater. 2007;13:40–45. doi: 10.22203/ecm.v013a04. discussion 45. [DOI] [PubMed] [Google Scholar]
- 23.Atarod M, Ludwig TE, Frank CB, et al. Cartilage boundary lubrication of ovine synovial fluid following anterior cruciate ligament transection: a longitudinal study. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2015;23:640–647. doi: 10.1016/j.joca.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Antonacci JM, Schmidt TA, Serventi LA, et al. Effects of equine joint injury on boundary lubrication of articular cartilage by synovial fluid: role of hyaluronan. Arthritis and rheumatism. 2012;64:2917–2926. doi: 10.1002/art.34520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neu CP, Reddi AH, Komvopoulos K, et al. Increased friction coefficient and superficial zone protein expression in patients with advanced osteoarthritis. Arthritis and rheumatism. 2010;62:2680–2687. doi: 10.1002/art.27577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ludwig TE, McAllister JR, Lun V, et al. Diminished cartilage-lubricating ability of human osteoarthritic synovial fluid deficient in proteoglycan 4: Restoration through proteoglycan 4 supplementation. Arthritis and rheumatism. 2012;64:3963–3971. doi: 10.1002/art.34674. [DOI] [PubMed] [Google Scholar]
- 27.Flannery CR, Zollner R, Corcoran C, et al. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis and rheumatism. 2009;60:840–847. doi: 10.1002/art.24304. [DOI] [PubMed] [Google Scholar]
- 28.Jay GD, Fleming BC, Watkins BA, et al. Prevention of cartilage degeneration and restoration of chondroprotection by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis and rheumatism. 2010;62:2382–2391. doi: 10.1002/art.27550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elsaid KA, Zhang L, Waller K, et al. The impact of forced joint exercise on lubricin biosynthesis from articular cartilage following ACL transection and intra-articular lubricin’s effect in exercised joints following ACL transection. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2012;20:940–948. doi: 10.1016/j.joca.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 30.Teeple E, Elsaid KA, Jay GD, et al. Effects of supplemental intra-articular lubricin and hyaluronic acid on the progression of posttraumatic arthritis in the anterior cruciate ligament-deficient rat knee. Am J Sports Med. 2011;39:164–172. doi: 10.1177/0363546510378088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vugmeyster Y, Wang Q, Xu X, et al. Disposition of human recombinant lubricin in naive rats and in a rat model of post-traumatic arthritis after intra-articular or intravenous administration. The AAPS journal. 2012;14:97–104. doi: 10.1208/s12248-011-9315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elsaid K, Z L, Shaman Z, Patel C, Schmidt TA, Jay GD. The Impact of Early Intra-Articular Administration of Interleukin-1 Receptor Antagonist on Lubricin Metabolism and Cartilage Degeneration in an Anterior Cruciate Ligament Transection Model. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2015;23:114–121. doi: 10.1016/j.joca.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neu CP, Khalafi A, Komvopoulos K, et al. Mechanotransduction of bovine articular cartilage superficial zone protein by transforming growth factor beta signaling. Arthritis and rheumatism. 2007;56:3706–3714. doi: 10.1002/art.23024. [DOI] [PubMed] [Google Scholar]
- 34.Abubacker SS, TA Full-Length Recombinant Human Proteoglycan 4 Interacts with Hyaluronan to Provide Cartilage Boundary Lubrication. Annals of Biomedical Engineering. 2015:1–10. doi: 10.1007/s10439-015-1390-8. [DOI] [PubMed] [Google Scholar]
- 35.Samsom MS, TA Characterization of full-length recombinant human Proteoglycan 4 as an ocular suface boundary lubricant. Experimental Eye Research. 2014;127:14–19. doi: 10.1016/j.exer.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 36.Minrong A, Mea W. Anti-Lubricin Monoclonal Antibodies Created Using Lubricin-Knockout Mice Immunodetect Lubricin in Several Species and in Patients with Healthy and Diseased Joints. PloS one. 2015;10:e0116237. doi: 10.1371/journal.pone.0116237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng G, McNary SM, Athanasiou KA, et al. The distribution of superficial zone protein (SZP)/lubricin/PRG4 and boundary mode frictional properties of the bovine diarthrodial joint. Journal of biomechanics. 2015;48:3406–3412. doi: 10.1016/j.jbiomech.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jay GD, Haberstroh K, Cha CJ. Comparison of the boundary-lubricating ability of bovine synovial fluid, lubricin, and Healon. Journal of biomedical materials research. 1998;40:414–418. doi: 10.1002/(sici)1097-4636(19980605)40:3<414::aid-jbm11>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 39.Ludwig TE, Hunter MM, Schmidt TA. Cartilage boundary lubrication synergism is mediated by hyaluronan concentration and PRG4 concentration and structure. BMC musculoskeletal disorders. 2015;16:386. doi: 10.1186/s12891-015-0842-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gleghorn JP, Bonassar LJ. Lubrication mode analysis of articular cartilage using Stribeck surfaces. Journal of biomechanics. 2008;41:1910–1918. doi: 10.1016/j.jbiomech.2008.03.043. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt TA, Sah RL. Effect of synovial fluid on boundary lubrication of articular cartilage. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2007;15:35–47. doi: 10.1016/j.joca.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Grad S, Gogolewski S, Alini M, et al. Effects of simple and complex motion patterns on gene expression of chondrocytes seeded in 3D scaffolds. Tissue engineering. 2006;12:3171–3179. doi: 10.1089/ten.2006.12.3171. [DOI] [PubMed] [Google Scholar]
- 43.Nugent-Derfus GE, Takara T, O’Neill JK, et al. Continuous passive motion applied to whole joints stimulates chondrocyte biosynthesis of PRG4. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2007;15:566–574. doi: 10.1016/j.joca.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt TA, Gastelum NS, Nguyen QT, et al. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis and rheumatism. 2007;56:882–891. doi: 10.1002/art.22446. [DOI] [PubMed] [Google Scholar]
- 45.Jay GD, Cha CJ. The effect of phospholipase digestion upon the boundary lubricating ability of synovial fluid. The Journal of rheumatology. 1999;26:2454–2457. [PubMed] [Google Scholar]
- 46.Seror J, Zhu L, Goldberg R, et al. Supramolecular synergy in the boundary lubrication of synovial joints. Nature communications. 2015;6:6497. doi: 10.1038/ncomms7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mirea DA, T-S AM, Matei CI, Munteanu B, Piednoir A, Rieu JP, Blanchin MG, Berthier Y. Role of the biomolecular interactions in the structure and tribological properties of synovial fluid. Tribology International. 2013;59:302–311. [Google Scholar]
- 48.Al-Sharif A, Jamal M, Zhang LX, et al. Lubricin/Proteoglycan 4 Binding to CD44 Receptor: A Mechanism of the Suppression of Proinflammatory Cytokine-Induced Synoviocyte Proliferation by Lubricin. Arthritis & rheumatology. 2015;67:1503–1513. doi: 10.1002/art.39087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kosinska MK, Ludwig TE, Liebisch G, et al. Articular Joint Lubricants during Osteoarthritis and Rheumatoid Arthritis Display Altered Levels and Molecular Species. PloS one. 2015;10:e0125192. doi: 10.1371/journal.pone.0125192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Musumeci G, Trovato FM, Loreto C, et al. Lubricin expression in human osteoarthritic knee meniscus and synovial fluid: a morphological, immunohistochemical and biochemical study. Acta histochemica. 2014;116:965–972. doi: 10.1016/j.acthis.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 51.Ungethuem U, Haeupl T, Witt H, et al. Molecular signatures and new candidates to target the pathogenesis of rheumatoid arthritis. Physiological genomics. 2010;42A:267–282. doi: 10.1152/physiolgenomics.00004.2010. [DOI] [PubMed] [Google Scholar]
- 52.Elsaid KA, Fleming BC, Oksendahl HL, et al. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis and rheumatism. 2008;58:1707–1715. doi: 10.1002/art.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teeple E, Elsaid KA, Fleming BC, et al. Coefficients of friction, lubricin, and cartilage damage in the anterior cruciate ligament-deficient guinea pig knee. J Orthop Res. 2008;26:231–237. doi: 10.1002/jor.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Young AA, McLennan S, Smith MM, et al. Proteoglycan 4 downregulation in a sheep meniscectomy model of early osteoarthritis. Arthritis research & therapy. 2006;8:R41. doi: 10.1186/ar1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krishnan R, Kopacz M, Ateshian GA. Experimental verification of the role of interstitial fluid pressurization in cartilage lubrication. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2004;22:565–570. doi: 10.1016/j.orthres.2003.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonnevie ED, Galesso D, Secchieri C, et al. Elastoviscous Transitions of Articular Cartilage Reveal a Mechanism of Synergy between Lubricin and Hyaluronic Acid. PloS one. 2015;10:e0143415. doi: 10.1371/journal.pone.0143415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jay GD. Characterization of a lubricating fraction of bovine synovial fluid I. Chemical and lubricating properties. Connective Tissue Research. 1992;28:71–88. doi: 10.3109/03008209209014228. [DOI] [PubMed] [Google Scholar]
- 58.Davis W, L SL, Sokoloff L. A proposed model of boundary lubrication synovial fluid: Structuring of boundary water. Journal of Biomechanical Engineering. 1979;101:185–192. [Google Scholar]


