Summary
A number of clinical studies have linked adiposity with increased cancer incidence, progression and metastasis, and adipose tissue is now being credited with both systemic and local effects on tumour development and survival. Adipocytes, a major component of benign adipose tissue, represent a significant source of lipids, cytokines and adipokines, and their presence in the tumour microenvironment substantially affects cellular trafficking, signalling and metabolism. Cancers that have a high predisposition to metastasize to the adipocyte-rich host organs are likely to be particularly affected by the presence of adipocytes. Although our understanding of how adipocytes influence tumour progression has grown significantly over the last several years, the mechanisms by which adipocytes regulate the meta-static niche are not well-understood. In this review, we focus on the omentum, a visceral white adipose tissue depot, and the bone, a depot for marrow adipose tissue, as two distinct adipocyte-rich organs that share common characteristic: they are both sites of significant metastatic growth. We highlight major differences in origin and function of each of these adipose depots and reveal potential common characteristics that make them environments that are attractive and conducive to secondary tumour growth. Special attention is given to how omental and marrow adipocytes modulate the tumour microenvironment by promoting angiogenesis, affecting immune cells and altering metabolism to support growth and survival of metastatic cancer cells.
Keywords: Adipose tissue, bone metastasis, MAT, omental metastasis, WAT
With many epidemiological studies raising awareness and correlating Western diet and metabolic disturbances with cancer progression and metastatic development, it is imperative to understand the molecular basis for adiposity-driven tumorigenesis. Overeating and sedentary lifestyles are clearly predominant reasons for elevated fat depots, but other physiological factors and medical conditions such as age and hormonal status are associated with adipocyte hypertrophy, adipogenesis and increased body mass index (1,2). General involvement of adipocytes in lipid storage and secretion of endocrine factors underscores the importance of adipose tissue in metabolism and inflammation (3–8). Our current body of knowledge indicates that various adipose depots exhibit important differences in adipogenic progenitors, metabolism and effects they exert on system organs. Herein, we will review how omentum, an organ that is naturally rich in adipocytes, and bone marrow, which becomes fatty in response to physiological and pathological factors, contribute to creating a niche that attracts and supports secondary metastatic tumour growth.
Metastatic microenvironment generated by white adipose tissue
According to National Cancer Institute, peritoneal metastases are the most common occurrences in the spread of ovarian, colorectal, gastric, pancreatic and uterine cancers (9), with the omentum often being the predominant metastatic site within the peritoneum (10,11). Direct intraperitoneal seeding is the most common route of dissemination of cancer cells to omentum because of the proximity of the primary abdominal tumours (10). Cancer cells that are shed from the primary tumour are initially spread via peritoneal fluid and eventually by the ascites accumulating in the abdominal cavity due to disruption of lymphatic system (12,13). Dissemination of ovarian tumours through the vasculature as a primary mode of metastasis is very rare, although pelvic and/or para-aortic lymph nodes can be involved in this process (13,14).
Omentum as a visceral white adipose tissue depot
To better understand what lures cancer cells to the omentum, we shall first review its structure. The peritoneum is a continuous membrane sheet lining the peritoneal cavity (15). The omentum is a visceral fold of the peritoneum subdivided into greater and lesser components. The lesser omentum extends from the smaller curvature of the stomach to the liver. The greater omentum is a fatty pad that extends from the large curvature of the stomach and, like an ‘apron’, and covers the majority of the abdominal organs converging into parietal peritoneum. This organ is made of two types of tissues. First is a highly vascularized connective tissue rich in white adipocytes and covered by a mesothelial cell layer that rests on a basement membrane, except where structures known as milky spots are located (15). The other tissue type is a fenestrated, membranous and translucent layer composed of two mesothelial cell layers wrapping collagen fibres and fibroblast-like cells and not resting on basement membrane (15). The main functions of the omentum as an organ are to store lipids, pool immune cells, adhere to peritoneum to localize inflammation (16,17), regulate fluid exchange in the peritoneal cavity and supply angiogenic and neurogenic factors (18,19).
Omentum is one of the six visceral white adipose tissue (WAT) depots: omental, mesenteric, perirenal, gonadal, epicardial and retroperitoneal, whose developmental origin is a subject of an ongoing debate (20,21). Visceral WAT forms shortly after birth following prenatal development of subcutaneous WAT (22). All six visceral depots derive from progenitors positive for Wilms’ tumour gene (Wt1) (20). Expression of Wt1 is specific for visceral WAT and does not trace to progenitors of subcutaneous WAT and brown adipose tissue (20). The Wt1-positive mesothelium, a major source of visceral WAT, is the key characteristic differentiating visceral fat depots from subcutaneous fat (20,23–25). There is heterogeneity among the various visceral depots with respect to Wt1 expression, with most of the epidymal but only a portion of retroperitoneal WAT arising from Wt1-positive cells. This notion of heterogeneity is further supported by the fact that a significant portion of interscapular and retroperitoneal WAT but not epidymal fat arise from Myf5-positive precursors of brown adipose tissue (21).
Omentum as a site of metastasis from ovarian cancer
With 80% of all serous ovarian carcinomas metastasizing to omentum, this organ appears to act as a very specific target for this type of tumour (11,26). Omentum is essentially a large fat pad and an endocrine tissue that can store lipids and serve as a potential source of energy for metastatic tumour cells (27). An experimental mouse model mimicking ovarian cancer metastasis by intraperitoneal injection of tumour cells revealed that ovarian cancer cells have high propensity to seed and proliferate in the omentum (11). High rate of homing towards the adipocytes in this model was attributed to a variety of factors, such as IL-8, IL-6, monocyte chemoattractant protein-1 and adiponectin, secreted by omental adipocytes. Omental fat cells were also shown to activate pro-survival pathways, p38 and stat3, in the ovarian cancer cells, indicating the potential importance of an adipocyte-rich environment for homing and growth of tumour cells in the omentum.
Apart from chemical signalling towards the tumour cells, fat cells are also known to affect cancer cell growth and behaviour through lipid-sharing. Specifically, in vitro co-culture of ovarian cancer cells with adipocytes was shown to result in transfer and accumulation of lipid droplets in the tumour cells (11). This observation was in line with the observed presence of lipid droplets in ovarian cancer cells bordering adipocytes in the human tissue. Importantly, this acquisition of adipocyte-supplied lipids appeared to be depot-specific with most accumulation occurring upon co-culture with omental and peritoneal adipocytes compared with co-culture with subcutaneous and bowel mesenteric adipocytes (11). The lipid transfer from adipocytes induced β-oxidation in tumour cells and enhanced their proliferation in vitro (11). Reciprocally, cancer cells affected metabolism in adipocytes by inducing hydrolysis of triglycerides stored in adipocyte lipid droplets into free fatty acids (FFA) and glycerol (11). Besides increased secretion of FFA and glycerol, activation of hormone-sensitive lipase (HSL), an enzyme central for lipolysis, and overexpression of perilipin A, a protein coating lipid droplets and activation of which is necessary for induced lipolysis (28), were detected (11). Essentially, it appears that cancer cells forced adipocytes to ‘give up’ their lipids, which ultimately translated into increased tumour burden. This process would eventually lead to transformation of omentum from a fat pad into a solid tumour with few remaining adipocytes, a process often referred to as ‘omental caking’. These observed molecular and histological changes upon adipocyte-tumour cell interactions cultivated the idea that cancer cells ‘consume’ the energy-rich lipids necessary for their survival and progression (29).
Omental adipocytes and metabolic effects on tumour cells
It appears that through paracrine secretions and the abundance of readily transferable lipids, adipocytes create a microenvironment that alters tumour metabolism and supports more aggressive phenotypes. In the majority of cancers, the rapid mode of proliferation forces tumour cells to modify their energy metabolism (30), by increasing de novo lipogenesis (31,32). The newly synthesized fatty acids are used for storage, membrane composition and signalling, and are central for cancer thriving. In contrast, tumours such as ovarian cancers that grow in adipose-rich microenvironments have unconventional metabolism characterized by reduced lipogenesis and increased rates of β-oxidation (11). These observations are in line with other studies demonstrating that exogenous lipids sustain cancer cell survival when de novo lipogenesis is inhibited (33,34). It appears increasingly clear that instead of ‘going through the trouble’ of making their own fuel in order to proliferate, tumour cells growing in lipid-rich adipose environments take advantage of having these fuel molecules catered to them (27,35). Thus, a ready source of energy may be a key reason behind their aggressive phenotype. It is also noteworthy that although not directly demonstrated in a context of omental metastases lipids have been shown to enhance the Warburg Effect in tumour cells (32,36–39). Because omental adipocytes are capable of inducing lipolysis in tumour cells (11), this lipolysis-generated glycerol has a potential to feed into the glycolytic pathway (40–42). This could offer an additional mechanism for enhanced tumour growth and metabolic adaptation in the metastatic niche.
Supporting adipocyte involvement in modulating tumour metabolism and progression was a discovery of significant overexpression of fatty acid-binding protein 4 (FABP4) (or aP2 protein) in omental metastases from ovarian cancer, particularly at the tumour–adipocyte interface (11). This was the first demonstration that this lipid chaperone, originally discovered in adipocytes and whose expression increases in response to fatty acid uptake and lipolytic stimulation (43–45), gets up-regulated in tumour cells exposed to adipocyte-derived lipids. Lipid exposure and cholesterol accumulation are known to induce FABP4 expression in other cell types, such as macrophages, where this chaperone contributes to foam cell formation and atherosclerotic plaque instability (43,46,47). Interestingly, FABP4 is also present in endothelial cells, where it appears to regulate cell proliferation (48) and its high expression in endothelial glioblastoma cells has been linked to the robust angiogenesis associated with this disease (49). Endothelial expression of FABP4 in adipocyte-rich environments such as omentum could have a potential role in growth and progression of metastatic ovarian cancers. Indeed, reduced tumour burden and lower metastatic potential in FABP4-deficient mice was found to be associated with inhibitory effects on tumour vasculature (11). These findings stress the importance of the adipocyte-rich tumour microenvironment and both tumour-derived and host-derived FABP4 in metastatic development and progression and call for further investigations.
Evidence of altered metabolic phenotype in clinical samples
To better appreciate the relevance of in vitro and in vivo animal studies, we performed Oncomine analyses and compared mRNA expression of genes linked to lipid metabolism in primary and metastatic ovarian cancers. A significant upregulation of genes encoding for FABP4 and CD36 (fatty acid translocase [FAT]), proteins involved in membrane transfer of fatty acids (43,50), was revealed in metastatic ovarian cancer when compared with primary tumours and confirmed in all four available datasets (Table 1). The changes in expression of specific isomers of fatty acid transport proteins (FATP1–6), which are known to regulate long-chain and very long-chain fatty acid uptake alongside CD36 (50,51), were not consistently significant across the analyzed datasets (data not shown). Both CD36 and FATP are known to be modulated by the microenvironment, particularly in obesity, and have been shown to synergistically enhance fatty acid uptake, although in some models CD36 was demonstrated to be far more effective than FATPs (50,52). The functional differences between CD36 and FATPs, especially in a context of tumour metabolism, are not known and need to be explored.
Table 1.
Oncomine analysis of mRNA expression of genes involved in fatty acid binding, uptake, synthesis and metabolism in patients with ovarian cancer
| Gene | Ovarian cancer dataset | Fold change | P value | n |
|---|---|---|---|---|
| FABP4 | Bittner [158] | 5.78 | 4.76E-8 | P: 166; M: 75 |
| Adib [159] | 37.42 | 0.004 | P: 6; M: 6 | |
| Anglesio [160] | 17.63 | 6.00E-5 | P: 74; M: 16 | |
| Tothill [161] | 10.51 | 3.91E-11 | P: 189; M: 54 | |
| CD36 | Bittner [158] | 2.43 | 5.75E-8 | P: 166; M: 75 |
| Adib [159] | 1.71 | 0.043 | P: 6; M: 6 | |
| Anglesio [160] | 5.02 | 9.31E-5 | P: 74; M: 16 | |
| Tothill [161] | 3.45 | 7.90E-10 | P: 189; M: 54 | |
| FASN | Bittner [158] | 1.35 | 7.61E-4 | P: 166; M: 75 |
| HSL | Anglesio [160] | 1.21 | 0.009 | P: 74; M: 16 |
| Tothill [161] | 1.15 | 3.93E-4 | P: 189; M: 54 | |
| PLIN2 | Anglesio [160] | 2.18 | 0.004 | P: 74; M: 16 |
| Tothill [161] | 1.46 | 0.001 | P: 189; M: 54 |
‘The Oncomine database (Oncomine™ v4.5: 729 datasets, 91,866 samples) was used for the analysis of primary (P) vs. metastatic (M) tumors. Following genes were analyzed: Fatty acid binding protein 4 (FABP4), Cluster of differentiation (CD36; FAT), Fatty acid synthase (FASN), Hormone sensitive lipase (HSL), Perilipin2 (PLIN2); n = number of samples. Data were ordered by “overexpression” and the threshold was adjusted to P-value <1E−4; fold change, 2 and gene rank, top 10%. For each database, only genes that met the criteria for significance are reported’.
In addition to fatty acid transport genes, we also analyzed the expression data for genes involved in lipogenesis (i.e. fatty acid synthase, FASN) and lipolysis (i.e. HSL and perilipin 2). Only modest changes in FASN levels were detected (Table 1), likely because in adipocyte-rich microenvironments like omentum, tumour cells rely more on lipid uptake from adipocytes than their own lipogenesis. The changes in lipolysis genes were also moderate. It is reasonable to assume that expression of FABP4 and CD36, proteins facilitating transport of fatty acids, was increased in both cancer cells and adipocytes and thus easier to detect; whereas lipolysis occurred predominantly in adipocytes, and depending on the stage of the tumour, the observed changes in expression might have been too modest to detect. Expression of these genes was also likely affected by the tumour heterogeneity. One noteworthy caveat of the aforementioned analyses is that the available datasets do not distinguish omentum from other potential metastatic sites. However, the fact that the observed differences in expression between primary and metastatic sites were reproducible across several datasets speaks to potential involvement of lipid transport and metabolism in metastatic progression. Apparent differences in the lipidome between subcutaneous and omental fat depots as well as tumours in patients with malignant or benign ovarian or endometrial tumours have been previously shown in a small subset of Israeli patients (53). Significantly lower concentrations of linoleic acid (LA), a polyunsaturated fatty acid (PUFA) were shown in both fat depots in cancer patients as compared with healthy controls. Moreover, the malignant tumours appeared to have a significantly lower saturated-to-unsaturated fatty acid ratio compared with the benign tumours, suggesting that LA is mobilized from both WAT depots to be utilized by the tumours resulting in overall desaturation of the cancer. This potential link between fatty acid metabolism and ovarian cancer progression is an understudied concept that warrants further investigation.
Immune microenvironment in adipocyte-driven omental metastasis
Closer histological investigation of the omental metastases led to the idea that adipocytes might not be the only incentive that attracts cancer cells to the omentum (29). The extensive vascular network of omentum converges in some areas into capillary beds comingled with aggregates of immune cells (macrophages, mast cells, B lymphocytes and T lymphocytes) and stromal cells forming ‘milky spots’ (54,55). Characteristically, milky spots are reminiscent of secondary lymphoid structures and are important representatives of the immune system in the peritoneal cavity (56,57). Initially viewed as defense structures against bacterial antigens (58–60), these highly vascularized areas have since been implicated as preferential sites for adhesion and proliferation of disseminated tumour cells of ovarian and colorectal origin (10,61–66). For tumours such as ovarian cancers, in which metastases occur primarily by direct seeding rather than through circulation, milky spots allow for rapid and selective attachment and provide an environment suitable for tumour growth and survival (10)
The initial tumour cell adhesion to the milky spots induces the influx of macrophages, which correlates with generation of new milky spots around tumour cell concentration (64,66). Studies utilizing a variety of immunodeficient strains of mice – Igh6, Nude, Rag1, and BN XID lacking functional B-cells, T-cells, T- and B-, or T-, B- and NK-cells respectively, but having intact macrophages – show that compromised B-, T- or NK-cell-mediated immunity does not affect abundance of milky spots or homing of tumour cells to these structures (29). The depletion of peritoneal macrophages, on the other hand, inhibits the metastatic potential of ovarian cancer potentially by affecting the expression of stromal vascular endothelial growth factor (VEGF) (67). Interestingly, among a variety of known peritoneal adipose depots that include omental, mesentery, uterine, gonadal and splenoportal – milky spots are only found in omental and splenoportal fat pads (29,68). In line with these findings, the i.p. injection of four different ovarian cancer cell lines into mice resulted in cancerous lesions forming predominantly in omental and splenoportal fat depots, as opposed to other depots, validating the importance of milky spots in tumour homing (29). This significantly increased migration of ovarian cells was attributed to factors secreted by omental and splenoportal fat depots (29). It appears that interaction of adipocytes with peritoneal macrophages might be important for the homing of the disseminating ovarian cancer cells and progression of metastasis. It has been demonstrated that adipocyte-supplied PUFAs serve as ligands for transcription factor peroxisome proliferator-activated receptor β/δ (PPARβ/δ) in macrophages, causing their polarization into tumour-associated phenotype (known as tumour-associated macrophage) (69). Accordingly, tumour-associated macrophages isolated from serous ascites of ovarian cancer patients contain lipid droplets with high composition of LA, and lower but high levels of AA and docasahexaenoic acid (DHA) PUFAs (69). This accumulation of fatty acids by macrophages leads to transcriptional deregulation and activation of pro-tumorigenic phenotype.
Adipocyte-induced angiogenesis
Beside the aggregates of immune cells, milky spots are also characterized by microvascular beds and are areas of active angiogenesis (10,65), a known contributor to tumour progression (55,70). High levels of neovascularization in milky spots have been attributed to secretion of vascular endothelial growth factor A predominantly by mesothelial cells of the omentum and to a lesser degree by omental macrophages (10). Isolated and in situ omental adipocytes were shown to secrete VEGF (10,71) and express additional angiogenesis-associated factors and receptors, such as CD105 (10) and VEGFR3 (65). Notably, the expression of VEGF appears to be induced when omental adipocytes undergo hypoxia (71), which is in line with the evidence of clearly hypoxic phenotype in a portion of mesothelial cells and macrophages isolated from murine omentum (10). Both hypoxia and hypoxia-induced neovascularization have been linked to invasiveness, survival and chemoresistance of many cancers including ovarian tumours (72,73). A specific role for adipocytes and adipocyte-derived fatty acids in neovascularization is further suggested by the lower levels of angiogenesis associated with reduced tumour burden in mice deficient for FABP4 (11). These observations are underscored by our Oncomine analyses, which reveal that in addition to upregulation of genes encoding FABP4 and CD36, metastatic ovarian tumours, representing primarily omental metastases, exhibit significantly increased expression of angiogenic genes (VEGFR1, VEGFR2, CD31 and CD34) compared with primary tumours (Table 2). This implies that although extensive vasculature of the omentum is probably sufficient for the initial tumour colonization and survival, angiogenic factors released by the tumour as well as the microenvironment in response to tumour presence further enhance neovascular growth to meet demands of increased tumour burden (65).
Table 2.
Oncomine (Oncomine v4.5) analysis of mRNA expression of genes involved in fatty acid binding, uptake, synthesis and metabolism in patients with metastatic ovarian cancer sites (M) compared with primary sites (P)
| Gene | Ovarian cancer | Fold change | P value | n |
|---|---|---|---|---|
| VEGFR1 | Anglesio [160] | 1.07 | 0.032 | P: 74; M: 16 |
| Tothill [161] | 1.16 | 0.031 | P: 189; M: 54 | |
| VEGFR2 | Bittner [158] | 1.25 | 0.001 | P: 166; M: 75 |
| Anglesio [160] | 1.36 | 0.006 | P: 74; M: 16 | |
| Tothill [161] | 1.26 | 2.87E-4 | P: 189; M: 54 | |
| CD31 | Bittner [158] | 1.86 | 4.76E-4 | P: 166; M: 75 |
| Tothill [161] | 1.46 | 0.026 | P: 189; M: 54 | |
| CD34 | Bittner [158] | 1.13 | 0.044 | P: 166; M: 75 |
| Tothill [161] | 1.08 | 0.040 | P: 189; M: 54 |
‘Following genes were analyzed: Vascular endothelial growth factor receptor-1 (VEGFR1) and -2 (VEGFR2), Cluster of differentiation 31 (CD31), and cluster of differentiation 34 (CD34); n = number of samples. Data were ordered by “overexpression” and the threshold was adjusted to P-value <1E−4; fold change, 2 and gene rank, top 10%. For each database, only genes that met the criteria for significance are reported’.
Taken together, it appears that upon dissemination, cancer cells are drawn to the milky spots, as sites of high neovascularization and immune cell presence conducive to initial tumour colonization and survival. Once adhered and viable, the abundance of energy from adipocytes allows for further tumour expansion. This ‘parasitic’ mode of survival of cancer cells reduces the number of adipocytes as tumour progresses (10,29) and naturally hypoxic omental cells drive the hypoxia-mediated angiogenesis (10). Hypoxia worsens as tumour burden increases, which further enhances angiogenesis and advances tumour progression even when fat depots are depleted.
Metastatic microenvironment generated by bone marrow adipose tissue
National Cancer Institute lists bone as a main site of metastases from prostate, breast, lung, thyroid, melanoma, kidney, bladder and uterine cancers (9). Although bone contains few adipocytes at birth, the abundance of marrow fat cells increases dramatically as we age (22). Other factors, including metabolic imbalance associated with obesity, caloric restriction and anorexia nervosa, administration of select diabetes treatment drugs, such as members of thiazolidinedione class, as well as chemotherapy and radiotherapy represent additional contributors to increased bone marrow adiposity (22,74,75). This is of importance, as in parallel, the factors contributing to the development of MAT are also linked to the poor prognosis for tumours with high affinity for bone, such as prostate (76–78) and breast cancers (79).
Defining marrow adipose tissue and its origin
In view of recent findings, the ontogeny of bone marrow adipocytes remains unclear. A recent study based on genome sequencing of subcutaneous WAT from patients who underwent allogenic transplantation with bone marrow or peripheral blood stem cells revealed evidence of donor genome in recipient WAT (80). This suggested the existence of mobile bone marrow-derived haematopoietic precursors of WAT, although whether the same precursors can give rise to MAT is not clear. Nevertheless, these findings put in question the previous notion of morphological structural differences in the origin of these two depots and introduced the idea that WAT and MAT might be deriving from the same progenitor populations (81). The additional evidence for common origin of MAT and WAT came from the observation that mesenchymal progenitors of bone marrow adipocytes and approximately half of MAT adipocytes can be traced by platelet-derived growth factor receptor-α, the same surface marker as progenitors of WAT (22). At the same time, these data also suggested that two distinct types of MAT precursors may exist, one common to MAT and WAT and one that is MAT-specific. Accordingly, only MAT and not WAT adipocytes express Osterix1 (22) a transcription factor involved in osteoblast differentiation (82,83). This not only speaks to the mesenchymal origin of at least a portion of marrow adipocytes but also indicates there are distinct differences between some populations of progenitors of MAT and WAT. As understanding of the origin of MAT is clearly an evolving field, the question of progenitors of different fat depots remains to be clarified.
Additional controversy related to origin of MAT comes from the fact that MATadipocytes and osteoblasts were long thought to share the same mesenchymal stem cell precursor (84); however, newly emerging findings now show that bone marrow has two mesenchymal progenitor populations: one giving rise to osteoblasts, stromal cells and adipocyte line-ages (22,85,86) and the other differentiating to all mesenchymal lineages but adipocytes (22,87). Numerous factors drive osteoblast differentiation: Runt-related transcription factor 2, Runt-related transcription factor 2 (Runx2), core binding factor β, core binding factor B (CbfB), Osx, activating transcription factor 4 (ATF4), and transcriptional co-activator with PDZ-binding motif (TAZ) (88). Osteoblasts are also sensitive to hormonal regulation, and because they express hormone receptors such as androgen receptors, oestrogen receptor-alpha and – beta (89), age-related hormonal decline (90) or androgen deprivation therapy (91) may contribute to bone loss by affecting osteoblast differentiation. There are also many transcription factors that can influence adipocyte differentiation, with peroxisome proliferatior-activated receptor gamma (PPARγ) and CCAAT/enhancer-binding protein a (C/EBPa) considered the master regulators (88). Expectedly, exogenous agonists of PPARγ stimulate adipogenesis, with some, depending on the nature and affinity of the ligand, inhibiting bone formation by suppressing osteogenesis- driving transcription factors (92). One example is Rosiglitazone, a potent ligand of PPARγ, which promotes bone loss and development of marrow adipocytes (93–96). A significant plasticity between osteogenesis and adipogenesis pathways is thought to exist in adult bone allowing certain factors to shift the balance in either direction (88,97,98). Specifically, Wnt signalling was identified as a cue for stimulation of osteoblast differentiation at the expense of adipogenesis through inhibition of PPARγ and C/EBPα (99,100). Regulation of Wnt is thought to be one of the key contributors to the inverse relationship between fat and bone formation (99). However, the possibility that different postnatal lineages for adipocytes and osteoblasts exist may explain the lack of correlation between increased MATand decreased bone density in various strains of mice (22,101) and in fatty bones induced by high-fat diet (102). Moreover, pharmacological inhibition of marrow adipogenesis with bisphenol-A-diglycidyl ether, or loss of function mutations in kit receptor resulting in the absence of marrow adipose tissue in mice do not inhibit bone loss due to type 1 diabetes or ovariectomy, respectively, thus further challenging the existence of a causal link between augmented marrow adipocytes and osteopenia (103,104).
Although adipocytes are scarce in the bone microenvironment at birth, adipogenesis causes progressive replacement of the red marrow in the distal skeletal regions with adipocyte-rich yellow marrow (reviewed in (88). The red marrow, which remains centralized to the axial skeleton, continues to house the majority of the haematopoietic cells but can still retain a large proportion of adipocytes. The localization in the skeleton is what appears to be the determining factor in differential development and regulation of MAT (101). Specifically, MAT adipocytes that are interspersed with the red, haematopoietic marrow (‘proximal’ regulated MAT, rMAT) appear to be different from those that make up the adipocyte-rich, yellow marrow (‘distal’ constitutive MAT, cMAT). This is in line with early studies, which showed distinctly different lipid composition between adipocytes localized to red as opposed to yellow marrow (105). Although there is still a lot to be uncovered about specific characteristics of rMAT and cMAT, we know so far that the rMAT adipocytes preferentially develop within the red marrow throughout life and can be depleted with 21-day cold exposure (101). They are also smaller in size, contain more saturated lipids, express lower levels of Cebpa and Cebpb and are lost in a PTRF (Polymerase I and Transcript Release Factor) knockout mouse model of congenital generalized lipodystrophy (101). Conversely, cMAT adipocytes develop shortly after birth and do not respond to 21-day cold exposure. They are larger, contain more unsaturated lipids relative to total lipid content, have elevated Cebpa and Cebpb, and are preserved in the PTRF knockout mouse (101). Degree of lipid saturation and expression of adipocyte genes in rMAT are similar to WAT. Thus, cMAT has increased lipid unsaturation and elevated expression of these genes relative to both rMAT and WAT depots. The important question that warrants further investigation is how these differences in rMAT and cMAT affect attraction and progression of metastatic cells in bone.
Role of pro-angiogenic factors in homing of cancer cells to the bone
Although many advances have been made in efforts to understand how the tumour microenvironment influences progression of skeletal metastases, deciphering what attracts circulating tumour cells to bone remains to be elucidated. Studies on bone colonization by the epithelial tumours, such as prostate and breast cancers, suggest that to initiate the metastatic cascade, primary tumour cells must undergo an epithelial-to-mesenchymal transformation (106). The mesenchymal phenotype includes invasive behaviour that allows cells to penetrate the surrounding stroma of the primary site, enter circulation and thus reach the bone. According to Stephen Paget's ‘seed and soil hypothesis’, cancer cells preferentially metastasize to specific organs in a ‘pre-determined’ fashion (107). In line with this original hypothesis, primary tumour cells have been shown to be ‘primed’ for metastasis by haematopoietic progenitor cells (HPCs) expressing vascular endothelial growth factor receptor 1 (VEGFR1) (108). These HPCs reside within specified niches of the bone marrow and are recruited to the primary site to drive angiogenesis and tumour growth. Following interaction with primary tumour cells, the clusters of VEGFR1+ HPCs cells home to the metastatic site and generate a ‘pre-metastatic niche’ that attracts disseminated tumour cells (DTCs) (109).
Once in the bone, DTCs adhere to the endothelium of the sinusoidal blood vessels throughout bone marrow (106). In fact, initial micrometastatic formations in the bone marrow are observed at the perivascular areas of the sinusoidal vessels (110). Our own Oncomine analysis of mRNA levels of pro-angiogenic genes in patients with prostate cancer data revealed significant upregulation of Flt1 gene in metastatic compared with the primary prostate cancer in three out of 10 studies (Table 3). Other angiogenic genes induced in metastatic prostate cancer were CD31 (in 2/3 datasets), CD34 (in 2/11 datasets) and VEGFR3 (in 5/9 datasets) (Table 3). Additional evidence that marrow adipocytes contribute to the upregulation of angiogenesis-supporting factors comes from our own studies in metastatic prostate tumour cells in vitro and in vivo (111). Specifically, we have shown that exposure of prostate cancer cells to marrow adipocytes induces expression of pro-angiogenic VEGF as well as genes coding for pro-inflammatory cytokines, CCL20, IL-1α and IL-1β (111). Our data also revealed an upregulation of hypoxia inducible factor-1α (111), a known regulator of angiogenesis, and a key driver of tumorigenesis (112). Collectively, neovascularization is an important factor in tumour colonization and growth in metastatic sites such as bone, and there is growing evidence that MAT via its effect on cancer cells might be promoting angiogenesis-mediated progression of skeletal metastases.
Table 3.
Oncomine (Oncomine v4.5) analysis of mRNA expression of genes involved in fatty acid binding, uptake, synthesis, and metabolism in patients with metastatic prostate cancer sites (M) compared with primary sites (P)
| Gene | Prostate cancer | Fold change | P value | n |
|---|---|---|---|---|
| VEGFR1 | Yu [162] | 1.20 | 7.87E-6 | P: 64; M: 24 |
| Varambally [163] | 6.17 | 0.001 | P: 7; M: 6 | |
| Chandran [164] | 2.10 | 1.00E-7 | P: 10; M: 21 | |
| VEGFR3 | Yu [162] | 1.26 | 6.49E-7 | P: 64; M: 24 |
| Magee [165] | 1.76 | 0.004 | P: 8; M: 3 | |
| Varambally [163] | 2.25 | 0.002 | P: 7; M: 6 | |
| Chandran [164] | 2.57 | 1.77E-10 | P: 10; M: 21 | |
| Taylor 3 [166] | 1.09 | 0.030 | P: 131; M: 19 | |
| CD31 | Taylor 3 [166] | 2.12 | 0.004 | P: 131; M: 19 |
| Varambally [163] | 2.68 | 0.002 | P: 7; M: 6 | |
| CD34 | Yu [162] | 1.10 | 5.74E-5 | P: 64; M: 24 |
| Ramaswamy [167] | 2.13 | 0.033 | P: 10; M:4 |
‘Following genes were analyzed: Vascular endothelial growth factor receptor-1 (VEGFR1) and -2 (VEGFR2), Cluster of differentiation 31 (CD31), and cluster of differentiation 34 (CD34); n = number of samples. Data were ordered by “overexpression” and the threshold was adjusted to P-value <1E−4; fold change, 2 and gene rank, top 10%. For each database, only genes that met the criteria for significance are reported’.
Cytokine/chemokine signalling and inflammatory microenvironment in metastatic disease in bone
Cancer homing to the bone has been extensively linked to specific chemokine signalling pathways, particularly those driven by CXCL12/CXCR4 and CXCL16/CXCR6 chemokine/receptor axes (113–115). Tumour cells expressing CXCR4 receptor migrate towards endothelial cells and osteoblasts, which secrete its ligand, CXCL12. Interestingly, although not investigated in a context of MAT, CXCL12 has been shown to be secreted by WAT adipocytes and to be involved in the recruitment of macrophages (116). Upon arrival in bone, many DTCs, most of which have escaped chemotherapy, stay dormant (117,118). Factors such as bone remodelling (119), hypoxia (120) and cytokines like parathyroid hormone-related protein, endothelin, Transforming Growth Factor β, platelet-derived growth factor, insulin-like growth factor 1 are known to influence metastatic progression, potentially by allowing tumour cells to escape dormancy (121). One important factor credited with the ability to keep metastatic breast cancer cells in dormant state is thrombospondin-1 (TSP-1), an anti-angiogenic molecule expressed by endothelial cells of bone marrow microvasculature and shown to act as a tumour suppressor (122). The deposits of TSP-1 disappear around sprouting tips of microvascular endothelium, and this coincides with a high concentration of pro-tumorigenic factors, such as active TGF-β1 and periostin around neovascular tips (122). Therefore, it appears that established microvessels inhibit meta-static potential of DTCs, whereas, in contrast, the neovascular tips promote tumour cell growth (122). Surprisingly, bone marrow-derived CD11b+Gr1+ cells and subcutaneous and visceral adipocytes were shown to express anti-angiogenic TSP-1 and its overexpression positively correlated with obesity (123–125). To add to the confusion, the expression of TSP-1 by adipocytes was shown to be significantly reduced by pioglitazone, a PPARγ agonist with insulin sensitizing activities (123). On the other hand, there is also evidence to suggest that obesity-associated induced expression of TSP-1 may mediate recruitment of macrophages to adipose tissue and release of pro-inflammatory cytokines contributing to obesity-accompanied low-grade systemic inflammation (126). TSP-1 increases expression of its receptor CD36 in bone marrow-derived macrophages (127) and activates macrophages via Toll-like receptor 4 (128). Therefore, aside from the earlier mentioned function in facilitating lipid transport, CD36 also appears to play a role in inflammation and angiogenesis (127). We have previously shown that culturing prostate cancer cells in bone marrow-derived adipocyte conditioned media results in mRNA overexpression of CD36 (111). Furthermore, our Oncomine analysis revealed significant overexpression of CD36 in metastatic prostate cancer when compared with primary tumour in four out of 10 available prostate cancer databases (Table 4). Although adipocytes clearly modulate tumour microenvironment to support aggressive phenotype of skeletal metastasis, the relationship between marrow adiposity and tumour cell escape from dormancy is not straightforward and remains to be further investigated.
Table 4.
Oncomine (Oncomine v4.5) analysis of mRNA expression of genes involved in fatty acid binding, uptake, synthesis, and metabolism in patients with metastatic prostate cancer sites (M) compared with primary sites (P)
| Gene | Prostate cancer | Fold change | P value | n |
|---|---|---|---|---|
| FABP4 | Grasso [168] | 2.16 | 0.024 | P: 59; M: 35 |
| Chandran [164] | 12.32 | 2.20E-6 | P: 10; M: 21 | |
| Yu [162] | 2.14 | 0.001 | P: 64; M: 24 | |
| Holzbeierlein [169] | 2.79 | 0.015 | P: 40; M: 9 | |
| Ramaswamy [167] | 6.37 | 0.013 | P: 10; M: 4 | |
| Ramaswamy 2 [170] | 5.86 | 0.018 | P: 10; M: 3 | |
| Tamura [171] | 2.23 | 0.005 | P: 25; M: 12 | |
| LaTulippe [172] | 6.87 | 0.003 | P: 23; M: 9 | |
| CD36 | Grasso [168] | 2.73 | 0.024 | P: 59; M: 35 |
| Varambally [163] | 2.95 | 5.34E-4 | P:7; M: 6 | |
| Chandran [164] | 2.98 | 0.001 | P: 10; M: 21 | |
| Yu [162] | 1.15 | 8.02E-6 | P: 64; M: 24 | |
| FASN | Grasso [168] | 2.49 | 6.67E-5 | P: 59; M: 35 |
| Chandran [164] | 1.66 | 0.041 | P: 10; M: 21 | |
| Yu [162] | 1.76 | 5.23E-4 | P: 64; M: 24 | |
| Holzbeierlein [169] | 1.67 | 0.029 | P: 40; M: 9 | |
| Ramaswamy [167] | 8.89 | 0.002 | P: 10; M: 4 | |
| Ramaswamy 2 [170] | 12.96 | 0.004 | P: 10; M: 3 | |
| Tamura [171] | 2.04 | 0.018 | P: 25; M: 12 | |
| HSL | Chandran [164] | 1.62 | 1.23E-7 | P:10; M: 21 |
| Lapointe [173] | 1.20 | 0.004 | P: 62; M: 9 | |
| LaTulippe [172] | 1.20 | 0.042 | P: 23; M: 9 | |
| Ramaswamy [167] | 5.96 | 0.027 | P: 10; M: 4 | |
| Grasso [168] | 1.80 | 2.93E-5 | P: 59; M: 35 | |
| Yu [162] | 1.10 | 7.50E-5 | P: 64; M: 24 | |
| PLIN2 | Chandran [164] | 1.98 | 8.28E-4 | P: 10; M: 21 |
| Vanaja [174] | 2.08 | 0.007 | P: 27; M: 5 | |
| Yu [162] | 1.77 | 9.59E-5 | P: 64; M: 24 | |
| FATP1 | Grasso [168] | 2.04 | 2.33E-7 | P: 59; M: 35 |
| FATP2 | Varambally [163] | 1.46 | 0.012 | P:7; M: 6 |
| FATP3 | Lapointe [173] | 1.66 | 0.005 | P: 62; M: 9 |
| Grasso [168] | 1.53 | 3.96E-5 | P: 59; M: 35 | |
| Varambally [163] | 2.11 | 0.014 | P:7; M: 6 | |
| FATP4 | Grasso [168] | 1.20 | 0.037 | P: 59; M: 35 |
| Varambally [163] | 1.58 | 0.002 | P:7; M: 6 | |
| FATP5 | Chandran [164] | 2.20 | 2.12E-8 | P: 10; M: 21 |
| FATP6 | Chandran [164] | 1.40 | 0.029 | P: 10; M: 21 |
‘Following genes were analyzed: Fatty acid binding protein 4 (FABP4), Cluster of differentiation (CD36; FAT), Fatty acid synthase (FASN), Hormone sensitive lipase (HSL), Perilipin2 (PLIN2); Fatty Acid Transporter Protein Family members (FATP 1-6); n = number of samples. Data were ordered by “overexpression” and the threshold was adjusted to P-value <1E−4; fold change, 2 and gene rank, top 10%. For each database, only genes that met the criteria for significance are reported’.
Role of adipogenesis in bone remodelling-mediated metastatic progression
It is becoming increasingly clear that the relationship between adipocytes and bone is complex, with some evidence of reciprocal association between increased adiposity and inhibition of osteoblast viability and function (129–132) and other studies revealing no apparent link between adipocyte numbers and bone density (103,104). It appears, however, that marrow adiposity may have definite promoting effects on tumour-driven osteolysis of the bone. Accelerated bone remodelling is one of the key factors associated with homing of tumour cells to the bone (133,134). Experimental treatment with calciotropic hormone (133) or oestrogen and androgen ablation (90) increases bone turnover, which in turn promotes tumour cell growth and occurrences of skeletal metastases (91,119). We have recently shown that bone marrow-derived adipocytes accelerate bone degradation by promoting osteoclastogenesis and augmenting osteoclast-specific expression of cathepsin K, MMP9 and calcineurin, proteins involved in bone remodelling (135). This process is at least partially mediated by adipocytic secretion of pro-inflammatory chemokines of CXC family, CXCL1 and CXCL2, whose levels are dramatically increased upon adipocyte interaction with prostate cancer cell line (PC3) and ARCAP(M) prostate carcinoma cells in bone. This is of importance in a context of metastatic tumour growth, as CXCL1 and CXCL2 are chemoattractants for macrophages, neutrophils and CD11b+Gr1+ cells (also known as myeloid-derived suppressor cells) (136,137). These immune cells express CXCR2 receptors and are capable of modulating tumour microenvironment and affecting progression by stimulating angiogenesis (138,139), suppressing anti-tumour immune responses (140) and promoting chemoresistance (137). This suggests the importance of bone marrow adiposity in bone remodelling associated with cancer-induced bone disease and opens avenues for further investigations.
Bisphosphonates are a class of drugs known primarily for their effects on osteoclast differentiation and survival (141) but are gaining recent attention with respect to their activity towards other cell types in bone (131,142–144). Of particular note is a recent study demonstrating that a single dose of bisphosphonate Zoledronic acid (ZOL) prevents ovariectomy-induced marrow adiposity in rats (131). This potential effect on adipocyte pool in bone could have clinical implications as ZOL has been administered to patients with prostate (91,145) and breast cancer (119) for the treatment and prevention of skeletal-related events and showed some promise based on the randomized study in castrate resistant prostate patients with bone metastases (146). Unfortunately, a recent study revealed that administration of ZOL, within 6 months of androgen deprivation therapy to castration sensitive men with bone metastases does not reduce risks for skeletal-related events (147). More positive results came from preclinical studies in mice bearing androgen receptor negative (PC3) tumours, where ZOL administration at the time of castration was shown to inhibit castration-triggered metastatic growth (91). Analogous to castration in males, ovariectomy in female mice was shown to induce bone loss and increase growth of breast cancer cells in bone, processes, which were inhibited by ZOL treatment (91,119). These observations are in line with the results of recent epidemiological study showing that use of bisphosphonates can reduce the rate of skeletal-related events only in postmenopausal women (148), supporting the idea that bone remodelling and accumulation of MAT in response to hormonal deprivation may be playing an important role in activation of DTCs.
Role of marrow adipose tissue in tumour metabolism
The important evidence that marrow adipocytes may have significant impact on progression of skeletal metastases came from our studies utilizing diet-induced marrow adiposity model (111). Intratibial injections of metastatic PC3 cells into high fat diet-induced fatty bones resulted in accelerated intraosseous tumour growth (111). Exposure of prostate cancer cells to marrow adipocytes in vitro led to a significant accumulation of lipid droplets in the tumour cells, a process that was accompanied by overexpression of three major regulators of lipid trafficking, FABP4, Perilipin 2 and CD36. In parallel, overexpression of FABP4 was also observed in the bone metastatic lesions as compared with benign and primary prostate cancer tissues (111). Co-culture of marrow adipocytes with prostate cancer cells turned out to be detrimental to adipocytes as observed by the loss of lipid droplets and cell shrinkage. We showed that adipocytes promote cancer cell proliferation and induce invasive behaviour in tumour cells, a process mediated, at least partially, by IL-1β- and FABP4-dependent mechanisms (111). These findings are in line with our Oncomine data, which show that metastatic prostate cancers exhibit highly increased expression of FABP4 (in eight out of 12 datasets) and CD36 (in 4/10 datasets) compared with primary tumours (Table 4). Although not consistently altered across all prostate cancer data sets, several of the FATP isomers are also significantly up-regulated in metastatic tissues. These data are in contrast to no observed significant changes in FATP transporters in omental metastases, which suggest there might be depot-specific differences in fatty acid transport mechanisms between the two sites that differentially affect metastatic progression. Other noteworthy changes between primary and metastatic tumours include the increased levels of lipolysis-associated genes, HSL (in 2 out of 4 datasets) and perilipin 2 (3/12 datasets), as well as lipogenesis-associated FASN (in 7/12 datasets) results further linking the presence of adipocytes in the microenvironment of cancer cells with altered metabolism.
The role of fatty acyl lipidome
To better understand the role of MAT in tumorigenesis, it is important to evaluate its lipidome. Analysis of normal human bone marrow fatty acid composition showed a wide range of saturated and unsaturated fatty acids (149). Oleic, palmitic and omega-6 PUFA, LAs, are just a few examples of the most abundantly available fatty acids in the bone marrow (149). Arachidonic acid (AA) was found less abundant in bone marrow stroma cells, marrow plasma (150) and in bone marrow mononuclear cells (151) where it was shown to be incorporated into triglycerides and phospholipids. Availability of fatty acids in the tumour microenvironment has a potential to affect tumour cell behaviour and growth. Accordingly, in vitro studies have shown that PC3 prostate carcinoma cells migrate towards bone marrow stromal cells and bone marrow-derived adipocytes (152,153). The detailed translocation of lipids from human bone marrow-derived adipocytes to prostate cancer cells was further revealed by Fourier transform infrared microspectroscopy (154). LA and AA were shown to enhance proliferation and cellular invasion of prostate cancer cells, potentially as a result of lipid transfer (155). Direct treatment of PC3 cells with AA showed activation of phosphatidylinositol 3-kinase (PI3K)/AkT and NF-kB signalling pathways leading to rise in gene expression of cyclooxygenase-2 (COX-2), IL-8, IL-1β, IL-6, TNF-α, LTA, CXCL1, PPARγ, GM-CSF, NFkB2, IkBα and ICAM-1 (CD54) (156). The uptake of AA by PC3 cells was shown to induce proliferation (156) and promote invasion, a process inhibited by blocking synthesis of prostaglandin E2 (PGE2) with omega-3 (ω-3/n-3) PUFAs (152). PGE2 is a major COX-2 metabolite reported to induce cellular proliferation, increase expression of COX-2 and stimulate endogenous synthesis of PGE2 (157). As an immunoregulator, PGE2 was reported to play a role in cancer stem cell repopulation and chemoresistance, further implicating AA metabolism in prostate cancer progression (158). Notably, metabolic processing of AA by lipoxygenase is known to result in the production of protumorigenic metabolites, and 5-LOX metabolites of n-6 PUFA, 5(S)-HETE and 5-oxoETE have been shown to inhibit apoptosis (159). Furthermore, overexpression of 12-LOX and the augmented levels of its metabolite 12(S)-HETE correlate with enhanced angiogenic potential and increased tumour burden (160,161). Although little is known about specific involvement of LA and AA metabolites in tumour progression in bone, the aforementioned studies suggest that bone-marrow adipocyte-derived n-6 PUFAs in MAT may serve an important role in driving metastatic bone disease, which warrants further investigation.
It is important to note that despite its negative publicity, omega-6 fatty acids remain important lipid molecules and their role in cancer should be viewed in a ratio to ω-3. Omega-3 PUFAs are represented by α-linolenic acid (α-LNA, n-3) and its long-chain metabolites, eicosapentaenoic acid (n-3) and DHA, n-3, dietary consumption of which is demonstrated to decrease the risk of prostate cancer (162). It has been suggested that n-3 competes with n-6 for Δ4 and Δ6-desaturases (reviewed in (163)). With n-3 having a higher affinity for these enzymes, increased consumption of omega-3 PUFA results in decreased conversion of LA into AA and consequently its metabolites (163). As a result, the cancer chemopreventive role of n-3 is usually described as its ratio to n-6 PUFAs (163). As expected, the level of PGE2, a derivative of AA, in rat bone marrow was significantly increased in rats on high ratio of n-6/n-3 fatty acid diet (164). Furthermore, the analysis of fatty acids in prostatic tissues showed significantly higher n-6/n-3 (shown as lower n-3/n-6) ratio in cancer samples compared with benign hyperplasia (165). Based on these findings, it appears that effects of marrow adiposity on tumour cells might be dependent on the individual lipidome.
Conclusions
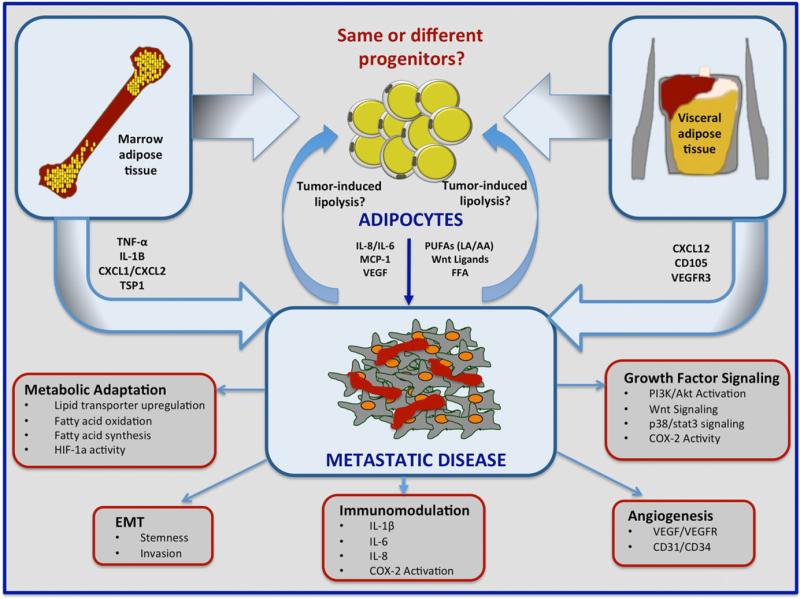
A key characteristic of a metastatic tumour is its dependence on the environment that surrounds it. Colonization and growth at the secondary site are a complex process involving reciprocal interactions between the tumour cells and the host microenvironment. Visceral WAT and MAT represent two distinct metastatic niches that have a common characteristic: they are adipocyte-rich depots that promote tumour progression by supplying lipids to disseminated cancer cells, stimulating growth factor signalling, modulating cancer cell metabolism and stemmness and priming the microenvironment for metastatic growth. Both WAT and MAT adipocytes evoke immunoregulatory effects on the environment by influencing the behaviour of immune cells and modulating the release of pro-inflammatory cytokines and lipid metabolites. They also appear to play a role in angiogenesis via direct release of pro-angiogenic factors or stimulation of their secretion by neighbouring cells, including the tumour cells. It is those effects on the host cells, in combination with direct impact on tumour cells via altered fatty acid metabolism, that provide an environment conducive to tumour adaptation and survival (Fig. 1). Little is known on whether heterogeneity of WAT and MAT has any effect on colonization and growth of tumour cells that homed to that site. There is evidence to suggest that more than one population of WAT and MAT adipocytes exist, some sharing common progenitors between the two fat depots and some of the origin that is distinct to a specific site. Time and additional research efforts will reveal whether the metabolic symbiosis between adipocytes and tumour cells at the metastatic site is driven by a specific population of adipocytes, and whether this opens avenues for therapeutic intervention.
Figure 1.
A schematic of proposed mechanisms underlying involvement of white adipose tissue and marrow adipose tissue in metastatic colonization and progression in omentum and bone. Omentum and bone are adipocyte-rich organs. Adipocytes in either depot have the propensity to fuel tumour growth and survival within the metastatic niche. Both depots are major sources of polyunsaturated fatty acids, Wnt ligands, free fatty acids, cytokines IL-6 and IL-8, MCP-1, and vascular endothelial growth factor (VEGF), all of which are known contributors to tumorigenesis. The specific marrow adipose tissue-derived factors such as TNF-α, IL-β, CXCL1, and CXCL2 as well as white adipose tissue-derived CXCL12, CD105, and VEGFR3, have also all been linked to metastatic disease. Tumour cells within an adipocyte-rich microenvironment have the ability to modulate adipocyte function by stimulating lipolysis and affecting secretion of pro-tumorigenic, pro-angiogenic and immunomodulatory factors. The interaction between adipocytes and tumour cells within the metastatic niche promotes metastatic progression through metabolic reprogramming or adaptation, immunomodulation, angiogenesis, epithelial-to-mesenchymal transformation (EMT) and growth factor/survival pathway signalling.
Acknowledgement
I.P. is supported by the NHI/NCI 1 R01 CA181189. O.A.M. is supported by RO1 DK095705, RO1 DK62876, R24 DK092759.
Footnotes
Conflict of interest statement
The authors disclose no potential conflicts of interest
References
- 1.Bays HE, Gonzalez-Campoy JM, Bray GA, et al. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev Cardiovasc Ther. 2008;6:343–368. doi: 10.1586/14779072.6.3.343. [DOI] [PubMed] [Google Scholar]
- 2.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 3.Wozniak SE, Gee LL, Wachtel MS, Frezza EE. Adipose tissue: the new endocrine organ? A review article. Dig Dis Sci. 2009;54:1847–1856. doi: 10.1007/s10620-008-0585-3. [DOI] [PubMed] [Google Scholar]
- 4.Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc. 2001;60:329–339. doi: 10.1079/pns200194. [DOI] [PubMed] [Google Scholar]
- 5.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 6.Wang P, Mariman E, Renes J, Keijer J. The secretory function of adipocytes in the physiology of white adipose tissue. J Cell Physiol. 2008;216:3–13. doi: 10.1002/jcp.21386. [DOI] [PubMed] [Google Scholar]
- 7.Conde J, Scotece M, Gomez R, et al. Adipokines: biofactors from white adipose tissue. A complex hub among inflammation, metabolism, and immunity. Biofactors. 2011;37:413–420. doi: 10.1002/biof.185. [DOI] [PubMed] [Google Scholar]
- 8.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 9.NCI Metastatic Canc. 2013 URL http://www.cancer.gov/types/metastatic-cancer.
- 10.Gerber SA, Rybalko VY, Bigelow CE, et al. Preferential attachment of peritoneal tumor metastases to omental immune aggregates and possible role of a unique vascular microenvironment in meta-static survival and growth. Am J Pathol. 2006;169:1739–1752. doi: 10.2353/ajpath.2006.051222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nieman KM, Kenny HA, Penicka CV, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He Y, Wu AC, Harrington BS, et al. Elevated CDCP1 predicts poor patient outcome and mediates ovarian clear cell carcinoma by promoting tumor spheroid formation, cell migration and chemoresistance. Oncogene. 2016;35:468–478. doi: 10.1038/onc.2015.101. [DOI] [PubMed] [Google Scholar]
- 13.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–1064. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan DS, Agarwal R, Kaye SB. Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet Oncol. 2006;7:925–934. doi: 10.1016/S1470-2045(06)70939-1. [DOI] [PubMed] [Google Scholar]
- 15.Wilkosz S, Ireland G, Khwaja N, et al. A comparative study of the structure of human and murine greater omentum. Anat Embryol (Berl) 2005;209:251–261. doi: 10.1007/s00429-004-0446-6. [DOI] [PubMed] [Google Scholar]
- 16.Ellis H. The aetiology of post-operative abdominal adhesions. An experimental study. Br J Surg. 1962;50:10–16. doi: 10.1002/bjs.18005021904. [DOI] [PubMed] [Google Scholar]
- 17.Hall JC, Heel KA, Papadimitriou JM, Platell C. The pathobiology of peritonitis. Gastroenterology. 1998;114:185–196. doi: 10.1016/s0016-5085(98)70646-8. [DOI] [PubMed] [Google Scholar]
- 18.Chamorro M, Carceller F, Llanos C, Rodriguez-Alvarino A, Colmenero C, Burgueno M. The effect of omental wrapping on nerve graft regeneration. Br J Plast Surg. 1993;46:426–429. doi: 10.1016/0007-1226(93)90050-l. [DOI] [PubMed] [Google Scholar]
- 19.Goldsmith HS. Role of the omentum in the treatment of Alzheimer's disease. Neurol Res. 2001;23:555–564. doi: 10.1179/016164101101198893. [DOI] [PubMed] [Google Scholar]
- 20.Chau YY, Bandiera R, Serrels A, et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol. 2014;16:367–375. doi: 10.1038/ncb2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez-Gurmaches J, Hung CM, Sparks CA, Tang Y, Li H, Guertin DA. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metab. 2012;16:348–362. doi: 10.1016/j.cmet.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berry R, Rodeheffer MS, Rosen CJ, Horowitz MC. Adipose tissue residing progenitors (adipocyte lineage progenitors and adi-pose derived stem cells (ADSC). Curr Mol Biol Rep. 2015;1:101–109. doi: 10.1007/s40610-015-0018-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol. 2013;15:302–308. doi: 10.1038/ncb2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Fox CS, Hickson DA, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab. 2010;95:5419–5426. doi: 10.1210/jc.2010-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doig T, Monaghan H. Sampling the omentum in ovarian neoplasia: when one block is enough. Int J Gynecol Cancer. 2006;16:36–40. doi: 10.1111/j.1525-1438.2006.00273.x. [DOI] [PubMed] [Google Scholar]
- 27.Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 2013;1831:1533–1541. doi: 10.1016/j.bbalip.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brasaemle DL, Subramanian V, Garcia A, Marcinkiewicz A, Rothenberg A. Perilipin A and the control of triacylglycerol metabolism. Mol Cell Biochem. 2009;326:15–21. doi: 10.1007/s11010-008-9998-8. [DOI] [PubMed] [Google Scholar]
- 29.Clark R, Krishnan V, Schoof M, et al. Milky spots promote ovarian cancer metastatic colonization of peritoneal adipose in experimental models. Am J Pathol. 2013;183:576–591. doi: 10.1016/j.ajpath.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulze A, Harris AL. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature. 2012;491:364–373. doi: 10.1038/nature11706. [DOI] [PubMed] [Google Scholar]
- 31.Currie E, Schulze A, Zechner R, Walther TC, Farese RV., Jr Cellular fatty acid metabolism and cancer. Cell Metab. 2013;18:153–161. doi: 10.1016/j.cmet.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baenke F, Peck B, Miess H, Schulze A. Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development. Dis Model Mech. 2013;6:1353–1363. doi: 10.1242/dmm.011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ros S, Santos CR, Moco S, et al. Functional metabolic screen identifies 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 as an important regulator of prostate cancer cell survival. Canc Discov. 2012;2:328–343. doi: 10.1158/2159-8290.CD-11-0234. [DOI] [PubMed] [Google Scholar]
- 34.Daniels VW, Smans K, Royaux I, Chypre M, Swinnen JV, Zaidi N. Cancer cells differentially activate and thrive on de novo lipid synthesis pathways in a low-lipid environment. PLoS One. 2014;9:e106913. doi: 10.1371/journal.pone.0106913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Power surge: supporting cells “fuel” cancer cell mitochondria. Cell Metab. 2012;15:4–5. doi: 10.1016/j.cmet.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz B, Yehuda-Shnaidman E. Putative role of adipose tissue in growth and metabolism of colon cancer cells. Front Oncol. 2014;4:164. doi: 10.3389/fonc.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson DG, Tonelli F, Alossaimi M, et al. The roles of sphingosine kinases 1 and 2 in regulating the Warburg effect in prostate cancer cells. Cell Signal. 2013;25:1011–1017. doi: 10.1016/j.cellsig.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tonelli F, Alossaimi M, Natarajan V, et al. The roles of sphingosine kinase 1 and 2 in regulating the metabolome and survival of prostate cancer cells. Biomolecules. 2013;3:316–333. doi: 10.3390/biom3020316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manzi L, Costantini L, Molinari R, Merendino N. Effect of dietary omega-3 polyunsaturated fatty acid DHA on glycolytic enzymes and Warburg phenotypes in cancer. Biomed Res Int. 2015;2015:137097. doi: 10.1155/2015/137097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaughan M. The production and release of glycerol by adipose tissue incubated in vitro. J Biol Chem. 1962;237:3354–3358. [PubMed] [Google Scholar]
- 41.Maeda N, Funahashi T, Shimomura I. Metabolic impact of adipose and hepatic glycerol channels aquaporin 7 and aquaporin 9. Nat Clin Pract Endocrinol Metab. 2008;4:627–634. doi: 10.1038/ncpendmet0980. [DOI] [PubMed] [Google Scholar]
- 42.Langin D. Control of fatty acid and glycerol release in adipose tissue lipolysis. C R Biol. 2006;329:598–607. doi: 10.1016/j.crvi.2005.10.008. discussion 653-5. [DOI] [PubMed] [Google Scholar]
- 43.Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov. 2008;7:489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furuhashi M, Tuncman G, Gorgun CZ, et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 2007;447:959–965. doi: 10.1038/nature05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hertzel AV, Bernlohr DA. The mammalian fatty acid-binding protein multigene family: molecular and genetic insights into function. Trends Endocrinol Metab. 2000;11:175–180. doi: 10.1016/s1043-2760(00)00257-5. [DOI] [PubMed] [Google Scholar]
- 46.Hardaway AL, Podgorski I. IL-1beta, RAGE and FABP4: targeting the dynamic trio in metabolic inflammation and related pathologies. Future Med Chem. 2013;5:1089–1108. doi: 10.4155/fmc.13.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Makowski L, Brittingham KC, Reynolds JM, Suttles J, Hotamisligil GS. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor gamma and IkappaB kinase activities. J Biol Chem. 2005;280:12888–12895. doi: 10.1074/jbc.M413788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elmasri H, Karaaslan C, Teper Y, et al. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J. 2009;23:3865–3873. doi: 10.1096/fj.09-134882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cataltepe O, Arikan MC, Ghelfi E, et al. Fatty acid binding protein 4 is expressed in distinct endothelial and non-endothelial cell populations in glioblastoma. Neuropathol Appl Neurobiol. 2012;38:400–410. doi: 10.1111/j.1365-2990.2011.01237.x. [DOI] [PubMed] [Google Scholar]
- 50.Balaban S, Lee LS, Schreuder M, Hoy AJ. Obesity and cancer progression: is there a role of fatty acid metabolism? Biomed Res Int. 2015;2015:274585. doi: 10.1155/2015/274585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doege H, Stahl A. Protein-mediated fatty acid uptake: novel insights from in vivo models. Physiology (Bethesda) 2006;21:259–268. doi: 10.1152/physiol.00014.2006. [DOI] [PubMed] [Google Scholar]
- 52.Schneider H, Staudacher S, Poppelreuther M, Stremmel W, Ehehalt R, Fullekrug J. Protein mediated fatty acid uptake: synergy between CD36/FAT-facilitated transport and acyl-CoA synthetase-driven metabolism. Arch Biochem Biophys. 2014;546:8–18. doi: 10.1016/j.abb.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 53.Yam D, Ben-Hur H, Dgani R, Fink A, Shani A, Berry EM. Subcutaneous, omentum and tumor fatty acid composition, and serum insulin status in patients with benign or cancerous ovarian or endometrial tumors. Do tumors preferentially utilize polyunsaturated fatty acids? Cancer Lett. 1997;111:179–185. doi: 10.1016/s0304-3835(96)04530-2. [DOI] [PubMed] [Google Scholar]
- 54.Shimotsuma M, Takahashi T, Kawata M, Dux K. Cellular subsets of the milky spots in the human greater omentum. Cell Tissue Res. 1991;264:599–601. doi: 10.1007/BF00319049. [DOI] [PubMed] [Google Scholar]
- 55.Krist LF, Eestermans IL, Steenbergen JJ, et al. Cellular composition of milky spots in the human greater omentum: an immunochemical and ultrastructural study. Anat Rec. 1995;241:163–174. doi: 10.1002/ar.1092410204. [DOI] [PubMed] [Google Scholar]
- 56.Mandache E, Moldoveanu E, Savi G. The involvement of omentum and its milky spots in the dynamics of peritoneal macrophages. Morphol Embryol (Bucur) 1985;31:137–142. [PubMed] [Google Scholar]
- 57.Rangel-Moreno J, Moyron-Quiroz JE, Carragher DM, et al. Omental milky spots develop in the absence of lymphoid tissue-inducer cells and support B and T cell responses to peritoneal antigens. Immunity. 2009;30:731–743. doi: 10.1016/j.immuni.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dux K, Rouse RV, Kyewski B. Composition of the lymphoid cell populations from omental milky spots during the immune response in C57BL/Ka mice. Eur J Immunol. 1986;16:1029–1032. doi: 10.1002/eji.1830160828. [DOI] [PubMed] [Google Scholar]
- 59.Funda D, Holub M, Sykora V. Development of the cellular response in the mouse omentum after intraperitoneal immunization. APMIS. 1993;101:939–945. doi: 10.1111/j.1699-0463.1993.tb00205.x. [DOI] [PubMed] [Google Scholar]
- 60.Van Vugt E, Van Rijthoven EA, Kamperdijk EW, Beelen RH. Omental milky spots in the local immune response in the peritoneal cavity of rats. Anat Rec. 1996;244:235–245. doi: 10.1002/(SICI)1097-0185(199602)244:2<235::AID-AR11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 61.Tsujimoto H, Takhashi T, Hagiwara A, et al. Site-specific implantation in the milky spots of malignant cells in peritoneal dissemination: immunohistochemical observation in mice inoculated intraperitoneally with bromodeoxyuridine-labelled cells. Br J Cancer. 1995;71:468–472. doi: 10.1038/bjc.1995.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hagiwara A, Takahashi T, Sawai K, et al. Milky spots as the implantation site for malignant cells in peritoneal dissemination in mice. Cancer Res. 1993;53:687–692. [PubMed] [Google Scholar]
- 63.Khan SM, Funk HM, Thiolloy S, et al. In vitro metastatic colonization of human ovarian cancer cells to the omentum. Clin Exp Metastasis. 2010;27:185–196. doi: 10.1007/s10585-010-9317-0. [DOI] [PubMed] [Google Scholar]
- 64.Krist LF, Kerremans M, Broekhuis-Fluitsma DM, Eestermans IL, Meyer S, Beelen RH. Milky spots in the greater omentum are predominant sites of local tumour cell proliferation and accumulation in the peritoneal cavity. Cancer Immunol Immunother. 1998;47:205–212. doi: 10.1007/s002620050522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sorensen EW, Gerber SA, Sedlacek AL, Rybalko VY, Chan WM, Lord EM. Omental immune aggregates and tumor metastasis within the peritoneal cavity. Immunol Res. 2009;45:185–194. doi: 10.1007/s12026-009-8100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lopes Cardozo AM, Gupta A, Koppe MJ, et al. Metastatic pattern of CC531 colon carcinoma cells in the abdominal cavity: an experimental model of peritoneal carcinomatosis in rats. Eur J Surg Oncol. 2001;27:359–363. doi: 10.1053/ejso.2001.1117. [DOI] [PubMed] [Google Scholar]
- 67.Robinson-Smith TM, Isaacsohn I, Mercer CA, et al. Macrophages mediate inflammation-enhanced metastasis of ovarian tumors in mice. Cancer Res. 2007;67:5708–5716. doi: 10.1158/0008-5472.CAN-06-4375. [DOI] [PubMed] [Google Scholar]
- 68.Takemori N, Hirai K, Onodera R, Saito N, Namiki M. Light and electron microscope study of splenoportal milky spots in New Zealand black mice: comparison between splenoportal milky spots and aberrant spleens. J Anat. 1995;186:287–299. [PMC free article] [PubMed] [Google Scholar]
- 69.Schumann T, Adhikary T, Wortmann A, et al. Deregulation of PPARbeta/delta target genes in tumor-associated macrophages by fatty acid ligands in the ovarian cancer microenvironment. Oncotarget. 2015;6:13416–13433. doi: 10.18632/oncotarget.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 71.Zhang QX, Magovern CJ, Mack CA, Budenbender KT, Ko W, Rosengart TK. Vascular endothelial growth factor is the major angiogenic factor in omentum: mechanism of the omentum-mediated angiogenesis. J Surg Res. 1997;67:147–154. doi: 10.1006/jsre.1996.4983. [DOI] [PubMed] [Google Scholar]
- 72.Choi HJ, Armaiz Pena GN, Pradeep S, Cho MS, Coleman RL, Sood AK. Anti-vascular therapies in ovarian cancer: moving beyond anti-VEGF approaches. Cancer Metastasis Rev. 2015;34:19–40. doi: 10.1007/s10555-014-9538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes Cancer. 2011;2:1117–1133. doi: 10.1177/1947601911423654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Georgiou KR, Hui SK, Xian CJ. Regulatory pathways associated with bone loss and bone marrow adiposity caused by aging, chemotherapy, glucocorticoid therapy and radiotherapy. Am J Stem Cells. 2012;1:205–224. [PMC free article] [PubMed] [Google Scholar]
- 75.Cawthorn WP, Scheller EL, Learman BS, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20:368–375. doi: 10.1016/j.cmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bassett WW, Cooperberg MR, Sadetsky N, et al. Impact of obesity on prostate cancer recurrence after radical prostatectomy: data from CaPSURE. Urology. 2005;66:1060–1065. doi: 10.1016/j.urology.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 77.Keto CJ, Aronson WJ, Terris MK, et al. Obesity is associated with castration-resistant disease and metastasis in men treated with androgen deprivation therapy after radical prostatectomy: results from the SEARCH database. BJU Int. 2012;110:492–498. doi: 10.1111/j.1464-410X.2011.10754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gong Z, Agalliu I, Lin DW, Stanford JL, Kristal AR. Obesity is associated with increased risks of prostate cancer metastasis and death after initial cancer diagnosis in middle-aged men. Cancer. 2007;109:1192–1202. doi: 10.1002/cncr.22534. [DOI] [PubMed] [Google Scholar]
- 79.von Drygalski A, Tran TB, Messer K, et al. Obesity is an independent predictor of poor survival in metastatic breast cancer: retrospective analysis of a patient cohort whose treatment included high-dose chemotherapy and autologous stem cell support. Int J Breast Canc. 2011;2011:523276. doi: 10.4061/2011/523276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ryden M, Uzunel M, Hard JL, et al. Transplanted bone marrow-derived cells contribute to human adipogenesis. Cell Metab. 2015;22:408–417. doi: 10.1016/j.cmet.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 81.Tavassoli M. Ultrastructural development of bone marrow adipose cell. Acta Anat (Basel) 1976;94:65–77. doi: 10.1159/000144545. [DOI] [PubMed] [Google Scholar]
- 82.Chen J, Shi Y, Regan J, Karuppaiah K, Ornitz DM, Long F. Osx-Cre targets multiple cell types besides osteoblast lineage in postnatal mice. PLoS One. 2014;9:e85161. doi: 10.1371/journal.pone.0085161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu Y, Strecker S, Wang L, et al. Osterix-cre labeled progenitor cells contribute to the formation and maintenance of the bone marrow stroma. PLoS One. 2013;8:e71318. doi: 10.1371/journal.pone.0071318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bianco P, Cao X, Frenette PS, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mizoguchi T, Pinho S, Ahmed J, et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell. 2014;29:340–349. doi: 10.1016/j.devcel.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15:154–168. doi: 10.1016/j.stem.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Worthley DL, Churchill M, Compton JT, et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160:269–284. doi: 10.1016/j.cell.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berendsen AD, Olsen BR. Osteoblast-adipocyte lineage plasticity in tissue development, maintenance and pathology. Cell Mol Life Sci. 2014;71:493–497. doi: 10.1007/s00018-013-1440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wiren KM, Chapman Evans A, Zhang XW. Osteoblast differentiation influences androgen and estrogen receptor-alpha and -beta expression. J Endocrinol. 2002;175:683–694. doi: 10.1677/joe.0.1750683. [DOI] [PubMed] [Google Scholar]
- 90.Reim NS, Breig B, Stahr K, et al. Cortical bone loss in androgen-deficient aged male rats is mainly caused by increased endocortical bone remodeling. J Bone Miner Res. 2008;23:694–704. doi: 10.1359/jbmr.080202. [DOI] [PubMed] [Google Scholar]
- 91.Ottewell PD, Wang N, Meek J, et al. Castration-induced bone loss triggers growth of disseminated prostate cancer cells in bone. Endocr Relat Cancer. 2014;21:769–781. doi: 10.1530/ERC-14-0199. [DOI] [PubMed] [Google Scholar]
- 92.Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci. 2009;66:236–253. doi: 10.1007/s00018-008-8429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology. 2005;146:1226–1235. doi: 10.1210/en.2004-0735. [DOI] [PubMed] [Google Scholar]
- 94.Grey A. Skeletal consequences of thiazolidinedione therapy. Osteoporos Int. 2008;19:129–137. doi: 10.1007/s00198-007-0477-y. [DOI] [PubMed] [Google Scholar]
- 95.Ackert-Bicknell CL, Shockley KR, Horton LG, Lecka-Czernik B, Churchill GA, Rosen CJ. Strain-specific effects of rosiglitazone on bone mass, body composition, and serum insulin-like growth factor-I. Endocrinology. 2009;150:1330–1340. doi: 10.1210/en.2008-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lecka-Czernik B, Moerman EJ, Grant DF, Lehmann JM, Manolagas SC, Jilka RL. Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology. 2002;143:2376–2384. doi: 10.1210/endo.143.6.8834. [DOI] [PubMed] [Google Scholar]
- 97.Bennett CN, Ouyang H, Ma YL, et al. Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation. J Bone Miner Res. 2007;22:1924–1932. doi: 10.1359/jbmr.070810. [DOI] [PubMed] [Google Scholar]
- 98.Cawthorn WP, Bree AJ, Yao Y, et al. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a beta-catenin-dependent mechanism. Bone. 2012;50:477–489. doi: 10.1016/j.bone.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kang S, Bennett CN, Gerin I, Rapp LA, Hankenson KD, Macdougald OA. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:14515–14524. doi: 10.1074/jbc.M700030200. [DOI] [PubMed] [Google Scholar]
- 100.Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116:1202–1209. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scheller EL, Doucette CR, Learman BS, et al. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun. 2015;6:7808. doi: 10.1038/ncomms8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Doucette CR, Horowitz MC, Berry R, et al. A high fat diet increases bone marrow adipose tissue (MAT) but does not alter trabecular or cortical bone mass in C57BL/6 J mice. J Cell Physiol. 2015;230:2032–2037. doi: 10.1002/jcp.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Botolin S, McCabe LR. Inhibition of PPARgamma prevents type I diabetic bone marrow adiposity but not bone loss. J Cell Physiol. 2006;209:967–976. doi: 10.1002/jcp.20804. [DOI] [PubMed] [Google Scholar]
- 104.Iwaniec UT, Turner RT. Failure to generate bone marrow adipocytes does not protect mice from ovariectomy-induced osteopenia. Bone. 2013;53:145–153. doi: 10.1016/j.bone.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tavassoli M. Marrow adipose cells. Histochemical identification of labile and stable components. Arch Pathol Lab Med. 1976;100:16–18. [PubMed] [Google Scholar]
- 106.van der Pluijm G. Epithelial plasticity, cancer stem cells and bone metastasis formation. Bone. 2011;48:37–43. doi: 10.1016/j.bone.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 107.Paget S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 108.Lyden D, Hattori K, Dias S, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 109.Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van der Horst G, van den Hoogen C, Buijs JT, et al. Targeting of alpha(v)-integrins in stem/progenitor cells and supportive micro-environment impairs bone metastasis in human prostate cancer. Neoplasia. 2011;13:516–525. doi: 10.1593/neo.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Herroon MK, Rajagurubandara E, Hardaway AL, et al. Bone marrow adipocytes promote tumor growth in bone via FABP4-dependent mechanisms. Oncotarget. 2013;4:2108–2123. doi: 10.18632/oncotarget.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Unruh A, Ressel A, Mohamed HG, et al. The hypoxia-inducible factor-1 alpha is a negative factor for tumor therapy. Oncogene. 2003;22:3213–3220. doi: 10.1038/sj.onc.1206385. [DOI] [PubMed] [Google Scholar]
- 113.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 114.Jung Y, Kim JK, Shiozawa Y, et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun. 2013;4:1795. doi: 10.1038/ncomms2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–1837. [PubMed] [Google Scholar]
- 116.Kim D, Kim J, Yoon JH, et al. CXCL12 secreted from adipose tissue recruits macrophages and induces insulin resistance in mice. Diabetologia. 2014;57:1456–1465. doi: 10.1007/s00125-014-3237-5. [DOI] [PubMed] [Google Scholar]
- 117.Pantel K, Muller V, Auer M, Nusser N, Harbeck N, Braun S. Detection and clinical implications of early systemic tumor cell dissemination in breast cancer. Clin Cancer Res. 2003;9:6326–6334. [PubMed] [Google Scholar]
- 118.Aft R, Naughton M, Trinkaus K, et al. Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trial. Lancet Oncol. 2010;11:421–428. doi: 10.1016/S1470-2045(10)70054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ottewell PD, Wang N, Brown HK, et al. Zoledronic acid has differential antitumor activity in the pre- and postmenopausal bone microenvironment in vivo. Clin Cancer Res. 2014;20:2922–2932. doi: 10.1158/1078-0432.CCR-13-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dunn LK, Mohammad KS, Fournier PG, et al. Hypoxia and TGF-beta drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PLoS One. 2009;4:e6896. doi: 10.1371/journal.pone.0006896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yin JJ, Pollock CB, Kelly K. Mechanisms of cancer metastasis to the bone. Cell Res. 2005;15:57–62. doi: 10.1038/sj.cr.7290266. [DOI] [PubMed] [Google Scholar]
- 122.Ghajar CM, Peinado H, Mori H, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15:807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Varma V, Yao-Borengasser A, Bodles AM, et al. Thrombospondin-1 is an adipokine associated with obesity, adi-pose inflammation, and insulin resistance. Diabetes. 2008;57:432–439. doi: 10.2337/db07-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ramis JM, Franssen-van Hal NL, Kramer E, et al. Carboxy-peptidase E and thrombospondin-1 are differently expressed in subcutaneous and visceral fat of obese subjects. Cell Mol Life Sci. 2002;59:1960–1971. doi: 10.1007/PL00012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Catena R, Bhattacharya N, El Rayes T, et al. Bone marrow-derived Gr1+ cells can generate a metastasis-resistant microenvironment via induced secretion of thrombospondin-1. Canc Discov. 2013;3:578–589. doi: 10.1158/2159-8290.CD-12-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li Y, Tong X, Rumala C, Clemons K, Wang S. Thrombospondin1 deficiency reduces obesity-associated inflammation and improves insulin sensitivity in a diet-induced obese mouse model. PLoS One. 2011;6:e26656. doi: 10.1371/journal.pone.0026656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li Y, Qi X, Tong X, Wang S. Thrombospondin 1 activates the macrophage Toll-like receptor 4 pathway. Cell Mol Immunol. 2013;10:506–512. doi: 10.1038/cmi.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Elbaz A, Wu X, Rivas D, Gimble JM, Duque G. Inhibition of fatty acid biosynthesis prevents adipocyte lipotoxicity on human osteoblasts in vitro. J Cell Mol Med. 2010;14:982–991. doi: 10.1111/j.1582-4934.2009.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gunaratnam K, Vidal C, Gimble JM, Duque G. Mechanisms of palmitate-induced lipotoxicity in human osteoblasts. Endocrinology. 2014;155:108–116. doi: 10.1210/en.2013-1712. [DOI] [PubMed] [Google Scholar]
- 131.Li GW, Xu Z, Chang SX, et al. Influence of early zoledronic acid administration on bone marrow fat in ovariectomized rats. Endocrinology. 2014;155:4731–4738. doi: 10.1210/en.2014-1359. [DOI] [PubMed] [Google Scholar]
- 132.Martin RB, Zissimos SL. Relationships between marrow fat and bone turnover in ovariectomized and intact rats. Bone. 1991;12:123–131. doi: 10.1016/8756-3282(91)90011-7. [DOI] [PubMed] [Google Scholar]
- 133.Guise TA, Mohammad KS, Clines G, et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res. 2006;12:6213s–6216s. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- 134.Schneider A, Kalikin LM, Mattos AC, et al. Bone turnover mediates preferential localization of prostate cancer in the skeleton. Endocrinology. 2005;146:1727–1736. doi: 10.1210/en.2004-1211. [DOI] [PubMed] [Google Scholar]
- 135.Hardaway AL, Herroon MK, Rajagurubandara E, Podgorski I. Marrow adipocyte-derived CXCL1 and CXCL2 contribute to osteolysis in metastatic prostate cancer. Clin Exp Metastasis. 2015;32:353–368. doi: 10.1007/s10585-015-9714-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.De Filippo K, Dudeck A, Hasenberg M, et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121:4930–4937. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- 137.Acharyya S, Oskarsson T, Vanharanta S, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–178. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shojaei F, Wu X, Malik AK, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b + Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 139.Yang L, DeBusk LM, Fukuda K, et al. Expansion of myeloid immune suppressor Gr + CD11b + cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 140.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.El-Amm J, Freeman A, Patel N, Aragon-Ching JB. Bone-targeted therapies in metastatic castration-resistant prostate cancer: evolving paradigms. Prostate Canc. 2013;2013:210686. doi: 10.1155/2013/210686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Basso FG, Silveira Turrioni AP, Hebling J, de Souza Costa CA. Zoledronic acid inhibits human osteoblast activities. Gerontology. 2013;59:534–541. doi: 10.1159/000351194. [DOI] [PubMed] [Google Scholar]
- 143.Li GW, Chang SX, Fan JZ, Tian YN, Xu Z, He YM. Marrow adiposity recovery after early zoledronic acid treatment of glucocorticoid-induced bone loss in rabbits assessed by magnetic resonance spectroscopy. Bone. 2013;52:668–675. doi: 10.1016/j.bone.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 144.Pozzi S, Vallet S, Mukherjee S, et al. High-dose zoledronic acid impacts bone remodeling with effects on osteoblastic lineage and bone mechanical properties. Clin Cancer Res. 2009;15:5829–5839. doi: 10.1158/1078-0432.CCR-09-0426. [DOI] [PubMed] [Google Scholar]
- 145.Morgans AK, Smith MR. Bone-targeted agents: preventing skeletal complications in prostate cancer. Urol Clin North Am. 2012;39:533–546. doi: 10.1016/j.ucl.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 147.Smith MR, Halabi S, Ryan CJ, et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance). J Clin Oncol. 2014;32:1143–1150. doi: 10.1200/JCO.2013.51.6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Coleman R, Powles T, Paterson A, et al. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386:1353–1361. doi: 10.1016/S0140-6736(15)60908-4. [DOI] [PubMed] [Google Scholar]
- 149.Sumida T. Clinical and experimental study on fatty acid composition of bone marrow lipid in hematologic disorders. Acta Med Nagasaki. 1965;9:222–241. [PubMed] [Google Scholar]
- 150.Denizot Y, Dulery C, Trimoreau F, Desplat V, Praloran V. Arachidonic acid and human bone marrow stromal cells. Biochim Biophys Acta. 1998;1402:209–215. doi: 10.1016/s0167-4889(97)00157-2. [DOI] [PubMed] [Google Scholar]
- 151.Denizot Y, Desplat V, Dulery C, Trimoreau F, Praloran V. Arachidonic acid and freshly isolated human bone marrow mononuclear cells. Mediators Inflamm. 1999;8:31–35. doi: 10.1080/09629359990694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Brown MD, Hart CA, Gazi E, Bagley S, Clarke NW. Promotion of prostatic metastatic migration towards human bone marrow stoma by Omega 6 and its inhibition by Omega 3 PUFAs. Br J Cancer. 2006;94:842–853. doi: 10.1038/sj.bjc.6603030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Brown MD, Hart C, Gazi E, Gardner P, Lockyer N, Clarke N. Influence of omega-6 PUFA arachidonic acid and bone marrow adipocytes on metastatic spread from prostate cancer. Br J Cancer. 2010;102:403–413. doi: 10.1038/sj.bjc.6605481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gazi E, Gardner P, Lockyer NP, Hart CA, Brown MD, Clarke NW. Direct evidence of lipid translocation between adipocytes and prostate cancer cells with imaging FTIR microspectroscopy. J Lipid Res. 2007;48:1846–1856. doi: 10.1194/jlr.M700131-JLR200. [DOI] [PubMed] [Google Scholar]
- 155.Rose DP. Effects of dietary fatty acids on breast and prostate cancers: evidence from in vitro experiments and animal studies. Am J Clin Nutr. 1997;66(6 Suppl):1513S–1522S. doi: 10.1093/ajcn/66.6.1513S. [DOI] [PubMed] [Google Scholar]
- 156.Hughes-Fulford M, Li CF, Boonyaratanakornkit J, Sayyah S. Arachidonic acid activates phosphatidylinositol 3-kinase signaling and induces gene expression in prostate cancer. Cancer Res. 2006;66:1427–1433. doi: 10.1158/0008-5472.CAN-05-0914. [DOI] [PubMed] [Google Scholar]
- 157.Tjandrawinata RR, Dahiya R, Hughes-Fulford M. Induction of cyclo-oxygenase-2 mRNA by prostaglandin E2 in human prostatic carcinoma cells. Br J Cancer. 1997;75:1111–1118. doi: 10.1038/bjc.1997.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kurtova AV, Xiao J, Mo Q, et al. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature. 2015;517:209–213. doi: 10.1038/nature14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ghosh J. Rapid induction of apoptosis in prostate cancer cells by selenium: reversal by metabolites of arachidonate 5-lipoxygenase. Biochem Biophys Res Commun. 2004;315:624–635. doi: 10.1016/j.bbrc.2004.01.100. [DOI] [PubMed] [Google Scholar]
- 160.Gao X, Grignon DJ, Chbihi T, et al. Elevated 12-lipoxygenase mRNA expression correlates with advanced stage and poor differentiation of human prostate cancer. Urology. 1995;46:227–237. doi: 10.1016/s0090-4295(99)80198-8. [DOI] [PubMed] [Google Scholar]
- 161.Nie D, Hillman GG, Geddes T, et al. Platelet-type 12-lipoxygenase in a human prostate carcinoma stimulates angiogenesis and tumor growth. Cancer Res. 1998;58:4047–4051. [PubMed] [Google Scholar]
- 162.Norrish AE, Skeaff CM, Arribas GL, Sharpe SJ, Jackson RT. Prostate cancer risk and consumption of fish oils: a dietary biomarker-based case-control study. Br J Cancer. 1999;81:1238–1242. doi: 10.1038/sj.bjc.6690835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rose DP, Connolly JM. Omega-3 fatty acids as cancer chemo-preventive agents. Pharmacol Ther. 1999;83:217–244. doi: 10.1016/s0163-7258(99)00026-1. [DOI] [PubMed] [Google Scholar]
- 164.Watkins BA, Li Y, Allen KG, Hoffmann WE, Seifert MF. Dietary ratio of (n-6)/(n-3) polyunsaturated fatty acids alters the fatty acid composition of bone compartments and biomarkers of bone formation in rats. J Nutr. 2000;130:2274–2284. doi: 10.1093/jn/130.9.2274. [DOI] [PubMed] [Google Scholar]
- 165.Mamalakis G, Kafatos A, Kalogeropoulos N, Andrikopoulos N, Daskalopulos G, Kranidis A. Prostate cancer vs hyperplasia: relationships with prostatic and adipose tissue fatty acid composition. Prostaglandins Leukot Essent Fatty Acids. 2002;66:467–477. doi: 10.1054/plef.2002.0384. [DOI] [PubMed] [Google Scholar]