Abstract
Kallistatin is an endogenous protein that exerts pleiotropic effects, including vasodilation and inhibition of angiogenesis, inflammation, oxidative stress, apoptosis, fibrosis, and tumor progression. Through its two functional domains – an active site and a heparin-binding site – kallistatin regulates differential signaling pathways and a wide spectrum of biological functions. Kallistatin's active site is key for inhibiting tissue kallikrein activity, and stimulating the expression of endothelial nitric oxide synthase (eNOS), sirtuin 1 (SIRT1) and suppressor of cytokine signaling 3 (SOCS3). Kallistatin via its heparin-binding site blocks signaling pathways mediated by growth factors and cytokines, such as vascular endothelial growth factor (VEGF), tumor necrosis factor-α (TNF-α), high mobility group box-1 (HMGB1), Wnt, transforming growth factor-β (TGF-β), and epidermal growth factor (EGF). Kallistatin gene or protein delivery protects against the pathogenesis of hypertension, heart and kidney damage, arthritis, sepsis, influenza virus infection, tumor growth and metastasis in animal models. Conversely, depletion of endogenous kallistatin by neutralizing antibody injection exacerbates cardiovascular and renal injury in hypertensive rats. Kallistatin levels are markedly reduced in rodents with hypertension, sepsis, streptozotocin-induced diabetes, and cardiac and renal injury. Kallistatin levels are also diminished in patients with liver disease, septic syndrome, diabetic retinopathy, severe pneumonia, inflammatory bowel disease, and obesity, prostate and colon cancer. Therefore, circulating kallistatin levels may serve as a new biomarker for human diseases. This review summarizes kallistatin's protective roles and mechanisms in vascular and organ injury, and highlights the therapeutic potential of kallistatin for multiple disease states.
Kallistatin Regulates Biological Functions via Its Structural Elements
Kallistatin was discovered in human plasma as a tissue kallikrein-binding protein (KBP).1 Sequence analysis indicated that KBP is a novel serine proteinase inhibitor (serpin), and was later designated “kallistatin” due to its ability to inhibit tissue kallikrein activity.2-9 Tissue kallikrein is a serine proteinase that cleaves low molecular weight kininogen substrate to release vasoactive kinin peptides.10 The tissue kallikrein-kinin system is involved in beneficial effects against hypertension, and cardiac, cerebral and renal injury.11 Kallistatin is mainly expressed in the liver, but is also widely distributed in tissues relevant to cardiovascular function, including the heart, kidney and blood vessel.9,12,13 The human kallistatin gene (SERPINA4) is located on chromosome 14q31-32.1 within a serpin gene cluster,7 and human kallistatin cDNA shares 44-46% homology with other serpins, such as α1-antitrypsin, α1-antichymotrypsin and protein C inhibitor.8 Kallistatin contains two important structural elements: an active site and a heparin-binding domain.14-16 Through these two distinct structural elements, kallistatin regulates the expression of multiple genes and controls the activation of several signaling pathways. Kallistatin's active site is crucial for: 1) inhibiting tissue kallikrein activity and tissue kallikrein/kinin-mediated processes; 2) increasing eNOS expression and activation; and 3) stimulating SIRT1 and SOCS3 expression. Kallistatin via its heparin-binding domain interacts with heparan sulfate proteoglycans, thereby antagonizing the following biological effects: 1) VEGF-mediated angiogenesis and vascular permeability; 2) TNF-α-induced nuclear factor (NF)-κB activation, inflammation, oxidative stress and apoptosis; 3) HMGB1-induced inflammation; 4) TGF-β-induced fibrosis and endothelial-mesenchymal transition (EndMT); 4) Wnt-mediated cancer cell proliferation, migration and invasion; and 5) EGF-mediated cancer cell migration and invasion. Thus, kallistatin regulates numerous signaling pathways unrelated to tissue kallikrein. Furthermore, kallistatin modulates a broad range of biological activities, such as blood pressure, angiogenesis, inflammation, oxidative stress, apoptosis, fibrosis, and cancer development.
Kallistatin Is a Novel Vasodilator
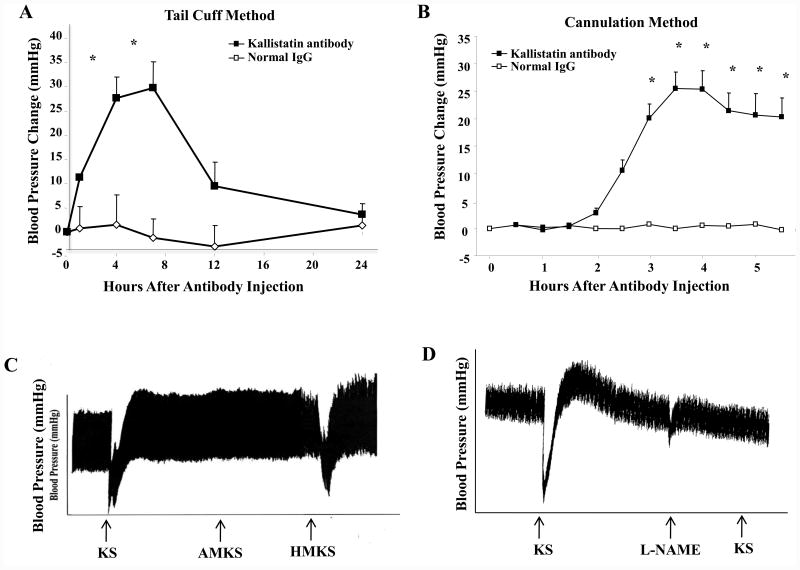
The expression and distribution of kallistatin in endothelial and smooth muscle cells of blood vessels implicate its role in vascular function.13 Rat KBP, the analogue of human kallistatin, is markedly reduced in the circulation of spontaneously hypertensive rats (SHR) compared to normotensive rats,3,17 suggesting that it may be involved in maintaining normal blood pressure. Kallistatin was identified to be a potent vasodilator, indicating its important role in vascular function.18 An intravenous bolus injection of purified kallistatin caused a rapid and transient reduction of blood pressure in both normotensive and hypertensive rats. Kallistatin administration also induced vasorelaxation in isolated aortic rings.18 Neither kallistatin's blood pressure-lowering effect nor its vasorelaxation ability was abolished by icatibant (Hoe140, a kinin B2 receptor antagonist), indicating that kallistatin-mediated vasodilation is unrelated to the tissue kallikrein-kinin system.18 In addition, kallistatin transgenic mice were found to have significantly lower blood pressure than control littermates.19 Likewise, expression of human kallistatin following adenovirus-mediated gene delivery caused a prolonged blood pressure reduction in SHR.20 Conversely, injection of anti-rat kallistatin antibody in normotensive rats induced a time-dependent blood pressure rise for several hours, as determined by both tail-cuff and cannulation methods, further implicating a role for endogenous kallistatin in blood pressure regulation (Fig. 1A & B). Kallistatin's active site is essential for its blood pressure-lowering effect, as both wild-type kallistatin and heparin-binding site mutant kallistatin, but not active site mutant kallistatin, induced vasodilation (Fig. 1C). Moreover, administration of a NOS inhibitor (Nω-nitro-L-arginine methylester, L-NAME) blocked the vasodilating activity of kallistatin, indicating that NO is involved in kallistatin's ability to reduce blood pressure (Fig. 1D). Indeed, kallistatin via its active site increases eNOS expression and activation, and thus NO production, in endothelial cells.21,22 Therefore, kallistatin through its active site induces vasorelaxation and decreases blood pressure, in part, through NO formation. These combined findings indicate that kallistatin is a new vasodilator and an endogenous blood pressure-lowering agent.
Fig. 1.
Endogenous kallistatin plays a role in blood pressure regulation of normotensive Wistar rats, and kallistatin's active site is essential for stimulating vasodilation via NO production. Injection of anti-rat kallistatin antibody increases blood pressure as determined by (A) tail-cuff and (B) cannulation methods. (C) Both wild-type kallistatin (KS) and heparin-binding site mutant kallistatin (HMKS), but not active site mutant kallistatin (AMKS), induce vasodilation. (D) Kallistatin's vasodilatory action is blocked by the nitric oxide synthase inhibitor, L-NAME.
Kallistatin Inhibits Angiogenesis and Tumor Progression
Angiogenesis is an important process in the evolution of inflammatory diseases, which may predispose patients to cancer development.23,24 Kallistatin has been shown to exert anti-tumor activity in breast, colon, stomach, lung and liver carcinomas by inhibiting angiogenesis, inflammation and cancer cell proliferation, and inducing cancer cell death.25-33 A single intramural injection of the kallistatin gene into pre-established breast cancer xenografts in mice resulted in significant suppression of tumor growth and reduction of blood vessel numbers.25 Systemic injection of lentivirus carrying the human kallistatin gene dramatically decreased cancer metastasis into lungs in association with reduced angiogenesis and inflammation, and also enhanced the survival of tumor-bearing mice.33 Since VEGF is critical in the development of new blood vessels and tumor growth,34 it is plausible that kallistatin may interfere with VEGF-mediated processes. Indeed, both wild-type kallistatin and active site mutant kallistatin, but not the heparin-binding site mutant, inhibited VEGF-induced endothelial cell proliferation, growth, migration, and capillary tube formation.35 These findings indicate that kallistatin, via its heparin-binding site, interacts with cell surface heparan sulfate proteoglycans, thereby blocking VEGF from binding to its receptor. Kallistatin through the heparin-binding domain also prevented TNF-α-induced NF-κB activation and VEGF expression in cultured endothelial cells.36,37 Moreover, kallistatin's active site is essential for stimulating eNOS activation and synthesis and NO formation21,38 which, in turn, is capable of inhibiting NF-κB activation.39 Therefore, kallistatin inhibits angiogenesis not only by interfering with VEGF-mediated effects, but also by down-regulating TNF-α-induced VEGF expression.
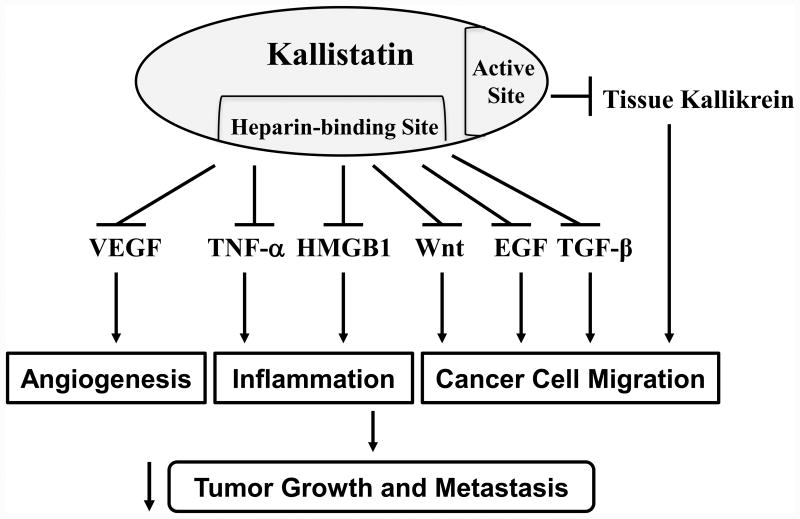
In addition to inhibiting angiogenesis, kallistatin's heparin-binding domain is crucial for antagonizing TNF-α- and HMGB1-induced inflammatory gene expression in endothelial cells.36,40 Kallistatin via its heparin-binding site blocks TGF-β-induced oxidative stress and miR-21 synthesis in endothelial cells, and thus EndMT,22 which is a major contributor to organ fibrosis and tumor progression.41 Likewise, kallistatin's heparin-binding site is essential for suppressing Wnt3a signaling and cancer cell proliferation.32 Kallistatin antagonizes Wnt3a signaling by forming a complex with the Wnt co-receptor low-density lipoprotein receptor 6 (LRP6), as demonstrated in retinal epithelial and breast cancer cells,42,43 thereby inhibiting Wnt3a-induced tumor cell proliferation, migration and invasion.43 Moreover, reduced kallistatin levels are correlated with βII-spectrin (SPTBN1), an adapter protein during Wnt signaling in hepatocellular carcinoma.44 Furthermore, kallistatin prevents the migration and invasion of prostate cancer cells elicited by tissue kallikrein (unpublished observations). Taken together, these findings indicate that kallistatin exerts multifaceted activities in protection against tumor growth and metastasis by inhibiting angiogenesis, inflammation, and cancer cell proliferation, migration and invasion. As shown in Fig. 2, kallistatin's heparin-binding site is essential for blocking signaling pathways stimulated by VEGF, TNF-α, HMGB1, Wnt, TGF-β, and EGF, whereas its active site is key for inhibiting tissue kallikrein-mediated effects.
Fig. 2.
Kallistatin, via its heparin-binding site and active site, protects against tumor growth and metastasis by blocking signaling pathways stimulated by vascular endothelial growth factor (VEGF), tumor necrosis factor (TNF)-α, high mobility group box-1 (HMGB1), Wnt, epidermal growth factor (EGF) and transforming growth factor (TGF)-β, and by inhibiting tissue kallikrein-mediated actions.
Kallistatin Is a Unique Anti-Inflammatory Agent
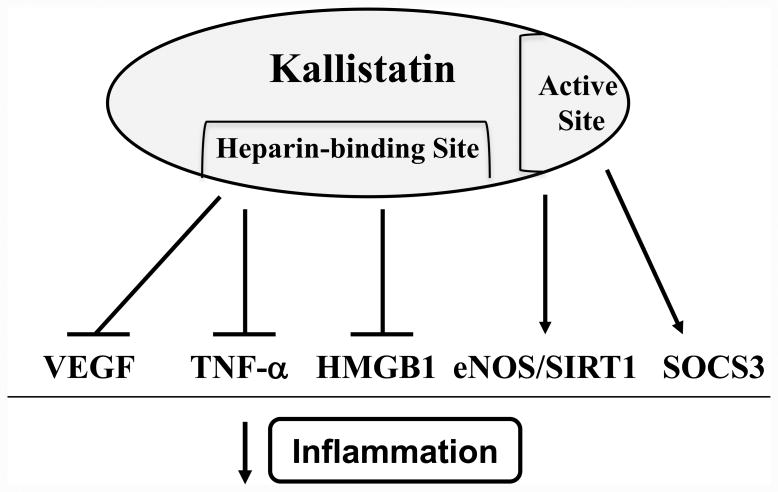
Inflammation is not only closely linked to cancer,23,24 but also to hypertension and organ injury.45 Recent studies demonstrate that inflammation, oxidative stress and immunity promote hypertensive end-organ damage.46,47 Stimuli such as angiotensin II and deoxycorticosterone (DOCA) acetate-salt lead to accumulation of activated immune cells, like T cells and monocytes/macrophages, in the kidney and blood vessels.47 Moreover, mice lacking NADPH oxidases are protected against angiotensin II-induced vascular inflammation and immune cell infiltration,48 which clearly establishes a link between oxidative stress and immune activation in hypertension. Numerous studies have indicated that kallistatin inhibits inflammatory responses in both normotensive and hypertensive animal models.49-53 Kallistatin gene delivery significantly reduced inflammatory responses and joint swelling in arthritic rats.49 Kallistatin administration also suppressed inflammatory cell infiltration and prevented complement factor C5a-induced paw edema and vascular leakage in mice.36 Local delivery of the kallistatin gene into rat heart enhanced cardiac performance and prevented inflammatory cell infiltration after acute myocardial ischemia/reperfusion (I/R).50 In salt-induced hypertensive rats, kallistatin therapy attenuated vascular and renal damage, and reduced oxidative stress and inflammation,38,51 while depletion of endogenous kallistatin augmented organ injury, and accentuated oxidative stress, inflammation and fibrosis.54 In addition, kallistatin treatment dramatically improved survival and reduced inflammation, oxidative stress, apoptosis and organ damage in mice with polymicrobial sepsis and endotoxemia.40,52,53 Mechanistically, kallistatin inhibits vascular inflammation by interacting with the transcription factor Kruppel-like factor 4 (KLF4), leading to increased eNOS expression and NO levels in endothelial cells.21 Kallistatin's active site is responsible for up-regulating eNOS and SIRT1 expression, and thus increasing NO production.22 NO, in turn, can inhibit the synthesis of cell adhesion molecules by preventing activation of the pro-inflammatory transcription factor NF-κB.39,55 Kallistatin, via its heparin-binding domain, also ameliorates inflammation by blocking VEGF-induced endothelial cell permeability, as well as TNF-α-induced NF-κB activation, p38 MAPK phosphorylation and inflammatory gene expression in vitro.36 Likewise, kallistatin's heparin-binding site was shown to block HMGB1-mediated synthesis of inflammatory genes in endothelial cells.40 Hence, kallistatin's heparin-binding domain is vital for inhibiting both early (TNF-α) and late (HMGB1) cytokine-induced inflammatory responses.36,40 In cultured macrophages, kallistatin via its active site induced the expression of SOCS3,52,56 a negative feedback regulator of inflammation, cancer development and progression.57 Thus, kallistatin through its active site and heparin-binding domain exerts anti-inflammatory actions by: 1) stimulating eNOS and SIRT1 synthesis and NO formation; 2) increasing SOCS3 expression; 3) antagonizing TNF-α- and HMGB1-mediated inflammatory gene expression; and 4) blocking VEGF-induced vascular permeability (Fig. 3). Together, these findings implicate kallistatin to be a novel anti-inflammatory agent that protects against hypertension and end-organ damage by regulating the activation of inflammatory and immune components.
Fig. 3.
Kallistatin, through its structural elements, exerts anti-inflammatory actions by antagonizing vascular endothelial growth factor (VEGF)-, tumor necrosis factor (TNF)-α-, and high mobility group box-1 (HMGB1)-mediated effects, and by inducing the expression of endothelial nitric oxide synthase (eNOS), sirtuin 1 (SIRT1) and suppressor of cytokine signaling 3 (SOCS3).
Kallistatin Acts As an Antioxidant to Protect Against Organ Injury
Imbalances between reactive oxygen species (ROS) formation and endogenous antioxidants can result in oxidative stress. Oxidative stress activates inflammatory pathways and triggers a series of events that lead to organ injury.24 Increased oxidative stress and reduced NO bioavailability are important contributing factors in the pathogenesis of hypertension and cardiovascular and renal diseases.58 Low kallistatin levels are associated with enhanced oxidative stress and organ damage in salt-induced hypertensive rats.51,59 Moreover, serum kallistatin levels are markedly decreased in animal models of hypertension, aortic constriction, STZ-induced diabetes, renal injury, myocardial I/R, and cerebral I/R (unpublished observations). Kallistatin gene transfer attenuated the pathogenesis of organ damage, inflammation and apoptosis in conjunction with decreased ROS formation and increased eNOS and NO levels in animal models of myocardial I/R, myocardial infarction (MI), and salt-induced hypertension.38,50,51,60 Conversely, depletion of endogenous kallistatin by neutralizing antibody injection compounded cardiovascular and renal damage, which was accompanied by elevated oxidative stress, inflammation, hypertrophy and fibrosis in DOCA-salt hypertensive rats.54 With its antioxidant activity, kallistatin administration protected against CCl4-induced liver fibrosis in rats,61 and lipopolysaccharide (LPS)-induced acute lung injury in mice.53 Kallistatin also decreased H2O2-mediated oxidative stress and down-regulated NAD(P)H oxidase expression in rat corneal epithelium and human hepatic stellate cells.61,62 In cultured endothelial cells, kallistatin via its heparin-binding site antagonized TNF-α and TGF-β-induced NADPH oxidase activity and expression, and thus ROS formation,22,36,38 whereas kallistatin's active site was crucial for stimulating the synthesis of the antioxidant proteins eNOS, SIRT1 and FoxO1.22 Furthermore, kallistatin exhibited antioxidant activity in cultured pterygium epithelial cells by increasing expression of superoxide dismutase 2 (SOD2),63 which is mediated by FoxO1.64 Many of kallistatin's antioxidant effects occur through NO, as it can inhibit NAD(P)H oxidase activity.65 Indeed, kallistatin, via stimulating NO formation, has been shown to reduce superoxide production and NAD(P)H oxidase activity induced by TNF-α, H2O2, or angiotensin II in cultured renal epithelial tubular and mesangial cells, cardiomyocytes, myofibroblasts and endothelial cells.21,36,38,51,60 The signaling mechanisms by which kallistatin exerts antioxidant activity are presented in Fig. 4.
Fig. 4.
Kallistatin, through its structural elements, exerts antioxidant actions by blocking tumor necrosis factor-α (TNF-α)- and transforming growth factor-β (TGF-β)-induced signaling, and stimulating endothelial nitric oxide synthase (eNOS) and sirtuin 1 (SIRT1) expression. Both eNOS and SIRT1 increase NO formation which, in turn, inhibits NAD(P)H oxidase. Moreover, FoxO1 activation by SIRT1 subsequently leads to superoxide dismutase 2 (SOD2) expression. Genistein, a tyrosine kinase inhibitor, blocks kallistatin's effects on eNOS, SIRT1 and FoxO1.
Kallistatin Reduces Vascular Injury by Promoting Endothelial Progenitor Cell Mobility, Viability and Function
EPCs are a continuous endogenous source of replenishment for damaged vessels, and serve to maintain vascular integrity in response to endothelial injury.66-68 Decreased numbers of circulating EPCs are found in patients with hypertension, chronic renal failure, coronary artery disease, diabetes, rheumatoid arthritis and sepsis.66,69-71 EPCs isolated from patients with hypertension and coronary artery disease also display an impaired migratory response.66 Reduced EPC numbers can be attributed to defective mobility and proliferation as well as accelerated apoptosis and/or senescence. Therefore, augmented mobilization of endogenous EPCs from bone marrow may be an alternative and effective means to promote vascular repair. Indeed, kallistatin gene delivery resulted in elevated circulating EPC number and reduced aortic oxidative stress and glomerular capillary loss in DOCA-salt hypertensive rats, whereas kallistatin deficiency further decreased EPC levels and exacerbated vascular oxidative stress and endothelial rarefaction.38,54,72 These findings indicate a novel mechanism of endogenous kallistatin in vascular repair by promoting EPC mobility and function. In vitro, kallistatin stimulated the proliferation, migration, adhesion and tube formation of EPCs via activation of phosphoinositide 3-kinase (PI3K)-Akt-eNOS and Akt-glycogen synthase kinase (GSK)-3β signaling pathways,72 which have both been demonstrated to promote EPC survival and function.73,74 Moreover, kallistatin inhibited TNF-α-induced apoptosis in EPCs.72 These findings indicate that kallistatin plays a protective role in vascular injury by enhancing vascularization and vascular repair through increasing EPC mobility, viability and function.
Kallistatin Attenuates Sepsis-Induced Organ Damage and Mortality
Sepsis is a systemic inflammatory response to infection that can lead to multi-organ dysfunction.75 Sepsis is a major contributor to morbidity and mortality of intensive care patients, and a leading cause of death worldwide. Because numerous signaling cascades are triggered during sepsis, selective blocking of inflammatory mediators is not sufficient to arrest this process.76 Kallistatin is a negative acute-phase protein, as kallistatin expression in the liver is rapidly decreased in rats after endotoxic shock,77 and circulating kallistatin levels are markedly diminished in patients with sepsis syndrome and liver disease.78 Interestingly, a kallistatin gene polymorphism is associated with a decreased risk of developing acute kidney injury in patients with septic shock.79 Studies of animal models of toxic shock have shown that kallistatin is protective against organ injury and lethality. Transgenic mice expressing rat kallistatin are highly resistant to LPS-induced mortality.80 Moreover, kallistatin gene transfer reduced mortality, bacterial counts, and inflammatory cell numbers, as well as skin and liver damage, in a mouse model of streptococcal infection.81 In addition, kallistatin treatment in mice with polymicrobial sepsis attenuated lethality, peritoneal bacterial counts, renal injury and inflammation, and splenic apoptosis.40 The protective effects of kallistatin in the kidney occurred in conjunction with reduced expression of TNF-α and HMGB1, and increased eNOS synthesis and NO levels. Furthermore, delayed kallistatin administration after the onset of sepsis attenuated mortality and multi-organ injury in mouse models of polymicrobial sepsis and endotoxemia.52 Kallistatin treatment inhibited systemic inflammation by reducing circulatory levels of TNF-α and HMGB1, and dramatically up-regulating SOCS3 expression in the kidney and lung.52 Kallistatin gene or protein delivery improved mortality and lung morphology in LPS-induced septic mice, and inhibited ROS-mediated inflammation and apoptosis in cultured lung epithelial cells.53 Kallistatin, via its heparin-binding site, blocked TNF-α- and HMGB1-mediated inflammatory gene expression in endothelial cells.36,40 Kallistatin's active site was found to be crucial for stimulating SOCS3 expression in macrophages through activation of a protein kinase C (PKC)-extracellular signal-regulated kinase (ERK) signaling pathway, illustrating a novel mechanism by which kallistatin protects against sepsis-induced organ damage.32,52 These findings indicate that kallistatin administration significantly enhances survival and protects against organ damage during sepsis.
Kallistatin Regulates Tissue Kallikrein-Mediated Biological Activities
As a tissue kallikrein inhibitor, kallistatin is capable of regulating its bioavailability in vivo.82 Kallistatin modulates several biological functions mediated by tissue kallikrein, such as blood pressure, angiogenesis, tumor development, and influenza infection. For example, purified kallistatin can induce a rapid and transient reduction of blood pressure and relaxation in isolated aortic rings, independent of tissue kallikrein.18 Kallistatin transgenic mice are hypotensive,19 and kallistatin gene delivery caused a prolonged blood pressure reduction for four weeks in SHR.20 Intramuscular injection of the kallistatin gene, however, reversed the hypotension of transgenic mice expressing human tissue kallikrein.83 Therefore, in addition to acting as a potent vasodilator, kallistatin is able to oppose the blood pressure-lowering effect of tissue kallikrein. Notably, since it took ten days for kallistatin to counter the hypotension in tissue kallikrein transgenic mice, it is possible that kallistatin's action is attributed to inhibiting renal-mediated sodium retention. Tissue kallikrein is also present in many tumors, such as those of the breast, lung, stomach, pancreas, pituitary, prostate, and uterus.84 The tissue kallikrein-kinin system is involved in tumor development, as icatibant administration suppressed angiogenesis, vascular permeability and tumor growth in a mouse tumor model.85 Tissue kallikrein has been shown to promote neovascularization and restore blood flow through kinin B2 receptor-Akt-GSK-3β and VEGF signaling pathways.86 Kallistatin, however, blocks the tissue kallikrein-induced migration and invasion of prostate cancer cells (unpublished observations). Thus, kallistatin may also regulate tissue kallikrein-mediated tumor metastasis. Finally, tissue kallikrein processes hemagglutinin and thus enhances infection by the influenza virus, whereas kallistatin gene transfer protects against influenza infection in mice by inhibiting tissue kallikrein-mediated hemagglutinin cleavage.87 This suggests a novel role of kallistatin in suppressing tissue kallikrein's effect on viral infection. These combined findings indicate that kallistatin modulates different biological actions mediated by tissue kallikrein.
Double-Edged Roles of Kallistatin
Kallistatin exerts protection in various biological functions, yet has unique double-edged actions in angiogenesis, apoptosis, and oxidative stress. First, kallistatin has both pro- and anti-angiogenic effects. Kallistatin inhibited angiogenesis by blocking VEGF-induced growth and migration of endothelial cells.25,35 However, kallistatin enhanced angiogenesis and vascular repair by promoting EPC migration, proliferation, viability and tube formation.72 Second, kallistatin has contradictory activities in apoptosis. Kallistatin administration attenuated apoptosis and cardiovascular and renal injury in rats with myocardial I/R or salt-induced hypertension.38,50 In addition, kallistatin prevented apoptosis and inflammation in mice with polymicrobial sepsis, and in cultured lung epithelial cells.40,50,53 Kallistatin blocked TNF-α-mediated apoptosis in cultured endothelial cells and EPCs.38,72 Conversely, kallistatin stimulated apoptosis in cultured retinal endothelial and colorectal and breast cancer cells.31,32,88 Third, kallistatin can act as a pro- and anti-oxidative agent. Kallistatin suppressed oxidative stress in cultured cardiomyocytes, myofibroblasts, endothelial cells and EPCs by increasing NO formation, and kallistatin's effect on oxidative stress was reversed by inhibition of NOS activity.38,60,72 On the other hand, kallistatin treatment exhibited marked bacterial killing activity in mice with streptococcal infection and polymicrobial sepsis, perceivably by increasing oxidative stress in peritoneal neutrophils.40,81 Thus, kallistatin displays double-edged actions in angiogenesis, apoptosis and oxidative stress, depending on cell types and pathological conditions.
Kallistatin-Binding Proteins
Kallistatin modulates several signaling pathways by binding to multiple proteins or extracellular molecules, including: 1) tissue kallikrein; 2) heparan sulfate proteoglycans; 3) the Wnt co-receptor LRP6; 4) the transcription factor KLF4; and 5) tyrosine kinase (Table 1). Through its active site, kallistatin binds to tissue kallikrein and inhibits its enzymatic activity.1-6 Kallistatin via its heparin-binding site prevents VEGF-induced angiogenesis by competing with VEGF binding to heparan sulfate proteoglycans on endothelial cell surfaces, thereby suppressing VEGF-mediated endothelial cell proliferation, migration and tube formation.35 Similarly, kallistatin antagonizes TNF-α- and HMGB1-mediated inflammatory gene expression via its heparin-binding domain.36,40 In addition, kallistatin's interaction with Wnt co-receptor LRP6 blocks canonical Wnt/β-catenin signaling and the growth and motility of breast cancer cells.43 The transcription factor KLF4 was identified as a kallistatin-binding protein on the surface of endothelial cells, which can result in enhanced eNOS expression and inhibition of vascular inflammation.21 Moreover, based on Scatchard plot analysis, kallistatin's vasodilating activity may be attributed to the presence of specific kallistatin-binding sites on aortic membranes.18 Additionally, kallistatin was shown to bind to Müller cells, leading to protection against oxidative stress.89 Furthermore, kallistatin stimulates eNOS and SIRT1 synthesis in endothelial cells, and induces SOCS3 expression in macrophages through activation of a cell surface tyrosine kinase, as the effect was blocked by genistein, a tyrosine kinase inhibitor;22,52 however, the identity of the cell surface kallistatin receptor remains to be determined. Taken together, these studies demonstrate that kallistatin's interaction with multiple proteins or molecules accounts for its regulation of various biological activities.
Table 1. Kallistatin-Binding Proteins.
| Tissue kallikrein |
| Heparan sulfate proteoglycan |
| Low density lipoprotein-like receptor (LRP)-6 |
| Kruppel-like factor 4 (KLF4) |
| Tyrosine kinase |
Kallistatin As a New Biomarker for Human Diseases
Kallistatin levels are decreased in animal models of hypertension, cardiovascular and renal injury, septic shock, diabetes, and hepatic neoplasia.3,51,59,77,90-92 Likewise, human kallistatin levels are significantly diminished in numerous diseases. Patients with liver disease, septic syndrome, inflammatory bowel disease, cirrhosis, severe pneumonia, and acute respiratory distress syndrome display decreased levels of kallistatin.53,78,93-95 Plasma kallistatin levels are also reduced in healthy African American youths with adiposity and cardiometabolic risk factors, indicating its potential role in metabolic disorders and perhaps the development of obesity.96 Moreover, kallistatin levels are decreased in the vitreous fluids of patients with diabetic retinopathy.97 Furthermore, circulating kallistatin levels are markedly reduced in patients with colon and prostate cancer.98 However, kallistatin levels in the circulation or tissues were shown to be elevated in patients with diabetic vascular complications and rheumatoid joints.99-101 These observations indicate that kallistatin may serve as a novel biomarker for human diseases, such as cardiovascular and metabolic disorders, obesity, and cancer.
Conclusions
Kallistatin plays important roles in protection against vascular and multi-organ damage. Kallistatin regulates a wide spectrum of biological activities, such as blood pressure reduction, inhibition of angiogenesis, inflammation, apoptosis, oxidative stress, fibrosis, and cancer growth and invasion. Moreover, kallistatin protects against pathogenesis of vascular and organ injury and tumor progression via its double-edged actions in angiogenesis, apoptosis and oxidative stress. In addition to regulating the bioavailability of tissue kallikrein, kallistatin by its active site and heparin-binding domain, modulates many important signaling pathways. Kallistatin treatment decreases the pathogenesis of hypertension, cardiovascular, renal and lung dysfunction, inflammatory arthritis, sepsis, and cancer progression in numerous animal models. In addition, kallistatin levels in plasma, body fluid or tissues are markedly reduced in animal models with hypertension, diabetes and organ injury. Kallistatin levels are also significantly lower in patients with liver disease, septic syndrome, severe pneumonia, diabetic retinopathy, inflammatory bowel disease, colon and prostate cancer, as well as in apparently healthy African American adolescents with adiposity. Therefore, kallistatin may serve as a new biomarker for the prediction of patient outcomes. Since kallistatin is an endogenous protein, minimal side effects are expected with kallistatin therapy. Thus, kallistatin treatment may potentially be used as a novel therapeutic agent for human diseases.
Acknowledgments
Sources of Funding: This work was supported by the National Institutes of Health grants HL118516 and HL44083.
Footnotes
Disclosures: None.
References
- 1.Chao J, Tillman D, Wang MY, Margolius HS, Chao L. Identification of a new tissue-kallikrein-binding protein. Biochem J. 1986;239:325–331. doi: 10.1042/bj2390325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen LM, Chao L, Mayfield RK, Chao J. Differential interactions of human kallikrein-binding protein and α1-antitrypsin with human tissue kallikrein. Biochem J. 1990;267:79–84. doi: 10.1042/bj2670079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chao J, Chai KX, Chen LM, Xiong W, Chao S, Woodley-Miller C, Wang LX, Lu HS, Chao L. Tissue kallikrein-binding protein is a serpin. I. Purification, characterization, and distribution in normotensive and spontaneously hypertensive rats. J Biol Chem. 1990;265:16394–16401. [PubMed] [Google Scholar]
- 4.Chai KX, Ma JX, Murray SR, Chao J, Chao L. Molecular cloning and analysis of the rat kallikrein-binding protein gene. J Biol Chem. 1991;266:16029–16036. [PubMed] [Google Scholar]
- 5.Chai KX, Chao J, Chao L. Molecular cloning and sequence analysis of the mouse kallikrein-binding protein gene. Biochim Biophys Acta. 1991;1129:127–130. doi: 10.1016/0167-4781(91)90227-d. [DOI] [PubMed] [Google Scholar]
- 6.Zhou GX, Chao L, Chao J. Kallistatin: a novel human tissue kallikrein inhibitor. Purification, characterization, and reactive center sequence. J Biol Chem. 1992;267:25873–25880. [PubMed] [Google Scholar]
- 7.Chai KX, Ward DC, Chao J, Chao L. Molecular cloning, sequence analyses, and chromosomal localization of human kallistatin gene. Genomics. 1994;23:370–378. doi: 10.1006/geno.1994.1513. [DOI] [PubMed] [Google Scholar]
- 8.Chai KX, Chen LM, Chao J, Chao L. Kallistatin: a novel human serine proteinase inhibitor. Molecular cloning, tissue distribution, and expression in Escherichia coli. J Biol Chem. 1993;268:24498–24505. [PubMed] [Google Scholar]
- 9.Chao J, Chao L. Biochemistry, regulation and potential function of kallistatin. Biol Chem Hoppe Seyler. 1995;376:705–713. [PubMed] [Google Scholar]
- 10.Bhoola KD, Figueroa CD, Worthy K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol Rev. 1992;44:1–80. [PubMed] [Google Scholar]
- 11.Chao J, Shen B, Gao L, Xia CF, Bledsoe G, Chao L. Tissue kallikrein in cardiovascular, cerebrovascular and renal diseases and skin wound healing. Biol Chem. 2010;391:345–355. doi: 10.1515/BC.2010.042. [DOI] [PubMed] [Google Scholar]
- 12.Chen LM, Song Q, Chao L, Chao J. Cellular localization of tissue kallikrein and kallistatin mRNAs in human kidney. Kidney Int. 1995;48:690–697. doi: 10.1038/ki.1995.339. [DOI] [PubMed] [Google Scholar]
- 13.Wolf WC, Harley RA, Sluce D, Chao L, Chao J. Localization and expression of tissue kallikrein and kallistatin in human blood vessels. J Histochem Cytochem. 1999;47:1–8. doi: 10.1177/002215549904700210. [DOI] [PubMed] [Google Scholar]
- 14.Chen VC, Chao L, Chao J. Reactive-site specificity of human kallistatin toward tissue kallikrein probed by site-directed mutagenesis. Biochim Biophys Acta. 2000;1479:237–246. doi: 10.1016/s0167-4838(00)00044-3. [DOI] [PubMed] [Google Scholar]
- 15.Chen VC, Chao L, Chao J. Roles of the P1, P2 and P3 residues in determining inhibitory specificity of kallistatin toward human tissue kallikrein. J Biol Chem. 2000;275:38457–38466. doi: 10.1074/jbc.M005605200. [DOI] [PubMed] [Google Scholar]
- 16.Chen VC, Chao L, Pimenta DC, Bledsoe G, Juliano L, Chao J. Identification of a major heparin-binding site in kallistatin. J Biol Chem. 2001;276:1276–1284. doi: 10.1074/jbc.M005791200. [DOI] [PubMed] [Google Scholar]
- 17.Chao J, Chao L. A major difference of kallikrein-binding protein in spontaneously hypertensive versus normotensive rats. J Hypertens. 1988;6:551–557. doi: 10.1097/00004872-198807000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Chao J, Stallone JN, Liang YM, Chen LM, Chao L. Kallistatin is a potent new vasodilator. J Clin Invest. 1997;100:11–17. doi: 10.1172/JCI119502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen LM, Ma JX, Liang YM, Chao L, Chao J. Tissue kallikrein-binding protein reduces blood pressure in transgenic mice. J Biol Chem. 1996;271:27590–27594. doi: 10.1074/jbc.271.44.27590. [DOI] [PubMed] [Google Scholar]
- 20.Chen LM, Chao L, Chao J. Adenovirus-mediated delivery of human kallistatin gene reduces blood pressure of spontaneously hypertensive rats. Hum Gene Ther. 1997;8:341–347. doi: 10.1089/hum.1997.8.3-341. [DOI] [PubMed] [Google Scholar]
- 21.Shen B, Smith RS, Jr, Hsu YT, Chao L, Chao J. Kruppel-like factor 4 is a novel mediator of kallistatin in inhibiting endothelial inflammation through increased eNOS expression. J Biol Chem. 2009;284:35471–35478. doi: 10.1074/jbc.M109.046813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo YM, Li PF, Bledsoe G, Yang ZR, Chao L, Chao J. Kallistatin inhibits TGF-β-induced endothelial-to-mesenchymal transition by differential regulation of microRNA-21 and eNOS expression. Exp Cell Res. 2015;337:103–110. doi: 10.1016/j.yexcr.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kundu JK, Surh YJ. Emerging avenues linking inflammation and cancer. Free Radic Biol Med. 2012;52:2013–2037. doi: 10.1016/j.freeradbiomed.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 24.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miao RQ, Agata J, Chao L, Chao J. Kallistatin is a new inhibitor of angiogenesis and tumor growth. Blood. 2002;100:3245–3252. doi: 10.1182/blood-2002-01-0185. [DOI] [PubMed] [Google Scholar]
- 26.Diao Y, Ma J, Xiao WD, Luo J, Li XY, Chu KW, Fung P, Habib N, Farzaneh F, Xu RA. Inhibition of angiogenesis and HCT-116 xenograft tumor growth in mice by kallistatin. World J Gastroenterol. 2007;23:4615–4619. doi: 10.3748/wjg.v13.i34.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu B, Lu L, Cai W, Yang X, Li C, Yang Z, Zhan W, Ma JX, Gao G. Kallikrein-binding protein inhibits growth of gastric carcinoma by reducing vascular endothelial growth factor production and angiogenesis. Mol Cancer Ther. 2007;6:3297–3306. doi: 10.1158/1535-7163.MCT-06-0798. [DOI] [PubMed] [Google Scholar]
- 28.Lu L, Yang Z, Zhu B, Fang S, Yang X, Cai W, Li C, Ma JX, Gao G. Kallikrein-binding protein suppresses growth of hepatocellular carcinoma by anti-angiogenic activity. Cancer Lett. 2007;257:97–106. doi: 10.1016/j.canlet.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Jiang X, Li H, Qiao H, Jiang H, Xu R, Sun X. Combining kallistatin gene therapy and meloxicam to treat hepatocellular carcinoma in mice. Cancer Sci. 2009;100:2226–2233. doi: 10.1111/j.1349-7006.2009.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tse LY, Sun X, Jiang H, Dong X, Fung PW, Farzaneh F, Xu R. Adeno-associated virus-mediated expression of kallistatin suppresses local and remote hepatocellular carcinomas. J Gene Med. 2008;10:508–517. doi: 10.1002/jgm.1180. [DOI] [PubMed] [Google Scholar]
- 31.Yao Y, Li L, Huang X, Gu X, Xu Z, Zhang Y, Huang L, Li S, Dai Z, Li C, Zhou T, Cai W, Yang Z, Gao G, Yang X. SERPINA3K induces apoptosis in human colorectal cancer cells via activating the Fas/FasL/caspase-8 signaling pathway. FEBS J. 2013;280:3244–3255. doi: 10.1111/febs.12303. [DOI] [PubMed] [Google Scholar]
- 32.Li P, Guo YM, Bledsoe G, Yang ZR, Chao L, Chao J. Kallistatin induces breast cancer cell apoptosis and autophagy by modulating Wnt signaling and microRNA synthesis. Exp Cell Res. 2016;340:305. doi: 10.1016/j.yexcr.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiau AL, Teo ML, Chen SY, Wang CR, Hsieh JL, Chang MY, Chang CJ, Chao J, Chao L, Wu CL, Lee CH. Inhibition of experimental lung metastasis by systemic lentiviral delivery of kallistatin. BMC Cancer. 2010;10:245. doi: 10.1186/1471-2407-10-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;8:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 35.Miao RQ, Chen V, Chao L, Chao J. Structural elements of kallistatin required for inhibition of angiogenesis. Am J Physiol Cell Physiol. 2003;284:C1604–C1613. doi: 10.1152/ajpcell.00524.2002. [DOI] [PubMed] [Google Scholar]
- 36.Yin H, Gao L, Shen B, Chao L, Chao J. Kallistatin inhibits vascular inflammation by antagonizing tumor necrosis factor-α-induced NF-κB activation. Hypertension. 2010;56:260–267. doi: 10.1161/HYPERTENSIONAHA.110.152330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang KF, Huang XP, Xiao GQ, Yang HY, Lin JS, Diao Y. Kallistatin, a novel anti-angiogenesis agent, inhibits angiogenesis via inhibition of the NF-κB signaling pathway. Biomed Pharmacother. 2014;68:455–461. doi: 10.1016/j.biopha.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Shen B, Gao L, Hsu YT, Chao L, Chao J. Kallistatin attenuates endothelial apoptosis through inhibition of oxidative stress and activation of Akt-eNOS signaling. Am J Physiol Heart Circ Physiol. 2010;299:H1419–H1427. doi: 10.1152/ajpheart.00591.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang MZ, Tsukahara H, Ohshima Y, Todoroki Y, Hiraoka M, Maeda M, Mayumi M. Effects of antioxidants and nitric oxide on TNF-alpha-induced adhesion molecule expression and NF-kappaB activation in human dermal microvascular endothelial cells. Life Sci. 2004;75:1159–1170. doi: 10.1016/j.lfs.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 40.Li P, Bledsoe G, Yang ZR, Fan H, Chao L, Chao J. Human kallistatin administration reduces organ injury and improves survival in a mouse model of polymicrobial sepsis. Immunology. 2014;42:216–226. doi: 10.1111/imm.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin F, Wang N, Zhang TC. The role of endothelial-mesenchymal transition in development and pathological process. IUBMB Life. 2012;64:717–723. doi: 10.1002/iub.1059. [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Zhang B, McBride JD, Zhou K, Lee K, Zhou Y, Liu Z, Ma JX. Antiangiogenic and antineuroinflammatory effects of kallistatin through interactions with the canonical Wnt pathway. Diabetes. 2013;62:4228–4238. doi: 10.2337/db12-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J, Yang Z, Li P, Bledsoe G, Chao L, Chao J. Kallistatin blocks Wnt/β-catenin signaling and cancer cell motility by binding to LRP6. Mol Cell Biochem. 2013;379:295–301. doi: 10.1007/s11010-013-1654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhi X, Lin L, Yang S, Bhuvaneshwar K, Wang H, Gusev Y, Lee MH, Kallakury B, Shivapurkar N, Cahn K, Tian X, Marshall JL, Byers SW, He AR. βII-Spectrin (SPTBN1) suppresses progression of hepatocellular carcinoma and Wnt signaling by regulation of Wnt inhibitor kallistatin. Hepatology. 2015;61:598–612. doi: 10.1002/hep.27558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li JJ, Fang CH, Hui RT. Is hypertension an inflammatory disease? Med Hypotheses. 2005;64:236–240. doi: 10.1016/j.mehy.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 46.Caillon A, Schiffrin EL. Role of inflammation and immunity in hypertension: Recent epidemiological, laboratory, and clinical evidence. Curr Hypertens Rep. 2016;18:21. doi: 10.1007/s11906-016-0628-7. [DOI] [PubMed] [Google Scholar]
- 47.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res. 2015;116:1022–1033. doi: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lob HE, Schultz D, Marvar PJ, Davisson RL, Harrison DG. Role of the NADPH oxidases in the subfornical organ in angiotensin II-induced hypertension. Hypertension. 2013;61:382–387. doi: 10.1161/HYPERTENSIONAHA.111.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang CR, Chen SY, Wu CL, Liu MF, Jin YT, Chao L, Chao J. Prophylactic adenovirus-mediated human kallistatin gene therapy suppresses rat arthritis by inhibiting angiogenesis and inflammation. Arthritis Rheum. 2005;52:1319–1324. doi: 10.1002/art.20991. [DOI] [PubMed] [Google Scholar]
- 50.Chao J, Yin H, Yao YY, Shen B, Smith RS, Jr, Chao L. Novel role of kallistatin in protection against myocardial ischemia-reperfusion injury by preventing apoptosis and inflammation. Hum Gene Ther. 2006;17:1201–1213. doi: 10.1089/hum.2006.17.1201. [DOI] [PubMed] [Google Scholar]
- 51.Shen B, Hagiwara M, Yao YY, Chao L, Chao J. Salutary effect of kallistatin in salt-induced renal injury, inflammation, and fibrosis via antioxidative stress. Hypertension. 2008;51:1358–1365. doi: 10.1161/HYPERTENSIONAHA.107.108514. [DOI] [PubMed] [Google Scholar]
- 52.Li P, Bledsoe G, Yang ZR, Fan H, Chao L, Chao J. Kallistatin treatment attenuates lethality and organ injury in mouse models of established sepsis. Crit Care. 2015;19:200. doi: 10.1186/s13054-015-0919-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin WC, Chen CW, Chao L, Chao J, Lin YS, Lin CF. Kallistatin protects against sepsis-related acute lung injury via inhibiting inflammation and apoptosis. Sci Rep. 2015;5:12463. doi: 10.1038/srep12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y, Bledsoe G, Hagiwara M, Shen B, Chao L, Chao J. Depletion of endogenous kallistatin exacerbates renal and cardiovascular oxidative stress, inflammation and organ remodeling. Am J Physiol Renal Physiol. 2012;303:F1230–F1238. doi: 10.1152/ajprenal.00257.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spiecker M, Darius H, Kaboth K, Hübner F, Liao JK. Differential regulation of endothelial cell adhesion molecule expression by nitric oxide donors and antioxidants. J Leukoc Biol. 1998;63:732–729. [PubMed] [Google Scholar]
- 56.Dai Z, Lu L, Yang Z, Mao Y, Lu J, Li C, Qi W, Chen Y, Yao Y, Li L, Chen S, Zhang Y, Cai W, Yang X, Gao G. Kallikrein-binding protein inhibits LPS-induced TNF-α by upregulating SOCS3 expression. J Cell Biochem. 2013;114:1020–1028. doi: 10.1002/jcb.24441. [DOI] [PubMed] [Google Scholar]
- 57.Inagaki-Ohara K, Kondo T, Ito M, Yoshimura A. SOCS, inflammation, and cancer. JAKSTAT. 2013;2:e24053. doi: 10.4161/jkst.24053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Montezano AC, Touyz RM. Molecular mechanisms of hypertension-reactive oxygen species and antioxidants: a basic science update for the clinician. Can J Cardiol. 2012;28:288–295. doi: 10.1016/j.cjca.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 59.Shen B, Chao L, Chao J. Pivotal role of JNK-dependent FOXO1 activation in downregulation of kallistatin expression by oxidative stress. Am J Physiol Heart Circ Physiol. 2010;298:H1048–H1054. doi: 10.1152/ajpheart.00826.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao L, Yin H, Smith RS, Jr, Chao L, Chao J. Role of kallistatin in prevention of cardiac remodeling after chronic myocardial infarction. Lab Invest. 2008;88:1157–1166. doi: 10.1038/labinvest.2008.85. [DOI] [PubMed] [Google Scholar]
- 61.Huang X, Wang X, Lv Y, Xu L, Lin J, Diao Y. Protection effect of kallistatin on carbon tetrachloride-induced liver fibrosis in rats via antioxidative stress. PLoS One. 2014;9:e88498. doi: 10.1371/journal.pone.0088498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou T, Zong R, Zhang Z, Zhu C, Pan F, Xiao X, Liu Z, He H, Ma JX, Liu Z, Zhou Y. SERPINA3K protects against oxidative stress via modulating ROS generation/degradation and KEAP1-NRF2 pathway in the corneal epithelium. Invest Ophthalmol Vis Sci. 2012;53:5033–5043. doi: 10.1167/iovs.12-9729. [DOI] [PubMed] [Google Scholar]
- 63.Zhu C, Pan F, Ge L, Zhou J, Chen L, Zhou T, Zong R, Xiao X, Dong N, Yang M, Ma JX, Liu Z, Zhou Y. SERPINA3K plays antioxidant roles in cultured pterygial epithelial cells through regulating ROS system. PLoS One. 2014;9:e108859. doi: 10.1371/journal.pone.0108859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Storz P. Forkhead homeobox type O transcription factors in the responses to oxidative stress. Antioxid Redox Signal. 2011;14:593–605. doi: 10.1089/ars.2010.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fujii H, Ichimori K, Hoshiai K, Nakazawa H. Nitric oxide inactivates NADPH oxidase in pig neutrophils by inhibiting its assembling process. J Biol Chem. 1997;272:32773–32778. doi: 10.1074/jbc.272.52.32773. [DOI] [PubMed] [Google Scholar]
- 66.Umemura T, Higashi Y. Endothelial progenitor cells: therapeutic target for cardiovascular diseases. J Pharmacol Sci. 2008;108:1–6. doi: 10.1254/jphs.08r01cp. [DOI] [PubMed] [Google Scholar]
- 67.Besler C, Doerries C, Giannotti G, Lüscher TF, Landmesser U. Pharmacological approaches to improve endothelial repair mechanisms. Expert Rev Cardiovasc Ther. 2008;6:1071–1082. doi: 10.1586/14779072.6.8.1071. [DOI] [PubMed] [Google Scholar]
- 68.Mikirova NA, Jackson JA, Hunninghake R, Kenyon J, Chan KW, Swindlehurst CA, Minev B, Patel AN, Murphy MP, Smith L, Alexandrescu DT, Ichim TE, Riordan NH. Circulating endothelial progenitor cells: a new approach to anti-aging medicine? J Transl Med. 2009;7:106. doi: 10.1186/1479-5876-7-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choi JH, Kim KL, Huh W, Kim B, Byun J, Suh W, Sung J, Jeon ES, Oh HY, Kim DK. Decreased number and impaired angiogenic function of endothelial progenitor cells in patients with chronic renal failure. Arterioscler Thromb Vasc Biol. 2004;24:1246–1252. doi: 10.1161/01.ATV.0000133488.56221.4a. [DOI] [PubMed] [Google Scholar]
- 70.Grisar J, Aletaha D, Steiner CW, Kapral T, Steiner S, Seidinger D, Weigel G, Schwarzinger I, Wolozcszuk W, Steiner G, Smolen JS. Depletion of endothelial progenitor cells in the peripheral blood of patients with rheumatoid arthritis. Circulation. 2005;111:204–211. doi: 10.1161/01.CIR.0000151875.21836.AE. [DOI] [PubMed] [Google Scholar]
- 71.Cribbs SK, Sutcliffe DJ, Taylor WR, Rojas M, Easley KA, Tang L, Brigham KL, Martin GS. Circulating endothelial progenitor cells inversely associate with organ dysfunction in sepsis. Intensive Care Med. 2012;38:429–436. doi: 10.1007/s00134-012-2480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao L, Li P, Zhang J, Hagiwara M, Shen B, Bledsoe G, Chang E, Chao L, Chao J. Novel role of kallistatin in vascular repair by promoting mobility, viability, and function of endothelial progenitor cells. J Am Heart Assoc. 2014;3:e001194. doi: 10.1161/JAHA.114.001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 74.Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rütten H, Fichtlscherer S, Martin H, Zeiher AM. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vincent JL, Opal SM, Marshall JC, Tracey KJ. Sepsis definitions: time for change. Lancet. 2013;381:774–775. doi: 10.1016/S0140-6736(12)61815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517–524. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 77.Chao J, Chen LM, Chai KX, Chao L. Expression of kallikrein-binding protein and alpha 1-antitrypsin genes in response to sex hormones, growth, inflammation and hypertension. Agents Actions Suppl. 1992;38:174–181. doi: 10.1007/978-3-0348-7321-5_23. [DOI] [PubMed] [Google Scholar]
- 78.Chao J, Schmaier A, Chen LM, Yang Z, Chao L. Kallistatin, a novel human tissue kallikrein inhibitor: levels in body fluids, blood cells, and tissues in health and disease. J Lab Clin Med. 1996;127:612–620. doi: 10.1016/s0022-2143(96)90152-3. [DOI] [PubMed] [Google Scholar]
- 79.Frank AJ, Sheu CC, Zhao Y, Chen F, Su L, Gong MN, Bajwa E, Thompson BT, Christiani DC. BCL2 genetic variants are associated with acute kidney injury in septic shock. Crit Care Med. 2012;40:2116–2123. doi: 10.1097/CCM.0b013e3182514bca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen LM, Chao L, Chao J. Beneficial effects of kallikrein-binding protein in transgenic mice during endotoxic shock. Life Sci. 1997;60:1431–1436. doi: 10.1016/s0024-3205(97)00094-5. [DOI] [PubMed] [Google Scholar]
- 81.Lu SL, Tsai CY, Luo YH, Kuo CF, Lin WC, Chang YT, Wu JJ, Chuang WJ, Liu CC, Chao L, Chao J, Lin YS. Kallistatin modulates immune cells and confers anti-inflammatory response to protect mice from group A Streptococcal infection. Antimicrob Agents Chemother. 2013;57:5366–5372. doi: 10.1128/AAC.00322-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xiong W, Tong C, Zhou G, Chao L, Chao J. In vivo catabolism of human kallikrein-binding protein and its complex with tissue kallikrein. J Lab Clin Med. 1992;119:514–521. [PubMed] [Google Scholar]
- 83.Ma JX, Yang Z, Chao J, Chao L. Intramuscular delivery of rat kallikrein-binding protein gene reverses hypotension in transgenic mice expressing human tissue kallikrein. J Biol Chem. 1995;270:451–455. doi: 10.1074/jbc.270.1.451. [DOI] [PubMed] [Google Scholar]
- 84.Mahabeer R, Bhoola KD. Kallikrein and kinin receptor genes. Pharmacol Ther. 2000;88:77–89. doi: 10.1016/s0163-7258(00)00080-2. [DOI] [PubMed] [Google Scholar]
- 85.Wu J, Akaike T, Maeda H. Modulation of enhanced vascular permeability in tumors by a bradykinin antagonist, a cyclooxygenase inhibitor, and a nitric oxide scavenger. Cancer Res. 1998;58:159–165. [PubMed] [Google Scholar]
- 86.Yao YY, Yin H, Shen B, Smith RS, Jr, Liu Y, Gao L, Chao L, Chao J. Tissue kallikrein promotes neovascularization and improves cardiac function by the Akt-glycogen synthase kinase-3beta pathway. Cardiovasc Res. 2008;80:354–364. doi: 10.1093/cvr/cvn223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leu CH, Yang ML, Chung NH, Huang YJ, Su YC, Chen YC, Lin CC, Shieh GS, Chang MY, Wang SW, Chang Y, Chao J, Chao L, Wu CL, Shiau AL. Kallistatin ameliorates influenza virus pathogenesis by inhibition of kallikrein-related peptidase 1-mediated cleavage of viral hemagglutinin. Antimicrob Agents Chemother. 2015;59:5619–5630. doi: 10.1128/AAC.00065-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gao G, Shao C, Zhang SX, Dudley A, Fant J, Ma JX. Kallikrein-binding protein inhibits retinal neovascularization and decreases vascular leakage. Diabetologia. 2003;46:689–698. doi: 10.1007/s00125-003-1085-9. [DOI] [PubMed] [Google Scholar]
- 89.Zhang B, Ma JX. SERPINA3K prevents oxidative stress induced necrotic cell death by inhibiting calcium overload. PLoS One. 2008;3:e4077. doi: 10.1371/journal.pone.0004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chao C, Madeddu P, Wang C, Liang Y, Chao L, Chao J. Differential regulation of kallikrein, kininogen, and kallikrein-binding protein in arterial hypertensive rats. Am J Physiol. 1996;271:F78–F86. doi: 10.1152/ajprenal.1996.271.1.F78. [DOI] [PubMed] [Google Scholar]
- 91.Hatcher HC, Ma JX, Chao J, Chao L, Ottlecz A. Kallikrein-binding protein levels are reduced in the retinas of streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci. 1997;38:658–664. [PubMed] [Google Scholar]
- 92.Luo Q, Siconolfi-Baez L, Annamaneni P, Bielawski MT, Novikoff PM, Angeletti RH. Altered protein expression at early-stage rat hepatic neoplasia. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1272–G1282. doi: 10.1152/ajpgi.00474.2006. [DOI] [PubMed] [Google Scholar]
- 93.Stadnicki A, Mazurek U, Plewka D, Wilczok T. Intestinal tissue kallikrein-kallistatin profile in inflammatory bowel disease. Int Immunopharmacol. 2003;3:939–944. doi: 10.1016/S1567-5769(03)00054-7. [DOI] [PubMed] [Google Scholar]
- 94.Cheng Z, Lv Y, Pang S, Bai R, Wang M, Lin S, Xu T, Spalding D, Habib N, Xu R. Kallistatin, a new and reliable biomarker for the diagnosis of liver cirrhosis. Acta Pharm Sin B. 2015;5:194–200. doi: 10.1016/j.apsb.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lin WC, Lu SL, Lin CF, Chen CW, Chao L, Chao J, Lin YS. Plasma kallistatin levels in patients with severe community-acquired pneumonia. Crit Care. 2013;17:R27. doi: 10.1186/cc12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhu H, Chao J, Kotak I, Guo D, Parikh SJ, Bhagatwala J, Dong Y, Patel SY, Houk C, Chao L, Dong Y. Plasma kallistatin is associated with adiposity and cardiometabolic risk in apparently healthy African American adolescents. Metabolism. 2013;62:642–646. doi: 10.1016/j.metabol.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ma JX, King LP, Yang Z, Crouch RK, Chao L, Chao J. Kallistatin in human ocular tissues: reduced levels in vitreous fluids from patients with diabetic retinopathy. Curr Eye Res. 1996;15:1117–1123. doi: 10.3109/02713689608995143. [DOI] [PubMed] [Google Scholar]
- 98.Chao J, Bledsoe G, Chao L. Kallistatin: a novel biomarker for hypertension, organ injury and cancer. Austin Biomark Diagn. 2015;2:1–5. [Google Scholar]
- 99.McBride J, Jenkins A, Liu X, Zhang B, Lee K, Berry WL, Janknecht R, Griffin C, Aston CE, Lyons T, Tomasek JJ, Ma JX. Elevated circulation levels of an anti-angiogenic SERPIN in patients with diabetic microvascular complications impairs wound healing through suppression of Wnt signaling. J Invest Dermatol. 2014;134:1725–1734. doi: 10.1038/jid.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jenkins AJ, McBride JD, Januszewski AS, Karschimkus CS, Zhang B, O'Neal DN, Nelson CL, Chung JS, Harper CA, Lyons TJ, Ma JX. Increased serum kallistatin levels in type 1 diabetes patients with vascular complications. J Angiogenes Res. 2010;2:19. doi: 10.1186/2040-2384-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang CR, Chen SY, Shiau AL, Wu CL, Jou IM, Chao L, Chao J. Upregulation of kallistatin expression in rheumatoid joints. J Rheumatol. 2007;34:2171–2176. [PubMed] [Google Scholar]