Abstract
Previous studies mainly focused on the role of the epidermal growth factor receptor (EGFR) in tumor cells, whereas the effects of the EGFR on immune responses has not been determined. Our study shows that the EGFR signaling pathway play a role in the regulation of regulatory T cells (Treg cells) in cancer patients. The EGF-like growth factor Amphiregulin (AREG) protein was frequently up-regulated in a tissue microarray, which was associated with worse overall survival. Additionally, in sera, tissue specimens, and effusions of lung or gastric cancer patients, up-regulated AREG protein enhanced the suppressive function of Treg cells. AREG maintained the Treg cell suppressive function via the EGFR/GSK-3β/Foxp3 axis in vitro and in vivo. Furthermore, inhibition of EGFR by the tyrosine kinase inhibitor gefitinib restored the activity of GSK-3β and attenuated Treg cell function. β-TrCP was involved in GSK-3β-mediated Foxp3 degradation, and mass spectrometry identified Lys356 as the ubiquitination site of Foxp3 by β-TrCP. These findings demonstrate the posttranslational regulation of Foxp3 expression by AREG in cancer patients through AREG/EGFR/GSK-3β signaling, which could lead to Foxp3 protein degradation in Treg cells and a potential therapeutic target for cancer treatment.
Keywords: epidermal growth factor receptor (EGFR), forkhead box P3 (FOXP3), glycogen synthase kinase 3 (GSK-3), phosphorylation, protein degradation, protein phosphorylation, ubiquitylation (ubiquitination), GSK-3β, lung adenocarcinoma, regulatory T cells
Introduction
The epidermal growth factor receptor (EGFR)5 is a transmembrane receptor that is activated by binding of its specific ligands. Stimulation of the EGFR through tyrosine kinase activity triggers a series of intracellular pathways that may result in cancer cell proliferation, blocking of apoptosis, activation of invasion and metastasis, and stimulation of tumor-induced neovascularization (1, 2). Importantly, the majority of human epithelial cancers are marked by functional activation of growth factors and receptors of the EGFR. Targeting the EGFR is currently available for the treatment of different types of epithelial cancers, such as non-small-cell lung cancer, squamous cell carcinoma of the head and neck, colorectal cancer, and pancreatic cancer (3–6). The treatment demonstrated that EGFR inhibitors bind to the extracellular domain of the EGFR when it is in the inactive configuration, compete for receptor binding by occluding the ligand-binding region, and thereby directly block ligand-induced EGFR tyrosine kinase activation on cancer cells (7, 8). However, the mechanisms of EGFR-targeted treatments in cancer patients remain poorly understood clinically. Recent studies indicated that the therapeutic effect of EGFR-targeted treatment can be observed both on cancer cells and treatment-induced immune responses (9, 10). The EGFR is demonstrated to be expressed on immune cells such as monocytes and plasma cells (11, 12). Additionally, the ligands of the EGFR, including epidermal growth factor and TGF-α, are released not only by epithelial cells but also by activated immune cells. Given these findings, the EGFR signaling pathway could play an important role in immune responses in cancer patients.
The EGF-like growth factor Amphiregulin (AREG) is an 84-amino acid glycoprotein that plays a vital role in physiological and cancerous tissues. AREG is also involved in the resistance to several cancer treatments. Recent studies demonstrated that AREG is involved in inflammation. Furthermore, the EGF-like growth factor AREG is expressed by activated Th2 cells, mast cells, eosinophils, and basophils (13–16). These studies suggest that the function of AREG might be to regulate immune responses.
Regulatory T (Treg) cells, crucial to maintain immune cell homeostasis, were shown to play a role in tumor development and progression (17). For instance, accumulation of Treg cells in a tumor lesion inhibited the tumor-specific T cell immunity that contributes to tumor cell growth in vivo (18, 19). More recently, AREG was shown to enhance the suppressive function of Treg cells via the EGFR signaling pathway (20). However, the precise mechanism underlying the pathogenic role of AREG/EGFR signaling in modulating Treg cell function is not fully defined.
Forkhead box P3 (Foxp3) is a protein involved in immune system responses and is required for Treg cell differentiation and function. Previous studies showed that deacetylation of Foxp3 is linked to impaired Treg cell function in autoimmune disorders (21), whereas phosphorylation and ubiquitination of Foxp3 affects its activity and Treg cell function (22, 23). Furthermore, our previous study suggested that GSK-3β (glycogen synthase kinase 3β) was able to inactivate Foxp3 protein (24). In light of these findings, we wanted to determine how AREG/EGFR signaling contributes to the regulation of immune responses, especially to Treg cells. In this study, we report that AREG/EGFR signaling enhances Foxp3 expression by inhibiting the GSK-3β/β-TrCP pathway. Foxp3 is destabilized as a consequence of its phosphorylation by GSK-3β and subsequent ubiquitination by β-TrCP. More importantly, investigation of the mechanisms that promote the stability of Foxp3 protein and functional plasticity of the Treg cell lineage helps to understand the limitation of cancer immune surveillance.
Results
Up-regulated Expression of AREG and Increased Level of Treg Cells in Specimens from Cancer Patients
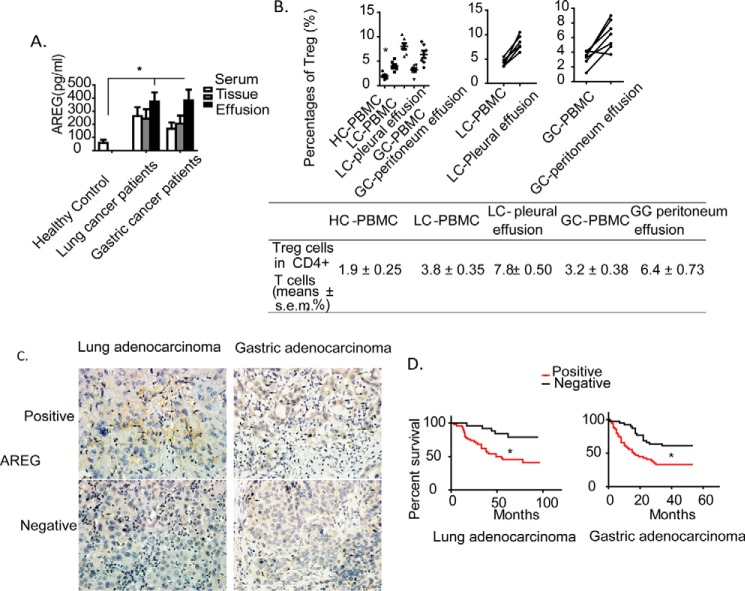
To determine the clinical association of AREG expression, we also assessed AREG expression in the blood and malignant pleural or peritoneal effusions from cancer patients (n = 7). Serum levels of AREG protein were higher in lung and gastric cancer patients than in age-matched healthy individuals. Elevated levels of AREG were also observed in the tumor tissues and effusions of these patients (Fig. 1A). In addition, fluorescence activated cell sorting (FACS) analysis revealed that the frequency of human Treg cells (CD4+CD25hi Foxp3+) was increased in tumor-derived effusions and peripheral blood mononuclear cells (PBMC) of lung cancer (LC-PBMC) and gastric cancer (GC-PBMC) compared with that of healthy controls (HC-PBMC) (Fig. 1B, quantitation shown in the bottom panel).
FIGURE 1.
Clinical association of AREG expression level and Treg cell ratio with survival of cancer patients. A, AREG expression levels in serum, tissue specimens, and effusions from HC donors and LC and GC patients. B, mean frequencies of CD4+CD25hiFoxp3+ Treg cells in CD4+ T cells from the indicated groups (top left, n = 7/group) and paired samples (top center and top right, n = 6/group). C, representative results of AREG immunoreactivity in tissue specimens of LC and GC patients. The arrows point to positive cells. D, Kaplan-Meier overall survival curves of AREG in LC and GC patients. *, p < 0.05.
To investigate the pathological relevance of AREG expression, we also analyzed the levels of AREG in tissue specimens of lung cancer (lung adenocarcinoma and bronchioloalveolar carcinoma; 75 cases) and gastric adenocarcinoma (90 cases). The data showed that AREG was highly expressed in 48 of 75 lung cancer tissues (64%) and 52 of 90 gastric cancer tissues (57.8%). AREG expression was associated with tumor stage in gastric cancer but not with tumor stage or lymph node metastasis in lung cancer (data not shown). Moreover, AREG expression was associated with poorer overall survival of lung and gastric cancer patients (Fig. 1, C and D). These results indicate that up-regulated expression of AREG protein and increased levels of Treg cells within CD4+ T cells in tumor-derived effusions and PBMC could play a suppressive role in Teff cell function.
AREG in Malignant Effusion Is Required for Maintaining Treg Cell Suppressive Function
To analyze Treg cell suppressive function on Teff cells, we isolated CD4+CD25hi T cells and CD4+CD25− Teff cells from peripheral blood or tumor-derived effusion mononuclear cells of cancer patients to evaluate the ability of CD4+CD25hi T cells to suppress the proliferation of activated CD4+CD25− T cells by an in vitro co-culture assay (the ratios between responder T cells (CD4+CD25− T cells) and Treg cells were 1:0 and 1:0.5) and then calculated the inhibition index (see “Experimental Procedures” for more details). CD4+CD25hi T and CD4+CD25− Teff cells sorted to high purity were used for the co-culture assay (Fig. 2A). Thus, CD4+CD25hi T cells (CD4+CD25hiFoxp3+ >95% purity) were considered to be Treg cells. Analysis of Treg cells by an immunosuppressive assay demonstrated that the suppressive function was largely enhanced in Treg cells isolated from cancer patients (Fig. 2B). Moreover, the addition of LC pleural effusions to Treg cell cultures derived from LC-PBMC enhanced the suppressive function of Treg cells in a dose-dependent manner (Fig. 2C). Notably, LC pleural effusion-enhanced Treg cell activities were neutralized by antibodies against AREG but not those against TNF-α, EGF, or TGF-α (Fig. 2D). Furthermore, dose-dependent addition of recombinant AREG enhanced the suppressive activity of Treg cells isolated from peripheral blood or tumor-derived effusion mononuclear cells of cancer patients (Fig. 2E). Collectively, these data suggest that AREG plays an important role in enhancing the suppressive function of Treg cells in cancer patients.
FIGURE 2.
AREG in malignant effusions is required for maintaining Treg cell suppressive function. A, representative FACS results of isolated Treg cells (CD4+CD25hi) from malignant effusions. The purity of Treg cells was >95%. B, the suppressive function of Treg cells is significantly impaired in malignant effusions. C, the proliferation inhibition ratio of CD4+CD25− T (Teff) cells (from LC-PBMC) by CD4+CD25hi T (Treg) cells from matched LC-pleural effusions at different dilutions. D, the proliferation inhibition ratio of CD4+CD25− T (Teff) cells (from LC-PBMC) by CD4+CD25hi T (Treg) cells from matched LC-pleural effusion at the optimal dilution (1:1), the LC-pleural effusion was pretreated with anti-TNF-α, anti-AREG, anti-TGF-α or anti-EGF, respectively. E, the proliferation inhibition ratio of CD4+CD25− T cells by Treg cells (from matched LC-PBMC) in response to recombinant AREG protein showing a dose-dependent effect. Data are mean ± S.D. of five independent experiments. *, p < 0.05.
Regulatory T Cells from Patients with Tumors Express the EGFR, and Blocking AREG or EGFR Signaling Inhibits Tumor Metastasis via Impairing Treg Cell Function
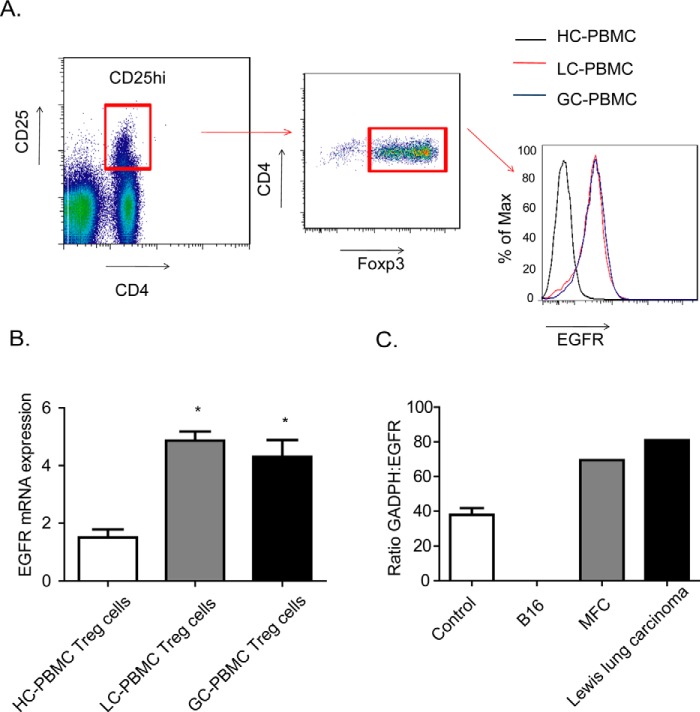
We asked how AREG signaling affects Treg cells, we first measured EGFR expression, a receptor for AREG, in CD4+CD25hi T cells by FACS and quantitative PCR. Notably, the expression of EGFR was substantially higher in Treg cells from GC-PBMC and LC-PBMC than from HC-PBMC (Fig. 3, A and B). The pivotal role of Treg cells in the generation of a tumor-intrinsic immunosuppressive environment is well established in the B16-luc melanoma model, which lacks EGFR expression (Fig. 3C).
FIGURE 3.
Treg cells as well as tumor cells express EGFR. A, representative FACS analysis of EGFR expression in CD4+CD25hi Treg cells derived from LC-PBMC and GC-PBMC. B, absolute EGFR expression is compared with β2 m expression and relative EGFR expression of CD4βCD25hi Treg cells are compared with different CD4 T cells in PMBC groups. Treg cells and CD4+ T cells were sorted by flow cytometry. C, mRNA from B16, MFC, Lewis lung carcinoma tumor cells and control mice lung tissue were purified, and quantitative RT-PCR was performed. Relative EGFR expression of different tumor and control cells was the mean of ratio GAPDH:EGFR. Data are mean ± S.D. of five independent experiments. MFC, mouse forestomach carcinoma. *, p < 0.05.
We compared this model with those expressing EGFR and determined the interactional role of AREG-EGFR in the regulation of Treg function. We immunized B16-luc-transplanted mice with TRP2180-188 tumor epitope-pulsed in vitro differentiated BMDC on days 5 and 7 after tumor transplantation. To facilitate sorting of mice Treg (CD4+Foxp3+) cells, we established a Foxp3-GFP transgenic C57BL/6 mouse model implanted with B16-luc melanoma. As reported, immunization alone had no effect on tumor growth in Foxp3-GFP transgenic C57BL/6 mice. Mice were treated with the EGFR tyrosine kinase inhibitor gefitinib or AREG antibody concomitantly with immunization every other day. Administration of the IgG antibody served as a control. As shown in Fig. 4, A and B, administration of gefitinib or anti-AREG antibody led to a decrease in the number of pulmonary metastasis nodes. Additionally, immunization combined treatment with gefitinib or anti-AREG antibody significantly enhanced the rejection of transplanted B16-luc melanoma, which transferred subcutaneously into the lower left flank of mice (data not shown). However, the number of CD4+Foxp3−GFP Treg cells isolated from the Foxp3−GFP transgenic mouse model by FACS was significantly decreased in the gefitinib or AREG treatment group compared with the control (Fig. 4C). The inhibition index of Treg cells isolated from the gefitinib or anti-AREG antibody treatment groups was decreased, which indicated that their suppressive functions were largely impaired (Fig. 4D). These data suggest that AREG/EGFR signaling enhances the suppressive function of Treg cells and promotes tumor metastasis in the mouse model.
FIGURE 4.
The AREG/EGFR pathway promotes tumor metastasis, Treg cell ratio, and suppressive function. A, representative results of the mouse tumor metastasis model in response to gefitinib or anti-AREG administration, as shown by pulmonary bioluminescence imaging. B, the mean lung photon flux in the mouse model of tumor metastasis in response to gefitinib or anti-AREG administration. C, the mean frequencies of Treg (Foxp3+CD4+) cells from LC-PBMC in response to gefitinib or anti-AREG administration. D, the proliferation inhibition ratio of CD4+CD25− T cells by Treg cells (from matched LC-PBMC) in response to gefitinib or anti-AREG administration. Data are mean ± S.D. of five independent experiments. *, p < 0.05.
AREG/EGFR Signaling Enhances Foxp3 Expression by Inhibiting GSK-3β Activity
We asked how AREG/EGFR signaling regulates the expression of Foxp3 in Treg cells. To determine whether GSK-3β associates with Foxp3, we detected a physical association between GSK-3β and Foxp3 in CD4+CD25hi Treg cells by immunoprecipitation using GSK-3β and Foxp3 antibodies (Fig. 5A). Our previous study showed, by mass spectrometry analysis, that GSK-3β phosphorylates Foxp3 at Ser270 and Ser274 (24). In this study, inhibition of GSK-3β expression by siRNA reduced the phosphorylation of Foxp3 in CD4+CD25hi Treg cells (Fig. 5B, quantitation blot shown in the right panel, p < 0.05), supporting that Foxp3 is a phosphorylation substrate of GSK-3β. In addition, the half-life of Foxp3 protein was significantly prolonged in CD4+CD25hi Treg cells treated with a GSK-3β siRNA (Fig. 5C). To this end, we found that AREG treatment induced higher phosphorylated GSK-3β and Foxp3 expression in Treg cells from LC-PBMC and GC-PBMC than from HC-PBMC (Fig. 5D), suggesting AREG/EGFR-mediated kinase may act on Foxp3. There were no differences in the level of GSK-3β expression in Treg cells among the groups (Fig. 5E). Based on the analysis of protein homology models from SWISS-MODEL annomination, consensus substrate sites of GSK-3β only among the AREG/EGFR kinases were identified in the Foxp3. Consistently, AREG treatment induced higher p-GSK-3β and Foxp3 expression in LC-PBMC and GC-PBMC Treg cells (CD4+CD25hi) than in HC-PBMC (quantitation blot shown in Fig. 5, E and F; p < 0.05). AREG stimulation, however, had no effect on Foxp3 mRNA levels (Fig. 5G). These findings raise the interesting possibility that AREG may regulate GSK-3β and phosphorylate Foxp3 to regulate the function of Treg cells.
FIGURE 5.
AREG modulates the GSK-3β/Foxp3 axis in Treg cells. A, interaction of GSK-3β and Foxp3 in CD4+CD25hi Treg cells. IP, immunoprecipitation. B, GSK-3β siRNA reduced phosphorylated Foxp3 (p270 and p274). Right panel, semiquantitative analysis of Ser270 and Ser274 phosphorylation of Foxp3 and protein expression of FLAG-Foxp3 and GSK-3β. C, measurement of Foxp3 protein half-life in control or GSK-3β siRNA knockdown CD4+CD25hi Treg cells. The half-life of Foxp3 was significantly increased in GSK-3β knockdown CD4+CD25hi Treg cells. CHX, cycloheximide. D and E, expression levels of GSK-3β, p-GSK-3β, and Foxp3 in CD4+CD25hi Treg cells in response to recombinant AREG (100 ng/ml). AREG significantly increased phosphorylation of GSK-3β, but not total GSK-3β protein level, in CD4+CD25hi Treg cells (isolated from LC- and GC-PBMC). F and G, AREG significantly increased the Foxp3 protein level, but not the Foxp3 mRNA level, in CD4+CD25hi Treg cells (isolated from LC- and GC-PBMC). H, the AREG-stimulated Foxp3 protein level in CD4+CD25hi Treg cells was decreased in response to gefitinib or anti-AREG administration. Data are mean ± S.D. of five independent experiments. *, p < 0.05.
Next we compared the effects of gefitinib and anti-AREG treatment by Western blotting analysis on FACS-sorted CD4Foxp3-GFP Treg and CD4CD25 Teff cells which were isolated from the spleens of B16-luc mice. Treatment with gefitinib or AREG antibody restored the activity of GSK-3β (as indicated by inhibition of GSK-3β phosphorylation) in B16 Treg cells but had no effect on the expression of GSK-β, p-GSK-β, or Foxp3 in Teff cells (Fig. 5H). These data suggest that AREG/EGFR signaling enhances the suppressive function of Treg cells through inhibition of GSK-3β activity.
Blockage of EGFR with Gefitinib Treatment Inhibits Treg Cell Function by Suppressing GSK-3β Activity
To assess the possible effect of the regulation of AREG on the EGFR/GSK-3/Foxp3 axis, we analyzed the levels of Foxp3 in CD4CD25hi Treg cells in PBMC deriving from cancer patients and found that gefitinib significantly reduced the expression of Foxp3 protein (Fig. 6A). In vitro, we analyzed the levels of GSK-3β and phosphorylated GSK-3β in HCC827 and PC-9 cells after treatment with gefitinib or AREG by Western blotting. Gefitinib treatment deceased phosphorylated-GSK-3β expression and enhanced GSK-3β activation (Fig. 6B). On the basis of these results, we propose that gefitinib treatment down-regulates the suppressive function of Treg cells. These data support a role for AREG/EGFR signaling in regulating the suppressive function of Treg cells by inhibiting the activity of GSK-3β.
FIGURE 6.
A, blockage of EGFR with gefitinib-inhibited Foxp3 expression in CD4+CD25hi Treg cells derived from LC-PBMC. B, blockage of EGFR with gefitinib-inhibited GSK-3β phosphorylation in HCC827 and PC-9 cells.
β-TrCP Involves the Degradation of GSK-3β-mediated Foxp3
It is well known that phosphorylation of proteins causes them to be degraded by the ATP-dependent ubiquitin/proteasome pathway. To determine whether GSK-3β-mediated phosphorylation of Foxp3 protein directly modulates its ubiquitination and subsequent degradation, HEK293T cells transfected with FLAG-tagged Foxp3 were treated with GSK-3β siRNA, and then cell lysates were immunoprecipitated with anti-ubiquitin antibody, followed by immunoblotting with anti-FLAG antibody to detect ubiquitinated Foxp3 proteins. As shown in Fig. 7A, we detected ubiquitin bound to Foxp3 protein, and the levels of ubiquitinated Foxp3 were noticeably decreased in the absence of GSK-3β (by siRNA). To determine whether Foxp3 is associated with β-TrCP, an E3 ligase that has been reported to pair with GSK-3β for Mcl-1 degradation, FLAG-tagged Foxp3 and Myc-tagged β-TrCP were cotransfected in HEK293T and HeLa cells. Cell lysates were immunoprecipitated with either FLAG or Myc antibody to purify Foxp3 or β-TrCP protein complex. We found that FLAG-tagged Foxp3 and Myc-tagged β-TrCP both physically interacted in HEK293T and HeLa cells, suggesting that β-TrCP may be involved in Foxp3 protein degradation (Fig. 7B). To determine whether β-TrCP ubiquitinates Foxp3 for degradation, we cotransfected FLAG-tagged Foxp3 and HA-ubiquitin with or without Myc-tagged β-TrCP into HEK293T cells, immunoprecipitated the ubiquitinated proteins with anti-HA, and then immunoblotted against anti-FLAG antibody to detect ubiquitinated Foxp3 protein. An increased level of ubiquitinated Foxp3 was detected following treatment with β-TrCP (Fig. 7C). The functional interaction between Foxp3 and β-TrCP was further characterized by mass spectrum analysis, which identified an ubiquitination site at Lys356 within the region between residues 348 and 358 of Foxp3 (348WAILEAPEKubQR358, Fig. 8A). To validate that β-TrCP ubiquitinates Foxp3 at Lys356 for subsequent degradation, we created a FLAG-Foxp3-L356R mutant. Compared with WT Foxp3, ubiquitination of the L356R mutant was blocked (Fig. 8B).
FIGURE 7.
β-TrCP is involved in GSK-3β-mediated Foxp3 degradation. A, blockage of GSK-3β with siRNA decreased Foxp3 ubiquitination in HEK293T cells. IP, immunoprecipitation. B, interaction of β-TrCP with Foxp3 in HeLa and HEK293T cells. C, β-TrCP increased Foxp3 ubiquitination in the presence of GSK-3β in HEK293T cells. Ub, ubiquitin.
FIGURE 8.

The ubiquitination site of Foxp3 is Lys356. A, mass spectrometry identifies Lys356 as the ubiquitination site of Foxp3 by β-TrCP in HEK293T cells. B, the Foxp3 mutant (Lys356) cannot be ubiquitinated in the presence of GSK-3β. IP, immunoprecipitation.
Discussion
Increased expression of AREG protein has been reported in many cancers, such as lung, colorectal, liver, breast, ovarian, and pancreatic cancers (25, 26). AREG has been considered as a novel secreted marker for increased cell invasion in cancer tissues (27). Zaiss et al. (13) reported that Treg cells expressed EGFR under inflammatory conditions and that AREG was of pivotal importance to ensure Treg cell-mediated immune regulation. Thus, we hypothesized that the AREG/EGFR signaling pathway may be involved in cancer progression through enhanced Treg cell function. The tumor microenvironment plays a key role in tumor immune escape. Foxp3-expressing Treg cells, which act as suppressor agents in the tumor microenvironment, limit anti-tumor responses and allow the persistence and growth of cancer. Importantly, the function of Foxp3-expressing Treg cells may persist in the tumor environment, mediating immune evasion. Thus, a mechanism that down-regulates Foxp3-expressing Treg cells would be important for the activation of an efficient anti-tumor response. Although many recent studies have focused extensively on how Foxp3 is induced in Treg cells, much less is known about how Foxp3 protein is negatively regulated and how that affects the suppressive function of Treg cells. In this study, we report that AREG/EGFR signaling down-regulates GSK-3β protein activity, leading to the stabilization of Foxp3 protein. As such, loss of Foxp3 protein phosphorylation because of GSK-3β inactivity renders Foxp3 protein resistant to β-TrCP ubiquitination-mediated degradation and, therefore, suppresses Treg activity. These data provide insights into the molecular mechanisms regulating Foxp3 protein expression and Treg function. These findings are also in line with the results reported by Zaiss et al. (13), in which the authors demonstrated that mast cell-derived AREG could directly enhance Treg cell function.
The ubiquitously expressed serine/threonine kinase GSK-3β regulates many components of the immune system (28, 29). Importantly, GSK-3β activation can impair T cell proliferation, differentiation, survival, and other functions via phosphorylation of key transcriptional factors such as NF-κB, cAMP-response element-binding protein, AP-1, STAT, nuclear factor of activated T cells (NFAT), Smads, and β-catenin (30). Thus, we hypothesized that GSK-3 may reduce the expression of Foxp3 in Treg cells. Here we provide strong evidence supporting a direct role for GSK-3β in the regulation of Foxp3 protein levels. In addition, GSK-3β exerts indirect regulatory control of Foxp3 via β-catenin and Bcl-xL so that treating Treg cells with a GSK-3β inhibitor prolonged the half-life of Foxp3 protein and increased the levels of Bcl-xL (31). Stabilization of β-catenin has been proven to improve Treg cell survival (32). Taken together, these findings indicate that GSK-3β plays a vital role in the regulation of Foxp3 protein expression.
Although many studies have focused on how Foxp3 is induced in Treg cells, much less attention has been paid to how Foxp3 protein is negatively regulated. Here we report that the E3 ligase β-TrCP is responsible for ubiquitinating Foxp3 in a process that leads to the degradation of the chief Treg cell transcription factor. Ubiquitination and degradation of Foxp3 are vital for cell signaling (33). It is well known that phosphorylation at one residue can prime ubiquitination at a nearby lysine. Previously, we demonstrated that GSK-3β phosphorylation was involved in this process by paring with the E3 ligase β-TrCP to target Mcl-1 for degradation (34). Given that Foxp3 is a phosphorylation substrate of GSK-3b, we then wondered whether Foxp3 is also a target of β-TrCp for degradation. In our study, we demonstrated that both exogenously and endogenously expressed Foxp3 physically interacted with β-TrCP (data not shown for the endogenous Foxp3 and β-TrCP interaction). In addition, GSK-3β-phosphorylated Foxp3 induces subsequent ubiquitination and degradation of Foxp3 by the E3 ligase β-TrCP. Using mass spectrum analysis, we identified one lysine residues that was ubiquitinated (Lys356). Furthermore, the mutation of Lys356 showed severely reduced ubiquitination levels, suggesting that β-TrCP targets Foxp3 at Lys356 for subsequent degradation.
Loss of Foxp3 protein expression was previously thought to have resulted from decreased transcription. More recently, a number of studies have described the importance of posttranslational modifications of Foxp3, including protein phosphorylation, acetylation, ubiquitination, sumoylation, and hydroxylation (35). These modifications, which are also recognized as important determinants for the dynamic regulation of transcription factors such as p53 and NF-κB, may elicit opposing or synergistic effects (36, 37). For example, deacetylation of Foxp3 protein has been linked to impaired function of Treg cells by affecting Foxp3 protein stability and its ability to bind to gene promoters. TGF-β signaling was identified to modulate the deacetylation of Foxp3, and TIP60, p300, and sirtuin-1 were subsequently shown to regulate Foxp3 acetylation (38, 39). It is well known that a substrate molecule with different phosphorylation sites may occupy multipotential states. A recent study from Zhang et al. (22) identified Ser418 of the Foxp3 protein as a potential key phosphorylation site responsible for impaired Treg cell function in rheumatoid arthritis, which is dephosphorylated by PP1 in response to TNF-α signaling. Moreover, Morawski et al. (40) reported that cyclin-dependent kinase 2 (CDK2) phosphorylates Foxp3 protein and alters its stability and activity and suggested that the cyclin-dependent kinase (CDK) motifs and nearby lysine residues cooperate to form a phosphodegron that regulates Foxp3 phosphorylation-dependent ubiquitination and degradation (22).
To date, a number of studies have shown that the tumor environment associated with EGFR signaling activates tumor-reactive T cells in tumor regression (41). The mast cell-derived AREG can directly enhance Treg cell function via EGFR-mediated signaling. However, the underlying molecular mechanism is poorly understood. By focusing on the events proximal to Foxp3, we demonstrated a previously unreported link between Foxp3 degradation and AREG/EGFR signaling through GSK-3β and β-TrCP. This study reveals a mechanism by which EGFR signaling regulates posttranslational modification of Foxp3 protein. AREG, which is expressed in various tissues, is up-regulated in a number of human cancers. In the B16-luc melanoma murine models, there was no significant difference between gefitinib treatment and anti-AREG antibody, suggesting that AREG/EGFR may play a dominant role in regulating Treg cells expressing EGFR. It is not clear how the various EGFR ligands perform functions differently. This study also establishes a unique function of AREG that is distinct from EGF and TGF.
This study revealed a novel molecular mechanism regulating the efficiency of Treg-cell-mediated immune modulation and identified a signaling pathway that mediates Foxp3 protein degradation in tumor microenvironment Treg cells (Fig. 9). Targeting the machinery responsible for Foxp3 loss, such as phosphorylation and ubiquitination by GSK-3β and β-TrCP, could be explored as a therapeutic strategy for the treatment of various types of human cancer.
FIGURE 9.

Schematic of the possible regulation of AREG on the EGFR/GSK-3β/Foxp3 axis.
Experimental Procedures
Patients and Specimens
Tissue specimens from 19 cancer patients (12 with lung adenocarcinoma, 5 of 12 with lung cancer treatment with an EGFR tyrosine kinase inhibitor (gefitinib), and 7 with gastric adenocarcinoma) were collected from Wuhan Union Hospital. All patients were diagnosed pathologically. This study was approved by Wuhan Union Hospital, and informed consent was obtained from all patients before sample collection. We also collected blood specimens and malignant pleural or peritoneal effusions from these patients. For control, we collected 12 subjects with matched sex and mean age from Wuhan Union hospital between October 2010 and December 2013. Malignant pleural or peritoneal effusions were immediately stored at −80 °C until use, and we processed cell pellets and blood samples using Ficoll-Hypaque centrifugation (Amersham Biosciences) to obtain mononuclear cells for cell culture.
Tissue Microarray and Immunohistochemistry
For immunohistochemistry, we purchased a tissue microarray (HLung-Ade90Sur-01 and HStm-Ade180Sur-02) from Shanghai Outdo Biotech Co. Ltd. (Shanghai, China) that contained a total of 75 cases of lung adenocarcinoma and bronchioloalveolar carcinoma and 90 cases of gastric adenocarcinoma. Immunohistochemistry was performed to detect AREG expression as described in a previous study (42). The staining data were scored using the H-score method, which combined the staining intensity and percentage of tumor cell stained as described in a previous study (42).
Cell Culture and Stable Gene Transfection
HEK293T, HeLa, HCC827, and PC-9 cell lines (HCC827 and PC-9 cell lines are EGFR-dependent non-small-cell lung cancer cells) were cultured in DMEM plus GlutaMax (Invitrogen) and 5% FBS, penicillin (10 units/ml), and streptomycin (10 mg/ml, Invitrogen) in a humidified incubator at 37 °C. Primary T cells were isolated from PBMC of patients and healthy people and cultured in RPMI 1640 with l-glutamine (Lonza, Versviers, Belgium) and 10% FBS, penicillin (10 units/ml), and streptomycin (10 mg/ml).
For gene transfection, pCGN-GSK-3βWT and pCGN-GSK-3βKD were kindly provided by Drs. A. Kikuchi, M. J. Birnhaum, and J. R. Woodgett. Foxp3 cDNA was cloned from pGEX6P-1 Foxp3 and pCMV5-MYC Foxp3 (kindly provided by Dr. Shimon Sakaguchi of the Department of Experimental Immunology, Immunology Frontier Research Center, Osaka University, Suita, Osaka, Japan) into pCDNA3.1 (+) containing a FLAG tag. After confirmation by DNA sequencing, we named it pCDNA3.1(+)-Foxp3. The pGEX6P-1 Foxp3 and pCDNA3.1 (+)-FLAG-Foxp3 mutants were constructed using a site-directed mutagenesis kit (Invitrogen). The GSK-3β siRNA plasmid (i.e. pKD-GSK-3β-v1) was purchased from Upstate Biotechnology (Lake Placid, NY). These plasmids were transfected into HEK293T, HeLa, or Treg cells, respectively, using Lipofectamine 2000 (Invitrogen). And the stable transfectants were selected by blasticidin (10 g/ml). Cycloheximide and MG132 (10 μm for 10 h) were purchased from Sigma-Aldrich (St. Louis, MO). GSK-3β activator (staurosporine, 50 μm) or GSK-3β inhibitors (SB216763, 5 μm or GSK-3β siRNA, Sigma-Aldrich) were added to the culture.
Western Blotting and Immunoprecipitation
Western blotting assays were performed as described previously by using the following antibodies (43): mouse anti-Foxp3 (eBioscience, San Diego, CA); rat anti-Foxp3, mouse or rabbit anti-GSK-3, antiphospho-(Ser9)-GSK-3, and rabbit anti-phosphorylated protein (Cell Signaling Technology, Beverly, MA); anti-FLAG and anti-HA (Santa Cruz Biotechnology, Santa Cruz, CA); and anti-actin, anti-tubulin, and anti-GAPDH (Sigma). The Pierce coimmunoprecipitation and ubiquitin enrichment experiments were performed according to the instructions of the manufacturer. According to our previous study (24), two specific antibodies against the two different phosphorylation sites of Foxp3 protein (Ser270 and Ser274) were generated by Beijing Protein Innovation Co., Ltd. (Beijing, China) and used in this study. Immunoblots were analyzed quantitatively with Image Quant software, version 5.2 (GE Healthcare). Relative Ser270 and Ser274 phosphorylation of Foxp3 were normalized to the expression of GAPDH. So were the protein expression of FLAG-Foxp3, Foxp3, GSK-3, and p-GSK-3.
Animal Experiments
C57BL/6(Foxp3-GFP) (H-2b) mice were purchased from the Institute of Laboratory Animal Sciences of the Chinese Academy of Medical Sciences (Beijing, China) and maintained in pathogen-free filter-top isolator cages. All animal experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee at Tongji Medical College (Wuhan, China). For the pulmonary metastasis assay, B16-luc cells (2 × 105 in 0.2 ml PBS) were inoculated into the lateral tail vein of 6-week-old C57BL/6 (Foxp3-GFP) mice. Pulmonary metastatic colonization in B16-luc was monitored by noninvasive bioluminescence. Mice were then immunized with TRP2180–188 peptide-loaded BMDC on days 5 and 7 after inoculation. From day 6 after tumor cell injection, mice were intraperitoneally injected every other day with either 10 mg/kg of body weight of gefitinib or an anti-AREG antibody (200 g). At day 21 after injection, the size of mouse metastasis tumor was determined by pulmonary bioluminescence imaging.
Cell Isolation and FACS
We first purified CD4+ T cells from fresh PBMC or malignant pleural or peritoneal effusions using the CD4+ T Cell Isolation Kit II (Miltenyi Biotec) and performed FACS to sort CD4+ CD25− and CD4+ CD25hi T cells from purified CD4+ T cells using FACSAria (BD Biosciences) to obtain a purity of >98% of the cell population. We also isolated T cells in the pooled mouse spleens and lymph nodes for the murine model and performed FACS to sort the CD4+CD25− and CD4+ Foxp3-GFP T cells. The final purity of these cell populations was 90–95% after validation by FACS. The methods used for the intracellular staining procedures were in accordance with those described in a previous study (44). The cells were stained with fluorochrome-conjugated mouse antibodies against CD4 (GK1.5), CD3 (17A2), CD25 (PC61.5), and Foxp3 (FJK-16s) (all from eBioscience). FACS was performed using the FACSCalibur instrument (BD FACSAria), and data were analyzed using the FlowJo version 7.6 software program (TreeStar, Ashland, OR).
Treg Suppression Assay
This assay was performed as described previously (22). In brief, CD4+ CD25hi Treg cells (inhibitor, 1 × 104 cells/well) in the presence of irradiated (2000 rads) T cells were co-cultured with CD4+ CD25− T cells (responder) stimulated with anti-CD3/CD28 Dynabeads (Invitrogen) and concanavalin A (ConA) in the presence of a TNF-α antibody, anti-AREG antibody, TGF-α antibody, EGFR tyrosine kinase inhibitor, or IgG control (all from R&D Systems). Treg cells were added to increase ratios relative to the CD4+ CD25− T cells for dose-response measurements. Cell proliferation was measured in triplicate by incorporation of tritiated thymidine over 18–20 h of co-culture. The results were expressed as percentage inhibition using the following formula: (1-(experimental counts per minute/control counts per minute))×100%. All of the cells were cultured in complete RPMI 1640 medium.
Statistical Analysis
The data were summarized as the mean ± S.E. and analyzed using log rank test (Mantel-Cox test) to generate a p value between two groups. p < 0.05 was considered statistically significant.
Author Contributions
S. W. and Y. Z. conducted most of the experiments, analyzed the results, and wrote most of the paper. Y. W., P. Y., and J. L. conducted mass experiments. H. L., Q. D., and J. X. conceived the idea for the project and wrote the paper with S. W. and Y. Z. All authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Acknowledgments
We thank all members of the Xia laboratory for insightful discussions.
This work was supported in part by National Nature Science Foundation of China Grants 81130056 and 81401323. The authors declare that they have no conflicts of interest with the contents of this article.
- EGFR
- EGF receptor
- AREG
- Amphiregulin
- Treg cell
- regulatory T cell
- PBMC
- peripheral blood mononuclear cell(s)
- LC
- lung cancer
- GC
- gastric cancer
- HC
- healthy control
- Teff
- effector T cell.
References
- 1. Ciardiello F., and Tortora G. (2008) EGFR antagonists in cancer treatment. N. Engl. J. Med. 358, 1160–1174 [DOI] [PubMed] [Google Scholar]
- 2. Avraham R., and Yarden Y. (2011) Feedback regulation of EGFR signalling: decision making by early and delayed loops. Nat. Rev. Mol. Cell Biol. 12, 104–117 [DOI] [PubMed] [Google Scholar]
- 3. Thienelt C. D., Bunn P. A. Jr, Hanna N., Rosenberg A., Needle M. N., Long M. E., Gustafson D. L., and Kelly K. (2005) Multicenter phase I/II study of cetuximab with paclitaxel and carboplatin in untreated patients with stage IV non-small-cell lung cancer. J. Clin. Oncol. 23, 8786–8793 [DOI] [PubMed] [Google Scholar]
- 4. Bonner J. A., Harari P. M., Giralt J., Azarnia N., Shin D. M., Cohen R. B., Jones C. U., Sur R., Raben D., Jassem J., Ove R., Kies M. S., Baselga J., Youssoufian H., Amellal N., et al. (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 354, 567–578 [DOI] [PubMed] [Google Scholar]
- 5. Cunningham D., Humblet Y., Siena S., Khayat D., Bleiberg H., Santoro A., Bets D., Mueser M., Harstrick A., Verslype C., Chau I., and Van Cutsem E. (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 351, 337–345 [DOI] [PubMed] [Google Scholar]
- 6. Crane C. H., Varadhachary G. R., Yordy J. S., Staerkel G. A., Javle M. M., Safran H., Haque W., Hobbs B. D., Krishnan S., Fleming J. B., Das P., Lee J. E., Abbruzzese J. L., and Wolff R. A. (2011) Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. J. Clin. Oncol. 29, 3037–3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hynes N. E., and Lane H. A. (2005) ERBB receptors and cancer: the complexity of targeted inhibitors. Nat. Rev. Cancer 5, 341–354 [DOI] [PubMed] [Google Scholar]
- 8. Li S., Schmitz K. R., Jeffrey P. D., Wiltzius J. J., Kussie P., and Ferguson K. M. (2005) Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 7, 301–311 [DOI] [PubMed] [Google Scholar]
- 9. Garrido G., Lorenzano P., Sánchez B., Beausoleil I., Alonso D. F., Pérez R., and Fernández L. E. (2007) T cells are crucial for the anti-metastatic effect of anti-epidermal growth factor receptor antibodies. Cancer Immunol. Immunother. 56, 1701–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferris R. L., Jaffee E. M., and Ferrone S. (2010) Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J. Clin. Oncol. 28, 4390–4399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen F., Liu Z., Wu W., Rozo C., Bowdridge S., Millman A., Van Rooijen N., Urban J. F. Jr, Wynn T. A., and Gause W. C. (2012) An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat. Med. 18, 260–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mekori Y. A., and Hershko A. Y. (2012) T cell-mediated modulation of mast cell function: heterotypic adhesion-induced stimulatory or inhibitory effects. Front. Immunol. 3, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zaiss D. M., Yang L., Shah P. R., Kobie J. J., Urban J. F., and Mosmann T. R. (2006) Amphiregulin, a TH2 cytokine enhancing resistance to nematodes. Science 314, 1746. [DOI] [PubMed] [Google Scholar]
- 14. Wang S. W., Oh C. K., Cho S. H., Hu G., Martin R., Demissie-Sanders S., Li K., Moyle M., and Yao Z. (2005) Amphiregulin expression in human mast cells and its effect on the primary human lung fibroblasts. J. Allergy Clin. Immunol. 115, 287–294 [DOI] [PubMed] [Google Scholar]
- 15. Matsushita N., Pilon-Thomas S. A., Martin L. M., and Riker A. I. (2008) Comparative methodologies of regulatory T cell depletion in a murine melanoma model. J. Immunol. Methods 333, 167–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qi Y., Operario D. J., Oberholzer C. M., Kobie J. J., Looney R. J., Georas S. N., and Mosmann T. R. (2010) Human basophils express amphiregulin in response to T cell-derived IL-3. J. Allergy Clin. Immunol. 126, 1260–1266.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nishikawa H., and Sakaguchi S. (2010) Regulatory T cells in tumor immunity. Int. J. Cancer 127, 759–767 [DOI] [PubMed] [Google Scholar]
- 18. Curiel T. J., Coukos G., Zou L., Alvarez X., Cheng P., Mottram P., Evdemon-Hogan M., Conejo-Garcia J. R., Zhang L., Burow M., Zhu Y., Wei S., Kryczek I., Daniel B., Gordon A., et al. (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 10, 942–949 [DOI] [PubMed] [Google Scholar]
- 19. Yang Z. Z., Novak A. J., Ziesmer S. C., Witzig T. E., and Ansell S. M. (2006) Attenuation of CD8+ T-cell function by CD4+CD25+ regulatory T cells in B-cell non-Hodgkin's lymphoma. Cancer Res. 66, 10145–10152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zaiss D. M., van Loosdregt J., Gorlani A., Bekker C. P., Gröne A., Sibilia M., van Bergen en Henegouwen P. M., Roovers R. C., Coffer P. J., and Sijts A. J. (2013) Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity 38, 275–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tao R., de Zoeten E. F., Ozkaynak E., Chen C., Wang L., Porrett P. M., Li B., Turka L. A., Olson E. N., Greene M. I., Wells A. D., and Hancock W. W. (2007) Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat. Med. 13, 1299–1307 [DOI] [PubMed] [Google Scholar]
- 22. Nie H., Zheng Y., Li R., Guo T. B., He D., Fang L., Liu X., Xiao L., Chen X., Wan B., Chin Y. E., and Zhang J. Z. (2013) Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-α in rheumatoid arthritis. Nat. Med. 19, 322–328 [DOI] [PubMed] [Google Scholar]
- 23. Chen Z., Barbi J., Bu S., Yang H. Y., Li Z., Gao Y., Jinasena D., Fu J., Lin F., Chen C., Zhang J., Yu N., Li X., Shan Z., Nie J., et al. (2013) The ubiquitin ligase Stub1 negatively modulates regulatory T cell suppressive activity by promoting degradation of the transcription factor Foxp3. Immunity 39, 272–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu X., Wang S., Han M., Song B., Ye P., Ma S., Li J., Chen F., Xu G., Ding Q., Xia J., and Li H. (2015) Critical link between glycogen synthase kinase 3β and forkhead box P3 in patients with chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 136, 1698–1700.e1–12 [DOI] [PubMed] [Google Scholar]
- 25. Berasain C., Castillo J., Perugorría M. J., Prieto J., and Avila M. A. (2007) Amphiregulin: a new growth factor in hepatocarcinogenesis. Cancer Lett. 254, 30–41 [DOI] [PubMed] [Google Scholar]
- 26. Yotsumoto F., Fukami T., Yagi H., Funakoshi A., Yoshizato T., Kuroki M., and Miyamoto S. (2010) Amphiregulin regulates the activation of ERK and Akt through epidermal growth factor receptor and HER3 signals involved in the progression of pancreatic cancer. Cancer Sci. 101, 2351–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Higginbotham J. N., Demory Beckler M., Gephart J. D., Franklin J. L., Bogatcheva G., Kremers G. J., Piston D. W., Ayers G. D., McConnell R. E., Tyska M. J., and Coffey R. J. (2011) Amphiregulin exosomes increase cancer cell invasion. Curr. Biol. 21, 779–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beurel E., Yeh W. I., Michalek S. M., Harrington L. E., and Jope R. S. (2011) Glycogen synthase kinase-3 is an early determinant in the differentiation of pathogenic Th17 cells. J. Immunol. 186, 1391–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang H., Brown J., Garcia C. A., Tang Y., Benakanakere M. R., Greenway T., Alard P., Kinane D. F., and Martin M. (2011) The role of glycogen synthase kinase 3 in regulating IFN-β-mediated IL-10 production. J. Immunol. 186, 675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Doble B. W., and Woodgett J. R. (2003) GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116, 1175–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Graham J. A., Fray M., de Haseth S., Lee K. M., Lian M. M., Chase C. M., Madsen J. C., Markmann J., Benichou G., Colvin R. B., Cosimi A. B., Deng S., Kim J., and Alessandrini A. (2010) Suppressive regulatory T cell activity is potentiated by glycogen synthase kinase 3β inhibition. J. Biol. Chem. 285, 32852–32859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ding Y., Shen S., Lino A. C., Curotto de Lafaille M. A., and Lafaille J. J. (2008) β-Catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nat. Med. 14, 162–169 [DOI] [PubMed] [Google Scholar]
- 33. van Loosdregt J., and Coffer P. J. (2014) Post-translational modification networks regulating FOXP3 function. Trends Immunol. 35, 368–378 [DOI] [PubMed] [Google Scholar]
- 34. Hunter T. (2007) The age of crosstalk: phosphorylation, ubiquitination, and beyond. Mol. Cell 28, 730–738 [DOI] [PubMed] [Google Scholar]
- 35. Kim H. P., and Leonard W. J. (2007) CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. J. Exp. Med. 204, 1543–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hollstein M., and Hainaut P. (2010) Massively regulated genes: the example of TP53. J. Pathol. 220, 164–173 [DOI] [PubMed] [Google Scholar]
- 37. Huang B., Yang X. D., Lamb A., and Chen L. F. (2010) Posttranslational modifications of NF-κB: another layer of regulation for NF-κB signaling pathway. Cell. Signal. 22, 1282–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Samanta A., Li B., Song X., Bembas K., Zhang G., Katsumata M., Saouaf S. J., Wang Q., Hancock W. W., Shen Y., and Greene M. I. (2008) TGF-β and IL-6 signals modulate chromatin binding and promoter occupancy by acetylated FOXP3. Proc. Natl. Acad. Sci. U.S.A. 105, 14023–14027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Loosdregt J., Vercoulen Y., Guichelaar T., Gent Y. Y., Beekman J. M., van Beekum O., Brenkman A. B., Hijnen D. J., Mutis T., Kalkhoven E., Prakken B. J., and Coffer P. J. (2010) Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood 115, 965–974 [DOI] [PubMed] [Google Scholar]
- 40. Morawski P. A., Mehra P., Chen C., Bhatti T., and Wells A. D. (2013) Foxp3 protein stability is regulated by cyclin-dependent kinase 2. J. Biol. Chem. 288, 24494–24502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Srivastava R. M., Lee S. C., Andrade Filho P. A., Lord C. A., Jie H. B., Davidson H. C., López-Albaitero A., Gibson S. P., Gooding W. E., Ferrone S., and Ferris R. L. (2013) Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin. Cancer Res. 19, 1858–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ding Q., Chang C. J., Xie X., Xia W., Yang J. Y., Wang S. C., Wang Y., Xia J., Chen L., Cai C., Li H., Yen C. J., Kuo H. P., Lee D. F., Lang J., et al. (2011) APOBEC3G promotes liver metastasis in an orthotopic mouse model of colorectal cancer and predicts human hepatic metastasis. J. Clin. Invest. 121, 4526–4536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ding Q., He X., Hsu J. M., Xia W., Chen C. T., Li L. Y., Lee D. F., Liu J. C., Zhong Q., Wang X., and Hung M. C. (2007) Degradation of Mcl-1 by β-TrCP mediates glycogen synthase kinase 3-induced tumor suppression and chemosensitization. Mol. Cell. Biol. 27, 4006–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xie A., Wang S., Zhang K., Wang G., Ye P., Li J., Chen W., and Xia J. (2011) Treatment with interleukin-12/23p40 antibody attenuates acute cardiac allograft rejection. Transplantation 91, 27–34 [DOI] [PubMed] [Google Scholar]