Abstract
A 62-year-old woman presented with a subarachnoid hemorrhage secondary to a ruptured right supraclinoid internal carotid artery blister aneurysm. She was treated in an emergent fashion with two flow diverting pipeline embolization devices (PED) deployed in a telescoping fashion. CT angiography performed for unrelated reasons at 7 months showed successful treatment of the aneurysm without evidence of residual aneurysm. However, a follow-up digital subtraction angiogram performed at 9 months showed a large aneurysm in a modified position compared with the original aneurysm. This is the first case of rapid regrowth of a supraclinoid blister aneurysm after successful treatment with a PED, and demonstrates the need for close follow-up for similar aneurysms treated with this novel device.
Keywords: Aneurysm, Device, Flow Diverter, Subarachnoid
Background
The use of flow diverting stents in the treatment of complex intracranial aneurysms, including blister aneurysms, has become increasingly popular in the past several years. Blister aneurysms have an aggressive course and historically limited treatment options. Pipeline embolization devices (PED) are cobalt chromium and platinum self-expanding mesh stents, which have a 30–35% metal surface area coverage. This results in altered flow within the parent vessel and disrupts blood flow to the aneurysm dome, promoting aneurysm thrombosis.1 These devices have shown efficacy in the treatment of ruptured blister aneurysms.2 3 Despite the fact that the safety and efficacy of PED have been established in the literature,4 there are well known delayed complications.5–7 However, reporting of long term complications is limited. Here we report the first case of a delayed, rapid growth of a supraclinoid aneurysm in the region of a previously successfully treated blister aneurysm.
Case presentation
A 62-year-old woman presented to the emergency department after a sudden collapse at work. Her initial Glasgow Coma Scale score was reported as 3. Emergent CT/CT angiography (CTA) demonstrated diffuse subarachnoid hemorrhage with a moderate amount of intraventricular blood. A small blister-type aneurysm was noted along the anterior aspect of the right supraclinoid internal carotid artery (ICA), measuring 2 mm in diameter (figure 1). Incidentally, she also had a fusiform appearance of the left terminal ICA, extending to the proximal segment of the left middle cerebral artery.
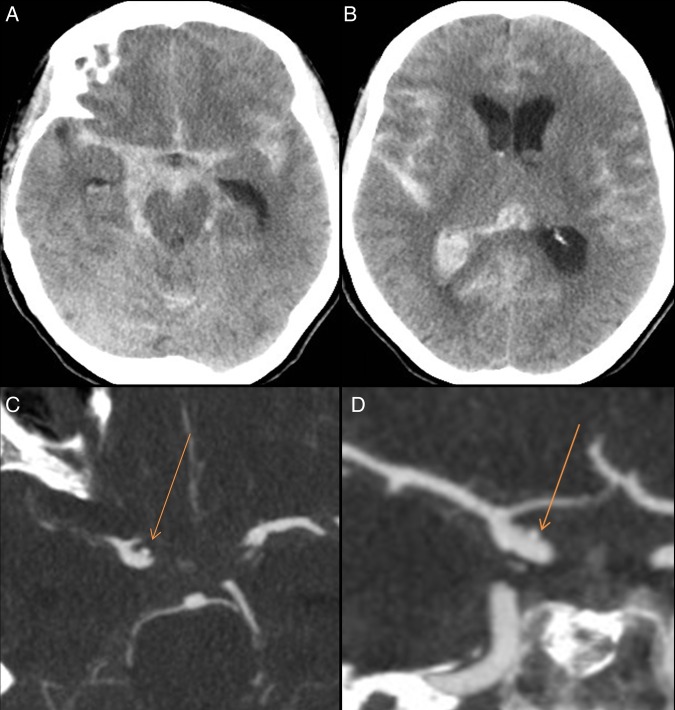
Figure 1.
Initial non-contrast CT (A, B) showing grade 4 subarachnoid hemorrhage, predominantly in the right suprasellar cistern. CT angiogram (C, D) showing a 2 mm blister involving the right supraclinoid internal carotid artery (arrow).
After she was stabilized, she was taken for a digital subtraction angiogram (DSA) with the intention to treat her aneurysm. In anticipation of treatment with a flow diverter, she was loaded with aspirin and clopidogrel. The angiogram revealed significant interval growth of the aneurysm, now measuring 7×6.5×5 mm (fundus×height×neck) (figure 2). Two telescoping PED (4×16 mm and 4×20 mm) (Covidien) were positioned to cover the aneurysm neck. A final control angiogram demonstrated adequate coverage of the aneurysm neck (figure 3).
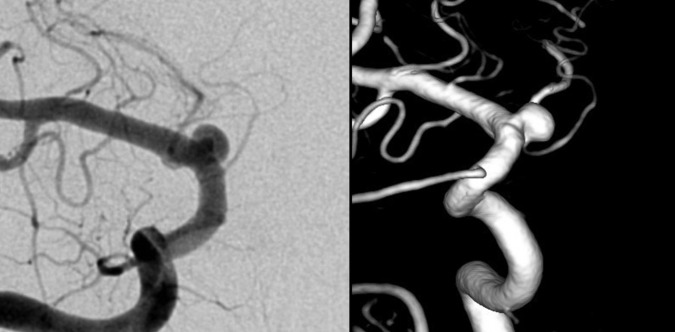
Figure 2.
Cerebral angiogram of the right internal carotid artery (ICA) (frontal image (left) and three-dimensional image (right)), showing significant interval growth of the right supraclinoid ICA aneurysm.
Figure 3.
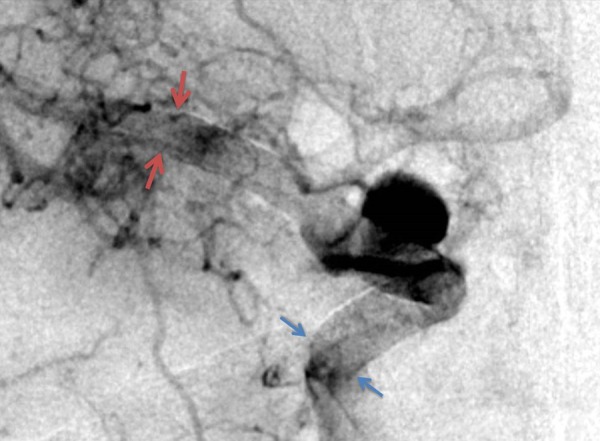
Final digital subtraction angiogram image after placing two telescoping pipeline flow diverters with good aneurysm neck coverage. Arrows demonstrate proximal and distal ends of the stent.
In the following days, the patient had several CTA scans performed for assessment of vasospasm. On post treatment day 6, the aneurysm was not seen on CTA. On post treatment day 12, an enlarging hyperdense focus in the right suprasellar cistern was found, consistent with a pseudoaneurysm. The next day she was brought for a DSA with the intention to treat the lesion. However, this angiogram was discordant with the recent CTA; the previously treated aneurysm in relation to the supraclinoid ICA and related to the PED was seen, but was significantly smaller in size (figure 4). There was no evidence of an enlarging pseudoaneurysm and no further treatment was undertaken.
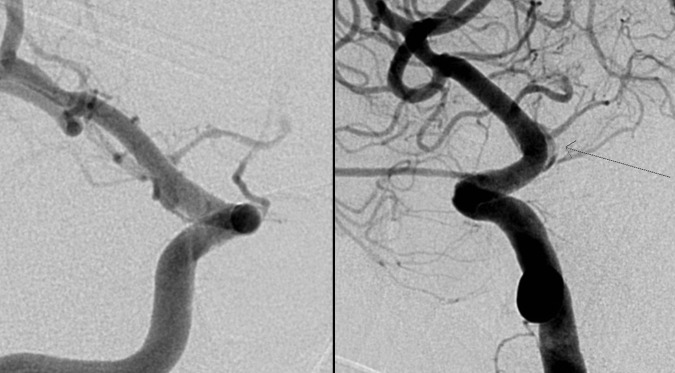
Figure 4.
Right internal carotid artery digital subtraction angiogram on day 13 showing no enlargement of the pseudoaneurysm (arrow).
The patient subsequently had a protracted course in hospital, with the development of hydrocephalus requiring a ventriculoperitoneal shunt, and the onset of a seizure disorder, managed medically. She was eventually discharged and continued to recover in the community, with some residual cognitive deficit.
CTA was performed 7 months after the initial treatment, during the investigation of a seizure. This demonstrated successful treatment of the supraclinoid aneurysm, with no evidence of residual (figure 5). While CTA is not the gold standard for assessing PED due to artifact, no evidence of residual was seen even on the late phases, suggesting the aneurysm was truly occluded at this time. As part of her routine follow-up, she had a repeat DSA at 9 months post-treatment. Interestingly, this demonstrated recurrence of the aneurysm, measuring 4.5×4 mm, adjacent to the origin of the anterior choroidal and posterior communicating artery (figure 6). At this time, she was booked for another angiogram and placement of a third PED, which was performed without complication.
Figure 5.
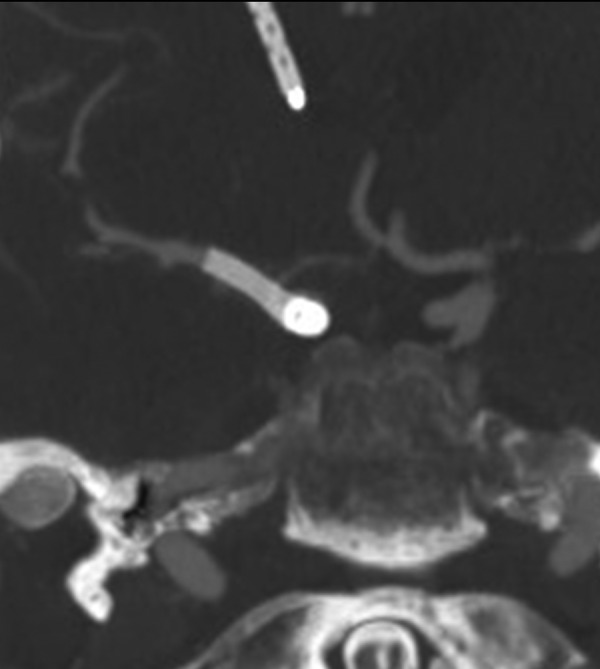
Seven month follow-up CT angiogram showing no residual aneurysm.
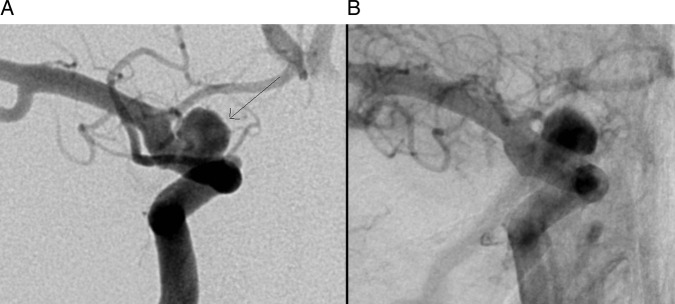
Figure 6.
Nine month follow-up digital subtraction angiogram shows recurrence of the right supraclinoid internal carotid artery aneurysm (arrow, A). Post deployment of the third pipeline flow diverter with contrast stagnation within the aneurysm (B).
Investigations
All angiograms were performed in the Foothills Medical Centre angiography suite.
Treatment
The patient was initially treated with two PED (Covidien) in a telescoping fashion. A third PED was placed 9 months after her initial treatment.
Outcome and follow-up
The patient recovered from her initial subarachnoid hemorrhage and by 9 months was fully independent with her activities of daily living. She will be followed with a repeat angiogram to assess treatment success of the third PED on her recurrent supraclinoid blister aneurysm. She continues on dual antiplatelet therapy.
Discussion
PED have been successfully used for the treatment of a variety of cerebral aneurysm classes, including blister aneurysms, and have delayed occlusion rates in the range of 69–100%.4 In the largest series of PED for the treatment of blister aneurysms, Yoon et al demonstrated complete obliteration of the aneurysm in 88% at 6 months.2 In a more recent series, Linfante et al showed complete occlusion of all nine surviving patients with an average follow-up of 15 months.3
However, PED do have well known late complications, and long term data are only just becoming available.8 Several influential studies assessing the efficacy and safety of PED in the treatment of cerebral aneurysms have only reported occlusion rates after 6 months.2 9 To our knowledge, there have been no cases of rapid recurrence after successful treatment.
In our case, we demonstrated a successfully treated supraclinoid blister aneurysm undergoing rapid growth between 7 and 9 months from initial PED deployment. This has several important implications, mostly regarding follow-up and reporting of occlusion data. Patients should be followed closely following treatment with a PED in order to identify this rare phenomenon, and studies reporting occlusion rates should ensure a minimum follow-up of 1 year. The occurrence of delayed aneurysm rupture has been hypothesized to be due to a complex cascade of biological effects, triggered by intra-aneurysmal thrombosis, which has a deleterious effect on the aneurysm wall.10 This case suggests that another possible cause for delayed rupture could be rapid regrowth of the aneurysm. Blister aneurysms, in particular, due to their dynamic nature and underlying pathophysiology, may lend themselves to late recurrences after treatment with flow diversion. Therefore, although flow diversion is becoming a more common treatment method for this type of aneurysm, since they are notoriously difficult to treat by other methods, special attention must be paid in terms of follow-up to ensure durability, despite previously documented angiographic occlusion.
Learning points.
Pipeline embolization devices (PED) are a relatively new technology, and their long term effects on aneurysm recurrence is still being defined.
Delayed, rapid recurrence of blister aneurysm is possible even after apparently successful treatment.
Aneurysms treated with PED should have close follow-up for at least 1 year, given the potential for delayed and rapid regrowth.
Footnotes
Correction notice: This article has been corrected since it first published Online First. Reference 2 has been updated.
Contributors: STL, ZA, and APM composed the article. APM, WM, and JHW participated in the care of the patient. All authors reviewed the article.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Fiorella D, Lylyk P, Szikora I et al. Curative cerebrovascular reconstruction with the pipeline embolization device: the emergence of definitive endovascular therapy for intracranial aneurysms. J Neurointerv Surg 2009;1:56–65. 10.1136/jnis.2009.000083 [DOI] [PubMed] [Google Scholar]
- 2.Linfante I, Mayich M, Sonig A et al. Flow diversion with pipeline embolic device as treatment of subarachnoid hemorrhage secondary to blister aneurysms: Dual-center experience and review of the literature. J Neurointerv Surg Published Online First: 13 Apr 2016. doi:10.1136/neurintsurg-2016-012287 10.1136/neurintsurg-2016-012287 [DOI] [PubMed] [Google Scholar]
- 3.Yoon JW, Siddiqui AH, Dumont TM et al. Feasibility and safety of pipeline embolization device in patients with ruptured carotid blister aneurysms. Neurosurgery 2014;75:419–29. 10.1227/NEU.0000000000000487 [DOI] [PubMed] [Google Scholar]
- 4.Briganti F, Leone G, Marseglia M et al. Endovascular treatment of cerebral aneurysms using flow-diverter devices: A systematic review. Neuroradiol J 2015;28:365–75. 10.1177/1971400915602803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan LA, Keigher KM, Munich SA et al. Thromboembolic complications with pipeline embolization device placement: impact of procedure time, number of stents and pre-procedure P2Y12 reaction unit (PRU) value. J Neurointerv Surg 2015;7:217–21. 10.1136/neurintsurg-2014-011111 [DOI] [PubMed] [Google Scholar]
- 6.Chalouhi N, Polifka A, Daou B et al. In-pipeline stenosis: incidence, predictors, and clinical outcomes. Neurosurgery 2015;77:875–9. 10.1227/NEU.0000000000000908 [DOI] [PubMed] [Google Scholar]
- 7.McTaggart RA, Santarelli JG, Marcellus ML et al. Delayed retraction of the pipeline embolization device and corking failure: pitfalls of pipeline embolization device placement in the setting of a ruptured aneurysm. Neurosurgery 2013;72(2 suppl operative):E245–50. [DOI] [PubMed] [Google Scholar]
- 8.Chiu AH, Cheung AK, Wenderoth JD et al. Long-term follow-up results following elective treatment of unruptured intracranial aneurysms with the pipeline embolization device. AJNR Am J Neuroradiol 2015;36:1728–34. 10.3174/ajnr.A4329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson PK, Lylyk P, Szikora I et al. The pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol 2011;32:34–40. 10.3174/ajnr.A2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulcsar Z, Houdart E, Bonafé A et al. Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. AJNR Am J Neuroradiol 2011;32:20–5. 10.3174/ajnr.A2370 [DOI] [PMC free article] [PubMed] [Google Scholar]