Abstract
Rationale
We investigated aging of human endogenous reparative capacity and aimed to clarify whether it is affected by presence of cardiovascular disease or its risk factors.
Objective
Circulating progenitor cell (PC) levels reflect endogenous regenerative potential. The effect on PC of healthy aging compared to aging with risk factors (RF) or cardiovascular disease (CVD) is unknown. We examined whether exposure to RF and CVD leads to an accelerated decline in circulating PC with increasing age.
Methods and Results
In 2,792 adult subjects, 498 were free of RFs (smoking, diabetes, hypertension or hyperlipidemia); 1036 subjects had 1–2 RF, and 1253 had ≥3 RFs and/or CVD. PC were enumerated by flow cytometry as CD45med+ mononuclear cells expressing CD34 and subsets co-expressing CD133, CXCR4, and vascular endothelial growth factor receptor-2 (VEGF2R) epitopes. Younger age, male gender and larger body size correlated with higher PC counts (p<0.01). After multivariable adjustment, both age and RF categories were independently associated with PC counts (p<0.05), with lower PC counts in older subjects and those with higher RF burden or CVD. PC counts remained unchanged with increasing age in healthy individuals. There were significant interactions between age and RF categories (p≤0.005), such that for younger subjects (<40 years), RFs were associated with increased PC counts, whereas for older subjects (>60 years), RFs and CVD were associated with lower PC counts.
Conclusion
Circulating PC levels do not decline with healthy aging; RF exposure at a younger age stimulates PC mobilization whereas continued exposure is associated with lower PC levels in later life. Over the lifespan, exposure to RFs and CVD is associated with an initial stimulation and subsequent decline in circulating PC levels, which reflect endogenous regenerative capacity.
Keywords: Aging, regenerative capacity, progenitor cells, cardiovascular disease risk factors, atherosclerosis
Subject Terms: Clinical Studies, Stem Cells, Vascular Biology
INTRODUCTION
Age is an overarching risk factor (RF) for development of the majority of chronic non-communicable diseases including cardiovascular diseases (CVD). Aside from prolonging exposure to atherosclerotic RFs, increasing age causes distinct changes in cardiovascular tissues that include arterial medial/intimal thickening and pathologic derangements in endothelial and myocardial cells that culminate in vascular and cardiac dysfunction. Superimposition of RFs accelerates these processes leading to premature aging of the cardiovascular system and early development of CVD.
A pivotal response to experimental vascular injury is the recruitment of bone marrow and tissue progenitor cells (PC) to participate in repair and regeneration. A rare population of bone-marrow mononuclear cells expressing the CD34 epitope are enriched for PC. They have the potential to differentiate into hematopoietic, endothelial, and other lineages and aid in vascular and myocardial repair and regeneration.1–5 CD34-expressing mononuclear cells include hematopoietic and endothelial PC, as well as non-hematopoietic (mesenchymal, lacking CD45 expression) PC.6 CD133 is a 5-transmembrane antigen of primitive stem cells that is lost during maturation, and CD34 negative cells expressing CD133 differentiate into CD34+ cells with greater proliferative activity. Thus, dual expression of these markers (CD34+/CD133+) identifies a PC-enriched subpopulation.3, 7 Co-expression of vascular endothelial growth factor receptor-2 (VEGFR2) appears to identify a rarer subpopulation of PC further enriched for either endothelial or hematopoietic progenitors.8–10 Finally, co-expression of CXCR4, which promotes homing of PC to stromal-derived factor-enriched hypoxic environments, may further characterize PC with capacity for tissue repair.11
Amplification of this reparative response by enhancing PC mobilization and bone-marrow mononuclear cell delivery has shown promise for cardiovascular tissue regeneration in clinical trials.12, 13 Acute tissue injury such as ischemia and infarction mobilizes PC into the circulation.14–20 Lower circulating PC counts and impaired PC activity measured by colony forming and migration assays have been reported in subjects with RFs for CVD in some but not all studies.21–23 It is also unclear in humans whether aging alters circulating PC numbers, whether exposure to the injurious effects of CVD RFs stimulates or inhibits PC, and whether presence of atherosclerotic CVD is associated with impairment of PC. Our aim was to investigate the relationship between healthy aging, and aging with exposure to CVD RFs or with established CVD, on circulating PC levels. We hypothesized that exposure to CVD RFs or CVD will modulate the age-related changes in PC levels compared to “healthy” aging in the absence of CVD or it RFs.
METHODS
Subjects
We recruited 2,792 adults from a database of studies conducted at the Emory Clinical Cardiovascular Research Institute and the Emory-Georgia Tech. Predictive Health Institute between 2006 and 2013. Informed consent was obtained from all subjects and all study protocols were approved by the Institutional Review Board of Emory University.
Cardiovascular RFs and CVD
Hypertension, hypercholesterolemia, and diabetes mellitus were defined according to the Joint National Committee, Adult Treatment Panel III and American Diabetes Association criteria, respectively, and smoking habits were recorded.24–26 For each subject, the number of the aforementioned concomitant RFs (0–4) was calculated. Peripheral artery disease (PAD) was defined as an ankle-brachial index <0.9 and CAD as >50% stenosis in one or more coronary arteries at angiography. Prior history of cerebrovascular accident (CVA) was documented. Subjects were divided into groups with no RFs (N=498), 1–2 RFs (N=1036), ≥3 RFs or overt CVD (CAD, PAD or CVA; N=1253).
Circulating progenitor cell counts
After an overnight fast, blood was collected in EDTA tubes and within 24 hours, incubated with fluorochrome-labeled monoclonal anti-human mouse antibodies to identify surface markers expressed on mononuclear cells before quantification using flow cytometry (detailed below). Peripheral blood mononuclear cell subsets that are considered to be enriched for hematopoietic and endothelial progenitors were measured in the CD45med cells expressing CD34+, CD133+ and VEGFR2+ surface markers, either singly or in combination. PC populations were enumerated as CD45med cells co-expressing CD34, using the ISHAGE criteria and modified to include antibodies directed against CD133, CXCR4 and VEGFR2 epitopes.27 We measured circulating numbers of CD34+, dual positive CD34+/CD133+, CD34+/CXCR4+ and CD34+/VEGFR2+, and triple positive CD34+/CD133+/CXCR4+ cell populations.
Flow cytometry
Peripheral blood PC were analyzed for the expression of surface antigens using direct flow cytometry (BD FACS Canto II Flow Cytometer). Three hundred µl of venous blood (anticoagulant: EDTA) was incubated with fluorochrome-labeled monoclonal mouse anti-human antibodies, namely, FITC-CD34 (BD Biosciences), PE-VEGF2R (R&D system - also known as “Kinase insert Domain Receptor-KDR”), APC-CD133 (Miltenyi) and PE-Cy7-conjugated anti-CXCR4 (EBioscience, clone 12G5 for 15 minutes. Red blood cells were removed by lysis in 1.5 ml of ammonium chloride lysis buffer which was added to the sample and incubated for an additional 10 minutes. The lysis process was stopped by adding 1.5 ml of staining medium (PBS with 3% heat-inactivated serum and 0.1% sodium azide) was added to stop lysing. Up to five million events were acquired from the Cytometer with Flowjo software (Treestar, Inc.) used for subsequent analysis of accumulated data. List mode files containing at least 3,000,000 events were collected so that analysis of rare sub-populations would contain an adequate number of events. Absolute numbers of each cell subset per milliliter were determined by multiplying the counts with the number of monocytes per milliliter of blood. (Online figure I)
Reproducibility testing
Twenty list mode samples were separately analyzed on two occasions by two technicians. The percent repeatability coefficients (%) were calculated as standard deviation (SD) of differences between pairs of measurements/mean of measurements*100. The repeatability coefficients for the various cell types were: CD34+ 2.9%, 4.8%, 6.5%, 7.5% and 21.6% for CD34+, CD34+/CD133+, CD34+/CXCR4+, CD34+/CD133+/CXCR4+ and CD34+/VEGF2R+; respectively.
Statistical analysis
Participant characteristics are summarized as means ± standard deviation or as counts and proportions for continuous and categorical variables, respectively. Subjects were categorized into three groups with none, 1–2 and ≥ 3 cardiovascular RFs. First, Spearman correlation coefficients between age and PC variables were determined in the entire population and each RF group. Prior to regression analysis, CD34+, CD34+/CD133+, CD34+/CXCR4+, and CD34+/CD133+/CXCR4+ were log-transformed to achieve normality. Univariate analyses were performed to assess the relationships between subject characteristics and risk factors and counts of PC sub-types, and partial correlation coefficients between PC and RF variables were obtained controlling for age, gender and BMI. Next, we investigated the association between PC and age, and whether the association depends on the number of risk factors by using a multiple linear regression model with the log-transformed PC variable as the response variable and age, risk group, and age×risk group interaction term as the explanatory variables. Associations between PCs and age were determined and compared by the risk groups, and the test for trend was also performed stratified by age= 55 years. Covariates included gender, BMI, and use of statin. P-values less than 0.05 were considered as statistically significant. All analyses were performed using SAS 9.3 (Cary, NC).
RESULTS
Demographic characteristics of subjects are shown in (Table 1). Mean age was 57±14 years (range: 18–98); 47.1% were women, 34.3% were African American and 40% had CAD. Cell populations expressing CD34 and subsets co-expressing CD34/CD133 and CD34/CXCR4 were highly correlated with each other, and had more modest correlations with cells expressing VEGFR2R+. (Online table I).
Table 1.
Demographic characteristics
| All N=2792 |
No RF N=498 |
1–2 RF N=1036 |
≥3 or CVD N=1253 |
p-value | |
|---|---|---|---|---|---|
| Age (years) | 57±14 | 44±14a | 56±13b | 64±12c | <.001 |
| Women (%) | 1315(47.1) | 303(60.8)a | 560(54.1)b | 464(37)c | <.001 |
| African Americans (%) | 956(34.3) | 153(30.7)a | 358(34.6)a | 443(35.4)a | 0.18 |
| Hypertension (%) | 1613(58.8) | — | 556(53.7)a | 1055(84.2)b | <.001 |
| Diabetes (%) | 708(25.4) | — | 131(12.6)a | 578(46.1)b | <.001 |
| Hyperlipidemia (%) | 1679(62) | — | 680(65.6)a | 1002(80)b | <.001 |
| Current Smoker (%) | 410(16.5) | — | 138(13.3)a | 271(21.6)b | <.001 |
| Coronary disease (%) | 912(40) | — | — | 938(74.9) | — |
| Statin use (%) | 1147(46.5) | — | 303(29.2)a | 839(67)b | <.001 |
| Body mass index (kg/m2) | 28.8±6.7 | 27±6a | 29±7b | 30±6b | <.001 |
| Body Surface Area (m2) | 2.0±.3 | 1.9±.3a | 1.9±.2b | 2.0±.2c | <.001 |
| Systolic blood pressure (mmHg) | 131±22 | 116±15a | 130±20b | 138±23c | <.001 |
| Diastolic blood pressure (mmHg) | 75±12 | 72±11a | 77±12b | 75±13c | <.001 |
| Mean arterial pressure (mmHg) | 94±14 | 86±11a | 95±13b | 96±14c | <.001 |
| Total cholesterol (mg/dL) | 179±43 | 174±29a | 195±43b | 165±45c | <.001 |
| Low density lipoprotein (mg/dL) | 100±36 | 95±24a | 111±36b | 92±37a | <.001 |
| High density lipoprotein (mg/dL) | 53±19 | 61±16a | 57±19b | 45±17c | <.001 |
| Triglycerides (mg/dL) | 121±89 | 88±62a | 120±71b | 142±111c | <.001 |
| Fasting blood glucose (mg/dL) | 106±37 | 87±10a | 99±28b | 120±45c | <.001 |
| C-Reactive Protein (mg/L) | 2.3±.4 | .70±1.8a | 1.7±5.9b | 7.0±12c | <.001 |
| CD34+ (cells/µL) | 2.0±1.2 | 2.1±1.3a | 2.1±1.4a | 1.87±1.1b | <.001 |
| CD34+/CD133+ (cells/µL) | .99±.8 | 1.0±.7a | 1.0±.7a | .90±.6b | <.001 |
| CD34+/CXCR4+ (cells/µL) | .97±.8 | .88±.6a | 1.0±.8b | .99±.7b | 0.06 |
| CD34+/CD133+/CXCR4+ (cells/µL) | .44±.3 | .40±.3a | .44±.3a, b | .45±.4b | 0.27 |
| CD34+/VEGF2R+ (cells/µL) | .13±.2 | .13±.2a | .13±.2a | .13±.2a | 0.22 |
Mean ± SD or counts (%) for continuous and categorical variables; respectively. RF: Risk factors (diabetes, hypertension, hyperlipidemia and smoking). P-values are derived from RF group comparison tests.
Values not sharing the same superscript are significantly different at p<.05 (e.g. valuea statistically differs from valueb, valuec and valueb, c at p<.05), and tests are adjusted for pairwise comparisons using the Bonferroni correction.
Age and PC counts
In univariate analyses, increasing age correlated with lower PC counts (p<0.001) for all PC populations (Table 2, Figure 1). Higher BMI also correlated with higher PC counts except for the CD34+/VEGFR2+ cell population, and men had higher PC counts compared to women, before and after adjustment for age and BMI. Age remained an independent predictor of PC after adjustment for gender and BMI. (Table 2).
Table 2.
Relationships between subject characteristics and PC counts
| CD34+ | CD34+/ CD133+ |
CD34+/ CXCR4+ |
CD34+/ CD133+/ CXCR4+ |
CD34+/ VEGF2R+ |
|
|---|---|---|---|---|---|
| Table 2A | |||||
| Age (year) | −0.8%** | −1.1%** | −0.4% * | −0.6%** | −0.5% * |
| Male vs. Female | 18.0%** | 14.0%** | 24.2%** | 24.5%** | 15.5% * |
| BMI (kg/m2) | 1.2%** | 1.7%** | 1.1%** | 1.6%** | −0.1% |
| Table 2B | |||||
| Diabetes (Yes vs. No) | 1.8% | 1.9% | 7.8% * | 6.5% * | −14.5% * |
| Hypertension (Yes vs. No) | 1.2% | 3.7% | 11.1% * | 15.5%** | 19.9% * |
| Hyperlipidemia (Yes vs. No) | 5.5% * | 6.3% * | 12.8%** | 13.3% * | 27.5%** |
| Smoking (Yes vs. No) | 2.5% | 3.8% | 6.9% | 12.5% * | −2.4% |
| Table 2C | |||||
| Mean BP (mmHg) | .06* | .05* | .08* | .07* | 0.03 |
| LDL (mg/dL) | .07* | .05* | .02 | 0.01 | 0.01 |
| HDL (mg/dL) | −.10** | −.11** | .04 | −.14** | 0.06 |
| Triglycerides (mg/dL) | .09* | .08* | .12** | .13** | 0.03 |
| Glucose (mg/dL) | .05* | .04* | .06* | .01 | −0.05 |
Table 2A: Univariate analysis showing percent differences of PC counts by age, gender and Body Mass Index (BMI). Table 2B: Estimated percent differences in PC counts by presence of individual risk factors after adjustment for age, gender and BMI. Table 2C: Partial correlation coefficients between PC subsets and BP, lipid and fasting glucose parameters controlling for age, gender and BMI. BP: Blood pressure. LDL: low density lipoprotein. HDL: High density lipoprotein.
p ≤ 0.05,
p < 0.0001.
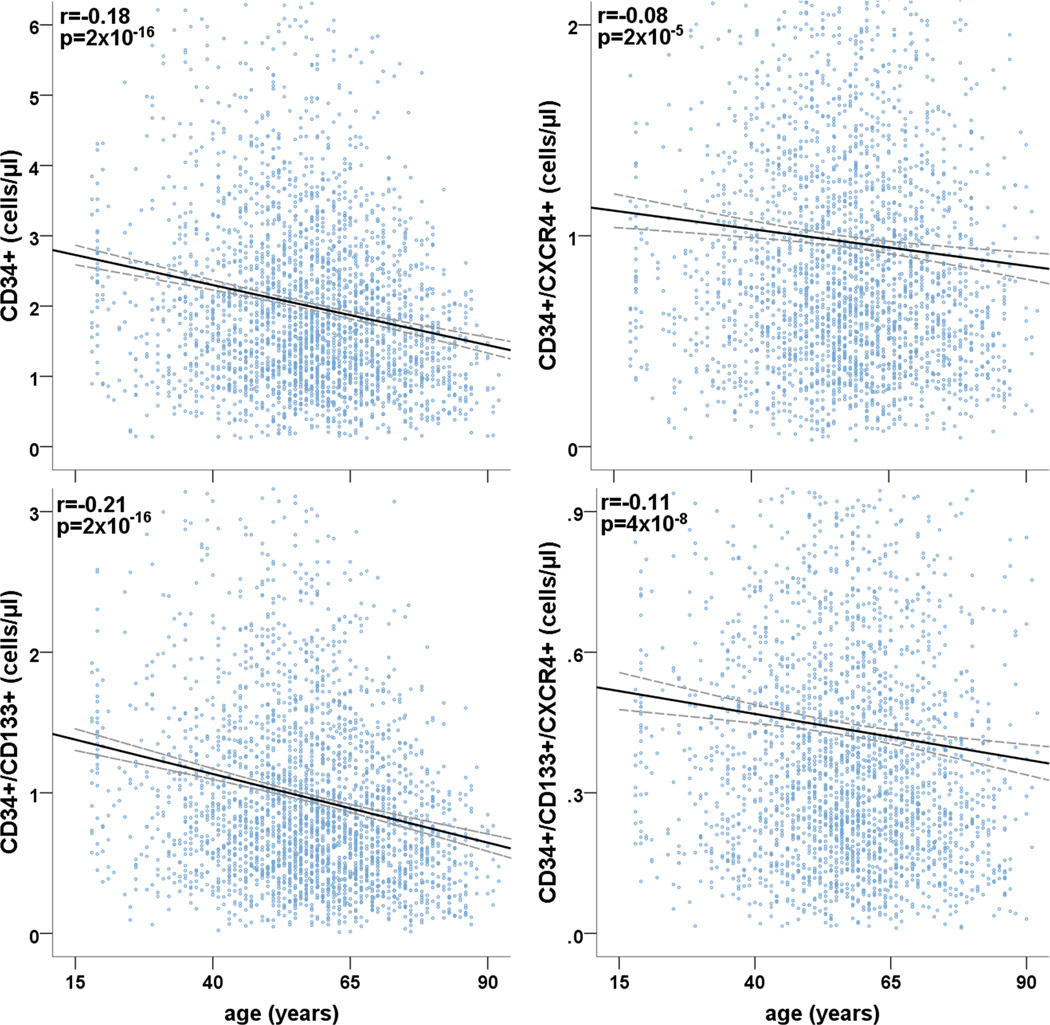
Figure 1. The relationship between age and PC.
r: correlation coefficient and associated p-value. Fit lines are solid and dashed lines represent the 95% confidence intervals. N=2792.
Cardiovascular risk factors and PC
Individual risk factors
In age, gender and BMI-adjusted analyses, presence of CVD risk factors was associated with higher circulating PC counts, and differences were statistically significant for several PC subtypes across individual risk factors. (Table 2B) For example, diabetics, hypertensives and those with hyperlipidemia exhibited 7.8%, 11.1% and 12.8%; respectively, higher counts of CD34+/CXCR4+ cells; as well as 6.5%, 15.5% and 13.3% more circulating CD34+/CD133+/CXCR4+ cell; respectively, compared to their counterparts. (Table 2B). Similarly, mean arterial pressure as well as fasting lipid and glucose correlated significantly with most PC subtypes, such that increasing blood pressure, dysglycemia and dyslipidemia were largely accompanied by higher circulating PC counts, after adjustment for age, gender and BMI. (Table 2C).
Risk factor burden and PC
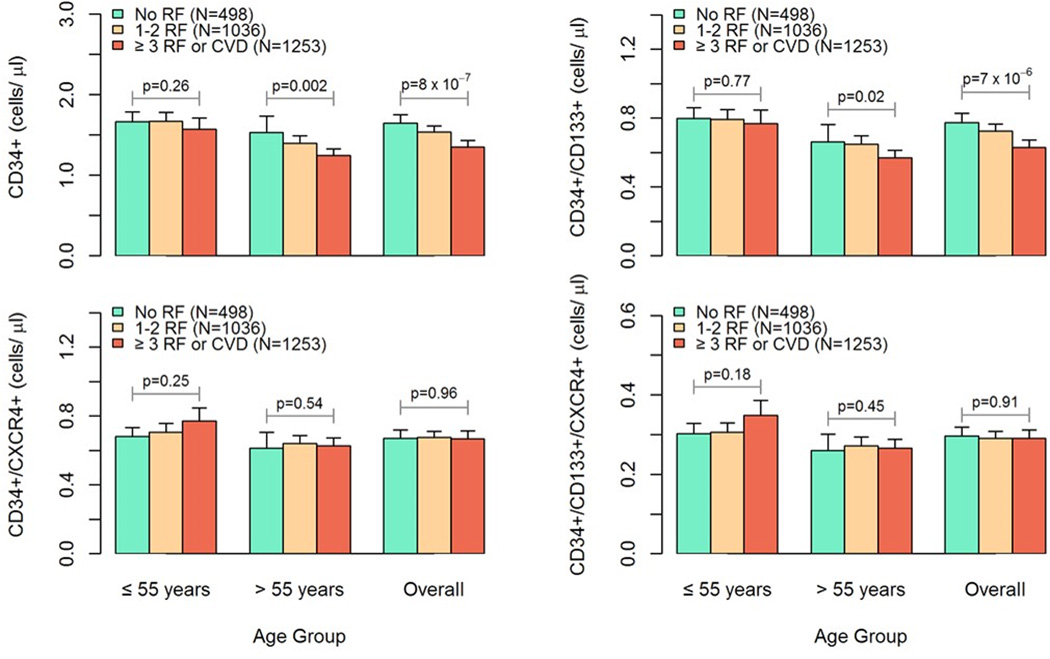
We examined the impact of cardiovascular RF burden on PC by categorizing subjects into those without RF, as well as subjects with 1 or 2 RF, or >2RF and CVD, based on the presence of hypertension, hypercholesterolemia, diabetes mellitus, and smoking, and/or overt CVD. Overall, increasing RF burden was associated with significantly higher circulating counts of CD34+ and CD34+/CD133+ cells (p<0.0001 for both) but with the other cell populations. (Figure 2). These associations were mainly driven by subjects 55 years of age or older, and were not evident in younger individuals, in which an opposite trend was in fact noted for CD34+/CXCR+ and CD34+/CD133+/CXCR+ cells (p=0.25 and 0.18; respectively). Thus, while increasing burden of cardiovascular RF correlated with diminished PC counts in older individuals, this was not observed in younger individuals. (Figure 2).
Figure 2. Cardiovascular risk factor burden and PC counts in younger vs. older subjects.
Effects of RF burden on PC counts in younger vs. older subjects. Estimated mean ± standard error PC counts with increasing cardiovascular risk factor burden in subjects > 55 years of age (N=1592) compared to those ≤ 55 years old (N=1200). Estimates were obtained by setting gender as female, BMI=28 kg/m2, and no statin use. P-values are for comparisons among three risk groups.
Relationship between age, RF burden and PC counts
Unadjusted analyses of the relationship between age and PC populations in all subjects and in groups with increasing RF burden appears in Table 3. While the age-related decline in circulating counts of all PC subtypes in the overall population, subjects with no RF (N=498) exhibited no significant differences in CD34+, CD34+/CXCR4+ or CD34+/VEGFR2+ counts (p=0.08, 0.17 and 0.15; respectively) with increasing age. (Table 3). Overall, the univariate correlations between age and PC counts were progressively more robust in groups with higher RF burden. (Table 3).
Table 3.
Relationship between age, PC counts and cardiovascular risk burden
| CD34+ | CD34+/ CD133+ |
CD34+/ CXCR4+ |
CD34+/ CD133+/ CXCR4+ |
CD34+/ VEGF2R+ |
|
|---|---|---|---|---|---|
| All (N=2787) | −0.18 | −0.21 | −0.08 | −0.11 | −0.07 |
| p=2×10−16 | p=2×10−16 | p=2×10−5 | p=4×10−8 | p=0.001 | |
| No RF (N=498) | −0.08 | −0.17 | −0.06 | −0.10 | 0.07 |
| p=0.08 | p=0.0001 | p=0.17 | p=0.02 | p=0.15 | |
| 1–2 RFs (N=1036) | −0.16 | −0.17 | −0.09 | −0.09 | −0.11 |
| p=2 × 10−7 | p=5 × 10−8 | p=0.01 | p=0.003 | p=0.0007 | |
| 3+ RFs (N=1253) | −0.23 | −0.25 | −0.16 | −0.20 | −0.06 |
| p=3×10−16 | p=2×10−16 | p=1×10−8 | P=1×10−11 | p=0.04 | |
Spearman correlation coefficients and corresponding p-values for the relationship between age and PC populations in all subjects and in groups with increasing RF burden. PC: Progenitor Cells. RF: Risk factors (diabetes, hypertension, hyperlipidemia and smoking)
Interaction analyses
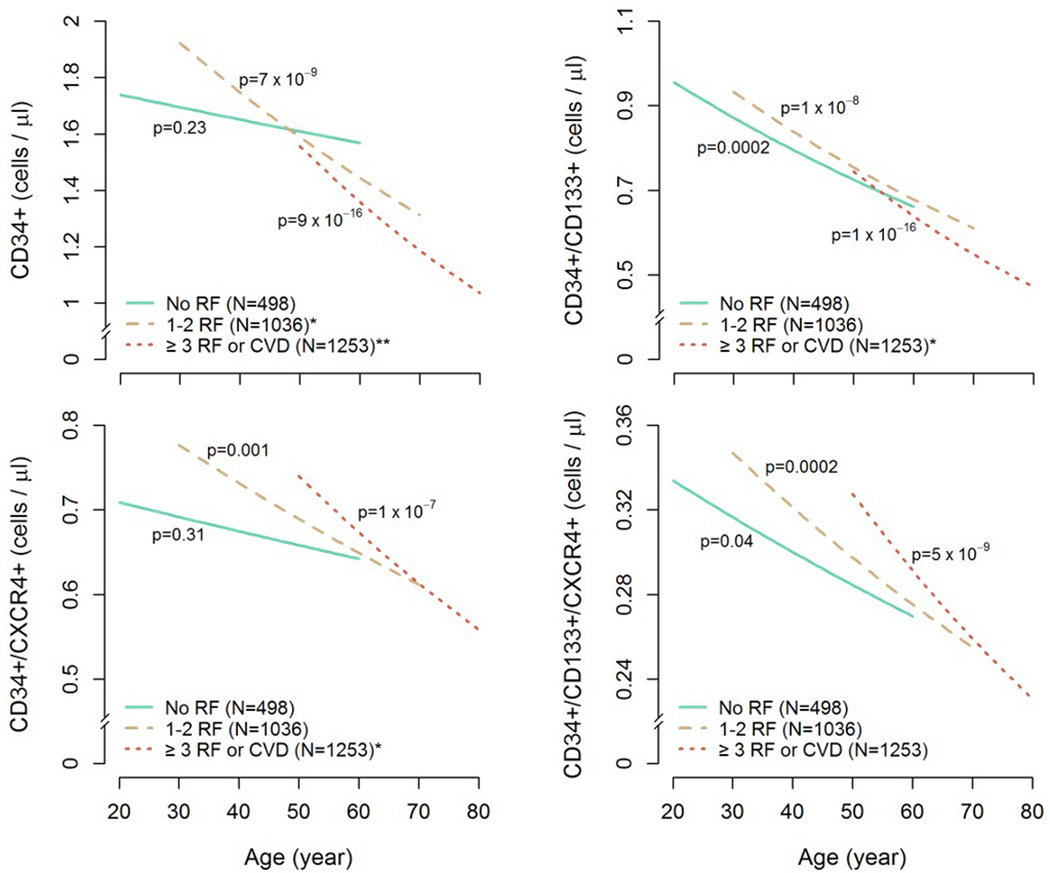
As the above findings suggested that age may exert substantial interaction effects on the relationship between RF burden and PC counts, we sought to determine whether age-related decline in PC counts is affected by RF burden after multivariate adjustment. Linear regression models with log-transformed PC counts as the response variable; and age, RF group, and age × RF group interaction term as the explanatory variables were constructed, with gender, BMI, and statin use entered as covariates. Full results of multiple linear regression analyses can be seen in the supplemental section. (Online table II).
Estimates of age-related decreases in PC counts with increasing age among the three risk groups, after multivariate adjustment, demonstrate significantly differing age-slopes for the three RF groups, confirming an interaction between age and RF burden on PC counts. (Figure 3). Similarly, the adjusted percent decline in PC counts per decade of increasing age for CD34+, CD34+/CD133+, CD34+/CXCR4+, and CD34+/CD133+/CXCR4+ cells among the three RF groups is presented in Table 4, and is significantly larger with increasing RF burden for all PC subtypes except for the CD34+/CD133+/CXCR4+ cell population. (Table 4). Thus, whereas, for each 10-year increase in age, the CD34+ cell counts decreased by 2.6% in those without RFs, subjects with 3 or more RF exhibited a significantly more precipitous decline of 12.6% per decade.
Figure 3. Effects of increasing risk factor burden on age-related decline of PC counts.
Decreases in PC counts with increasing age among the three risk groups. P-values for the age effect on PC counts are shown for each risk group. All analyses were adjusted for gender, BMI, and statin use. *p ≤ 0.05 compared with no RF group, **p < 0.0001 compared with no RF group. RF: Risk factors
Table 4.
Age-related decline in PC counts and cardiovascular risk factor burden
| Risk Group |
Estimate | 95% Confidence Intervals |
p-value | p-value* | ||
|---|---|---|---|---|---|---|
| CD34+ | 0 RF | −2.6% | −6.6% | 1.7% | 0.23 | 0.0003 |
| 1–2 RF | −9.1% | −12.0% | −6.1% | <.0001 | ||
| 3+ RF | −12.6% | −15.4% | −9.9% | <.0001 | ||
| CD34+/CD133+ | 0 RF | −8.8% | −13.1% | −4.3% | 0.0002 | 0.01 |
| 1–2 RF | −10.1% | −13.2% | −6.7% | <.0001 | ||
| 3+ RF | −14.1% | −17.1% | −11.0% | <.0001 | ||
| CD34+/CXCR4+ | 0 RF | −2.5% | −6.9% | 2.3% | 0.31 | 0.04 |
| 1–2 RF | −5.8% | −9.2% | −2.4% | 0.001 | ||
| 3+ RF | −9.0% | −12.1% | −5.7% | <.0001 | ||
| CD34+/CD133+/CXCR4+ | 0 RF | −5.2% | −10.0% | −0.2% | 0.04 | 0.13 |
| 1–2 RF | −7.4% | −11.0% | −3.6% | 0.0002 | ||
| 3+ RF | −11.0% | −14.4% | −7.5% | <.0001 | ||
| CD34+/VEGF2R+ | 0 RF | 11.0% | 1.3% | 21.7% | 0.03 | 0.003 |
| 1–2 RF | −8.4% | −14.4% | −2.1% | 0.01 | ||
| 3+ RF | −6.3% | 12.0% | −0.2% | 0.04 | ||
Estimated differences in mean CD34+, CD34+/CD133+, CD34+/CXCR4+, CD34+/CD133+/CXCR4+ and CD34+/VEGF2R+ per 10-years age increase for the three risk groups, after adjusting for gender, BMI, and statin use (N=2787).
Test for difference in age-related changes in PC counts among three risk groups.
DISCUSSION
In a large population study that included healthy subjects, those with RFs and overt CVD, we demonstrate that age-related differences in circulating PC numbers are significantly modulated by CVD risk burden, independent of gender, body size and statin use. Although there was an overall age-related decline in PC counts in the entire population, the presence of CVD RFs significantly modified this relationship such that healthy subjects with no RFs exhibited no significant changes or small declines in PC counts with increasing age, while groups with higher CVD RF burden had progressively steeper age-related declines in most PC subsets. Furthermore, whereas the presence of RFs in subjects younger than 55 years was not associated with decreases in blood PC counts, significantly lower numbers of circulating PC were observed in older subjects with higher RF burden. Taken together, these novel findings suggest that protracted exposure to injurious effects of CVD and its RFs over the lifespan might result in exhaustion in circulating PC.
Several techniques are available for assessing PC number and function, including evaluation of in vitro growth and mobility potential.3, 22 Measurement of the number of circulating PC as in this study is nevertheless readily available, is reproducible, and amenable for performance in large scale studies. We employed a robust flow-cytometric technique for evaluation of PC-enriched populations. In addition to CD34+ cells, we examined sub-populations that co-expressed CD133, VEGF2R, and CXCR4 in order to identify cell populations that are enriched for various putative progenitors. CD133 co-expression identifies an early progenitor subset, VEGF2R expression identifies PCs enriched for endothelial progenitors, and the CXCR4 receptor binds to the highly chemotactic and angiogenic stromal derived factor-1 responsible for PC homing.28, 29 The large number and wide age range of subjects in each risk category permitted examination of PC counts at different ages in healthy subjects and in those with RFs or with established CVD. Notably, hematopoietic PC populations expressing CD34 and those co-expressing CD133+ and/or CXCR4+ showed a similar decline with increasing age and RF burden. In contrast, VEGF2R+ cells that are enriched for endothelial PC, exhibited either none or weak associations that may be partly due to their relatively rare numbers and/or relatively higher measurement variability.
The notion that cardiovascular risk influences aging of regenerative capacity in humans is supported by experimental studies demonstrating age-related decline in peripheral blood and bone marrow PC, both numerically and functionally, that is accelerated in the atherosclerotic milieu. Also, the atheroprotective property of bone marrow PC was shown to be exhausted with increasing age and exposure to RFs in a murine model of atherosclerosis resulting in deficient vascular repair.30, 31 Human studies also support our findings. Lower frequency of circulating CD34+ cells with increasing CVD risk burden was reported in subjects recruited in the Framingham Heart Study with a mean age of 66 years, findings similar to our observations in the >55 years of age cohort.32 Our study however also included a large number of younger and healthier subjects, revealing the relationship between PC and RFs in the younger cohorts. Our findings of higher CD34+ counts in younger subjects, men and in hypertensives, as well as with increasing body size, higher triglycerides and lower high-density lipoprotein levels have been previously reported, and we extend these results to other circulating PC subpopulations co-expressing CD133 and CXCR4.32 Overall, emerging experimental and clinical data suggest that recruitment of bone marrow PC and mobilization into the circulation is a homeostatic response to vascular injury that is preserved in young and healthy individuals and diminishes with aging and chronic RF exposure. Moreover, depressed PC numbers and function in the presence of CVD is associated with adverse outcomes.33–35 Furthermore, PC mobilization after tissue injury such as acute myocardial infarction, or in response to cytokine administration is blunted with increasing age and RF exposure in humans, and the potential of transplanted bone marrow PC to effect neovascularization is impaired with aging.36–39
Our findings are relevant for the field of regenerative medicine. Herein, we demonstrate that PC counts in the circulation, as an index of endogenous regenerative capacity, should be assessed after accounting for an individual’s age, gender, body size, and RF burden. Specifically, older subjects with the combination of RFs and lower PC content in their blood may be appropriate subjects for novel cell-based therapeutic studies that involve PC mobilization or bone marrow isolation and expansion.12, 13, 40 In the context of CVD risk prediction, we have previously demonstrated that lower counts of circulating PC in patients with suspected CAD were prospectively associated with increased morbidity and mortality.34 Similarly, whether lower counts in younger individuals confer future protection from CVD needs further investigation.
Limitations
The cross-sectional design of our study precludes definitive conclusions regarding the causal relationships between PC and CVD RFs. Similarly, we cannot conclude that aging with RFs leads to exhaustion of circulating PC, although these findings have been validated in experimental models. The contribution of other comorbidities or organ dysfunction, as well as effects of pharmacologic therapy were not evaluated in this study and will be worthy of further investigations in longitudinal studies.
Conclusion
Our results suggest that CVD RFs modulate aging of human regenerative capacity, whereby increasing RF burden is associated with a greater age-related decline in PC counts, which may be due to exhaustion of endogenous regenerative capacity. In subjects free of CVD RFs or CVD, circulating levels of hematopoietic PC do not change appreciably with increasing age. Exposure to injurious CVD RFs at a young age is not associated with decreased PC counts; however, with aging, continued exposure to RFs leads to reduction in PC levels and consequent exhaustion of endogenous regenerative capacity.
Supplementary Material
Novelty and Significance.
What Is Known?
Bone marrow and tissue progenitor cells (PC) participate in vascular repair and regeneration.
Some studies have reported that tissue injury mobilizes PC into the circulation and depressed PC activity in the presence of cardiovascular disease (CVD).
It is unclear whether “healthy aging” with significant CVD risk affects the mobilization of PC and their circulating counts.
What New Information Does This Article Contribute?
The interaction between age and risk factor burden significantly affects their relationship with circulating levels of PC.
Healthy aging is accompanied by non-significant changes in PC counts and worsening risk factor burden is associated with a precipitous age-related decline in the circulating levels of most PC subsets.
Cardiovascular disease risk accelerates the aging of human regenerative capacity and healthy aging is characterized by preservation of PC activity.
Age is an overarching risk factor for the development of CVD. However, it is unclear how aging alters circulating PC numbers, and whether atherosclerosis impairs PC. We investigated the relationship between healthy aging as well as aging with CVD risk burden or established CVD on PC levels. We found that increasing CVD risk burden was accompanied by a precipitous age-related decline in circulating PC counts, reflecting exhaustion of endogenous regenerative capacity. Conversely, in subjects free of CVD risk factors circulating levels of PC do not change appreciably with age. These findings suggest that for CVD risk prediction PC activity should be assessed in the context of an individual’s age, gender, body size, and risk factor burden.
Acknowledgments
SOURCES OF FUNDING
This work was supported by the Marcus and Woodruff Foundations, Atlanta, Georgia; and the Georgia Tech/Emory University Predictive Health Institute. PHS Grant UL1 TR000454 from the Clinical and Translational Science Award Program, National Institutes of Health, National Center for Research Resources.
Abbreviations
- CVD
Cardiovascular disease
- RF
Risk factor
- PC
Progenitor cells
- VEGFR2
Vascular endothelial growth factor receptor-2 (VEGFR2)
- CVA
Cerebrovascular accident
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 3.Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, Schafer B, Hossfeld DK, Fiedler W. In vitro differentiation of endothelial cells from ac133-positive progenitor cells. Blood. 2000;95:3106–3112. [PubMed] [Google Scholar]
- 4.Urbich C, Dimmeler S. Endothelial progenitor cells: Characterization and role in vascular biology. Circulation research. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 5.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, Kearney M, Zak V, Asahara T, Losordo DW. Cd34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 6.Waller EK, Olweus J, Lund-Johansen F, Huang S, Nguyen M, Guo GR, Terstappen L. The "common stem cell" hypothesis reevaluated: Human fetal bone marrow contains separate populations of hematopoietic and stromal progenitors. Blood. 1995;85:2422–2435. [PubMed] [Google Scholar]
- 7.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. Ac133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 8.Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110:624–637. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, Bhavsar JR, Yoder MC, Haneline LS, Ingram DA. Human cd34+ac133+vegfr-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Experimental hematology. 2007;35:1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Alaiti MA, Ishikawa M, Costa MA. Bone marrow and circulating stem/progenitor cells for regenerative cardiovascular therapy. Translational research : the journal of laboratory and clinical medicine. 2010;156:112–129. doi: 10.1016/j.trsl.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Seeger FH, Rasper T, Koyanagi M, Fox H, Zeiher AM, Dimmeler S. Cxcr4 expression determines functional activity of bone marrow-derived mononuclear cells for therapeutic neovascularization in acute ischemia. Arterioscler Thromb Vasc Biol. 2009;29:1802–1809. doi: 10.1161/ATVBAHA.109.194688. [DOI] [PubMed] [Google Scholar]
- 12.Al Mheid I, Quyyumi AA. Cell therapy in peripheral arterial disease. Angiology. 2008;59:705–716. doi: 10.1177/0003319708321584. [DOI] [PubMed] [Google Scholar]
- 13.Poole J, Mavromatis K, Binongo JN, Khan A, Li Q, Khayata M, Rocco E, Topel M, Zhang X, Brown C, Corriere MA, Murrow J, Sher S, Clement S, Ashraf K, Rashed A, Kabbany T, Neuman R, Morris A, Ali A, Hayek S, Oshinski J, Yoon YS, Waller EK, Quyyumi AA. Effect of progenitor cell mobilization with granulocyte-macrophage colony-stimulating factor in patients with peripheral artery disease: A randomized clinical trial. Jama. 2013;310:2631–2639. doi: 10.1001/jama.2013.282540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leone AM, Rutella S, Bonanno G, Contemi AM, de Ritis DG, Giannico MB, Rebuzzi AG, Leone G, Crea F. Endogenous g-csf and cd34+ cell mobilization after acute myocardial infarction. International journal of cardiology. 2006;111:202–208. doi: 10.1016/j.ijcard.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 15.Wojakowski W, Tendera M, Michalowska A, Majka M, Kucia M, Maslankiewicz K, Wyderka R, Ochala A, Ratajczak MZ. Mobilization of cd34/cxcr4+, cd34/cd117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110:3213–3220. doi: 10.1161/01.CIR.0000147609.39780.02. [DOI] [PubMed] [Google Scholar]
- 16.Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 17.Roberts N, Xiao Q, Weir G, Xu Q, Jahangiri M. Endothelial progenitor cells are mobilized after cardiac surgery. The Annals of thoracic surgery. 2007;83:598–605. doi: 10.1016/j.athoracsur.2006.09.087. [DOI] [PubMed] [Google Scholar]
- 18.Fox A, Smythe J, Fisher N, Tyler MP, McGrouther DA, Watt SM, Harris AL. Mobilization of endothelial progenitor cells into the circulation in burned patients. The British journal of surgery. 2008;95:244–251. doi: 10.1002/bjs.5913. [DOI] [PubMed] [Google Scholar]
- 19.Gill M, Dias S, Hattori K, Rivera ML, Hicklin D, Witte L, Girardi L, Yurt R, Himel H, Rafii S. Vascular trauma induces rapid but transient mobilization of vegfr2(+)ac133(+) endothelial precursor cells. Circ Res. 2001;88:167–174. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- 20.Sepp D, Franz D, Triftshaeuser N, Ott I, Esposito-Bauer L, Feurer R, Seifert CL, Thaler M, Hemmer B, Poppert H. Mobilization of cd133+ progenitor cells in patients with acute cerebral infarction. PloS one. 2014;9:e70796. doi: 10.1371/journal.pone.0070796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu T, She Q, Jiang Y, Su L, Yin Y. Level of cd14+-endothelial progenitor cells is not associated with coronary artery disease or cardiovascular risk factors. Age. 2008;30:319–326. doi: 10.1007/s11357-008-9074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. The New England journal of medicine. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 23.Fadini GP, Maruyama S, Ozaki T, Taguchi A, Meigs J, Dimmeler S, Zeiher AM, de Kreutzenberg S, Avogaro A, Nickenig G, Schmidt-Lucke C, Werner N. Circulating progenitor cell count for cardiovascular risk stratification: A pooled analysis. PloS one. 2010;5:e11488. doi: 10.1371/journal.pone.0011488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lenfant C, Chobanian AV, Jones DW, Roccella EJ Joint National Committee on the Prevention DE, Treatment of High Blood P. Seventh report of the joint national committee on the prevention, detection, evaluation, and treatment of high blood pressure (jnc 7): Resetting the hypertension sails. Hypertension. 2003;41:1178–1179. doi: 10.1161/01.HYP.0000075790.33892.AE. [DOI] [PubMed] [Google Scholar]
- 25.Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P, Frohlich J, Albers JJ. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. The New England journal of medicine. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 26.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P Expert Committee on the D, Classification of Diabetes M. Follow-up report on the diagnosis of diabetes mellitus. Diabetes care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 27.Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ishage guidelines for cd34+ cell determination by flow cytometry. International society of hematotherapy and graft engineering. Journal of hematotherapy. 1996;5:213–226. doi: 10.1089/scd.1.1996.5.213. [DOI] [PubMed] [Google Scholar]
- 28.Mheid IA, Corrigan F, Shirazi F, Veledar E, Li Q, Alexander WR, Taylor WR, Waller EK, Quyyumi AA. Circadian variation in vascular function and regenerative capacity in healthy humans. Journal of the American Heart Association. 2014;3 doi: 10.1161/JAHA.114.000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng H, Fu G, Dai T, Huang H. Migration of endothelial progenitor cells mediated by stromal cell-derived factor-1alpha/cxcr4 via pi3k/akt/enos signal transduction pathway. Journal of cardiovascular pharmacology. 2007;50:274–280. doi: 10.1097/FJC.0b013e318093ec8f. [DOI] [PubMed] [Google Scholar]
- 30.Zhu S, Liu X, Li Y, Goldschmidt-Clermont PJ, Dong C. Aging in the atherosclerosis milieu may accelerate the consumption of bone marrow endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2007;27:113–119. doi: 10.1161/01.ATV.0000252035.12881.d0. [DOI] [PubMed] [Google Scholar]
- 31.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108:457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 32.Cohen KS, Cheng S, Larson MG, Cupples LA, McCabe EL, Wang YA, Ngwa JS, Martin RP, Klein RJ, Hashmi B, Ge Y, O'Donnell CJ, Vasan RS, Shaw SY, Wang TJ. Circulating cd34(+) progenitor cell frequency is associated with clinical and genetic factors. Blood. 2013;121:e50–e56. doi: 10.1182/blood-2012-05-424846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt-Lucke C, Rossig L, Fichtlscherer S, Vasa M, Britten M, Kamper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: Proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 34.Patel RS, Li Q, Eapen DJ, Ghasemzadeh N, Moss-Owens L, Manocha P, Rahman A, Al Kassem H, Veledar E, Taylor WR, Zafari AM, Samady H, Vaccarino V, Waller EK, AA Q. Circulating cd34+ progenitor cell levels predict adverse cardiovascular outcomes. Ahead of Print. 2013 [Google Scholar]
- 35.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. The New England journal of medicine. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 36.Abkowitz JL, Robinson AE, Kale S, Long MW, Chen J. Mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood. 2003;102:1249–1253. doi: 10.1182/blood-2003-01-0318. [DOI] [PubMed] [Google Scholar]
- 37.Korbling M, Haas R, Knauf W, Holle R, Hunstein W. Therapeutic efficacy of autologous blood stem cell transplantation (absct): The role of cytotoxic/cytokine stem cell mobilization. Bone marrow transplantation. 1990;5(Suppl 1):39–40. [PubMed] [Google Scholar]
- 38.Lavazais E, Pogu S, Sai P, Martignat L. Cytokine mobilization of bone marrow cells and pancreatic lesion do not improve streptozotocin-induced diabetes in mice by transdifferentiation of bone marrow cells into insulin-producing cells. Diabetes & metabolism. 2007;33:68–78. doi: 10.1016/j.diabet.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Povsic TJ, Najjar SS, Prather K, Zhou J, Adams SD, Zavodni KL, Kelly F, Melton LG, Hasselblad V, Heitner JF, Raman SV, Barsness GW, Patel MR, Kim RJ, Lakatta EG, Harrington RA, Rao SV. Epc mobilization after erythropoietin treatment in acute st-elevation myocardial infarction: The reveal epc substudy. Journal of thrombosis and thrombolysis. 2013;36:375–383. doi: 10.1007/s11239-013-0944-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quyyumi AA, Waller EK, Murrow J, Esteves F, Galt J, Oshinski J, Lerakis S, Sher S, Vaughan D, Perin E, Willerson J, Kereiakes D, Gersh BJ, Gregory D, Werner A, Moss T, Chan WS, Preti R, Pecora AL. Cd34(+) cell infusion after st elevation myocardial infarction is associated with improved perfusion and is dose dependent. American heart journal. 2011;161:98–105. doi: 10.1016/j.ahj.2010.09.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.