Abstract
Background
Antibiotic resistance is a challenge in Long Term Care Facilities (LTCFs). The objective was to demonstrate that a novel, minimally invasive program not interfering with activities of daily living (ADL) or socialization could lower methicillin-resistant Staphylococcus aureus (MRSA) disease.
Methods
This was a prospective, cluster-randomized, non-blinded trial initiated at three LTCFs. During Year 1 units were stratified by type of care and randomized to intervention or control. In Year 2 all units were converted to intervention consisting of universal decolonization using intranasal mupirocin and a chlorhexidine bath performed twice (two decolonization/bathing cycles one month apart) at the start of the intervention period. Subsequently, after initial decolonization, all admissions were screened on site using real-time PCR and those MRSA positive were decolonized, but not isolated. Units received annual instruction on hand hygiene. Enhanced bleach wipe cleaning of flat surfaces was done every four months.
Results
16,773 tests were performed. The MRSA infection rate decreased 65% between the baseline (44 infections during 365,809 patient-days) and Year 2 (12 during 287,847 patient-days; p<0.001); significant reduction was observed at each of the LTCFs (p<0.03).
Discussion and Conclusion
On-site MRSA surveillance with targeted decolonization resulted in a significant decrease in clinical MRSA infection among LTCF residents.
Keywords: Long Term Care Facility (LTCF), Methicillin-resistant Staphylococcus aureus (MRSA), Real-time PCR, Healthcare-Associated Infection, Decolonization therapy, MDRO control
INTRODUCTION
Infections in Long Term Care Facilities (LTCFs) are frequent and result in morbidity, mortality, hospital readmission, and substantial cost to the healthcare system (1). There are approximately 16,000 LTCFs, or nursing homes, in the United States that are the residence for 1.5 million persons. In this population of older and generally frailer individuals, some 2 million infections occur each year, often from antibiotic resistant bacteria (1). By 2030 it is estimated the LTCF population will grow to 5.3 million people, indicating the urgent need for addressing this healthcare-associated infection (HAI) problem (2). Common bacterial infections in LTCFs are methicillin-resistant Staphylococcus aureus (MRSA), antibiotic resistant Gram-negative bacilli and vancomycin-resistant enterococci (VRE) that cause urinary, respiratory, and skin-soft tissue infection (1). A recent critical review investigating prevention of MRSA clinical disease in LTCFs found only a single published controlled trial as of August 2013, which included 32 nursing homes and evaluated the effect of infection control education and training on MRSA prevalence (3). There was no impact on MRSA prevalence as part of the intervention.
We undertook the Detection, Education, Research and Decolonization without Isolation in Long-term care (DERAIL MRSA) program with a focus on MRSA. Our goal of this demonstration project was to reduce the MRSA clinical disease rate in LTCF residents (e.g., patients), as has successfully done in Acute Care (4, 5). Our hypothesis was that this could be done using a novel approach tailored to the LTCF setting without negatively impacting the socialization needs and the activities of daily living (eating, bathing, dressing, toileting, transferring (walking) and continence; ADL) in these patients (residents) who have the LTCF as their home, and often permanent residence.
METHODS
Trial Design and Participants
The overall Methods to the DERAIL MRSA pragmatic intervention design were straightforward. We first removed the target pathogen (MRSA) from the population by decolonizing all the intervention unit residents and then tested (using a rapid method) all new admissions on-site followed by decolonization of those positive going to the intervention units. All nursing unit personnel received education on the nature of pathogen transmission, the need for effective cleaning and disinfection of healthcare facility surfaces/equipment, and the importance of hand hygiene, which are considered standard practices for adequate MRSA control (3, 6).
The original design was an IRB-approved, prospective, cluster randomized, clinical trial performed in 12 nursing units (between 850–900 beds in total) at three separate LTCFs with an approximate total of 4,200 annual admissions. Nursing units for the three facilities each belonged to one of three categories: skilled nursing, rehabilitation, or dementia care that were included in the cluster randomization. These 12 units were randomly assigned to intervention or control. All persons residing (cared for) in those units were eligible for participation – there were no exclusions. Isolation, or contact precautions, was not part of this trial so the intervention did not interfere with any movement or daily activities of the residents. A systematic review of the program and outcome measures was done at the end of Year 1 before Year 2 began.
A point prevalence survey for MRSA nasal colonization was performed at the beginning of the study (March 2011) and then repeated five additional times (Table 1). At each of these times, environmental decontamination of flat surfaces in all rooms, common areas, and equipment with bleach was conducted over a one week period. Beginning in April 2011 all admissions were tested for MRSA nasal colonization. Discharge testing for MRSA colonization was performed on patients during January–March 2012 and again during the same 3 months in 2013. All residents on the intervention units were decolonized twice at the onset of the study, once at the beginning and again at the end of March 2011. This was repeated for all residents (intervention and control units) during March 2012. For the first year, all admissions that were MRSA colonized were decolonized at the time of admission to an intervention unit. This was done for all admissions (intervention and control) during Year 2. Also, during Year 2 all MRSA positive residents were retested after decolonization and this process was continued until they had a negative test for MRSA in their nares or were discharged. Table 1 is a complete timeline of the project events that includes the number of patients involved at each point.
Table 1.
Project timeline and number of patients (residents) tested during each project event.
| Event | PP 1* and Decol. of Intervention Unit residents | All admit testing for MRSA | PP 2* and All admit testing for MRSA | All admit testing for MRSA | PP 3* and All admit testing for MRSA | All admit testing for MRSA | All admit and discharge testing for MRSA | PP 4* and Decol. of all Unit residents; All admit and discharge testing for MRSA | All admit testing for MRSA | PP 5* and All admit testing for MRSA | All admit testing for MRSA | All admit and discharge testing for MRSA | PP 6* and All admit and discharge testing for MRSA |
| Number included | 675 | 1420 | 1178 | 1434 | 1209 | 451 | 1320 | 1413 | 2249 | 1209 | 1355 | 1475 | 1385 |
| Date | MarϮ 2011 | Apr through Jun | Jul | Aug through Oct | Nov | Dec | Jan and Feb 2012 | Mar | Apr through Aug | Sep | Oct through Dec | Jan and Feb 2013 | Mar |
PP = Point Prevalence
Jan = January, Feb = February, Mar = March, Apr = April, Jun = June, Jul = July, Aug = August, Sep = September, Oct = October, Nov = November, Dec = December
All residents were concurrently followed for clinical infectious disease using defined criteria following the surveillance definitions of Stone and colleagues (7), with the data recorded on a standard form. These represented infections not present on admission but occurring >2 calendar days after admission. Two research Infection Control Preventionists recorded this data designed for detecting and monitoring infections in LTCF. Residents with active MRSA clinical infection were placed in contact precautions (isolation) according to the established practice of the LTCFs; isolation was not used for asymptomatic MRSA colonization only.
At the time of the 12-month analysis it was found that there was a non-significant reduction in clinical disease attributed to the cluster randomized design being not appropriate for LTCF. There was too much resident and staff intermingling between all units each day, and thus the decision was made to make all units intervention sites for Year 2 in order to test if the original intervention plan would have a significant impact. The critical reasons for continuing a second year were to demonstrate sustainability of the intervention for MRSA colonization reduction and to monitor change in clinical disease in the LTCFs.
The study was approved by our Institutional Review Board (Protocol EH10-198). This study is registered (trial number NCT01302210) at: http://clinicaltrials.gov/ct2/show/NCT01302210?term¼MRSAþinþLongþTermþCare&rank¼1).
Microbiologic Methods
A pre-moistened double swab of both nares was collected for nasal colonization testing, and a new single swab was used on any open wounds. Real-time PCR (qPCR) was performed at each LTCF using one of the nasal swabs and processed in the Cepheid GeneXpert® system using their FDA cleared Xpert® MRSA assay. The testing was done on site at each LTCF and processed either by the nursing personnel working at each facility or by the research personnel hired as part of this study. The other nasal swab and any wound swabs were transferred to the research laboratory for culture. Surveillance testing has proven safe in over 500,000 patients who have received it as reported by Robicsek and colleagues (4). Subjects had the right to decline (opt-out design) this testing should they desire to do so. All MRSA isolates were tested for the presence of mupirocin (mupA) resistance. They were also banked for later subspecies typing using pulsed-field gel electrophoresis (PFGE) of isolates detected during the point prevalence surveys. During Year 1 of the study, additional swabs for culture were collected from throat, axilla, inguinal areas, and the peri-rectum of a subset of patients providing written, informed consent to determine the efficiency of nasal qPCR. This was done as a means of validating the utility of nasal swabs alone tested by qPCR for MRSA surveillance in the LTCF population (8).
PFGE was performed in the research laboratory to i) determine the clonality of MRSA strains introduced into the LTCFs, ii) the pattern of potential spread within the facilities, and iii) the association of any mupirocin resistance with specific MRSA clonal groups. We used standard methods that have been published (9–11). Mupirocin (high-level) resistance was determined from MRSA isolates with a qPCR method previously validated in our laboratory (12).
Standard Decolonization Regimen (Intervention Units)
A 5 day regimen of mupirocin calcium 2%, twice daily (applied to the nares and any open wounds), plus at least one chlorhexidine gluconate 4% body wash was used. Medications were applied by the patient, a caregiver, or one of the research ICPs. This regimen has proven safe in more than 55,000 patients who have received it in prior published work to control staphylococcal infections since 2005 (4). Patients who had MRSA strains resistant to mupirocin were given retapamulin 1% in the same manner as mupirocin.
Intensive Decolonization Regimen (Intervention units)
The therapy was used during Year 2 for those not decolonized with nasal treatment alone. This regimen consisted of minocycline (100 mg orally twice daily for 5 days), rifampin (600 mg orally once daily for 5 days), 2% mupirocin ointment applied to the anterior nares twice per day for 7 days, and a bath or shower with 4% chlorhexidine once per week for 2 weeks (13). Since this is a more aggressive decolonization regimen with potential adverse events from the systemic medications, only those providing written informed consent were given this therapy.
Statistical Considerations
With 850–900 beds, and assuming a reasonable intracluster correlation coefficient of 0.03 or less, this cluster-randomized trial was powered to have >80% power to demonstrate a 50% reduction in disease prevalence in the intervention arm (two-sided alpha 0.05). The primary outcome measure was MRSA the number of MRSA clinical infections. MRSA infection rates in each time period were estimated overall and within each site per 10,000 patient-days. Differences between sites and between time periods were evaluated using chi-square or Fisher’s exact test for proportions and Poisson tests for comparing rates. For assessing trends in mupirocin resistance, results from the point prevalence surveys were modeled using logistic regression with LTCF, time, and the interaction between LTCF and time included as independent variables. R version 3.1.1 was used for analysis.
RESULTS
A total of 16,773 swab samples were collected over two years. Less than 1% of the patients or their families in this ‘Op-Out design’ declined participation. Schora et al. have reported that the program significantly reduced MRSA colonization (14). For that analysis the baseline MRSA colonization rate was 16.64%. In Year 1, the colonization rate of intervention units dropped to 11.61% (p = .028) and remained at 17.85% in the control units (p = .61; significance compared with baseline). The intervention unit rate difference compared with control was also significant (p = .001). In Year 2, the overall combined colonization rate was 10.55% (p < .001) compared with baseline. During Year 2, fifteen residents received the intensive decolonization regimen after failing the standard regimen twice; 13 (87%) were decolonized by this treatment. The overall decolonization success suggested that there should be a reduction in clinical disease since MRSA colonization had decreased (15).
There were six long term stay units (274 beds) and three short stay units (115 beds) assigned to the intervention group. The control group had six long term stay units (299 beds) and four short stay units (174 beds). In the long term stay units the mean length of stay (LOS) ranged from 187 days to 13.3 years. For the short stay units the average LOS ranged from 17.5 to 26.1 days. The overall rate of MRSA infections significantly decreased between the baseline and Year 2 (Table 2). This amounted to a 65% lowering of MRSA clinical infection (reduced by .78 infections per 10,000 patient days; p< .001). A significant reduction (p ≤ .022) in MRSA clinical infection also was observed at each of the three LTCFs (Table 2).
Table 2.
MRSA infection rates in each time period, overall and by study site.
| Baseline | Year 1 | Year 2 | ||
|---|---|---|---|---|
| Control | Intervention | Decolonize All | ||
| Total Combined Facilities | ||||
| Total Patient Days | 365,809 | 165,052 | 129,113 | 287,847 |
| Total Number of MRSA Infections | 44 | 9 | 14 | 12 |
| MRSA Infections/10,000 patient-days | 1.20 | 0.55 | 1.08 | 0.42† |
| LTCF 1 | ||||
| Total Patient Days | 73,836 | 23,060* | 36,067* | 57,679 |
| Total Number of MRSA Infections | 16 | 2 | 5 | 4 |
| MRSA Infections/10,000 patient-days | 2.17 | 0.87 | 1.39 | 0.69‡ |
| LTCF 2 | ||||
| Total Patient Days | 128,287 | 56,518* | 44,407* | 97,371 |
| Total Number of MRSA Infections | 18 | 2 | 6 | 7 |
| MRSA Infections/10,000 patient-days | 1.40 | 0.35 | 1.35 | 0.72§ |
| LTCF 3 | ||||
| Total # of Patient Days | 163,686 | 85,474 | 48,639 | 132,797 |
| Total Number of MRSA Infections | 10 | 5 | 3 | 1 |
| MRSA Infections/10,000 patient-days | 0.61 | 0.58 | 0.62 | 0.08¥ |
Estimated based on relative distribution of beds between intervention and control units.
Difference between baseline and year 2 significant at p < .001;
Difference between baseline and year 2 significant at p = .022;
Difference between baseline and year 2 significant at p < .001;
Difference between baseline and year 2 significant at p = .009
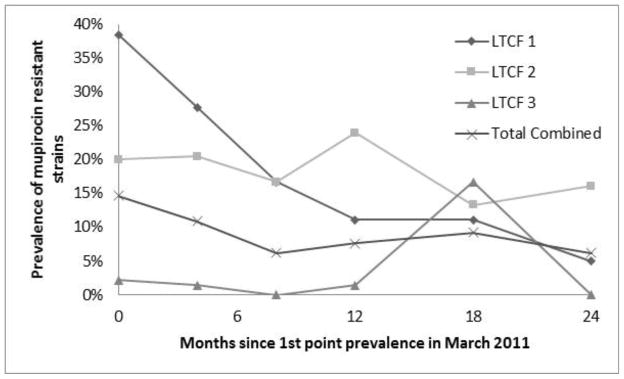
Mupirocin resistance rates (shown in Figure 1) in the recovered MRSA were significantly different between the LTCFs in March 2011 (Chi-square, 2 df = 12.7, p = .002). There was a significant downward trend in resistance over time (Chi-square, 1 df = 4.1, p = .042), and this trend was not significantly different between LTCFs (interaction Chi-square, 2 df = 3.9, p = .145).
Figure 1.
Trend of mupirocin resistance in MRSA recovered from patients at the three LTCFs over time.
No clonal associations existed between mupirocin resistant MRSA strains. The clones (e.g., pulsotypes) are depicted in Table 3. Of interest is that while individual MRSA clones predominated at each facility, they were not the same clone (14). All three of the facilities maintain a close contact with our Infection Control group at the Acute Care organization with two of the three developing a contractual relationship for supervising and managing their Infection Control program.
Table 3.
Distribution of MRSA pulsotypes for the three Long Term Care facilities (LTCFs).
| MRSA pulso-type | PP1 | PP2 | PP3 | PP4 | PP5 | Pp6 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LTCF No. | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| % USA 100 | 66.7 | 38 | 89.8 | 76.5 | 34.9 | 76.7 | 92.3 | 32.4 | 67.1 | 88.9 | 29.2 | 59.7 | 71.4 | 20 | 65.4 | 52.6 | 30.6 | 54.8 |
| % USA 300 | 8.3 | 6 | 2 | 5.9 | 4.7 | 5.5 | 0 | 18.9 | 23.7 | 0 | 8.3 | 22.2 | 14.3 | 0 | 34.6 | 15.8 | 0 | 35.7 |
| % USA 500 | 0 | 18 | 0 | 0 | 4.7 | 0 | 0 | 8.1 | 0 | 0 | 20.8 | 0 | 0 | 13.3 | 0 | 0 | 8.3 | 0 |
| % USA 1000 | 0 | 18 | 0 | 0 | 34.9 | 0 | 0 | 32.4 | 0 | 0 | 33.3 | 0 | 0 | 56.7 | 0 | 0 | 47.2 | 0 |
| % EMRSA-15 | 0 | 10 | 0 | 0 | 14 | 0 | 0 | 5.4 | 0 | 0 | 4.2 | 0 | 0 | 10 | 0 | 0 | 2.8 | 0 |
| % other | 25 | 10 | 8.2 | 17.7 | 7 | 17.8 | 7.7 | 2.7 | 9.2 | 11.1 | 4.2 | 18.1 | 14.3 | 0 | 0 | 31.6 | 11.1 | 9.5 |
DISCUSSION
We performed a two year project whose purpose was to demonstrate that Long Term and Acute Care working together could lower infections in the LTCF setting, with MRSA control being the target for proof of concept. We were able to implement a simple and sustainable program for reducing MRSA clinical infection in the Long Term Care setting that did not interfere with the daily activities of the residents and had no adverse events on any of the participants. A prior report found decolonization of those with asymptomatic MRSA carriage led to reduced nasal carriage prevalence (14). Importantly, in another study, lower nasal colonization prevalence was significantly associated with less MRSA clinical disease (15).
In 2013 the Centers for Disease Control and Prevention published a roadmap for elimination of healthcare-associated infections for Long Term Care (6), but no specifics were given for dealing with MDRO pathogens such as MRSA. In 2008 the Society for Healthcare Epidemiology of America (SHEA) and the Association for Professionals in Infection Control and Epidemiology (APIC) published a guideline for infection prevention in Long Term Care (16). Recommended practices for controlling the spread of MDROs included i) a formal Infection Control Program, ii) surveillance of ongoing infections, iii) a plan for outbreak control, iv) isolation consistent with current (Acute Care) guidelines, v) effective hand hygiene, vi) patient and employee health programs, and vii) antibiotic stewardship (15). The major comment regarding MRSA stated ‘When MRSA becomes endemic within a facility, elimination is highly unlikely’. A following recommendation from APIC specifically addressing MRSA elimination recommended performing a MRSA risk assessment for clinical disease rate along with monitoring the MRSA status of new admissions and enhanced environmental cleaning (17). Of interest is that while MRSA decolonization was mentioned, it is stated that ‘there is insufficient evidence regarding the efficacy of decolonization to promote its use as a routine intervention’. This is likely due to an earlier LTCF study where decolonization therapy was only recommended during outbreaks of clinical MRSA infection (18). Importantly, these same authors reported on a subsequent trial where mupirocin decolonization appeared effective and disease trends lowered, with one of their conclusions being that additional studies on mupirocin decolonization in LTCFs were needed to assess the benefit for reducing clinical disease (19).
Bowler and colleagues reported on the impact of decolonizing nursing home residents in a before:after trial performed in a rural community that had a significant impact on reducing MRSA clinical disease in the affiliated Acute Care hospital associated with the LTCFs (13). They decolonized MRSA colonized residents of five nursing homes and then continued active surveillance cultures and decolonization of positive patients subsequently admitted. After the intervention, the prevalence of MRSA colonization decreased by 67% (p < .001), and the incidence rate of nosocomial MRSA infection at the Acute Care regional facility significantly decreased (p < .01) from 0.64 infections per 1,000 patient-days before the interventions to 0.32 infections per 1,000 patient-days two years after the intervention (12), an outcome higher than ours for clinical disease but a reduction consistent with our results. The largest study similar to ours attempting to lower MRSA colonization prevalence in a large nursing home cohort was a cluster-randomized trial between June 2010 and December 2011 in the state of Vaud, Switzerland (20). Screening for MRSA carriage was done at study entry and 12 months thereafter. Additionally, all newly admitted or readmitted residents underwent MRSA screening. The outcome was unexpected in that the mean absolute colonization prevalence decreased significantly by 3.0% in the intervention LTCFs and 2.3% in the control facilities, which corresponded to a non-significant 0.7% decrease attributable to the intervention (p = .66). The authors noted that low screening compliance in some facilities may have negatively impacted the outcome along with the observation that colonization rates at the onset of the study were lower than anticipated (8.9%), suggesting that MRSA prevalence may have been decreasing independently and confounded the outcome of this investigation (20). Also, screening compliance was less than ours (<87% vs >99%), and the MRSA detection methods were not defined – factors that impact MRSA control (21). These investigators did not measure clinical MRSA disease rates (20). Other approaches to MRSA control in LTCFs have been poorly studied with no clear evidence of success (3). The single well done report evaluating enhanced infection control training and education found no change in MRSA prevalence (22). Our investigation suggests a positive clinical impact is possible using rapid surveillance of patients with decolonization of carriers, and is supported by sophisticated simulation models of the problem (23–24), as well as by a report from a rehabilitation center where MRSA clinical disease was significantly reduced after implementing a screening and decolonization program (25).
There were findings in our LTCF study that might appear unexpected. One was that transmission (acquisition during stay in the facility) increased between year 2 and year 3, with the significant increase occurring in one of the LTCFs, and the overall rate going from 1.7% to 3.5% of patients tested (14). While this appears to be a meaningful increase in transmission events, the length of stay in LTCFs is much longer than in Acute Care and converting these numbers to transmission per 1,000 patient days demonstrates the increase to be from 0.095 transmissions per 1,000 days to 0.146 transmissions per 1,000 days; a rate well below that of 2 to 2.7 events per 1,000 patient days recently reported for a successful MRSA control program in Acute Care (26). Since we had no baseline transmission data we cannot compare that to the 2 years of intervention, but during both Year 1 and Year 2 the transmission rate was below that necessary to realize a reduction in MRSA clinical disease in the Acute Care setting. Another concern is in another reported study where prescription of mupirocin for nasal decolonization did not lead to a reduction of disease in those colonized (27). However, this result was for patients already colonized with MRSA and who received a single course of nasal mupirocin decolonization. Our aim in this current LTCF study was to reduce transmission to non-colonized patients as a means of reducing MRSA clinical disease, not to reduce infection via decolonization of those already colonized. Somewhat counterintuitively we demonstrated a reduction in mupirocin resistance during the trial. While universal decolonization would be expected to raise resistance to mupirocin (28), this was only done twice, one month apart at the beginning of each year to decolonize all residents, and following that all decolonization was done on a targeted basis. Also, retapamulin was used for those harboring mupirocin resistant strains, and patients in Year 2 were repeatedly decolonized until nasal swab test negative. This practice would be expected to slowly remove all MRSA, including mupirocin resistant strains, from the healthcare setting and result in lowered resistance to mupirocin – an outcome that appeared to occur.
Also of interest was the various clones or pulsotypes found at the three LTCFs (Table 3). USA 100, a typical hospital-associated clone of MRSA, initially dominated at all three facilities. However, by the end of the two year study a new clone USA 1000 was dominant at LTCF 2. In the past this clone has occurred infrequently and been associated with wound infections in persons who abuse drugs (29), so finding this clone in a LTCF is unexpected. Also unexpected was detection of the EMRSA-15 clone in this same facility. This particular MRSA clone has been associated with widespread and persistent MRSA infection in England, Europe and Asia-Pacific (30), and it is important that we did not see spread of this clone outside of LTCF 2.
The main limitation of our study was the initial design with the unanticipated failure of a cluster randomized study approach to perform adequately. While we had realized the daily community gathering of patients, we had not anticipated the magnitude of this intermingling and its impact on separating outcome measures between intervention and control units (31). However, this was recognized at the end of Year 1, and after consultation with the involved LTCFs, we all agreed that changing the study design to a before:after demonstration project was appropriate as it would permit the assessment of the proposed MRSA intervention at three distinct LTCFs. Another potential limitation was that we only performed nasal surveillance for MRSA, but we tested multiple body sites on a subset of the population and found more than 80% of the MRSA carriers were detected using nasal swab samples tested by qPCR (8), which led to a successful reduction in clinical disease.
A concern that might be raised is the cost of a surveillance program in relationship to benefit. Peterson and Schora recently reviewed the utility of MRSA active surveillance programs using qPCR and estimated the cost:benefit as part of their analysis (32). They demonstrated that the main cost of MRSA control is contact precautions, with an expense of over $50 per day. In the program we describe the total cost of decolonization is approximately $10. The other expense is the price of MRSA testing, which also can be as high as $50. However, if testing is required before LTCF admission, then this cost is not borne by the facility. Finally, there is some expense related to healthcare worker time. Nasal swabbing takes about 1 minute as does application of the nasal mupirocin. Bathing with chlorhexidine is straightforward since baths are routinely given and the only change is substituting chlorhexidine for the soap routinely used. The mean excess cost of a healthcare-associated MRSA clinical infection was $24,000 (32). This is based on the acute care cost, but it is an expense to the healthcare system regardless of where the resources are used. After adjusting for patient days, we avoided 23 MRSA infections when comparing baseline data to the final year of the program, which translates to a saved expense of $552,000 – well in excess of the mupirocin and chlorhexidine cost. We did not study patient satisfaction as part of our investigation and this is a useful endeavor for future research. In this LTCF study fewer than 1% of patients or their families declined participation. Also, in our acute care facilities we have received only one complaint from more than 500,000 patients tested, suggesting that MRSA surveillance is accepted.
Our study demonstrates that MRSA clinical disease can be reduced in LTCFs and indicates that future research in this area is worthwhile. A pragmatic program where the colonization status of new residents entering long term care is known so that those found positive can be decolonized is possible and clearly beneficial. Additional novel practices for managing the wide range of potential MDRO HAIs in the LTCF setting are urgently needed.
CONCLUSIONS
We have demonstrated that rapid, active surveillance for MRSA nasal carriage with targeted decolonization, coupled with enhanced environmental cleaning, results in a meaningful and significant decrease in clinical MRSA infection among LTCF residents without limiting socialization or ADL. Partnering Acute Care with Long Term Care in the national approach to improved healthcare is useful and should be part of future policy planning.
Highlights.
MRSA surveillance with targeted decolonization resulted in a significant 65% decrease in clinical infection.
A two year project successfully demonstrated that Long Term and Acute Care can work together to lower infections.
Decolonization of those with asymptomatic MRSA carriage led to reduced nasal carriage prevalence.
Mupirocin resistance was reduced during the trial that included determination of decolonization success.
Acknowledgments
This study was reported, in part at ID Week 2014 (Smith B, Boehm S, Beaumont J, Robicsek A, Patel P, Schora D, Burdsall D, Peterson K, Fausone M, Peterson L. Control of methicillin-resistant Staphylococcus aureus (MRSA) in long term care is possible while maintaining patient socialization without isolation. ID Week 2014. October 8–12, 2014. Philadelphia, PA. 637. Last accessed on 8/29/2015 at: https://idsa.confex.com/idsa/2014/webprogram/Paper47340.html).
Funding: This project was funded by grant number R18 HS19968 from the Agency for Healthcare Research and Quality (AHRQ), U.S. Department of Health and Human Services. This study is registered (trial number NCT01302210) at: http://clinicaltrials.gov/ct2/show/NCT01302210?term¼MRSAþinþLongþTermþCare&rank¼1).
Footnotes
Role of the Funder: Only the authors had a role in the study design, data analysis, data interpretation, or writing of this report. The corresponding authors had full access to all the data and had final responsibility for the decision to submit for publication. The opinions are those of the authors and do not reflect the official position of AHRQ or the U.S. Department of Health and Human Services.
Conflict of Interest Statement: No authors had any conflict of interest that pertains to this study and report. For potential conflicts of interest, LRP has received speaking honoraria from Becton Dickinson, Cepheid, Roche, and CareFusion. LRP has received research funding from Becton Dickinson, Cepheid, Nanosphere, 3M, GeneWEAVE and Roche. The other authors declare they have no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Montoya A, Mody L. Common infections in nursing homes: A review of current issues and challenges. Aging Health. 2011;7:889–899. doi: 10.2217/AHE.11.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knickman J, Snell E. The 2030 problem: caring for aging baby boomers. Health Serv Res. 2002;37:849–884. doi: 10.1034/j.1600-0560.2002.56.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes C, Tunney M, Bradley MC. Infection control strategies for preventing the transmission of meticillin-resistant Staphylococcus aureus (MRSA) in nursing homes for older people. Cochrane Database of Systematic Reviews. 2013;(11) doi: 10.1002/14651858.CD006354.pub4. Art. No.: CD006354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robicsek A, Beaumont JL, Paule SM, Hacek DM, Thomson RB, Jr, Kaul KL, King P, Peterson LR. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Int Med. 2008;148:409–418. doi: 10.7326/0003-4819-148-6-200803180-00003. [DOI] [PubMed] [Google Scholar]
- 5.Jain R, Kralovic SM, Evans ME, Ambrose M, Simbartl LA, Obrosky DS, Render ML, Freyberg RW, Jernigan JA, Muder RR, Miller LJ, Roselle GA. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364:1419–1430. doi: 10.1056/NEJMoa1007474. [DOI] [PubMed] [Google Scholar]
- 6.National Action Plan to Prevent Health Care-Associated Infections. Road Map to Elimination. [Last accessed January 31, 2016];Chapter 8: Long-Term Care Facilities. 2013 Apr;:194–239. http://health.gov/hcq/pdfs/hai-action-plan-ltcf.pdf.
- 7.Stone ND, Ashraf MS, Calder J, Crnich CJ, Drinka KP, Gould CV, Juthani-Mehta M, Lautenbach E, Loeb M, MacCannell T, Malani PN, Mody L, Mylotte JM, Nicolle LE, Roghmann M-C, Schweon SJ, Simor AE, Smith PW, Stevenson KB, Bradley SF for the Society for Healthcare Epidemiology Long-Term Care Special Interest Group. Surveillance definitions of infections in Long-Term Care Facilities: Revisiting the McGeer criteria. Infect Contr Hosp Epid. 2012;33:965–977. doi: 10.1086/667743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schora DM, Boehm S, Das S, Patel PA, Schora K, Peterson KE, Grayes A, Hines C, Burdsall D, Robicsek A, Peterson LR. Advan Prevent Contr HAIs. Agency for Healthcare Research and Quality; Rockville, MD. : 2014. [Last accessed November 9 2015]. Detection of methicillin-resistant Staphylococcus aureus (MRSA) from multiple body sites of residents at long-term care facilities. http://www.ahrq.gov/professionals/quality-patient-safety/patient-safety-resources/resources/advances-in-hai/hai-article15.html. [Google Scholar]
- 9.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol. 2003;41:5113–5120. doi: 10.1128/JCM.41.11.5113-5120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaggi P, Paule SM, Peterson LR, Tan TQ. Characteristics of Staphylococcus aureus infections, Chicago Pediatric Hospital. Emerg Infec Dis. 2007;13:311–314. doi: 10.3201/eid1302.060295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paule SM, Robicsek A, Suseno M, Kaul KL, Peterson LR. Incidence of mupirocin resistance in methicillin-resistant Staphylococcus aureus (MRSA) during universal surveillance and decolonization [abstract C2- 1149]. Program and abstracts of the 46th Interscience Conference on Antimicrobal Agents and Chemotherapy; September 27–30, 2006; San Francisco, CA. 2006. [Google Scholar]
- 13.Bowler WA, Bresnahan J, Bradfish A, Fernandez C. An integrated approach to methicillin-resistant Staphylococcus aureus control in a rural, regional-referral healthcare setting. Infect Control Hosp Epidemiol. 2010;31:269–275. doi: 10.1086/650445. [DOI] [PubMed] [Google Scholar]
- 14.Schora DM, Boehm S, Das S, Patel PA, O’Brien J, Hines C, Burdsall D, Beaumont J, Peterson K, Fausone M, Peterson LR. Impact of Detection, Education, Research And decolonization without Isolation in Long term care on MRSA (DERAIL MRSA) colonization and transmission at three long term care facilities. Am J Infect Control. 2014;42(10 Suppl):S269–S273. doi: 10.1016/j.ajic.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Ridgway JP, Peterson LR, Brown EC, Du H, Hebert C, Thomson RB, Jr, Kaul KL, Robicsek A. Clinical significance of methicillin-resistant Staphylococcus aureus colonization on hospital admission: one-year infection risk. PLoS One. 2013;8(11):e79716. doi: 10.1371/journal.pone.0079716. eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith PW, Bennett G, Bradley S, Drinka P, Lautenbach E, Marx J, Mody L, Nicolle L, Stevenson K Society for Healthcare Epidemiology of America (SHEA); Association for Professionals in Infection Control and Epidemiology (APIC) SHEA/APIC Guideline: Infection prevention and control in the long-term care facility. Am J Infect Control. 2008;36:504–535. doi: 10.1016/j.ajic.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rebmann T, Aureden K Association for Professionals in Infection Control and Epidemiology. Preventing methicillin-resistant Staphylococcus aureus transmission in long-term care facilities: an executive summary of the APIC Elimination Guide. Am J Infect Control. 2011;39:235–238. doi: 10.1016/j.ajic.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 18.McNeil SA, Mody L, Bradley SF. Methicillin-resistant Staphylococcus aureus. Management of asymptomatic colonization and outbreaks of infection in long-term care. Geriatrics. 2002;57:16–18. 21–24, 27. [PubMed] [Google Scholar]
- 19.Mody L, Kauffman CA, McNeil SA, Galecki AT, Bradley SF. Mupirocin-based decolonization of Staphylococcus aureus carriers in residents of 2 long-term care facilities: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2003;37:1467–1474. doi: 10.1086/379325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellini C, Petignat C, Masserey C, Büla C, Burnand C, Rousson C, Blanc DS, Zanetti G. Universal screening and decolonization for control of MRSA in nursing homes: A cluster randomized controlled study. Infect Contr Hosp Epi. 2015:1–8. doi: 10.1017/ice.2014.74. Published online: 12 January 2015. [DOI] [PubMed] [Google Scholar]
- 21.Peterson LR, Diekema DJ. To screen or not to screen for methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2010;48:683–689. doi: 10.1128/JCM.02516-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baldwin NS, Gilpin DF, Tunney MM, Kearney MP, Crymble L, Cardwell C, Hughes CM. Cluster randomized controlled trial of an infection control education and training intervention programme focusing on meticillin-resistant Staphylococcus aureus in nursing homes for older people. J Hosp Infect. 2010;76:36–41. doi: 10.1016/j.jhin.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Gurieva TV, Bootsma MC, Bonten MJ. Decolonization of patients and health care workers to control nosocomial spread of methicillin-resistant Staphylococcus aureus: a simulation study. BMC Infect Dis. 2012;12:302. doi: 10.1186/1471-2334-12-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chamchod F, Ruan S. Modeling the spread of methicillin-resistant Staphylococcus aureus in nursing homes for elderly. PLoS One. 2012;7(1):e29757. doi: 10.1371/journal.pone.0029757. Epub 2012 Jan 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Widner A, Nobles DL, Faulk C, Vos P, Ramsey KM. The impact of a “search and destroy” strategy for the prevention of methicillin-resistant Staphylococcus aureus infections in an inpatient rehabilitation facility. PM&R. 2014;6:121–126. doi: 10.1016/j.pmrj.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Peterson LR, Wright MO, Beaumont JL, Komutanon V, Patel PA, Schora DM, Schmitt BH, Robicsek A. Nonimpact of decolonization as an adjunctive measure to contact precautions for the control of methicillin-resistant Staphylococcus aureus transmission in acute care. Antimicrob Agents Chemother. 2016;60:99–104. doi: 10.1128/AAC.02046-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robicsek A, Beaumont JL, Thomson RB, Jr, Govindarajan G, Peterson LR. Topical therapy for methicillin-resistant Staphylococcus aureus colonization: impact on infection risk. Infect Control Hosp Epidemiol. 2009;30:623–632. doi: 10.1086/597550. [DOI] [PubMed] [Google Scholar]
- 28.Walker ES, Levy F, Shorman M, David G, Abdalla J, Sarubbi FA. A decline in mupirocin resistance in methicillin-resistant Staphylococcus aureus accompanied administrative control of prescriptions. J Clin Microbiol. 2004;42:2792–2795. doi: 10.1128/JCM.42.6.2792-2795.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts JC. Classification of epidemic community-acquired methicillin-resistant Staphylococcus aureus by anatomical site of isolation. Biomed Res Int. 2014;2014:904283. doi: 10.1155/2014/904283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Das S, Anderson CJ, Grayes A, Mendoza K, Harazin M, Schora DM, Peterson LR. Nasal carriage of Epidemic Methicillin-Resistant Staphylococcus aureus -15 (EMRSA-15) observed in three Chicago-area Long Term Care Facilities. Antimicrob Agents Chemother. 57:4551–4553. doi: 10.1128/AAC.00528-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donner A, Klar N. Pitfalls of and controversies in cluster randomization trials. Am J Public Health. 2004;94:416–422. doi: 10.2105/ajph.94.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson LR, Schora DM. MRSA Control in the 21st Century: Laboratory Involvement Affecting Disease Impact and Economic Benefit from Large Population Studies. J Clin Microbiol. 2016:54. doi: 10.1128/JCM.00698-16. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]