Abstract
OBJECTIVE:
The goal of this study was to describe family history and inheritance patterns in patients with periodic fever, aphthous stomatitis, pharyngitis, cervical adenitis (PFAPA) syndrome.
METHODS:
We performed a case–control study to compare the family histories of patients with PFAPA recruited from Vanderbilt University Medical Center and matched healthy control subjects from a pediatric primary care practice in Nashville, Tennessee, by using a structured questionnaire. Characteristics of paired case subjects, control subjects, and their family members were compared by using McNemar’s test and Wilcoxon signed-rank tests.
RESULTS:
Eighty PFAPA index case subjects and 80 control subjects were recruited. Eighteen PFAPA case subjects (23%) had ≥1 family member with PFAPA. Parents of PFAPA index case subjects were more likely to have recurrent pharyngitis (36% vs 16%; P < .001) and recurrent aphthous stomatitis (46% vs 28%; P = .002) compared with parents of control subjects. Siblings of case subjects had a higher prevalence of PFAPA (10% vs 2%; P = .04), recurrent pharyngitis (24% vs 10%; P = .03), and recurrent aphthous stomatitis (27% vs 7%; P = .003) compared with siblings of control subjects.
CONCLUSIONS:
A portion of PFAPA case subjects seems to be familial, implying an inherited genetic predisposition to the disorder and/or shared environmental exposures. First-degree relatives (parents and siblings) of patients with PFAPA have a higher prevalence of recurrent pharyngitis and aphthous stomatitis than relatives of control subjects, which suggests that these disorders represent reduced penetrance phenotypes of PFAPA. Further characterization of the genetics and inflammatory profiles of these patients and their relatives is warranted.
What’s Known on This Subject:
Although the majority of case subjects of periodic fever, aphthous stomatitis, pharyngitis, cervical adenitis (PFAPA) syndrome are considered sporadic, familial case subjects are reported. Patterns of familial clustering require additional study and may provide insights into the etiology of the disease.
What This Study Adds:
We identified many familial case subjects of PFAPA and found a high prevalence of recurrent pharyngitis and aphthous stomatitis among family members of children with PFAPA. These disorders may represent reduced penetrance phenotypes of PFAPA.
Periodic fever, aphthous stomatitis, pharyngitis, cervical adenitis (PFAPA) syndrome was first described in 1987 in the United States.1 Since then, numerous cases have been reported worldwide, and PFAPA is considered the most common periodic fever syndrome of childhood.2–4 To date, no predisposing genetic mutation has been reported in patients with PFAPA.
The objectives of the present study were as follows: (1) to determine inheritance patterns in familial case subjects of PFAPA; and (2) to compare the prevalence of PFAPA, recurrent pharyngitis, recurrent aphthous stomatitis, recurrent otitis media, and tonsillectomy in families of patients with PFAPA and families of matched healthy control subjects.
Methods
Approval for this case–control study was obtained from the Vanderbilt University Institutional Review Board. Written consent was obtained from all participants.
Criteria for PFAPA
Participants were classified as having either “definite PFAPA” or “probable PFAPA” if they met prespecified criteria. Criteria were based on the original criteria proposed in 1989 with further refinement to improve diagnostic specificity.5,6 Those subjects with “definite PFAPA” were defined as having the following features: (1) recurrent, stereotypical episodes of fever; (2) age of episode onset <10 years; (3) episodes lasting 2 to 7 days; (4) episodes occurring every 2 to 10 weeks with at least 6 episodes in 1 year; (5) aphthous stomatitis, pharyngitis, and/or cervical adenitis during episodes; (6) regular timing of episodes; (7) asymptomatic intervals between episodes; (8) normal growth and development; and (9) exclusion of cyclic neutropenia, monogenic fever syndromes, and recurrent upper respiratory tract infections based on history and/or laboratory testing. Those with “probable PFAPA” had all of the aforementioned features but lacked either regular episode timing or the presence of aphthous stomatitis, cervical adenitis, or pharyngitis during episodes (but not both). In addition, 2 pediatric infectious disease physicians reviewed the histories of patients with probable PFAPA to determine whether the individual would be diagnosed with PFAPA in the clinical setting.
Recruitment of PFAPA Index Case Subjects
Patients seen at Vanderbilt University Medical Center in Nashville, Tennessee, from 2000 to 2014 with an International Classification of Diseases, Ninth Revision, code for recurrent fever (087.9) were identified. Investigators reviewed charts of these patients to identify those diagnosed with PFAPA. These participants are referred to as PFAPA index case subjects (probands) because they are the first affected individuals identified in each family. Patients diagnosed with PFAPA and/or their parents/guardians were contacted by telephone. A structured interview regarding patient demographic characteristics, symptoms associated with PFAPA, and medical history was conducted. Index case subjects who were adopted were excluded.
Recruitment of Control Subjects
Control subjects were recruited from University Pediatrics, a pediatric primary care practice in Nashville affiliated with Vanderbilt University Medical Center. Patients were eligible for recruitment if they attended the clinic for a well-child appointment, were aged >5 years as of April 1, 2015, and were age-, ethnicity-, and race-matched to a PFAPA case subject. Age was considered matched if the control child’s age was within 3 years of a case subject’s age. Only patients aged >5 years were eligible to be control subjects because onset of PFAPA occurs by 5 years of age in most patients. Control subjects were matched 1:1 with case subjects. Eligible children and/or their parents were approached during their appointment or given flyers regarding the study and contacted by telephone at a later time. A structured interview was conducted regarding history of symptoms compatible with PFAPA. Control subjects were excluded from enrollment if they were adopted or had a diagnosed systemic autoimmune disorder, immunodeficiency, or genetic disorder.
Family History
History of recurrent pharyngitis (also referred to as “strep,” “tonsillitis,” or “sore throat”), recurrent otitis media, myringotomy tubes, tonsillectomy, recurrent aphthous stomatitis, autoimmune conditions, and kidney failure was obtained about first-degree relatives (parents and full siblings) of both index case subjects and control subjects. Half-siblings were not included. First-degree relatives were further questioned about their symptoms if they had any of the following conditions: (1) recurrent pharyngitis >5 times per year; (2) repeated pharyngitis at an unknown frequency; (3) tonsillectomy for unknown reasons; and/or (4) recurrent, stereotypical fevers. If the family member could not recall a detailed symptom history, their parent/guardian was contacted after permission of the index case subject was obtained. For PFAPA case subjects, additional history was obtained about second- and third-degree family members with recurrent fever to identify familial case subjects. Family members were classified as having definite or probable PFAPA based on the aforementioned criteria.
Statistical Analysis
Those subjects with definite or probable PFAPA were combined as “PFAPA” in the analysis. Characteristics of PFAPA case and control subjects were compared by using McNemar’s test for dichotomous variables and Wilcoxon signed-rank tests for continuous variables. We compared the prevalence of PFAPA and the following 6 oropharyngeal disorders in the families of index case subjects and control subjects as ascertained according to history details: PFAPA, recurrent pharyngitis, recurrent aphthous stomatitis, tonsillectomy, recurrent otitis media, and myringotomy tubes. Only case and control subjects with at least 1 sibling were matched to compare sibling histories. The percentage of parents and siblings with each condition was compared in matched case subjects and control subjects by using Wilcoxon signed-rank tests. Among familial case subjects of PFAPA, concordance of episode characteristics between family member pairs was assessed by using Spearman’s test.
Data were collected and managed by using REDCap (Vanderbilt University, Nashville, TN), a secure Web-based application that records data for research studies. Data were analyzed by using SPSS version 23 (IBM SPSS Statistics, IBM Corporation, Armonk, NY).7
Results
PFAPA Case Subjects
A total of 80 PFAPA index case subjects were recruited (Supplemental Fig 2A). Characteristics of PFAPA index case subjects are shown in Table 1 and Supplemental Table 3. Nine (11%) patients had genetic testing for monogenic fever syndromes, and 7 (9%) had serial complete blood cell counts sampled to test for cyclic neutropenia. Two patients had mutations in the LPIN2 gene that were not considered pathogenic (S579P and E601K). No patients with periods of neutropenia were noted. Fifty-three percent of case subjects were perceived by their parents to have fewer infectious illnesses (excluding PFAPA episodes) compared with other children.
TABLE 1.
Characteristics of PFAPA Index Case Subjects and Healthy Control Subjects
| Characteristic | PFAPA Index Case Subjects (n = 80) | Healthy Control Subjects (n = 80) |
|---|---|---|
| Average age, y | 8.9 | 9.4 |
| Sex, % | ||
| Female | 53 | 55 |
| Male | 47 | 45 |
| Ethnicity and race, % | ||
| Non-Hispanic white | 94 | 94 |
| Hispanic white | 3 | 3 |
| Non-Hispanic black | 3 | 3 |
| Non-Hispanic Asian | 1 | 1 |
| Average age of mother, y | 38.9 | 40.2 |
| Average age of father, y | 40.9 | 41.3 |
| Average sibling-years (sum of sibling ages), y | 12.0 | 10.3 |
| Maternal education, % | ||
| High school or below | 34 | 4 |
| College or above | 66 | 96 |
| Paternal education, % | ||
| High school or below | 31 | 13 |
| College or above | 69 | 87 |
| Source of historical informationa, % | ||
| Self | 1 | 5 |
| Mother | 96 | 89 |
| Father | 16 | 19 |
| Other guardian | 1 |
Percentages do not add to 100% because >1 entity could be selected.
Familial Cases of PFAPA
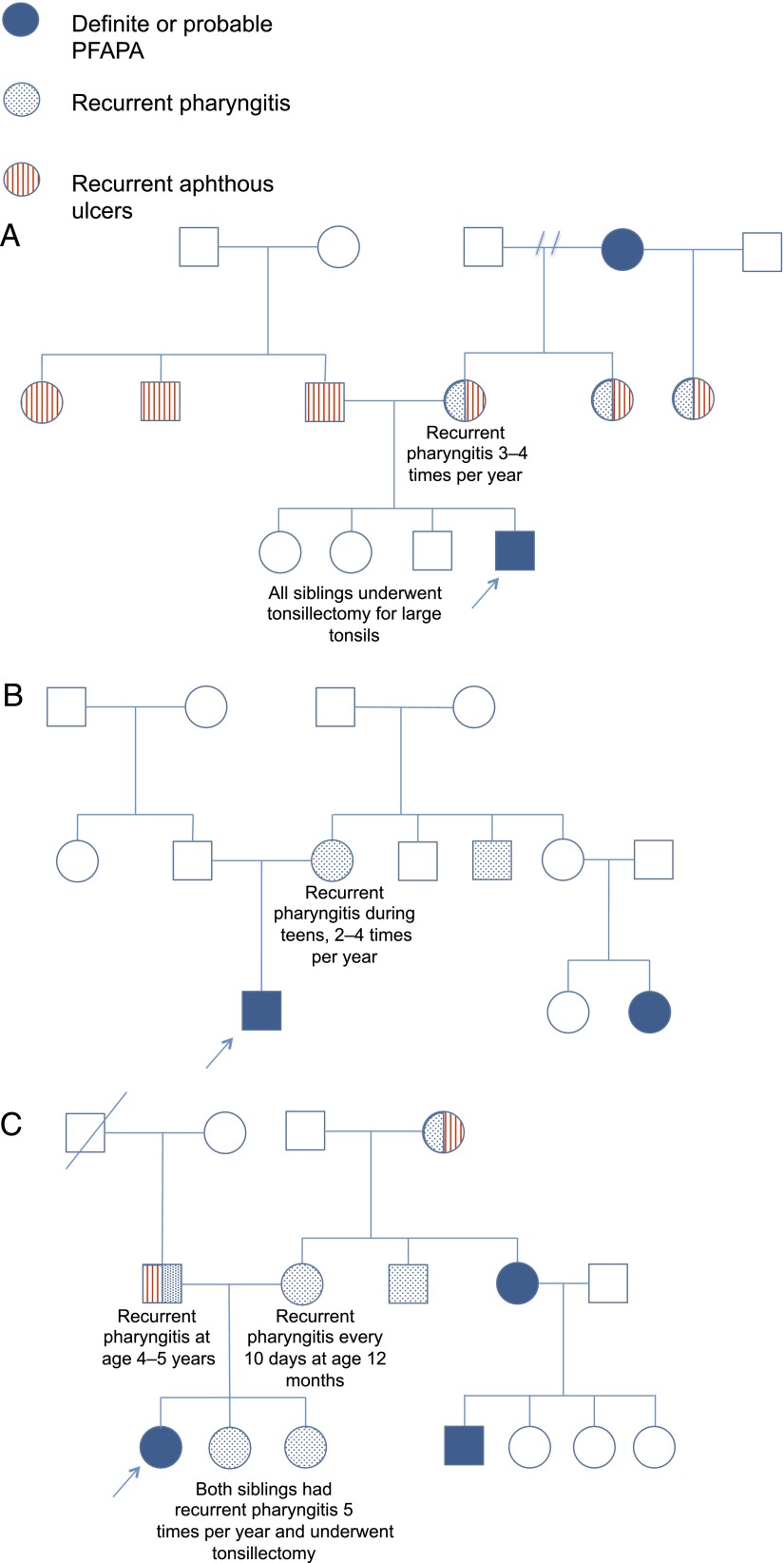
More than one-fifth of PFAPA index case subjects (18 of 80 [23%]) had at least 1 family member with symptoms consistent with PFAPA; in these 18 pedigrees, a total of 41 affected relatives were identified. Eight were siblings (including 1 monozygotic twin), 8 were parents (5 mothers, 3 fathers), 4 were aunts or uncles, 2 were cousins, and 1 was a grandparent. The male-to-female ratio of the 41 affected members was 1:0.8. Eight pedigrees showed an autosomal dominant inheritance pattern with an affected parent and child. In another 9 pedigrees, at least 1 parent had recurrent pharyngitis and/or aphthous ulcers. In 1 pedigree, no first-degree relatives had PFAPA, recurrent pharyngitis, or aphthous ulcers. In 1 family, both the mother and paternal uncle were affected; this pedigree was the only one in which individuals with PFAPA were identified on both the maternal and paternal side. Figure 1 displays pedigrees with affected second- or third-degree relative pairs (cousin-index case or grandparent-index case). In all of these pedigrees, the presumed obligate carrier of the PFAPA trait had recurrent pharyngitis with fever but at a lower or higher frequency than required in our criteria for PFAPA.
FIGURE 1.
Representative pedigrees of PFAPA cases with family history of PFAPA.The solid blue circles and squares represent individuals who met the diagnostic criteria for definite or probable PFAPA. Blue speckled circles and squares represent individuals who had recurrent pharyngitis but did not meet the diagnostic criteria for PFAPA. Circles and squares with red vertical lines represent individuals with recurrent aphthous ulcers who did not meet the diagnostic criteria for PFAPA. In these pedigrees, presumed obligate carriers of the PFAPA trait have recurrent pharyngitis but do not meet the criteria for PFAPA, suggesting that some family members have reduced penetrance phenotypes.
The concordance of episode characteristics among affected members within each family was determined. Among all 27 relationship pairs, 56% had concordance in the presence of aphthous stomatitis during fever episodes. Relative pairs had no significant correlations in the length of the interval between episodes (Spearman’s r = 0.37; P = .06) or age of onset (Spearman’s r = 0.09; P = .65). To assess for environmental factors related to PFAPA, when controlling for degree of relationship (first- versus second-degree), family members who lived in the same city as the PFAPA index case subjects were not significantly more likely to have PFAPA, recurrent pharyngitis, or recurrent aphthous stomatitis than those who lived elsewhere (adjusted odds ratio, 1.5 [95% confidence interval, 0.99–2.14]; P = .06).
Control Subjects
Control recruitment is shown in Supplemental Fig 2B. Eight control participants had recurrent stereotypical fever episodes that met the diagnostic criteria for definite or probable PFAPA and were excluded, but none had a formal diagnosis of PFAPA. The average age of episode onset of these patients was 40 months. Most (88%) reported pharyngitis, 38% reported cervical adenitis, and 38% reported aphthous stomatitis during fever episodes, whereas 3 reported stereotypic episodes of fever at regular intervals.
Comparison of Family History
Characteristics of PFAPA index case subjects and control subjects are shown in Table 1. Fifty parents of PFAPA index case subjects had a history of recurrent pharyngitis or tonsillectomy and required further history details from a grandparent. We were able to contact 74% (37 of 50) of grandparents to obtain additional details of history. Among control subjects, we were able to reach 47% (8 of 17) of grandparents from whom further history details were needed.
Table 2 compares the medical issues of PFAPA index case subjects and control subjects and of their parents and siblings. PFAPA index case subjects were more likely to have undergone tonsillectomy (43% vs 4%; P < .001) and have recurrent aphthous ulcers (71% vs 29%; P < .001) than control subjects. Parents of PFAPA index case subjects were more likely to have recurrent pharyngitis or PFAPA (36% vs 16%; P < .001), recurrent aphthous stomatitis (46% vs 28%; P = .002), or a combination of recurrent pharyngitis and aphthous stomatitis (22% vs 7%; P < .001) compared with control parents. Nearly two-thirds of PFAPA index case subjects had at least 1 parent with recurrent pharyngitis or PFAPA compared with one-third of control subjects (P < .001). PFAPA index case subjects had significantly more siblings with PFAPA (10% vs 2%; P = .04), recurrent pharyngitis or PFAPA (24% vs 10%; P = .03), recurrent aphthous stomatitis (27% vs 7%; P = .003), or a combination of recurrent pharyngitis and aphthous stomatitis (15% vs 3%; P = .01). Siblings of case subjects were also more likely to have undergone tonsillectomy (21% vs 7%; P = .01) than siblings of control subjects.
TABLE 2.
Prevalence of Medical Conditions in PFAPA Index Case Subjects Versus Control Subjects and Parents and Siblings of PFAPA Index Case Subjects Versus Control Subjects
| Characteristic | PFAPA Index Cases | Control Subjects | P |
|---|---|---|---|
| % of index case/control subjects with | n = 80 | n = 80 | |
| Recurrent aphthous stomatitis | 71% | 29% | <.001 |
| Tonsillectomy | 43% | 4% | <.001 |
| Recurrent otitis media | 30% | 29% | 1.000 |
| Myringotomy tubes | 23% | 20% | .83 |
| % parents of index case subjects/control subjects with | n = 160 parents | n = 160 parents | |
| PFAPA | 5% | 1% | .06 |
| Recurrent pharyngitis or PFAPA | 36% | 16% | <.001 |
| Recurrent pharyngitis, not PFAPA | 32% | 15% | .001 |
| Tonsillectomy | 22% | 16% | .27 |
| Recurrent aphthous ulcers | 46% | 28% | .002 |
| Recurrent pharyngitis/PFAPA and recurrent aphthous ulcers | 22% | 7% | <.001 |
| PFAPA, recurrent pharyngitis, or recurrent aphthous ulcers | 60% | 38% | <.001 |
| Recurrent otitis media | 17% | 10% | .06 |
| Myringotomy tubes | 4% | 6% | .62 |
| % of siblings of index case subjects/control subjects with | n = 96 siblings | n = 107 siblings | |
| PFAPA | 10% | 2% | .04 |
| Recurrent pharyngitis or PFAPA | 24% | 10% | .03 |
| Recurrent pharyngitis, not PFAPA | 14% | 8% | .33 |
| Tonsillectomy | 21% | 7% | .01 |
| Recurrent aphthous ulcers | 27% | 7% | .003 |
| Recurrent pharyngitis/PFAPA AND recurrent aphthous ulcers | 15% | 3% | .01 |
| PFAPA, recurrent pharyngitis, or recurrent aphthous ulcers | 38% | 12% | <.001 |
| Recurrent otitis media | 24% | 21% | .77 |
| Myringotomy tubes | 15% | 13% | .95 |
Similar numbers of PFAPA index case subjects and control subjects had at least 1 first-degree relative with autoimmune disease (9% vs 16%, respectively; P = .26) or tonsillar hypertrophy/sleep-disordered breathing (15% vs 10%; P = .50). Only 1 first-degree relative of a PFAPA case had renal failure; this father underwent nephrectomy after having repeated bouts of nephrolithiasis. No relatives reported amyloid A amyloidosis; 1 paternal grandfather of a PFAPA case subject had amyloidosis due to multiple myeloma.
Discussion
We systematically analyzed family history in a large cohort of patients with PFAPA compared with matched healthy control subjects. We found that first-degree relatives of patients with PFAPA had a higher prevalence of recurrent pharyngitis and/or recurrent aphthous stomatitis than relatives of healthy control subjects. In addition, nearly one-quarter of patients with PFAPA had at least 1 family member who met the clinical criteria for PFAPA.
Although the heritability of PFAPA is unclear, several familial case subjects have been reported in the literature.8–11 In descriptions of cohorts of patients with PFAPA, 6% to 45% of patients were reported to have at least 1 family member with “recurrent fever,” but symptom histories were not characterized in more detail nor corroborated with symptom histories from affected family members.3,5,12,13 In the present study, we systematically obtained family history and confirmed symptoms with parents of affected individuals when possible. In 1 previous report, the analysis of 14 pedigrees suggested autosomal dominant inheritance and penetrance of 50%.14 Efforts to identify a causative single gene through whole exome sequencing and genome-wide linkage analysis were unsuccessful in this group, leading the investigators to believe that the disorder is oligogenic.
Because parents and siblings of patients with PFAPA had a higher prevalence of recurrent pharyngitis and aphthous stomatitis than those of healthy control subjects, these disease entities may represent reduced penetrance phenotypes of PFAPA. Moreover, analysis of pedigrees with affected second- or third-degree relatives revealed seemingly affected first-degree relatives with recurrent pharyngitis and/or aphthous ulcers (who did not meet criteria for PFAPA), providing further evidence that the spectrum of PFAPA may be broader than initially thought. Although the pathogenesis of PFAPA is unknown, it is considered an autoinflammatory syndrome due to its episodic nature and periodicity, activation of innate immune pathways during flares, and rapid improvement with corticosteroid administration.15 If recurrent pharyngitis and aphthous stomatitis share a common pathogenesis with PFAPA in these families, these disease entities may also be autoinflammatory in some individuals. This finding also suggests that some patients with recurrent stereotypical episodes of pharyngitis at frequencies lower than expected for PFAPA may have autoinflammatory pharyngitis and not repeated infectious pharyngitis as typically diagnosed.
Because tonsillectomy is curative in many patients with PFAPA, the origin of the inflammatory stimulus in PFAPA may be in the tonsils, which are mucosal lymphoid organs.16–18 A lymphocytic infiltrate is observed early in the histopathologic progression of recurrent aphthous ulcers as well.19 Therefore, both PFAPA and recurrent aphthous stomatitis may result from dysregulated inflammation in mucosal lymphoid tissue in the oropharynx. Interestingly, >50% of patients with PFAPA reported having fewer infections than other children, raising the possibility that PFAPA confers immunologic protection against other infectious ailments. These observations underscore the need to better understand the biological mechanism of PFAPA.
Although some consider PFAPA a sporadic illness, we identified many familial case subjects of PFAPA, which highlights the importance of asking not only about family history of “recurrent fever” but also about recurrent “sore throat,” “strep throat,” and “tonsillitis” to obtain a complete family history. Symptoms of PFAPA can easily be construed as recurrent pharyngitis by patients and physicians if pharyngitis is considered the predominant symptom rather than fever. This scenario is compounded by variations in the nomenclature used by patients and physicians. Because almost two-thirds of children with PFAPA had a parent with recurrent pharyngitis or PFAPA, a family history of these disorders may support a diagnosis of PFAPA and should be systematically sought in patients evaluated for PFAPA.
Most of the familial case subjects identified in our cohort seemed to exhibit autosomal dominant inheritance. However, if recurrent pharyngitis and aphthous ulcers are considered reduced penetrance phenotypes, in many pedigrees, both maternal and paternal sides seemed to have affected members. Therefore, other inheritance patterns are possible, including additive dominant, pseudo-dominant, and polygenic.
Familial aggregation does not prove genetic origin of disease; family members may have common environmental exposures that lead to disease. Concordance of symptom characteristics was poor among family members, suggesting that disease characteristics are not dictated by genetics alone and may be influenced by environmental factors as well. However, living in the same city as a PFAPA case did not significantly increase the odds of a relative having PFAPA, recurrent pharyngitis, or aphthous ulcers, making shared environmental stimuli less plausible. Whether the odds ratio would become statistically significant with a larger sample size remains to be determined. Genomic analysis of patients with PFAPA is necessary to understand the interplay of genetics and environment.
Nearly 10% of patients recruited to be in the control group had a clinical history meeting the criteria for definite or probable PFAPA. Because the classification of PFAPA in our study was made solely according to history, we cannot confirm the diagnosis with certainty. However, the high frequency of this phenotype in a primary care practice suggests that PFAPA may be underdiagnosed and a fairly common syndrome in this population.
The major limitation of our study is recall bias. Relatives of patients with PFAPA may be more aware of related diseases in their family members than control subjects. The magnitude of recall bias is likely reduced in comparisons of sibling histories because siblings are younger and parents are typically more aware of their children’s medical problems than that of their own childhood. Conversely, other respondents were unable to recall sufficient information to fully support a diagnosis of PFAPA, and thus PFAPA may be underdiagnosed in this cohort as well. To minimize recall bias, we administered the same structured survey about family history to case subjects and control subjects and obtained history from grandparents about parents whenever possible. The second major limitation is the reliance on history alone to diagnose PFAPA. In the clinical setting, we examine patients during episodes, carefully scrutinize episode dates, and may obtain laboratory testing to rule out upper respiratory tract infections and monogenic fever syndromes. This type of detailed information was often not available to us, likely reducing the specificity of the diagnosis; however, we did review patient charts for this information when available. Third, we did not require regular episode timing to diagnose “probable PFAPA” due to frequent lack of recall of this finding. We also did not require the presence of associated features to diagnose “probable PFAPA” if all other diagnostic features, including regularity, were met. It is arguable that these variants may not truly represent PFAPA. However, such patients have been reported in other studies and respond promptly to steroids and tonsillectomy, treatments used in PFAPA.4,5,20,21 Fourth, genetic testing was not obtained in all PFAPA patients to rule out monogenic periodic fever syndromes, but most participants were followed up for several years and did not develop symptoms concerning for monogenic syndromes.
Conclusions
Our results indicate that family history of recurrent pharyngitis, recurrent aphthous ulcers, and PFAPA can be used to support the diagnosis of PFAPA. These common disorders may have an autoinflammatory pathogenesis in some patients. Further study of the biological mechanism of PFAPA is necessary to better understand the relationships of these disorders.
Acknowledgments
We thank Brandi Anderson, Dr Rachel Mace, and the other nurses and physicians at University Pediatrics for allowing us to recruit control subjects at their practice and informing their patients about the study. We are also grateful to Dr Buddy Creech and Dr Isaac Thomsen for their critical review of study design and to Dr Natalia Jimenez-Truque for her advice on statistical analysis.
Glossary
- PFAPA
periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis syndrome
Footnotes
Dr Manthiram conceptualized and designed the study, collected data, performed the statistical analysis, and drafted the initial manuscript; Ms Nesbitt helped design survey instruments, coordinated data collection of control participants, and critically reviewed the manuscript; Dr Morgan designed key elements of the study and critically reviewed the manuscript; Dr Edwards conceptualized and designed key elements of the study, contributed to writing the manuscript, and critically reviewed the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the Childhood Infection Research Program (1T32AI095202-01) and Conducting Child Health Care Research in Vulnerable Populations (1T32HD060554-05) National Institutes of Health T32 training grants from the Vanderbilt University School of Medicine. Support for REDCap provided by a Vanderbilt Institute for Clinical and Translational Research grant (UL1 TR000445 from the National Center for Advancing Translational Sciences/National Institutes of Health) from the National Institutes of Health (NIH). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Marshall GS, Edwards KM, Butler J, Lawton AR. Syndrome of periodic fever, pharyngitis, and aphthous stomatitis. J Pediatr. 1987;110(1):43–46 [DOI] [PubMed] [Google Scholar]
- 2.Padeh S, Brezniak N, Zemer D, et al. Periodic fever, aphthous stomatitis, pharyngitis, and adenopathy syndrome: clinical characteristics and outcome. J Pediatr. 1999;135(1):98–101 [DOI] [PubMed] [Google Scholar]
- 3.Førsvoll J, Kristoffersen EK, Øymar K. Incidence, clinical characteristics and outcome in Norwegian children with periodic fever, aphthous stomatitis, pharyngitis and cervical adenitis syndrome; a population-based study. Acta Paediatr. 2013;102(2):187–192 [DOI] [PubMed] [Google Scholar]
- 4.Hofer M, Pillet P, Cochard MM, et al. International periodic fever, aphthous stomatitis, pharyngitis, cervical adenitis syndrome cohort: description of distinct phenotypes in 301 patients. Rheumatology (Oxford). 2014;53(6):1125–1129 [DOI] [PubMed] [Google Scholar]
- 5.Wurster VM, Carlucci JG, Feder HM Jr, Edwards KM. Long-term follow-up of children with periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis syndrome. J Pediatr. 2011;159(6):958–964 [DOI] [PubMed] [Google Scholar]
- 6.Marshall GS, Edwards KM, Lawton AR. PFAPA syndrome. Pediatr Infect Dis J. 1989;8(9):658–659 [DOI] [PubMed] [Google Scholar]
- 7.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antón-Martín P, Ortiz Movilla R, Guillén Martín S, et al. PFAPA syndrome in siblings. Is there a genetic background? Eur J Pediatr. 2011;170(12):1563–1568 [DOI] [PubMed] [Google Scholar]
- 9.Sampaio IC, Rodrigo MJ, Monteiro Marques JG. Two siblings with periodic fever, aphthous stomatitis, pharyngitis, adenitis (PFAPA) syndrome. Pediatr Infect Dis J. 2009;28(3):254–255 [DOI] [PubMed] [Google Scholar]
- 10.Adachi M, Watanabe A, Nishiyama A, et al. Familial cases of periodic fever with aphthous stomatitis, pharyngitis, and cervical adenitis syndrome. J Pediatr. 2011;158(1):155–159 [DOI] [PubMed] [Google Scholar]
- 11.Valenzuela PM, Majerson D, Tapia JL, Talesnik E. Syndrome of periodic fever, aphthous stomatitis, pharyngitis, and adenitis (PFAPA) in siblings. Clin Rheumatol. 2009;28(10):1235–1237 [DOI] [PubMed] [Google Scholar]
- 12.Cochard M, Clet J, Le L, et al. PFAPA syndrome is not a sporadic disease. Rheumatology (Oxford). 2010;49(10):1984–1987 [DOI] [PubMed] [Google Scholar]
- 13.Thomas KT, Feder HM Jr, Lawton AR, Edwards KM. Periodic fever syndrome in children. J Pediatr. 1999;135(1):15–21 [DOI] [PubMed] [Google Scholar]
- 14.Di Gioia SA, Bedoni N, von Scheven-Gête A, et al. Analysis of the genetic basis of periodic fever with aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome. Sci Rep. 2015;5:10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stojanov S, Lapidus S, Chitkara P, et al. Periodic fever, aphthous stomatitis, pharyngitis, and adenitis (PFAPA) is a disorder of innate immunity and Th1 activation responsive to IL-1 blockade. Proc Natl Acad Sci USA. 2011;108(17):7148–7153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Licameli G, Lawton M, Kenna M, Dedeoglu F. Long-term surgical outcomes of adenotonsillectomy for PFAPA syndrome. Arch Otolaryngol Head Neck Surg. 2012;138(10):902–906 [DOI] [PubMed] [Google Scholar]
- 17.Dytrych P, Krol P, Kotrova M, et al. Polyclonal, newly derived T cells with low expression of inhibitory molecule PD-1 in tonsils define the phenotype of lymphocytes in children with periodic fever, aphtous stomatitis, pharyngitis and adenitis (PFAPA) syndrome [published correction appears in Mol Immunol. 2015;66(2):428]. Mol Immunol. 2015;65(1):139–147 [DOI] [PubMed] [Google Scholar]
- 18.Garavello W, Pignataro L, Gaini L, Torretta S, Somigliana E, Gaini R. Tonsillectomy in children with periodic fever with aphthous stomatitis, pharyngitis, and adenitis syndrome. J Pediatr. 2011;159(1):138–142 [DOI] [PubMed] [Google Scholar]
- 19.Graykowski EA, Barile MF, Lee WB, Stanley HR Jr. Recurrent aphthous stomatitis. Clinical, therapeutic, histopathologic, and hypersensitivity aspects. JAMA. 1966;196(7):637–644 [DOI] [PubMed] [Google Scholar]
- 20.Król P, Böhm M, Sula V, et al. PFAPA syndrome: clinical characteristics and treatment outcomes in a large single-centre cohort. Clin Exp Rheumatol. 2013;31(6):980–987 [PubMed] [Google Scholar]
- 21.Gattorno M, Caorsi R, Meini A, et al. Differentiating PFAPA syndrome from monogenic periodic fevers. Pediatrics. 2009;124(4). Available at: www.pediatrics.org/cgi/content/full/124/4/e721 [DOI] [PubMed] [Google Scholar]