Abstract
Despite advances in the detection, pathological diagnosis and therapeutics of lung cancer, many patients still develop advanced, incurable and progressively fatal disease. As physicians, the duties to cure sometimes, relieve often and comfort always should be a constant reminder to us of the needs that must be met when caring for a patient with lung cancer. Four key areas of end-of-life care in advanced lung cancer begin with first recognizing ‘when a patient is approaching the end of life’. The clinician should be able to recognize when the focus of care needs to shift from an aggressive life-sustaining approach to an approach that helps prepare and support a patient and family members through a period of progressive, inevitable decline. Once the needs are recognized, the second key area is appropriate communication, where the clinician should assist patients and family members in understanding where they are in the disease trajectory and what to expect. This involves developing rapport, breaking bad news, managing expectations and navigating care plans. Subsequently, the third key area is symptom management that focuses on the goals to first and foremost provide comfort and dignity. Symptoms that are common towards the end of life in lung cancer include pain, dyspnoea, delirium and respiratory secretions. Such symptoms need to be anticipated and addressed promptly with appropriate medications and explanations to the patient and family. Lastly, in order for physicians to provide quality end-of-life care, it is necessary to understand the ethical principles applied to end-of-life-care interventions. Misconceptions about euthanasia versus withholding or withdrawing life-sustaining treatments may lead to physician distress and inappropriate decision making.
Keywords: hospice care, lung neoplasm, medical ethics, palliative care, physician–patient relations, terminal care
Introduction
Lung cancer is the most common cancer as well as the most frequent cause of cancer death in the world today [World Health Organization, 2013]. Despite advances in the detection, pathological diagnosis and therapeutics of lung cancer, many patients still develop advanced, incurable and progressively fatal disease. For a majority of patients with lung cancer, the diagnosis is often made at stages III and IV where survival is still very poor, with an overall 5-year survival rate of 9.5% to 16.8%. Data from the UK showed that 67.6% of all lung cancer patients had stage III and IV disease at initial diagnosis. In the USA, SEER data from 2004 to 2010 recorded 79% of lung cancer patients were in stage III and IV at diagnosis [Cancer Research UK, 2013; National Cancer Institute SEER program, 2014]. In China, a population-based study of non-small cell lung cancer in Shanghai, recorded 76.4% of patients diagnosed from 2011 to 2013 were at stage III or IV [Fan et al. 2015]. Although there have been tremendous advances in the treatment of lung cancer, particularly in non-small cell lung cancer with the progress in tyrosine-kinase inhibitors, this has led to only modest improvements in overall survival. Hence, it is still fair to state that at this present time, the vast majority of lung cancer patients will eventually die from their illness within 5 years of diagnosis.
Research has also shown that over the years, there has been an increasing trend amongst oncologists to continue aggressive cancer care in patients facing the last month of life and with the rapid advances in cancer therapeutics, this trend is likely to continue [Earle et al. 2004]. It is therefore essential that any clinician who manages patients with lung cancer should be familiar and sensitive to the needs of patients facing the end of life as this comprises a large proportion of lung cancer care.
When is a patient at the ‘end of life’ and what is ‘end-of-life care’?
Clinicians, researchers and healthcare policy makers often try to compartmentalize care into neat definitions with clear-cut time frames so as to identify needs and define roles. However, with end-of-life care (EOLC), is there a time frame to define when this should occur? At present, there is no consensus on the definition or time frame for the ‘end of life’. Generally, many consider end of life to be the last few days to 1–2 weeks of life. In the UK, the General Medical Council (GMC) guidance refers to patients approaching the end of life when they are likely to die within the next 12 months [GMC, 2010; Izumi et al. 2012]. In the USA, Medicare defines the need for hospice care at the end of life as the last 6 months of life [National Hospice and Palliative Care Organization, 2014]. Many of the definitions using a time frame are based on the survival duration of a patient; however, survival is something that can never be predicted with great accuracy and is really a retrospective outcome. Hence, if a clinician were to use such definitions to identify patients requiring EOLC they would need a crystal ball or a clairvoyant in order to ensure the right patient received appropriate care. Therefore, when we focus on a rigid definition, using the model of dichotomous intent (Figure 1), many patients requiring EOLC will be missed and a lot of physical, psychosocial and spiritual needs unmet.
Figure 1.
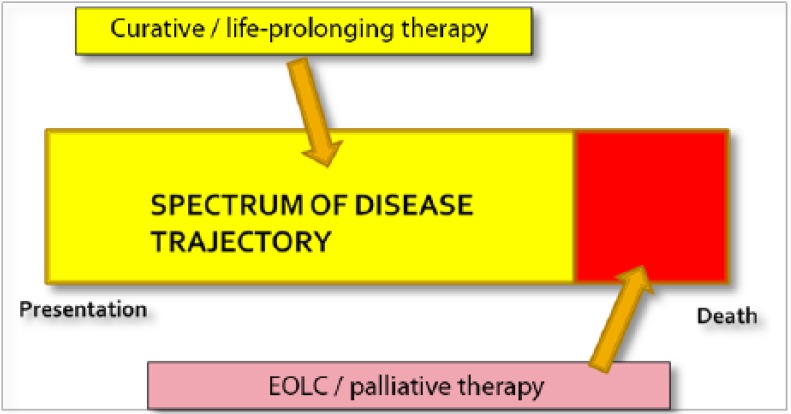
Model of dichotomous intent.
The definition of EOLC should therefore be based on the needs of a patient rather than a time frame. This can be seen in the model of integrated curative–palliative intent (Figure 2). Hence, regardless of the time frame and the active interventions that may be ongoing, patients with problems and needs associated with this life-threatening condition will be treated appropriately.
Figure 2.
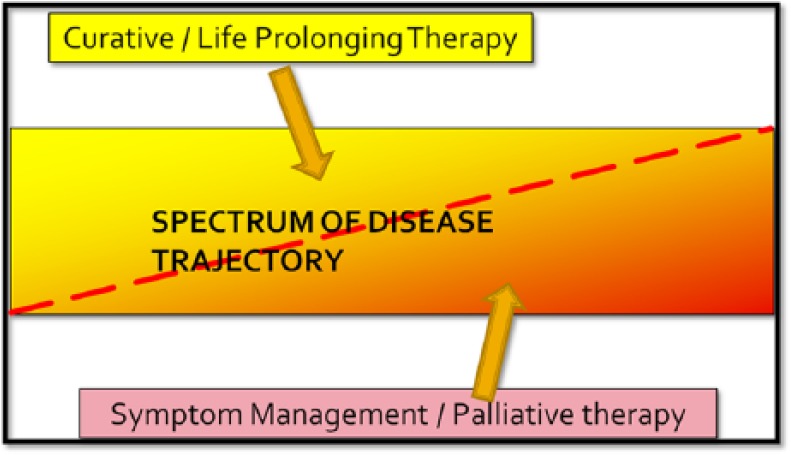
Integrated curative–palliative model.
Qualitative research has shown that amongst the priorities of patients and families facing problems of serious life-limiting conditions are [Steinhauser et al. 2000]:
Pain and symptom management: requires attention to the physical distress caused by the illness at the end of life;
Clear decision making: requires good communication skills and information to patient and family;
Affirmation of the whole person: requires attitudes, behaviours and compassion that promote dignity in patients;
Preparation for death: requires honest communication and support;
Completion: concerns fulfilling achievable goals at the end of life.
Clinicians should be aware that important needs of a patient at the end of life are not merely confined to physical needs but must also include spiritual, existential and psychosocial needs as well.
Hence, a definition of EOLC should be described in terms of fulfilling these needs of patients and families at the end of life. At present, there is no clearly accepted definition for EOLC; however, the National Council for Palliative Care in the UK developed a working definition in 2006, describing it based on the role it plays [National Council for Palliative Care, 2011; Department of Health, 2008]:
Care that helps all those with advanced, progressive, incurable illness to live as well as possible until they die;
It enables the supportive and palliative care needs of both patient and family to be identified and met throughout the last phase of life and into bereavement;
It includes management of pain and other symptoms and provision of psychological, social, spiritual and practical support.
The disease trajectory of a patient with advanced cancer has been described as an initial slow progression of the disease where patients will have minimal symptoms and maintain a fairly good overall performance status until they gradually reach a point where they begin to develop more symptoms that then leads to a more rapid decline that tends to spiral downwards till the end of life [Murray et al. 2005]. Therefore, looking at the disease trajectory of advanced lung cancer patients, the period in which EOLC is most needed will be in that period where a patient’s performance status declines rapidly as they approach an incurable and rapidly progressive phase of the illness (Figure 3).
Figure 3.
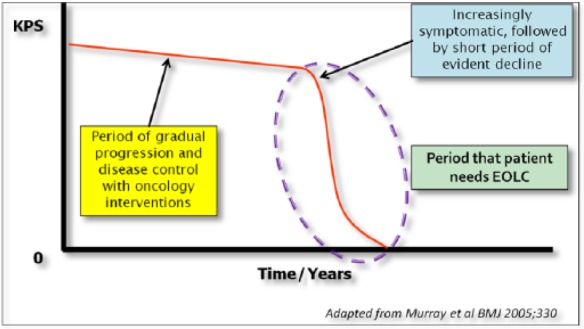
Disease trajectory of advanced lung cancer.
Karnofsky Performance Scale (KPS).
Having discussed when patients are in need of EOLC, as the scope of EOLC can be very broad, this paper will focus mainly on the practical issues of EOLC in patients approaching the last few days of life.
Caring for patients in the last days of life
Many clinicians find themselves at a loss as to what to do when patients approach this phase of their illness particularly if they have not been trained or exposed to palliative care. It is important, however, to be able to manage this phase of life well because a great deal of suffering can occur at this time, not only for the patient, but also for those who love and care about the person who is dying. Dame Cicely Saunders, who was the founder of the modern hospice movement said, ‘How people die lives on in the memories of those who live on.’ Hence, all clinicians should feel a sense of obligation to their patients to ensure that beyond all the interventions that have been exhausted, there will always be hope in a peaceful and dignified death.
In general, there are four key areas of concern when managing patients who are approaching the last days of life. If clinicians are able to put these four key areas into perspective at the time when patients are approaching their last days, this may help as a practical guide to better care at the end of life.
The four key areas of concern include:
Recognizing the dying phase;
Communication with the patient, family and loved ones;
Provision of symptom management;
Ethical decision making.
The first step is to be able to recognize that the patient is in the dying phase based on clinical evidence as well as understanding the disease trajectory and prognosis. Once one recognizes that a patient is in the dying phase, the next step is to communicate our understanding of the situation with family and loved ones. This is a vital step and it must be remembered that although we might know a patient is obviously dying, the family and loved ones may be oblivious of this. Talking about expectations and setting goals of care, focusing on comfort and dignity are most important to help prepare the setting for subsequent EOLC. So, after communicating the essential issues with the family and loved ones, it is then necessary for the clinician to be able to ‘walk the talk’ and to really manage and care for the patient to optimize comfort and dignity. Step three, therefore, is to provide adequate symptom management and also deal with important psychosocial and spiritual issues that need to be addressed in order to optimize the situation and hopefully facilitate a good death. Finally, the fourth step is to understand the ethical basis of decision making and management at the end of life. This is of utmost importance, as clinicians need to be comfortable with managing patients in this critical period of life. Without a clear understanding of the ethical principles and policies that guide management plans at the end of life, such care may lead to conflict and dilemmas among healthcare professionals. We will now look at each area in detail.
Key area one: recognizing the dying phase
Recognizing the dying phase is the first and very important step in providing good EOLC. Although this may seem like a very basic and intuitive skill, it cannot be assumed as apparent in all healthcare professionals and many a time, the reason for poor management at the end of life is the result of failure to recognize the dying phase. When clinicians are able to recognize the dying phase in their patients, it allows them to start important discussions with patients and family regarding death and dying. Among these discussions would include issues on end-of-life choices and preferences of the patient and family in order to avoid futile interventions. Also, discussions to prepare family members to anticipate death and understand the dying process are important to allow pre-emptive bereavement to take place and reduce the shock and burden of death. Lastly, discussion amongst the medical team members should take place to reprioritise goals of care and shift the focus of care to ensuring comfort and dignity, rather than prolonging suffering. The consequences of failing to recognize the dying phase as stated by Ellershaw and Ward would include the following [Ellershaw and Ward, 2003]:
The patient loses trust in the doctor as his or her condition is deteriorating without acknowledgement that this is happening;
The family is unaware that death is imminent;
The patient dies with uncontrolled symptoms, leading to a distressing and undignified death;
At death, cardiopulmonary resuscitation may be inappropriately initiated;
Cultural and spiritual needs are not met;
The patient and family are dissatisfied.
There are a numerous prognostic tools available to help clinicians recognise the disease trajectory of patients with chronic life-limiting illnesses, such as the Palliative Prognostic Score, which uses parameters such as performance status, clinical symptoms and blood parameters to estimate the likelihood of a patient surviving more than 1 month [Glare and Sinclair, 2008]. Such tools are useful, particularly in assisting clinicians plan management; and may alert one to have important discussions with patients regarding cessation of aggressive treatments. However, in the last few days of life, the dying phase can be recognized using several clinical signs and symptoms, which, together, have been recognized as the ‘syndrome of imminent dying’ [Ferris et al. 2003; Weissman, 2005]. These include:
Extremely lethargic: progressively weak with reducing mobility till bed bound;
Reducing cognition and consciousness: from drowsy and confused to delirious, and finally, comatose;
Poor oral intake: has increasing difficulty tolerating oral medications and food till only able to take sips of fluid with frequent aspiration;
Changes in respiration: this includes patterns of Cheyne–Stokes breathing, frequent apnoea and finally mandibular breathing;
Terminal secretions: rattling, gurgling sound on breathing due to secretions vibrating in airways as air passes during breathing;
Decreasing vital signs: reducing blood pressure, oxygen saturation, peripheral cyanosis, feeble pulses and mottling.
Morita and colleagues, who studied the duration of survival upon the onset of these signs of imminent dying, reported the median survival of patients with terminal secretions was 57 hours, mandilbular breathing was 7.6 hours, cyanosed extremities was 5.1 hours and pulseless radial artery was 2.6 hours [Morita et al. 1998].
Key area two: communicating with the patient and family
Research has shown that physicians often feel helpless and uncomfortable when communicating prognostic information to patients and families. Providing physical treatment is seen as being easier than having to confront distraught patients and families with end-of-life discussions [SUPPORT, 1995]. In two prospective cohort studies [Wright et al. 2008; Mack et al. 2012], it was found that less than one-third of oncologists have end-of-life discussions with their patients. The lack of end-of-life discussions was associated with an increase in use of aggressive care, such as chemotherapy and intensive care unit care in the last 1 month of life. This ultimately leads to poorer patient satisfaction, psychological morbidity and poorer quality of life.
Conversely, when clinicians engage in honest end-of-life discussions with their patients, it leads to better decision making, lower anxiety and depression levels, as well as better quality of life for both patients and families [Steinhauser et al. 2001; Fallowfield et al. 2002; Wright et al. 2008]. Hence, communication is a vital part of the provision of good EOLC. Once the clinician has recognized the dying phase in a patient, it is essential that this knowledge be communicated appropriately and compassionately to the family so that they too will recognise the situation and be prepared.
The general principles and steps in communicating with patients and families when discussing end-of-life issues can be remembered by the acronym, PREPARED, as developed by Clayton and colleagues [Clayton et al. 2007]:
P Prepare and understand all the updated information on the patient’s condition and status
R Rapport: relate to person; show empathy and compassion
E Expectations: elicit patient and caregiver expectations and preference for information
P Provide information in simple, clear language
A Acknowledge emotions and concerns
R Realistic hope
E Encourage questions
D Document discussion in medical records
Of these steps, the most important of all would be to develop good rapport with the family. Good rapport can be regarded as the ‘passport’ of communication of which, without it, one should not attempt to venture into discussions of very serious nature. This is simply because, talking to a family about anticipating the death of a loved one is a very life-changing and serious matter that requires the family to trust and believe what it is they are hearing. Without establishing good rapport prior to this, the family may have doubts as to the credibility and accuracy of the information provided [Lim, 2012].
In order to develop good rapport, clinicians need to be able to relate to the person they are communicating with; firstly, by starting with a good introduction, and then explaining how they are involved with the care of the patient. The clinician should then make it apparent that he or she is knowledgeable of the case and is up to date with the latest issues. This is where preparation before the discussion is important. One of the easiest ways to lose rapport with the family is to display ignorance of the case at hand by mentioning information that is inaccurate.
The next important skill to help develop the family rapport is the ability to listen. Listening is a skill that requires not only hearing what the person is saying, but also being attentive to their body language, tone of voice and truly understanding what the other person is communicating. Clinicians need to listen in order to understand what the perceptions of the family are regarding the patient’s condition. Once their perceptions are understood, one can then communicate in a way that addresses their needs and shows empathy that will further enhance the relationship. In trying to understand families’ perceptions, one should explore the following areas:
Insight: What does the family understand at present, and what has been explained so far by other doctors?
Concerns: What are their fears, and what issues require clarification?
Expectations: What do they hope for, and what are their goals of care? What do they assume will be done for the patient?
The simple acronym of ICE may be used to remember this.
Key area three: providing symptom management in the last days
Among the symptoms encountered in the last days of life of patients with advanced lung cancer, common priority symptoms include the following [Ellershaw et al. 2001; Stone et al. 2001; Kvale et al. 2007]:
Pain
Dyspnoea
Cough
Restlessness and delirium
Terminal secretions
Clinicians should be vigilant to recognise these symptoms and then be knowledgeable and skilful in managing these symptoms which will contribute to good EOLC.
Table 1.
Examples of the end-of-life communication process.
| Communicating with family members | Focus |
|---|---|
| Mrs A: ‘Doctor, can you please tell me how my husband is
doing?’ Doctor: ‘Yes, of course, I know you must be very worried about him.’ |
Showing empathy goes a long way in developing rapport |
| Doctor: ‘Over the past few days, have any of the other
doctors spoken to you about his condition? What do you
recall of the things the other doctors have
explained?’ Mrs A: ‘They just said he is not doing well, but never mentioned anything about his chances of getting better.’ |
Checking insight helps you get on the same wavelength as the family |
| Doctor: ‘Looking at how your husband has been these past few
days, how do you think things are going?’ Mrs A: ‘He just seems to be getting weaker and weaker. He’s not getting better, is he?’ Doctor: ‘I’m afraid that is correct, he is not getting better and the cancer is making him weaker by the day. I know this must be hard for you Mrs A, but I’m afraid your husband is dying.’ |
Exploring expectations helps you assess how deep into the
subject you need to go Sometimes it may merely require you to confirm what is already suspected |
| Doctor: ‘Tell me, what worries you the most?’ | Explore concerns and never assume what a person wants to know |
| Mrs A: ‘Can’t you do anything to keep him alive? You have to
do something doctor, PLEASE!’ Doctor: ‘I know you must love him very much and the thought of losing him must be so painful.’ |
Respond emphatically to emotional statements to show you acknowledge the feelings expressed |
| Doctor: ‘Did your husband ever talk about what he would or would not want for himself if he were to become very sick like he is right now?’ | At a later point, once good rapport has been established, and insight, expectations and concerns explored, consider opening up discussions on advanced care plans, such as resuscitation and preferred place of death, etc. |
Pain
Pain is a common symptom in patients with advanced cancer. Epidemiological studies show that about 70% of patients with advanced cancer suffer from pain [Teunissen et al. 2007]. The management of pain should therefore be provided throughout the course of the cancer patient’s illness and even during the last days. However, during the last days of a patient, the ability to speak and swallow oral medications often decreases, and it is a common mistake amongst clinicians to assume that in this phase, patients no longer require pain-relieving medications. Functional positron emission tomography scans of the brain have shown that even patients with a minimally conscious state can perceive pain [Boly et al. 2008]. Hence, the recommended practice is that all pain medications should be continued at the same dose, even when the patient enters a less conscious terminal state.
Such pain-relieving medication may easily be continued by converting medications such as oral morphine to subcutaneous morphine or transdermal fentanyl. The conversion of subcutaneous morphine to oral morphine is achieved using a factor of between 2 and 3, while a 25 mcg/h fentanyl patch delivers a dose equivalent to about 75 mg of oral morphine in a day. Breakthrough doses of subcutaneous morphine (which will be a dose of between 1/12 to 1/6 of the 24-hour morphine-equivalent dose) should be made available in case of additional pain. This may be identified by observing for nonverbal pain-related behaviour, such as facial grimacing and groaning, especially on movement [Ministry of Health, 2010].
Dyspnoea
Dyspnoea is a very common symptom in advanced lung cancer, and towards the end of life, this symptom generally persists and often escalates [Kvale et al. 2007; Iyer et al. 2014]. Next to pain, it is a symptom that clearly contributes to poor quality of life and a lot of distress in the last days. Apart from lung parenchymal damage and airway obstruction from extensive lung cancer, there are numerous other causes of dyspnoea in patients with advanced lung cancer, including superior vena cava obstruction (SVCO), pleural effusion, superimposed chest infection, concurrent anemia, cardiac failure, metabolic acidosis, pulmonary embolism and generalized muscle weakness.
In general, the initial approach to managing dyspnoea would be to correct what is correctable such as providing oxygen, treating infections, draining pleural effusions or using corticosteroids for patients with SVCO [Twycross et al. 2009b]. However, for patients who are in the last days of life, it may not always be feasible or possible to correct these causes, hence, providing adequate symptomatic relief is the mainstay of management.
Opioids have significant benefit in the symptomatic management of dyspnoea in patients with advanced cancer. In patients who are opioid naïve, it is advisable to start with low doses of 2–3 mg oral morphine, or 1 mg subcutaneous morphine 6–8 hourly and when necessary. If the patient is tolerating the morphine with no adverse effects, the dose may then be gradually titrated to 4 hourly and further increased, according to the need. For patients who are already on an opioid for pain, it would be appropriate to increase the overall dose of the opioid by 20–30% and provide breakthrough doses of 1/12–1/6 of the total 24-hour dose as necessary, if breathlessness is still severe despite the increased dose [Twycross et al. 2009b; Abernethy et al. 2003; Jennings et al. 2002].
Although evidence is still lacking in patients who are very distressed and anxious due to dyspnoea, adding a benzodiazepine can be beneficial to reduce the distress and anxiety that often compound the perception of dyspnoea. If patients display severe anxiety with episodes of panic, sublingual lorazepam 0.5–1 mg can be useful in reducing the anxiety. Subcutaneous midazolam 2.5–5 mg may also be used as an alternative. For patients who are constantly distressed, a continuous subcutaneous infusion of midazolam 10–20 mg over 24 hours is commonly added to opioid therapy [Currow et al. 2013; Simon et al. 2010].
Cough
Cough is a common symptom in advanced lung cancer and in the months and weeks before a patient reaches the terminal phase, it can be a source of great discomfort and distress. The approach to this symptom generally depends on the cause of the cough and whether it is productive of sputum or dry.
For productive cough, which is associated with purulent sputum, antibiotics may be useful if there is an infective component causing this symptom. To promote mucus clearance, a simple measure is to use nebulized saline. Mucolytic agents such as bromhexine, N-acetylcysteine and guaifenesin may also be useful. If there is associated bronchoconstriction, consider nebulized bronchodilators. If there is significant haemoptysis, antifibrinolytic agents such as tranexamic acid may be used. Palliative radiotherapy may also be considered if haemoptysis is severe.
For dry cough, which is troublesome, and in patients who are in the last days of life and too weak to cough, the mainstay of symptom management would be to suppress the cough in order to allow the patient to rest. Cough suppressants include linctus codeine and low-dose strong opioids such as morphine, hydrocodone, oxycodone or methadone. In severe situations, nebulized lignocaine may be considered; however, caution should be taken as it may cause bronchospasm. Sedation may also be considered at this stage to alleviate severe distress if other measures have failed [Molassiotis et al. 2010; Twycross et al. 2009b].
Delirium and restlessness
Delirium and restlessness are common symptoms at the end of life, and it can be an extremely troublesome and exhausting problem for both the family and hospital staff. As cancer progresses and the patient’s condition deteriorates, an early symptom of delirium may be the reversal of the sleep–wake cycle, whereby family will complain that the patient tends not to sleep well at night but is sleeping mostly in the day. As the condition worsens, the patient may develop hypoactive or hyperactive symptoms. This may include symptoms of confused behaviour, incoherent speech, nonpurposeful movements and trying to get up and down from the bed [Breitbart and Alici, 2008].
When faced with this problem of delirium, one should first consider simple investigations to rule out some common but reversible causes of delirium such as constipation, hypoglycaemia, electrolyte imbalances, hypercalcemia, sepsis or adverse drug effects. In the absence of any other reversible cause, one may then consider the diagnosis of terminal delirium, which is that caused by the terminal state of the patient. Family may often find this frightening, as their loved one may seem to be losing his or her mind; it is important to explain to them that this is a sign that the ‘brain function is gradually shutting down’.
Treatment of delirium can be managed using an antipsychotic drug, such as haloperidol as a first line. This may be given orally or subcutaneously with a typical dose of 1–5 mg at night and 1 mg prn. Apart from haloperidol, newer atypical antipsychotic agents may also be used such as olanzapine, quetiapine® or risperidone.
Patients with hyperactive delirium may be extremely agitated, and at times, it may be necessary to sedate the patient using a short-acting benzodiazepine such as midazolam. Commonly a small dose of 2.5–5 mg may be used subcutaneously, as and when needed; however, it may also be given as a continuous subcutaneous infusion, gradually titrated to a dose of between 0.5 and 2 mg per hour if continuous sedation is desired. It should be remembered, however, that benzodiazepines should not be used as monotherapy for agitated delirium, as it may result in paradoxical worsening of the agitation and should be used in combination with antipsychotic agents [Twycross et al.2009a].
Terminal secretions
Terminal secretions have also been known as the term ‘death rattle’, and it is a sign that inevitably heralds the last few hours or short days of life [Morita et al. 1998]. It has a sound resembling that of a chesty secretion and may sometimes be mistaken for the sound of acute pulmonary oedema. It is therefore important for clinicians to be able to recognise this symptom, and differentiate it from other conditions so as to avoid futile and unnecessary investigations such as chest X-rays, intravenous antibiotics and ECG monitoring. The key to recognizing this symptom is mainly in understanding the patient’s prognosis and disease trajectory. Hence, if this is a patient whom we know is approaching a terminal phase, we would most certainly recognize the sound as that of the ‘death rattle’ rather than acute pulmonary oedema.
This symptom is another very distressing symptom to family members as it often gives them an impression that their loved one is ‘drowning’ on their secretions [Wee et al. 2006]. It is therefore important to explain to them that this is due to pooled secretions in the airways, which cause noisy vibrations as air passes through, but does not obstruct nor cause respiratory distress to the patient. It is also important to explain that suctioning is not helpful in this case, as it will not reduce the rattle but may in fact cause more distress to the patient. Deep suctioning may in fact lead to vagal stimulation which could cause sudden bradycardia and asystole in the patient who is already in a terminal phase. Although death is an anticipated event, for a patient to suddenly stop breathing during a procedure of suctioning may, in fact, invite great dissatisfaction amongst family members.
Treatment of this symptom therefore will be to use anticholinergic medications, which will help to reduce the amount of secretions and thus reducing the rattling sound. Usual medications which are used in this case include the following [Twycross et al. 2009a]:
Subcutaneous hyoscine butylbromide (buscopan) 20 mg 4–8 hourly and prn
Subcutaneous hyoscine hydrobromide 400 mcg 4–8 hourly and prn
Subcutaneous glycopyrrolate 200 mcg 4–8 hourly and prn
These medications may also be given as a 24-hour continuous subcutaneous infusion.
Key area four: ethical decision making at the end of life
Making clinical decisions for patients with advanced cancer who are approaching the end of life can be challenging, and at times, pose moral dilemmas for the clinical team. This is because as health professionals, there is a perceived duty to preserve and uphold the sanctity of life. At the same time, clinicians also have a duty to act in the best interest of the patient. The ‘sanctity of life’ doctrine which has been a primary principle in biomedical ethics till today, is a principle that was carried down from Judeo–Christian and Hippocratic traditions centuries ago [Baranzke, 2012]. In terms of caring for patients at the end of life, about a century ago, the state of medical science was at a level where often, there was little doubt in accepting when death was inevitable and decisions to allow irreversible pathology to progress naturally were easy to make. Today, however, due to the advances in medical technology, more and more life-prolonging interventions are being discovered and therefore, finding the right balance between quantity and quality of life now has become a major challenge to EOLC. Clinicians should therefore be familiar with the common ethical principles that are applicable to clinical decision making at the end of life, as this allows them to practise good EOLC with clarity and a clear conscience. Certain end-of-life issues that may lead to doubts in clinicians include:
Withholding and withdrawing life-sustaining treatment, such as cardiopulmonary resuscitation, ventilator support, artificial hydration and nutrition;
Sedative medications used at the end of life;
Confusion in terminology of euthanasia.
Often, the main concern of a clinician will be, ‘Does the management provided to my patient at the end of life hasten death and will it approximate to euthanasia?’ Because of such concerns, clinicians may fear medico-legal implications and experience conflict within themselves when deciding on how to manage a patient at the end of life, and this may lead to poorer EOLC [Swanson and McCrary, 1996; Marik et al. 1999; Miccinesi et al. 2005].
Withholding and withdrawing medical interventions
Ethically, there is no difference between the actions of withdrawing a medical intervention and withholding a medical intervention. Hence, stopping a life-sustaining therapy is no different to not starting it. Both actions are ethically acceptable when treatment is [Ko and Blinderman, 2015; British Medical Association, 2009; Ackermann, 2000]:
Futile;
Not in the patient’s best interest;
Refused by the patient.
The guiding principles in this situation include that of autonomy and beneficence. It is clear that patients have every right to refuse medical interventions, even though they may be beneficial. However, the problem arises when a patient is not able to express that autonomy, and there is no clear advance directive. In this situation, communication with a surrogate decision maker to determine the patient’s preferences for care is the normal practice. This can be challenging at times because surrogate decision makers, who are often close relatives, may make emotional decisions, which represent their own preferences and possibly not that of the patient. Hence, it is important to explain to the surrogate decision maker that their choice should be that which approximates to the patient’s own preference [Ko and Blinderman, 2015; GMC, 2010].
Clinicians should also bear in mind very clearly that it is not necessary to offer all forms of life-sustaining therapies; the professional opinion is that such therapies would not yield any obvious benefit. Medical futility may be defined quantitatively, referring to an intervention with very remote chances of resulting in benefit; or it may be defined qualitatively, meaning the intervention is unlikely to achieve patient-centred goals. In clinical practice however, futility may be difficult to define, as all individuals, the patient, clinician and family may have different views about what is beneficial and what the goals of care should be. Therefore, a useful guide to help clinicians decide on what is considered futile would be to determine the goals of care in every individual case before considering an intervention. If the intervention is unlikely to achieve these goals in any way, then futility should be considered [Schneiderman, 2011].
One of the most difficult areas concerning withdrawal and withholding medical interventions is the issue of artificial hydration and nutrition. It has been argued that hydration and nutrition are considered basic requirements of care, of which any human being should never be deprived. However, artificial hydration and nutrition through feeding tubes or the intravenous route are not as basic as normal oral feeding and drinking. Many of the ethical dilemmas of withdrawal of artificial hydration and nutrition, however, stem from its use in patients with persistent vegetative states, where such interventions allow patients to survive for years, and its withdrawal can be seen as a deliberate act to shorten life [Brody et al. 2010]. However, in the context of a patient with advanced cancer who is in the last days of life, it is quite clear that evidence has shown there is no survival benefit from artificial hydration and nutrition [Good et al. 2008a, 2008b]. The inability to maintain nutrition through the oral route in the setting of advanced cancer, and declining function, are markers of the dying process; and providing good mouth care and keeping the oral cavity clean and moist is the best form of palliation. Witholding artificial hydration and nutrition in such case is therefore appropriate [Slomka, 2003; Twycross et al. 2009a].
Although clinicians ethically have the right to withhold or withdraw medical interventions on the basis of medical futility, it should be remembered that these decisions should still be discussed with the family to ensure they understand the reason and basis of such decisions. If family, however, refuse to accept these decisions and demand for the intervention to continue, clinicians should refer the case to a colleague for a second opinion and who may accept to continue the care or further negotiate the management with the family [GMC, 2010; Ko and Blinderman, 2015].
Using sedative medications at the end of life
One of the common fears clinicians have with using sedative medications such as morphine or benzodiazepines at the end of life is hastening death. It is common belief that by using such medications in an already frail and weak patient, these drugs may lead to hypotension, respiratory depression and death. Because of these fears, clinicians are often reluctant to provide adequate relief for pain, and sedation for restlessness in the terminal phase. Evidence, however, has shown that such medications can indeed be given to patients in the terminal phase without causing any significant reduction in survival, when used appropriately for relieving symptoms at the end of life [Maltoni et al. 2012; Morita et al. 2001, 2005; Sykes and Thorns, 2003]. However, if sedative medications such as morphine or benzodiazepines did in fact hasten death, the use of such medications can still be justified, based on the principle of ‘double effect’ and the principle of proportionality [De Graeff and Dean, 2007; Juth et al. 2010; Krakauer et al. 2000].
The principle of ‘double effect’ states that where an action intended to have a good effect can achieve this effect only at the risk of producing a harmful effect, then this action is ethically permissible, provided it satisfies the following conditions:
The action is good in itself;
The intention is solely to produce the good effect;
The good effect is not achieved through the bad effect;
There is sufficient reason to permit the bad effect.
This principle therefore distinguishes the use of sedating medications at the end of life from euthanasia, as in this case, the intention is to relieve pain, dyspnoea or restlessness, and by no means intends to deliberately cause harm to the patient. When such sedating medications are used, they are administered in small doses and titrated till just enough is given to achieve the desired effect, which demonstrates ethical practice, based on the principle of proportionality.
Euthanasia, on the other hand, cannot be justified by these principles, as the practice of euthanasia employs means that are beyond what is necessary to merely relieve symptoms and the intent is to cause immediate death of the patient. Therefore, even if a doctor were to claim his intention is to relieve suffering, the practice of euthanasia is an example of achieving a good effect through a bad effect [Materstvedt et al. 2003].
Confusing terminology of euthanasia
Terminology such as ‘active euthanasia’, ‘passive euthanasia’, ‘voluntary euthanasia’ and ‘nonvoluntary euthanasia’, which have been commonly used in literature, have led to clinicians feeling confused and unsure about what practices actually constitute euthanasia. The term ‘passive euthanasia’ has often been used to describe withholding or withdrawing futile medical interventions while ‘active euthanasia’ and ‘voluntary euthanasia’ synonymously describe the active and deliberate administration of an intervention that leads to the death of a patient who has voluntarily requested it. ‘Nonvoluntary euthanasia’ describes the act of deliberately administering an intervention that leads to the death of a patient who has not voluntarily requested such an intervention due to lack of capacity. In order to clarify some of these often misleading terms, the European Association of Palliative Care published a consensus statement defining euthanasia as: ‘A doctor intentionally killing a person by the administration of drugs, at that person’s voluntary and competent request.’ They further clarified that euthanasia must always be active and the term ‘passive euthanasia’ is a contradiction of terms. Euthanasia must also always be voluntary and ‘nonvoluntary’ euthanasia is, in fact, murder. The key word in the definition of euthanasia is therefore the word ‘intentional’. The intent is to kill a person and successful outcome is for immediate death to occur [Matersvedt et al. 2003; Matersvedt and Bosshard, 2015].
Doctors should therefore be clear on the definition of euthanasia and not confuse it with ethically appropriate managements at the end of life, such as withdrawing and withholding futile medical interventions and palliative sedation at the end of life.
Conclusion
Despite advances in the management of lung cancer, the majority of patients still succumb to their illness within 5 years of diagnosis, and death from lung cancer can be associated with many symptoms and poor quality of life. Clinicians should therefore be more aware of the needs of such patients and learn the basic skills required to care for patients at this stage of their illness.
Although there has been significant development in the field of palliative medicine worldwide, it is still evident that many patients requiring palliative care are unable to access it due to various reasons, such as a lack of knowledge and skills amongst healthcare professionals, lack of availability of essential medications, especially opioids, and a lack of healthcare systems in place to provide such care [World Palliative Care Alliance, 2014]. Even in areas where palliative care services are readily available, there are still barriers that prevent patients from receiving good EOLC care, such as a reluctance among clinicians to refer patients to palliative care services and also patients’ reluctance to be referred, due to negative perceptions of palliative care and its meaning [Wentlandt et al. 2012].
In May 2014, the World Health Organization adopted a resolution for the strengthening of palliative care as a component of comprehensive care throughout the life course that emphasized the need for palliative care to be integrated into every country’s healthcare system to ensure access to all in need [World Health Organization, 2014]. For this resolution to become a reality, all healthcare professionals caring for patients with serious illnesses, including lung cancer, should have the basic knowledge and skills to improve care towards the end of life in situations where cure is no longer a possibility.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest: The author declares no conflicts of interest in preparing this article.
References
- Abernethy A., Currow D., Frith P., Fazekas B., McHugh A., Bui C. (2003) Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ 327: 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann R. (2000) Withholding and withdrawing life-sustaining treatment. Am Fam Physician 62: 1555–1560. [PubMed] [Google Scholar]
- Baranzke H. (2012) ‘Sanctity-of-life’—a bioethical principle for a right to life? Ethic Theory Moral Prac 15: 295–308. [Google Scholar]
- Boly M., Faymonville M., Schnakers C., Peigneux P., Lambermont B., Philips C. (2008) Perception of pain in the minimally conscious state with PET activation: an observational study. Lancet Neurol 7: 1013–1020. [DOI] [PubMed] [Google Scholar]
- Breitbart W., Alici Y. (2008) Agitation and delirium at the end of life: ‘We couldn’t manage him.’ JAMA 300: 2898–2910. [DOI] [PubMed] [Google Scholar]
- British Medical Association (2009) End of life decisions: views of the BMA. Available at: http://bma.org.uk/practical-support-at-work/ethics/ethics-a-to-z (accessed 16 July 2014).
- Brody H., Hermer L., Scott L., Grumbles L., Kutac J., McCammon S. (2010) Artificial nutrition and hydration: the evolution of ethics, evidence, and policy. J Gen Intern Med 2: 1053–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Research UK (2013) Lung cancer survival statistics. Available at: http://www.cancerresearchuk.org/cancer-info/cancerstats/types/lung/survival/ (accessed 19 October 2014).
- Clayton J., Hancock K., Butow P., Tattersall M., Currow D. (2007) Clinical practice guidelines for communicating prognosis and end-of-life issues with adults in the advanced stages of a life-limiting illness, and their caregivers. MJA 186: S77–S108. [DOI] [PubMed] [Google Scholar]
- Currow D., Johnson M., White P., Abernethy A. (2013) Evidence-based intervention for chronic refractory breathlessness: practical therapies that make a difference. Br J Gen Pract 63: 609–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Graeff A., Dean M. (2007) Palliative sedation therapy in the last weeks of life: a literature review and recommendations for standards. J Pall Med 10: 67–81. [DOI] [PubMed] [Google Scholar]
- Department of Health. (2008) End of Life Care Strategy –promoting high quality care for all adults at the end of life. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/136431/End_of_life_strategy.pdf (accessed 13 October 2014).
- Earle C., Neville B., Landrum M., Ayanian J., Block S., Weeks J. (2004) Trends in the aggressiveness of cancer care near the end of life. J.Clin Oncol. 22: 315–321. [DOI] [PubMed] [Google Scholar]
- Ellershaw J., Ward C. (2003) Care of the dying patient: the last hours or days of life. BMJ 326:30–34 [PMC free article] [PubMed] [Google Scholar]
- Ellershaw J., Smith C., Overill S., Walker S., Aldridge J. (2001) Care of the dying: setting the standards for symptom control in the last 48 hours of life. J Pain Symptom Manage 21: 12–17. [DOI] [PubMed] [Google Scholar]
- Fallowfield L., Jenkins V., Beveridge H. (2002) Truth may hurt but deceit hurts more: communication in palliative care. Palliat Med 16: 297–303. [DOI] [PubMed] [Google Scholar]
- Fan H., Shao Z., Xiao Y., Xie Z., Chen W., Xie H., et al. (2015) Incidence and survival of non-small cell lung cancer in Shanghai: a population-based cohort study. BMJ Open 5: e009419 Available at: http://bmjopen.bmj.com/content/5/12/e009419.long (accessed 10 May 2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris F., Von Gunten C., Emanuel L. (2003) Competency in end-of-life care: last hours of life. J Pall Med 6: 605–613. [DOI] [PubMed] [Google Scholar]
- Glare P., Sinclair C. (2008) Palliative Medicine Review: prognostication. J Pall Med 11: 84–103 [DOI] [PubMed] [Google Scholar]
- Good P., Cavenagh J., Mather M., Ravenscroft P. (2008a) Medically assisted hydration for adult palliative care patients. Cochrane Database of Systematic Rev 2: CD006273. [DOI] [PubMed] [Google Scholar]
- Good P., Cavenagh J., Mather M., Ravenscroft P. (2008b) Medically assisted nutrition for palliative care in adult patients. Cochrane Database of Systematic Rev 4: CD006274. [DOI] [PubMed] [Google Scholar]
- General Medical Council. (2010) Treatment and care towards the end of life: good practice in decision making. Available at: http://www.gmc-uk.org/End_of_life.pdf_32486688.pdf (accessed 17 July 2014).
- Iyer S., Roughley A., Rider A., Taylor-Stokes G. (2014) The symptom burden of non-small cell lung cancer in the USA: a real world cross-sectional study. Support Care Cancer 22: 181–187. [DOI] [PubMed] [Google Scholar]
- Izumi S., Nagae H., Sakurai C., Imamura E. (2012) Defining end-of-life care from perspective of nursing ethics. Nursing Ethics 19: 608–618. [DOI] [PubMed] [Google Scholar]
- Jennings A., Davies A., Higgins J., Gibbs J., Broadley K. (2002) A systematic review of the use of opioids in the management of dyspnoea. Thorax 57: 939–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juth N., Lindblad A., Lynöe N., Sjöstrand M., Helgesson G. (2010) European Association for Palliative Care framework for palliative sedation: an ethical discussion. BMC Palliative Care 9: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko D., Blinderman C. (2015) Withholding and withdrawing life-sustaining treatment. In: Cherny N., Fallon M., Kassa S., Portenoy R., Currow D. (eds.), Oxford Textbook of Palliative Medicine, 5th Edition. Oxford: Oxford University Press. [Google Scholar]
- Krakauer E., Penson R., Truog R., King L., Chabner B., Lynch T. (2000) Sedation for intractable distress of a dying patient: acute palliative care and the principle of double effect. The Oncologist 5: 53–62. [DOI] [PubMed] [Google Scholar]
- Kvale P., Selecky P., Prakash U. (2007) Palliative care in lung cancer: ACCP evidence based clinical practice guidelines (2nd edition). Chest 132: 368S–403S. [DOI] [PubMed] [Google Scholar]
- Lim R. (2012) Communication at the end of life. In: Lee F., Yau W., Pok A. (eds.), The Handbook of Geriatric Medicine, Kuala Lumpur: Geriatric Unit Hospital Kuala Lumpur. [Google Scholar]
- Mack J., Cronin A., Keating N., Taback N., Huskamp H., Malin J., et al. (2012) Associations between end of life discussion characteristics and care received near death: a prospective cohort study. J.Clin Oncol. 30: 4387–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltoni M., Scarpi E., Rosati M., Derni S., Fabbri L., Martini F., et al. (2012) Palliative sedation in end-of-life care and survival: a systematic review. J Clin Oncol 30: 1378–1383. [DOI] [PubMed] [Google Scholar]
- Marik P., Varon J., Lisbon A., Reich H. (1999) Physicians’ own preferences to the limitation and withdrawal of life-sustaining therapy. Resuscitation 42: 197–201. [DOI] [PubMed] [Google Scholar]
- Materstvedt L., Bosshard G. (2015) Euthanasia and palliative care. In: Cherny N., Fallon M., Kassa S., Portenoy R., Currow D. (eds.), Oxford Textbook of Palliative Medicine, 5th edition. Oxford: Oxford University Press. [Google Scholar]
- Materstvedt L., Clark D., Ellershaw J., Førde R., Gravgaard A, Müller-Busch H., et al. (2003) Euthanasia and physician-assisted suicide: a view from an EAPC ethics task force. Pall Med 17: 97–101. [DOI] [PubMed] [Google Scholar]
- Miccinesi G., Fischer S., Pace E., Onweteaka-Philipsen B., Cartwright C., van der Heide A., et al. (2005) Physicians attitudes towards end of life decisions: a comparison between seven countries. Soc Sci Med 60: 1961–1974. [DOI] [PubMed] [Google Scholar]
- Ministry of Health, Malaysia (2010) Clinical practice guidelines: management of cancer pain. Available at: http://www.moh.gov.my/attachments/6098.pdf (accessed 19 October 2014).
- Molassiotis A., Smith J., Bennett M., Blackhall F., Taylor D., Zavery B., et al. (2010) Clinical expert guidelines for the management of cough in lung cancer: report of a UK task group on cough. Cough 6: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T., Chinone Y., Ikenaga M., Miyoshi M., Nakaho T., Nishitateno K. (2005) Efficacy and safety of palliative sedation therapy: a multicenter, prospective, observational study conducted on specialized palliative care units in Japan. J Pain Symptom Manage 30: 320–328. [DOI] [PubMed] [Google Scholar]
- Morita T., Ichiki T., Tsunoda J., Inoue S., Chihara S. (1998) A prospective study on the dying process in terminally ill cancer patients. Am J Hosp Palliat Care 15: 217–222. [DOI] [PubMed] [Google Scholar]
- Morita T., Tsunoda J., Inoue S., Chihara S. (2001) Effects of high dose opioids and sedatives on survival in terminally ill cancer patients. J Pain Symptom Manage 21: 282–289. [DOI] [PubMed] [Google Scholar]
- Murray S., Kendall M., Boyd K., Sheikh A. (2005) Illness trajectories and palliative care. BMJ 330: 1007–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute, SEER Program. (2014) SEER Stat Fact Sheet: lung and bronchus cancer. Available at: http://seer.cancer.gov/statfacts/html/lungb.html (accessed 19 October 2014).
- National Council for Palliative Care. (2011) Commissioning End of Life Care: act & early. Available at: http://www.ncpc.org.uk/publications (accessed 13 October 2014).
- National Hospice and Palliative Care Organization. (2014) CMS-Medicare hospice regulations. Available at: http://www.nhpco.org/cms-medicare-hospice-regulations (accessed 20 October 2014).
- Schneiderman L. (2011) Defining medical futility and improving medical care. J Bioeth Inq 8: 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S., Higginson I., Booth S., Harding R., Bausewein C. (2010) Benzodiazepines for the relief of breathlessness in advanced malignant and non-malignant diseases in adults. Cochrane Database of Systematic Rev: CD007354. [DOI] [PubMed] [Google Scholar]
- Slomka J. (2003) Withholding nutrition at the end-of-life: Clinical and ethical issues. Cleve Clin J Med 70: 548–552. [DOI] [PubMed] [Google Scholar]
- Steinhauser K., Christakis N., Clipp E., McNeilly M., Granbow S., Parker J. (2001) Preparing for the end of life: preferences of patients, families, physicians and other care providers. J Pain Symptom Manage 22: 727–737. [DOI] [PubMed] [Google Scholar]
- Steinhauser K., Clipp E., McNeilly M., Christakis N., McIntyre L., Tulsky J. (2000) Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA 284: 2476–2482. [DOI] [PubMed] [Google Scholar]
- Stone P., Rees E., Hardy J. (2001) End of life care in patients with malignant disease. Eur J Cancer 37: 1070–1075. [DOI] [PubMed] [Google Scholar]
- Swanson J., McCrary S. (1996) Medical futility decisions and physicians’ legal defensiveness: the impact of anticipated conflict on thresholds for end-of-life treatment. Soc Sci Med 42: 125–132. [DOI] [PubMed] [Google Scholar]
- Sykes N., Thorns A. (2003) The use of opioids and sedatives at the end of life. The Lancet Oncology 4: 312–318. [DOI] [PubMed] [Google Scholar]
- Teunissen S., Wesker W., Kruitwagen C., De Haes H., Voest E., De Graeff A. (2007) Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage 34: 94–104. [DOI] [PubMed] [Google Scholar]
- The SUPPORT principal investigators (1995) A controlled trial to improve care for seriously ill hospitalized patients. JAMA 274: 1591–1598. [PubMed] [Google Scholar]
- Twycross R., Wilcock A., Toller C. (2009a) Last days. In: Symptom management in advanced cancer, 4th Edition, Nottingham: Palliativedrugs.com. [Google Scholar]
- Twycross R., Wilcock A., Toller C. (2009b) Respiratory symptoms. In: Symptom management in advanced cancer, 4th Edition, Nottingham: Palliativedrugs.com. [Google Scholar]
- Wee B., Coleman P., Hillier R., Holgate S. (2006) The sound of death rattle II: how do relatives interpret the sound? Palliat Med 20: 177–181. [DOI] [PubMed] [Google Scholar]
- Weissman D. (2005) Fast facts and concepts. Syndrome of imminent death, 2nd edition. Available at: http://www.eperc.mcw.edu/EPERC/FastFactsIndex/ff_003.htm.EPERC (accessed 16 October 2014).
- Wentlandt K., Krzyanowska M., Swami N., Rodin G., Le L., Zimmermann C. (2012) Referral practices of oncologists to specialised palliative care. J Clin Oncol. 30: 4380–4386. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2013) International Agency for Research on Cancer. Press release No.223, latest world cancer statistics. Available at: http://www.iarc.fr/en/media-centre/pr/2013/pdfs/pr223_E.pdf (accessed 19 October 2014).
- World Health Organization (2014) Strengthening of palliative care as a component of comprehensive care throughout the life course. Available at: http://apps.who.int/gb/ebwha/pdf_files/WHA67/A67_R19-en.pdf (accessed 19 June 2015). [DOI] [PubMed]
- World Palliative Care Alliance (2014) Global atlas of palliative care at the end of life. Available at: http://www.thewhpca.org/resources/global-atlas-on-end-of-life-care (accessed 22 March 2015).
- Wright A., Zhang B., Ray A., Mack J., Trice E., Balboni T., et al. (2008) Associations between end of life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 300: 1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]


