Abstract
Background
Tobramycin is frequently used for treatment of bronchopneumonia in patients with cystic fibrosis (CF). Variability in tobramycin clearance (CL) is high in this population with few reliable approaches to guide dosing.
Objectives
We sought to evaluate the pharmacokinetics of once-daily intravenous tobramycin in patients with CF and test the influence of covariates on tobramycin CL, including serum creatinine (SCr) and urinary biomarkers: neutrophil gelatinase-associated lipocalin (NGAL), retinol-binding protein (RBP) and kidney injury molecule-1 (KIM-1).
Methods
This was a prospective, observational cohort study of children/young adults with CF receiving once-daily intravenous tobramycin from October 2012 to May 2014 at Cincinnati Children's Hospital Medical Center. Therapeutic drug monitoring data were prospectively obtained. Population pharmacokinetic analyses were performed using non-linear mixed-effects modelling.
Results
Thirty-seven patients (median age 15.3 years, IQR 12.7–19.5) received 62 tobramycin courses. A one-compartment model with allometrically scaled weight for tobramycin CL and volume of distribution (V) best described the data. Urinary NGAL was associated with tobramycin CL (P < 0.001), as was urinary RBP (P < 0.001). SCr, estimated glomerular filtration rate and urinary KIM-1 were not significant covariates. The population pharmacokinetic parameter estimates were CL = 8.60 L/h/70 kg (relative standard error 4.3%) and V = 31.3 L/70 kg (relative standard error 4.7%).
Conclusions
We describe urinary biomarkers as predictors of tobramycin CL using a population pharmacokinetic modelling approach. Our findings suggest that patient weight and urinary NGAL or RBP could be used to individualize tobramycin therapy in patients with CF.
Introduction
Intravenous tobramycin is often used in the management of cystic fibrosis (CF) pulmonary exacerbations because of its bactericidal activity against Gram-negative pathogens.1 From a pharmacokinetic/pharmacodynamic (PK/PD) standpoint, aminoglycosides demonstrate concentration-dependent killing2 while prolonged and increased drug exposure increase the risk of nephrotoxicity.3 Most clinicians utilize once-daily intravenous bolus infusions of tobramycin during treatment of bronchopneumonia to combat the more resistant pathogens encountered in the CF population and allow for adequate drug clearance (CL) prior to readministration.
Serum creatinine (SCr) is a suboptimal marker of kidney injury and function in patients with CF, whose muscle mass is often reduced.4,5 Estimated glomerular filtration rate (eGFR) frequently underestimates renal impairment in patients with CF and the relationship between SCr-based estimates of renal function and aminoglycoside CL in this population is poor.6 Individualized PK monitoring can improve efficacy and minimize toxicity of aminoglycosides,7–9 but SCr measurement may add little to guide aminoglycoside dosing in patients with CF.
Urinary kidney injury biomarkers are clinically useful for early detection, risk stratification and prognostication of acute kidney injury (AKI).10 However, these biomarkers can also provide functional information based on the mechanism of excretion and site of interaction within the kidney. Neutrophil gelatinase-associated lipocalin (NGAL), increased in the urine following a variety of kidney insults,11–15 is reabsorbed by proximal tubule cells16 via the same endocytic receptor as aminoglycosides, megalin.17,18 Urinary excretion is increased secondary to nephrotoxic proximal tubule injury causing impaired reabsorption.19,20 Retinol-binding protein (RBP) is a hepatically synthesized chaperone for vitamin A transport to tissues that is freely filtered by the glomerulus and reabsorbed via megalin in the proximal tubule.21 It is a sensitive marker of tubule dysfunction22,23 and in patients with CF may be increased following a course of aminoglycoside therapy even though changes in SCr are not apparent.24 Kidney injury molecule-1 (KIM-1) is a proximal tubule transmembrane protein25,26 and urinary levels are elevated in the setting of both ischaemia and toxin administration, specifically gentamicin.25,27–29
Urinary biomarkers have not been studied extensively in the CF population.24,30 The relationships between tobramycin PK parameters, specifically total body CL, and urinary biomarker concentrations have also not been previously explored. Since tobramycin is renally eliminated, urinary biomarkers could augment current individualized PK monitoring strategies to estimate tobramycin renal drug handling. We hypothesized that RBP and NGAL, which are elevated when tubular reabsorption is impaired and utilize the same proximal tubule receptor as tobramycin, will be inversely related to tobramycin CL, while KIM-1, which is a marker of cytotoxicity, will not be directly correlated with CL. We hypothesized that NGAL and RBP will lead to improvement in the population model fit for CL compared with KIM-1 and sought to assess these relationships using a population PK modelling approach.
Patients and methods
Study population
We conducted a prospective, observational pilot study of children and young adults with CF admitted to Cincinnati Children's Hospital Medical Center (CCHMC) over a 20 month period (October 2012 to May 2014). Patients were eligible if they had a documented diagnosis of CF, were admitted for a pulmonary exacerbation and received intravenous tobramycin once daily for ≥5 days. Subjects were excluded if they had received a lung transplant, were receiving immunosuppressant medications, were admitted to the ICU or if they failed to provide urine samples on ≥70% of inpatient days. Subjects could participate during multiple admissions if their preceding tobramycin course was completed >7 days prior to admission.
Ethics
Informed consent/assent was obtained from patients and their parents/guardians as appropriate. The study protocol was approved by the Institutional Review Board at CCHMC (IRB #2012–1231). This study was conducted in accordance with the principles of the Declaration of Helsinki.
Dosing regimen and sampling
Recommendations for dosing of tobramycin were made by inpatient clinical pharmacists, who were not directly involved with this study. Initial starting doses were based on doses used during previous courses or 10 mg/kg/dose if the patient had not recently received tobramycin. All doses were given as a 30 min infusion. Serum tobramycin drug concentrations were obtained at ∼3 and ∼10 h post-dose on day 1 of therapy in all patients, per standard of care; additional levels were obtained with dose adjustments and at the discretion of the medical team. A subset of subjects (n = 32) had tobramycin levels obtained every 3 days during hospitalization along with standard-of-care labs when drawn within 12 h of a preceding dose.
Urine specimens were collected once daily from enrolment through completion of the hospital tobramycin course. Only urine samples collected on days when tobramycin measurements were collected were included in analyses (see below). Samples were stored at 4°C for up to 72 h. Specimens were centrifuged at 3000 g for 15 min and the supernatants divided into nine 1 mL specimens. Aliquots were then frozen at −80°C and stored until analysis was performed. SCr was measured daily in all patients receiving tobramycin as part of routine patient care at our institution.
Study procedures
Each urine sample was tested for concentrations of NGAL, KIM-1, RBP and creatinine in the CCHMC Center for Acute Care Nephrology biomarker laboratory. NGAL and RBP were measured via ELISA using human-specific commercially available assays (AntibodyShop, Grusbakken, Denmark for NGAL and ALPCO, Salem, NH, USA for RBP). KIM-1 was measured by an ELISA that has been constructed using commercially available reagents (R&D Systems, Minneapolis, MN, USA) as previously described.31 Urine creatinine (UCr) was measured by modified Jaffe reaction (Dimension® Xpand plus HM Clinical Analyzer, Siemens Diagnostics, Tarrytown, NY, USA). Investigators performed all measurements blindly and were unaware of subjects' clinical characteristics. SCr and tobramycin measurements were performed by the CCHMC clinical laboratory via coupled enzymatic reaction (Siemens Dimension Vista® 1500 Chemistry Analyzer) and turbidimetric inhibition immunoassay (Dimension Vista®, Siemens Diagnostics, Tarrytown, NY, USA), respectively. The lower limit of detection of tobramycin measurements was 0.3 mg/L. The assay error pattern was described as SD (mg/L) = 0.0486 + 0.0341 · C + 0.0006 · C2, where SD is the standard deviation of the assay in mg/L, C represents the measured tobramycin concentration and C2 is the square of C.
Population PK model
We developed a population PK model for tobramycin using non-linear mixed-effects modelling with NONMEM® (version 7.2, ICON, Dublin, Ireland). The first-order conditional estimation with the interaction option was used to estimate the PK parameters and their variability. For the structural model, one- and two-compartment models with first-order elimination were tested. The three-quarter allometric scaling method [(weight/70)0.75] was utilized to account for variability in body weight when estimating tobramycin CL; the scaling coefficient for volume of distribution (V) was 1.0. The interindividual variability for the PK parameters was determined with an exponential error model. The residual error was described by a combined additive and proportional model, but other residual error models such as exponential, additive or proportional were also examined. Model selection was based on goodness-of-fit plots with assessment of the objective function value (OFV).
We assessed the following covariates: age, gender, height, weight, lean body weight, SCr, eGFR, urine biomarker concentrations and urine biomarker concentrations corrected for UCr (biomarker/UCr). For days on which biomarkers were unavailable (n = 11/197), missing values were imputed using the last observation from the same patient. eGFR was examined using age-appropriate estimating equations: the Schwartz formula32 for subjects ≤18 years of age and the Cockcroft–Gault formula33 for subjects >18 years of age; each equation was also tested separately for all subjects regardless of age. Covariates were selected using a forward addition, backward elimination process. Covariates that improved the model fit on univariate analysis by decreasing the OFV by ≥10.8, which corresponds to a P value ≤0.001, remained in the final model.
Model validation
Model validation was performed using bootstrap analysis and a prediction-corrected visual predictive check (PC-VPC). For VPC, 1000 virtual datasets were simulated using the final model. The 5th, 50th and 95th percentiles of simulated concentrations were plotted against the observed data for comparison. The final PK model was evaluated using a non-parametric bootstrapping analysis of 1000 samples selected to calculate the 95% CIs for the population estimates. The model was considered reliable if the PK parameter estimates were within the 95% CIs. Summary statistics were described using means (with SD) or medians (with IQR), as appropriate, for the study population.
PD relationships
To assess the relationship between NGAL and tobramycin area under the curve (AUC0–24), deterministic simulation was performed based on the final model by NONMEM 7.2. Several studies have suggested that a cut-off NGAL value of 100 ng/mL has been associated with an increased risk of AKI in adults34,35 and children.36 Simulations were conducted using the final model with NGAL concentrations that correspond to the lowest, median, cut-off level for AKI (100 ng/mL) and highest NGAL levels reported in our study cohort. A typical patient weighing 50 kg was selected and 10 mg/kg tobramycin was given to the patients via 30 min intravenous infusion. The simulated AUCs were calculated by the linear log trapezoidal rule.
We next examined the relationships between biomarkers and PK parameter estimates (CL, Cmax and AUC0–24) via analyses performed using Stata 13.1 (StataCorp, College Station, TX, USA). We first used validated clinical PK software (MW/PHARM, Mediware, The Netherlands)37 to perform population model-based Bayesian estimation of individual PK parameter estimates for each subject each day.38,39 Cmax was determined as the concentration immediately after the end of the infusion. The one-compartment model with allometric scaling for weight to describe CL and V (Model 2, Table S1, available as Supplementary data at JAC Online) was used as the a priori model in the PK software program. Available patient data (age, height, weight and gender), SCr, drug doses and drug levels were used to generate PK estimates in the software program.
To examine the relationship between Cmax, AUC0–24 and biomarkers across individuals, we tested the correlation between subject means using Pearson correlation tests accounting for repeated measurements.40 Biomarkers were log-transformed due to non-normal distribution of values. To account for repeated measurements among subjects, a weighted average correlation coefficient was calculated. Frequency weights were based on the number of repeated measurements per subject per tobramycin course and P values computed according to the number of tobramycin courses included in the analysis (n = 60).
Finally, to further explore the relationship between daily CL estimates and NGAL, we performed nested linear mixed-effects regression of CL on NGAL accounting for correlation within subjects and individual admissions. The primary outcome was log-transformed NGAL and the primary exposure was CL. We also tested the effects of other covariates on NGAL including day of therapy, dose (mg/kg and mg), Cmax, admission number during the study, age (years), body weight (kg), gender, aminoglycoside courses in the previous 10 years, presence of CF-related diabetes and receipt of two or more concomitant nephrotoxins during the tobramycin course. Covariates that were significantly associated with NGAL values on univariate analysis were combined into a final multivariable model. Robust standard errors and an independent covariance structure were used.
Results
The final study cohort consisted of 37 subjects receiving 62 intravenous tobramycin courses. Twenty-one (57%) received a single tobramycin course, 12 (32%) received two courses and 4 (11%) received three or more courses. There were a total of 307 tobramycin drug concentration measurements over the study period: 103 were drawn between 2 and 4 h post-dose, 167 were drawn between 9 and 11 h post-dose and the remaining 37 serum concentrations were drawn at other times; 5 samples were below the level of detection. The demographics and clinical characteristics of the study population are shown in Table 1. All patients were Caucasian. The median duration of tobramycin was 10 days (IQR 7–13). The most commonly coadministered antibiotics were ceftazidime (n = 18, 29%), meropenem (n = 18, 29%), a fluoroquinolone (n = 13, 21%), ticarcillin/clavulanate (n = 12, 19%) and linezolid (n = 10, 16%).
Table 1.
Patient demographics and clinical characteristics
| Variable | Unique subjectsa (N = 37) | All admissions (N = 62) |
|---|---|---|
| Female gender, n (%) | 24 (65) | 43 (69) |
| Age (years)b, median (IQR) | 15.3 (12.7–19.5) | 14.7 (12.5–19.2) |
| Weight (kg)b, median (IQR) | 49.0 (40.4–57.0) | 46.9 (33.8–56.2) |
| Height (cm)b, median (IQR) | 161 (150–165) | 159 (145.5–164.0) |
| SCr (mg/dL), median (IQR) | 0.50 (0.41–0.64) | 0.49 (0.41–0.62) |
| NGAL (ng/mL), median (IQR) | 25.4 (9.6–54.4) | 22.7 (10.1–50.0) |
| KIM-1 (pg/mL), median (IQR) | 318.0 (167.7–530.9) | 309.8 (161.3–530.9) |
| RBP (ng/mL), median (IQR) | 73.1 (23.5–171.0) | 42.2 (19.0–144.8) |
| UCr (mg/dL), median (IQR) | 48.0 (27.9–77.2) | 44.3 (26.2–73.8) |
| Initial dose (mg)b, median (IQR) | 560 (450–640) | 563 (450–640) |
| Initial dose (mg/kg)b, median (IQR) | 11.0 (10.0–12.4) | 11.2 (10.2–14.0) |
| Tobramycin serum concentrations per subject over the entire study, median (IQR) | NA | 10 (6–17) |
NA, not applicable.
aSummary statistics based on first tobramycin course.
bAt time of tobramycin start.
PK analysis
A one-compartment model best described the concentration–time data for our population. Weight was an independent covariate on both V and CL. Derivation of the final population PK model to describe CL is shown in Table S1. Inclusion of age and female gender as covariates for CL did not significantly decrease the OFV, nor did SCr or eGFR. NGAL, NGAL/UCr, RBP and RBP/UCr all significantly reduced the OFV (P < 0.001) from the base model, while KIM-1, KIM-1/UCr and UCr did not (P > 0.01). NGAL provided the largest reduction in the OFV (−19.0). The addition of other covariates (biomarkers) to a model including NGAL did not further significantly improve model fit (P > 0.001). There was no collinearity between patient weight and NGAL (R2 = 0.076).
The final parameter estimates derived from the final model are shown in Table S2. Minimization and the covariance step were successful for the final model. Overall, the mean estimates of V and CL were 31.3 L/70 kg and 8.60 L/h/70 kg, respectively. The model estimates for CL were minimally different when restricted to the first tobramycin course per subject: 8.69 (first course only) versus 8.60 (all courses) L/h/70 kg. There were no significant differences in NGAL during course 1 versus courses 2–6 (P = 0.13).
Model evaluation
Goodness-of-fit/diagnostic plots are shown in Figures S1 and S2. There was good agreement between observed and predicted concentrations for both the population and individual predicted concentrations. The mean population parameter estimates obtained from the bootstrap procedures were similar to the estimates from the final model and were within the bounds of the 95% CIs, indicating little bias in the parameter estimates. PC-VPC demonstrated good agreement between observed and predicted concentrations by time after dose (Figure S3).
PD relationships
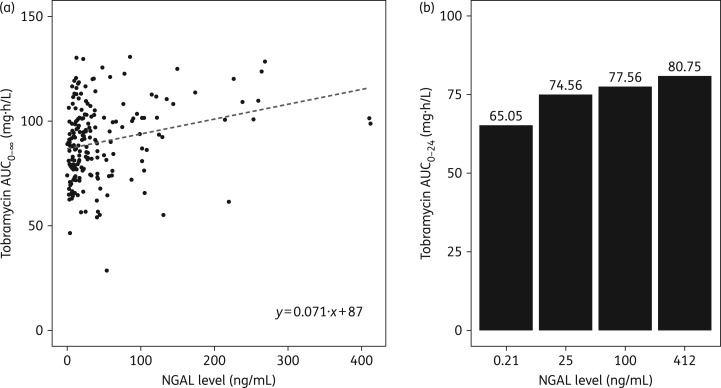
To further visualize the impact of NGAL on overall tobramycin exposure, we plotted the individual AUC0–∞ estimations of each subject versus NGAL concentrations (Figure 1a). Simulations were conducted to estimate tobramycin AUC0–24 using the final model with NGAL concentrations of 0.21, 25, 100 and 412 ng/mL, which correspond to NGAL levels that are the lowest, the median, a commonly used cut-off level for AKI34,35 and the highest reported in our study cohort, respectively (Figure 1b). As shown, tobramycin AUC increases with higher urine NGAL levels.
Figure 1.
Estimated AUC against urinary NGAL. (a) Scatterplot of estimated AUC0–∞ versus measured NGAL for individual subjects (N = 197 measurements). (b) Estimated tobramycin AUC0–24 using the final population PK model in 1000 simulations of NGAL concentrations of 0.21, 25, 100 and 412 ng/mL, which correspond to the lowest, median, cut-off level for AKI and highest NGAL levels reported in our study cohort, respectively.
PK parameters were estimated using Bayesian estimation with a validated clinical PK software program and our base one-compartment model (Table S1, Model 2) on 186 patient days with both biomarker and tobramycin concentrations available. The median Cmax and AUC0–24 over all study days were 26.9 mg/L (IQR 23.5–29.6) and 86.4 mg · h/L (IQR 75.5–98.5), respectively. The median biomarker values over all study days were: NGAL, 22.7 ng/mL (IQR 10.1–50.0); NGAL/UCr, 49.2 ng/mg (IQR 27.8–102.8); RBP, 42.2 ng/mL (IQR 19.0–144.8); RBP/UCr, 103.5 ng/mg (IQR 54.1–237.5); KIM-1, 309 pg/mL (IQR 161.3–530.9); and KIM-1/UCr, 604.3 pg/mg (IQR 407.1–1066.0). Median biomarker values were not significantly different when restricted only to the first tobramycin course for all subjects (data not shown). The between-subject correlation of subjects' weighted average PK parameter estimates and biomarker concentrations, after accounting for repeated measurements during tobramycin courses, are shown in Table 2. There was weak correlation between NGAL and dose (r = 0.25, P = 0.05), but not weight-adjusted dose (r = −0.18, P = 0.20). There was an inverse correlation between tobramycin CL and NGAL (r = −0.36, P = 0.01).
Table 2.
Between-subject correlations of weighted average biomarker concentrations and individual PK parameter estimatesa
|
Cmax |
AUC0–24 |
|||
|---|---|---|---|---|
| Biomarkerb | regression coefficient | P value | regression coefficient | P value |
| NGAL | 0.07 | 0.57 | 0.46 | <0.001 |
| NGAL/UCr | 0.19 | 0.15 | 0.33 | 0.009 |
| RBP | −0.19 | 0.16 | 0.15 | 0.25 |
| RBP/UCr | −0.15 | 0.26 | 0.03 | 0.80 |
| KIM-1 | 0.19 | 0.14 | 0.13 | 0.32 |
| KIM-1/UCr | 0.29 | 0.02 | −0.08 | 0.53 |
aAnalyses performed using 186 PK estimates and biomarker pairs, accounting for repeated measurements during 60 tobramycin courses.
bBiomarkers log-transformed for comparisons.
Results of univariate mixed-effects linear regression of clinical factors on NGAL are shown in Table S3. Dosage, Cmax, day of therapy, prior aminoglycoside courses and admission number during the study were not associated with NGAL. On multivariate analyses (Table 3), CL was inversely associated with NGAL after accounting for other significant covariates (age and receipt of two or more nephrotoxins): a decrease in CL of 1 L/h/70 kg corresponded to 14.5% higher NGAL values (95% CI 5.4%–24.7%; P < 0.01).
Table 3.
Multivariable linear mixed-effects model of covariates on log-transformed NGAL accounting for correlation within subjects and admissions
| Variablea | Estimatea (95% CI) | P value |
|---|---|---|
| CL (L/h/70 kg) | 0.855 (0.773–0.946) | 0.002 |
| Age (years) | 1.067 (1.014–1.124) | 0.01 |
| Receipt of ≥2 concomitant nephrotoxins | 1.881 (1.356–2.608) | <0.001 |
| Intercept | 30.257 (7.590–120.619) | <0.001 |
aBiomarkers were log-transformed for regression and coefficients reported have been exponentiated (10x) for display in this table. The percentage change in biomarker (y) per 1 U increase in the covariate can be calculated as y% = 100 × (x−1) where x is the coefficient reported above.
Discussion
This pilot study explored the relationship between urinary kidney injury biomarkers and tobramycin PK among a population of children and young adults with CF receiving once-daily tobramycin for a pulmonary exacerbation. Inclusion of urinary NGAL as a covariate on tobramycin CL led to the most significant improvement in our population model's fit. Further, when controlling for other factors that may influence NGAL, decreased CL was significantly associated with higher NGAL values. This association has not been previously described.
A number of population PK models for tobramycin have been developed for children with CF.41–44 The population estimate for CL in our study was similar to previously described models, although V was larger. The larger V may have been secondary to increased total body water: concurrent to the study, CCHMC pulmonology service patients received intravenous fluids prior to the initiation of tobramycin until their urine specific gravity was <1.010 as part of a quality improvement project to reduce AKI. As with previous models, we found that body weight was a significant covariate for both CL and V. Meanwhile, inclusion of SCr and eGFR did not improve our model fit for tobramycin CL. SCr and creatinine-based equations do not reliably estimate kidney function in patients with CF;4 therefore, it is not surprising that they are poor predictors of tobramycin CL as well. The inclusion of urinary biomarkers, in particular NGAL, did lead to an improved model for tobramycin CL. After NGAL was included in the model, the addition of other biomarkers did not further improve the model fit.
Our findings may have important clinical implications. Patients with CF are often infected with resistant Gram-negative organisms and large tobramycin doses may be necessary to achieve adequate peak concentrations when the MIC is >1 mg/L.45 Measurement of urinary NGAL could supplement traditional therapeutic drug monitoring practices and provide reassurance that appropriate tobramycin CL is sustained throughout therapy. Additionally, measurements of urinary NGAL in conjunction with tobramycin levels may allow for improved estimation of an individual patient's CL and facilitate AUC-driven dose adjustments. We did not find a significant association between tobramycin dose and NGAL, suggesting that the relationship between AUC and NGAL may be driven by CL (AUC = dose/CL). Although patients in our study had stable tobramycin CL over therapy, serial monitoring of urinary biomarkers can provide a non-invasive means to detect changes in renal elimination of tobramycin and avoid development of AKI. Future studies will be needed to further elucidate the clinical value of urinary biomarkers in the CF population.
We did not detect an association between biomarkers collected prior to tobramycin start and severity of lung disease as measured by pulmonary function testing (data not shown). In a study by Zughaier et al.,46 serum NGAL concentrations were higher in patients with CF compared with healthy controls, but values were similar when compared among patients with stable disease versus those experiencing a pulmonary exacerbation. Therefore, determination of normal serum and urinary NGAL values in patients with CF is necessary to promote its clinical usefulness.
We believe that our findings are also biologically plausible. First, the positive association between NGAL and tobramycin AUC0–24 may reflect an exposure–toxicity relationship where increased AUC0–24 resulting from large doses leads to direct tubular injury. However, no patients had a doubling of their creatinine (data not shown), suggesting possible subclinical injury detected best by urinary NGAL.47 Alternatively, increased biomarkers may reflect impaired CL and hence higher AUC. NGAL, RBP and tobramycin are all substrates for the same endocytic receptor in the proximal tubule, megalin, and increased excretion of urinary NGAL and RBP during tobramycin therapy may result from impaired tubular reabsorption. Although the effects of aminoglycoside exposure on the kidney are complex,48 tubular dysfunction is the initiating event leading to aminoglycoside nephrotoxicity. Therefore, the inverse relationship between NGAL and tobramycin CL is rational. Future studies involving patients with a range of underlying kidney function may further elucidate the mechanism underlying our findings.
There are limitations to our study. First, the biological variability in urinary biomarkers among patients with CF has not been studied. We measured urinary biomarkers once daily, but the timing between tobramycin administration and urinary biomarker measurement was not assessed. Fluctuations in biomarkers or tobramycin CL occurring over the course of a day may have affected the relationship between the biomarkers and CL. However, this would likely bias the association between biomarkers and PK parameters towards the null and should not discredit the relationship between NGAL, RBP and CL. Second, our study was conducted as part of routine clinical care. Most tobramycin concentrations fell in two time periods: 2–4 or 9–11 h. Additional sampling times would be needed to estimate PK parameters using a two-compartment model. Lastly, inclusion of urinary biomarkers into a model of antimicrobial population PK is a novel approach. We believe that there is biological plausibility to our findings, as detailed above. However, studies are needed that further explore and validate the PK/PD relationship described here; investigation of serum and urinary biomarkers may provide further evidence for these associations.
Urinary biomarkers hold promise for prognostication and early detection of kidney injury. They also may provide information relating to functional processes at sites within the kidney. We describe for the first time that inclusion of urinary biomarkers, most specifically NGAL, in a population model for tobramycin CL improves model fit more so than traditional renal function markers of creatinine and eGFR among children and young adults with CF. Future studies are needed to validate and further explore the relationships between urinary biomarkers and tobramycin PK/PD in CF and other populations.
Funding
K. J. D. was supported by a Thrasher Research Fund Early Career Award (Thrasher Award number 9189). K. J. D. and M. D. were supported by the National Institute of Child Health and Human Development of the National Institutes of Health under award number 5T32HD069054, Cincinnati Training Program in Pediatric Clinical and Developmental Pharmacology. The study was also supported by a CCHMC Cystic Fibrosis Research and Development Program Pilot & Feasibility program grant (to K. J. D.). J. P. C. receives grant support from the National Heart, Lung, and Blood Institute of the National Institutes of Health (NHLBI-FOA-HL 12–035) and the Cystic Fibrosis Foundation (R457-CR11, AMIN09YO). S. L. G. is supported in part by the Agency for Healthcare Research and Quality Center for Education and Research on Therapeutics grant (AHRQ CERT 1U19HS021114).
Transparency declarations
None to declare.
Supplementary data
Acknowledgements
Portions of this work were presented as abstracts at CRRT 2014 (abstract no. 46), San Diego, CA, USA, March 2014, Pediatric Academic Societies Meeting (abstract no. 2908.100), Vancouver, Canada, May 2014 and IDWeek 2014 (abstract no. 399), Philadelphia, PA, USA, October 2014.
We thank Amanda Dressman, NP and Jeanne Weiland, NP for their help with subject recruitment and coordination of study procedures and Qing Ma for performance of urinary biomarker measurements.
References
- 1.Flume PA, Mogayzel PJ Jr, Robinson KA et al. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med 2009; 180: 802–8. [DOI] [PubMed] [Google Scholar]
- 2.Mouton JW, Jacobs N, Tiddens H et al. Pharmacodynamics of tobramycin in patients with cystic fibrosis. Diagn Microbiol Infect Dis 2005; 52: 123–7. [DOI] [PubMed] [Google Scholar]
- 3.Bertino JS Jr, Booker LA, Franck PA et al. Incidence of and significant risk factors for aminoglycoside-associated nephrotoxicity in patients dosed by using individualized pharmacokinetic monitoring. J Infect Dis 1993; 167: 173–9. [DOI] [PubMed] [Google Scholar]
- 4.Al-Aloul M, Jackson M, Bell G et al. Comparison of methods of assessment of renal function in cystic fibrosis (CF) patients. J Cyst Fibros 2007; 6: 41–7. [DOI] [PubMed] [Google Scholar]
- 5.Soulsby N, Greville H, Coulthard K et al. What is the best method for measuring renal function in adults and children with cystic fibrosis? J Cyst Fibros 2010; 9: 124–9. [DOI] [PubMed] [Google Scholar]
- 6.Alghanem S, Paterson I, Touw DJ et al. Influence of multiple courses of therapy on aminoglycoside clearance in adult patients with cystic fibrosis. J Antimicrob Chemother 2013; 68: 1338–47. [DOI] [PubMed] [Google Scholar]
- 7.Drusano GL, Louie A. Optimization of aminoglycoside therapy. Antimicrob Agents Chemother 2011; 55: 2528–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Streetman DS, Nafziger AN, Destache CJ et al. Individualized pharmacokinetic monitoring results in less aminoglycoside-associated nephrotoxicity and fewer associated costs. Pharmacotherapy 2001; 21: 443–51. [DOI] [PubMed] [Google Scholar]
- 9.van Lent-Evers NA, Mathot RA, Geus WP et al. Impact of goal-oriented and model-based clinical pharmacokinetic dosing of aminoglycosides on clinical outcome: a cost-effectiveness analysis. Ther Drug Monit 1999; 21: 63–73. [DOI] [PubMed] [Google Scholar]
- 10.Devarajan P. Biomarkers for the early detection of acute kidney injury. Curr Opin Pediatr 2011; 23: 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra J, Mori K, Ma Q et al. Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol 2004; 24: 307–15. [DOI] [PubMed] [Google Scholar]
- 12.Gaspari F, Cravedi P, Mandala M et al. Predicting cisplatin-induced acute kidney injury by urinary neutrophil gelatinase-associated lipocalin excretion: a pilot prospective case–control study. Nephron Clin Pract 2010; 115: c154–60. [DOI] [PubMed] [Google Scholar]
- 13.Mishra J, Dent C, Tarabishi R et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005; 365: 1231–8. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch R, Dent C, Pfriem H et al. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol 2007; 22: 2089–95. [DOI] [PubMed] [Google Scholar]
- 15.Wheeler DS, Devarajan P, Ma Q et al. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit Care Med 2008; 36: 1297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mori K, Lee HT, Rapoport D et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest 2005; 115: 610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hvidberg V, Jacobsen C, Strong RK et al. The endocytic receptor megalin binds the iron transporting neutrophil-gelatinase-associated lipocalin with high affinity and mediates its cellular uptake. FEBS Lett 2005; 579: 773–7. [DOI] [PubMed] [Google Scholar]
- 18.Moestrup SK, Cui S, Vorum H et al. Evidence that epithelial glycoprotein 330/megalin mediates uptake of polybasic drugs. J Clin Invest 1995; 96: 1404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuwabara T, Mori K, Mukoyama M et al. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int 2009; 75: 285–94. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki D, Yamada A, Umeno H et al. Comparison of the course of biomarker changes and kidney injury in a rat model of drug-induced acute kidney injury. Biomarkers 2011; 16: 553–66. [DOI] [PubMed] [Google Scholar]
- 21.Christensen EI, Moskaug JO, Vorum H et al. Evidence for an essential role of megalin in transepithelial transport of retinol. J Am Soc Nephrol 1999; 10: 685–95. [DOI] [PubMed] [Google Scholar]
- 22.Bernard AM, Vyskocil AA, Mahieu P et al. Assessment of urinary retinol-binding protein as an index of proximal tubular injury. Clin Chem 1987; 33: 775–9. [PubMed] [Google Scholar]
- 23.Ferguson MA, Vaidya VS, Bonventre JV. Biomarkers of nephrotoxic acute kidney injury. Toxicology 2008; 245: 182–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glass S, Plant ND, Spencer DA. The effects of intravenous tobramycin on renal tubular function in children with cystic fibrosis. J Cyst Fibros 2005; 4: 221–5. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Vaidya VS, Brown RP et al. Comparison of kidney injury molecule-1 and other nephrotoxicity biomarkers in urine and kidney following acute exposure to gentamicin, mercury, and chromium. Toxicol Sci 2008; 101: 159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiusolo A, Defazio R, Zanetti E et al. Kidney injury molecule-1 expression in rat proximal tubule after treatment with segment-specific nephrotoxicants: a tool for early screening of potential kidney toxicity. Toxicol Pathol 2010; 38: 338–45. [DOI] [PubMed] [Google Scholar]
- 27.Vaidya VS, Ford GM, Waikar SS et al. A rapid urine test for early detection of kidney injury. Kidney Int 2009; 76: 108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen F, Smith R, Gu YZ et al. Toxicoepigenetic alteration of the kidney injury molecule 1 gene in gentamicin-exposed rat kidney. Toxicol Sci 2010; 117: 375–80. [DOI] [PubMed] [Google Scholar]
- 29.Sieber M, Hoffmann D, Adler M et al. Comparative analysis of novel noninvasive renal biomarkers and metabolomic changes in a rat model of gentamicin nephrotoxicity. Toxicol Sci 2009; 109: 336–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lahiri T, Guillet A, Diehl S et al. High-dose ibuprofen is not associated with increased biomarkers of kidney injury in patients with cystic fibrosis. Pediatr Pulmonol 2014; 49: 148–53. [DOI] [PubMed] [Google Scholar]
- 31.Chaturvedi S, Farmer T, Kapke GF. Assay validation for KIM-1: human urinary renal dysfunction biomarker. Int J Biol Sci 2009; 5: 128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz GJ, Munoz A, Schneider MF et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 2009; 20: 629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41. [DOI] [PubMed] [Google Scholar]
- 34.Parikh CR, Coca SG, Thiessen-Philbrook H et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol 2011; 22: 1748–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verna EC, Brown RS, Farrand E et al. Urinary neutrophil gelatinase-associated lipocalin predicts mortality and identifies acute kidney injury in cirrhosis. Dig Dis Sci 2012; 57: 2362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bennett M, Dent CL, Ma Q et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol 2008; 3: 665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Proost JH, Meijer DK. MW/Pharm, an integrated software package for drug dosage regimen calculation and therapeutic drug monitoring. Comput Biol Med 1992; 22: 155–63. [DOI] [PubMed] [Google Scholar]
- 38.Rougier F, Claude D, Maurin M et al. Aminoglycoside nephrotoxicity: modeling, simulation, and control. Antimicrob Agents Chemother 2003; 47: 1010–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rougier F, Ducher M, Maurin M et al. Aminoglycoside dosages and nephrotoxicity: quantitative relationships. Clin Pharmacokinet 2003; 42: 493–500. [DOI] [PubMed] [Google Scholar]
- 40.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: part 2—correlation between subjects. BMJ 1995; 310: 633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sherwin CM, Zobell JT, Stockmann C et al. Pharmacokinetic and pharmacodynamic optimisation of intravenous tobramycin dosing among children with cystic fibrosis. J Pharmacokinet Pharmacodyn 2014; 41: 71–9. [DOI] [PubMed] [Google Scholar]
- 42.Hennig S, Norris R, Kirkpatrick CM. Target concentration intervention is needed for tobramycin dosing in paediatric patients with cystic fibrosis—a population pharmacokinetic study. Br J Clin Pharmacol 2008; 65: 502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hennig S, Standing JF, Staatz CE et al. Population pharmacokinetics of tobramycin in patients with and without cystic fibrosis. Clin Pharmacokinet 2013; 52: 289–301. [DOI] [PubMed] [Google Scholar]
- 44.Massie J, Cranswick N. Pharmacokinetic profile of once daily intravenous tobramycin in children with cystic fibrosis. J Paediatr Child Health 2006; 42: 601–5. [DOI] [PubMed] [Google Scholar]
- 45.Butterfield JM, Lodise TP, Beegle S et al. Pharmacokinetics and pharmacodynamics of once-daily administration of intravenous tobramycin in adult patients with cystic fibrosis hospitalized for an acute pulmonary exacerbation. Antimicrob Agents Chemother 2013; 57: 5175–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zughaier SM, Tangpricha V, Leong T et al. Peripheral monocytes derived from patients with cystic fibrosis and healthy donors secrete NGAL in response to Pseudomonas aeruginosa infection. J Investig Med 2013; 61: 1018–25. [DOI] [PubMed] [Google Scholar]
- 47.Haase M, Devarajan P, Haase-Fielitz A et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol 2011; 57: 1752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mingeot-Leclercq MP, Tulkens PM. Aminoglycosides: nephrotoxicity. Antimicrob Agents Chemother 1999; 43: 1003–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.