Abstract
BACKGROUND
This single arm, open-label trial was designed to evaluate the activity of apitolisib (GDC-0980), a dual PI3K/mTOR inhibitor, in patients with advanced endometrial cancer (EC).
METHODS
Patients with recurrent or persistent EC treated with 1–2 prior lines of chemotherapy but no prior PI3K/mTOR inhibitor received oral apitolisib 40 mg daily during 28-day cycles until progression or intolerable toxicity. Type I/II diabetic patients requiring insulin were excluded. The primary endpoints were progression-free survival (PFS) at 6 months and objective response rate (ORR).
RESULTS
A total of 56 women were enrolled including 13 (23%) with well-controlled diabetes. Discontinuation reasons were disease progression (24, 43%), adverse events (13, 23%), and withdrawal by subject (12, 21%). Grade 3/4 apitolisib-related adverse events were hyperglycemia (46%), rash (30%), colitis (5%), and pneumonitis (4%). PFS at 6 months was 20% (KM estimate 95% CI: 7%–33%). ORR was 6% (confirmed). Median PFS was 3.5 months (95% CI: 2.7–3.7 months); median OS was 15.7 months (95% CI: 9.2–17.0 months). Nineteen patients discontinued prior to first tumor assessment. Dose reductions were required for 4 (31%) diabetic and 18 (42%) non-diabetic patients. Comprehensive molecular profiling of 46 evaluable archival tumor samples showed 57% of patients had at least one alteration in PIK3CA, PTEN, or AKT1. All three patients with a confirmed response had at least one alteration in a PI3K pathway gene.
CONCLUSION
Anti-tumor activity of 40 mg apitolisib daily was limited by tolerability, especially in diabetic patients. Patients with PI3K pathway mutation may have derived enhanced benefit from apitolisib.
Keywords: apitolisib, GDC-0980, MAGGIE, endometrial cancer
INTRODUCTION
The phosphatidylinositol 3-kinase/mammalian target of rapamycin (PI3K/mTOR) pathway plays a central role in regulating physiological processes including growth, survival, proliferation, and metabolism, so that its aberrant activation results in the development of malignant disease.1, 2 In endometrial cancer, genomic characterization has identified the PI3K/mTOR pathway as being central to pathogenesis,3 and analyses of large series of tumors by the Cancer Genome Atlas (TCGA) Research Network have found this pathway to be activated in >80% of primary surgical endometrial cancer specimens.4 This disease results in 8,590 women dying in the US annually.5 While carboplatin and paclitaxel are the standard first-line treatment regimen for advanced recurrent or persistent disease,6, and with the exception of megesteol acetate for the palliative treatment of advanced endometrial carcinoma, there are no FDA-approved treatments for patients who progress on first-line therapy.
Based on genetic lesions associated with dysregulation of PI3K-signaling in endometrial cancer, single-target mTOR inhibitors have been evaluated in clinical trials in advanced/recurrent disease and have resulted in clinical benefit rates (CBR) of 21%–83%.7–10 Such encouraging activity has generated interest in expanding to dual-target inhibitors targeting PI3K as well as mTOR. Notably though, the availability of extensive molecular correlates for response to therapeutic inhibitors in endometrial cancer is lacking. Here, we report final results of a phase II clinical trial of single agent apitolisib, a potent pan-inhibitor of class I PI3K (inhibiting both wild-type and commonly mutated p110α isoforms) and mTORC1 and mTORC2 kinases.11 We assessed the activity of apitolisib in patients with recurrent or persistent endometrial cancer, and provide extensive biomarker analysis as well. To date, no PI3K/mTOR dual-target inhibitor has been approved for cancer although several molecules are under investigation.
MATERIALS AND METHODS
This was a phase 2, open-label trial. The primary objective was to assess the activity of apitolisib in patients with recurrent or persistent endometrial cancer as measured by progression-free survival (PFS) at 6 months and objective response rate (ORR). As pre-specified secondary endpoints, we also evaluated toxicity, median PFS, and median overall survival (OS). Molecular biomarkers were evaluated for correlation with response to therapy. Pharmacokinetics of apitolisib was also assessed (Supplemental Information).12 The study protocol was approved by the institutional review boards at all participating institutions. All patients gave written informed consent. The study (ClinicalTrials.gov: NCT01455493) was conducted according to good clinical practice and the Declaration of Helsinki and its amendments.
Patients and Population
Patients with recurrent or persistent endometrial cancer that was refractory to curative therapy or established treatments were enrolled from 23 centers within the United States. Patients with carcinosarcoma or sarcoma were excluded. Other eligibility criteria included age ≥18 years, Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, measurable disease by RECIST 1.1,13 one but no more than two prior chemotherapy regimens, and adequate organ function. Fasting blood glucose requirements were ≤135 or <160 mg/dL and HbA1c <7.0% or <8.5% for patients without or with type II diabetes, respectively. The latter group also required stable regimen of oral-anti-hyperglycemic therapy without insulin usage for at least 3 weeks prior to first study treatment. Pretreatment hematologic, renal, and hepatic function tests were required to be grade 0 or 1 according to National Cancer Institute Common Terminology Criteria for Adverse Events [CTCAE] (version 4.0).
Treatment Plan, Safety Monitoring, and Response Evaluation
Patients received apitolisib on an outpatient basis as a daily 40 mg oral dose, starting on day 1 of each 28-day cycle and continuing until disease progression, intolerable toxicity, elective withdrawal from the study, or study completion. Dose administration could be temporarily suspended for up to 28 days if patients experienced toxicity related to the study drug, assessed using CTCAE version 4.0. Patients who missed >28 consecutive days of scheduled study treatment because of drug-related adverse events (AEs) were discontinued from the study. Protocol mandated expedited reporting of target adverse events such as hyperglycemia, pneumonitis and rash and management guidelines were provided in the protocol for pneumonitis and hyperglycemia, as described in supplemental information. A committee reviewed safety outcomes periodically.
Efficacy assessments were performed every 8 weeks from the initiation of study treatment until disease progression and evaluated according to RECIST v1.1. The primary efficacy endpoints were objective tumor response (the occurrence of a complete response [CR] or partial response [PR]) and PFS at 6 months (the time from first treatment with apitolisib to disease progression or to an event of death from any cause within 30 days of last study treatment). In order to be considered an objective tumor response for the primary endpoint, confirmation by repeat imaging performed ≥28 days after the criteria for response were first met was required. For the purpose of exploratory analyses, patients with a 30% or better reduction in target tumor size but without a follow-up confirmatory scan were considered unconfirmed responders.
An internal monitoring committee, convened three times during the conduct of the study to evaluate interim safety and pharmacokinetic data. Based on the available safety information, the dose reduction and management guidelines in place, no changes to the conduct of the study were recommended during conduct of the trial.
Biomarker Studies
Biomarker analyses were conducted on archival tumor tissue using a targeted next-generation sequencing platform, MMP-seq, as previously described.14 The assay utilized a tiled PCR-based enrichment strategy to amplify 963 amplicons covering 88 oncogenes and tumor suppressors, followed by sequencing on the Illumina GAIIx platform. The assay gave coverage of PIK3CA, PTEN, AKT1, PIK3R1, PIK3R2, and a number of other PI3K- and mTOR-related genes relevant to this patient population. PTEN immunohistochemistry (antibody clone CST 138G6) was performed as previously described.15 Copy number alterations for ERBB2, IGF1R, MET, MYC, and CCNE1 were assessed by real-time quantitative PCR using an Eva Green detection system.16
Statistical Design and Analysis
This study was designed to estimate ORR and PFS rate at 6 months with reasonable precision to contrast the results with historical data with ineffective agents in a similar patient population, with ORR <10% and PFS rate at 6 months <20%. In particular, the study was designed to mirror the patient population, sample size, and primary clinical endpoints of other single-arm cohort studies of endometrial cancer patients conducted by the Gynecologic Oncology Group (GOG).17, 18 However, this study was not designed to have adequate power to distinguish between a minimum clinically meaningful difference and null rates. An estimate and 95% confidence interval (CI) were calculated for ORR, as well as for Kaplan-Meier estimates of PFS at 6 months, median PFS, and median OS. The primary analysis occurred 6 months after approximately 50 efficacy-evaluable patients had been enrolled.
Patients without post-baseline tumor assessments were considered non-responders. Data for patients without documented disease progression or death within 30 days of last study treatment were censored for PFS at the date of the last tumor assessment (or, if no tumor assessments were performed after the baseline visit, at the date of first treatment with apitolisib plus 1 day). Data for patients who withdrew from the study or who were lost to follow-up were censored at the last date of tumor assessment at which the patient was known to be progression-free. OS was defined as the time from first treatment with apitolisib until death by any cause.
Descriptive statistics were used to summarize the demographic and clinical characteristics of patients, and apitolisib Cmin and Cmax parameters. Safety analyses included all patients who received at least one dose of study treatment. Pharmacokinetic analyses included all patients who received at least one dose of study treatment and who had blood samples suitable for analysis. Efficacy analyses included all patients who received at least one prior systemic therapy and who received at least one dose of study treatment.
RESULTS
Study Population and Efficacy
From October 2011 to February 2014, 56 patients enrolled (Figure 1), whose baseline characteristics are provided in Table 1. One of the 56 enrolled patients was not evaluable due to not having received at least one prior chemotherapeutic regimen, although this patient was included in the safety and pharmacokinetic analyses. The median patient observation time from treatment initiation until the last available assessment for each patient was 11.6 months. The majority of the efficacy-evaluable patients (52/55 patients; 95%) were non-responders. Objective response was 6%: three patients, all without diabetes, had follow-up scans confirming CR in two patients and PR in one patient (Table 1). One patient with a confirmed CR had target lymph node involvement and therefore did not have 100% reduction in RECIST target lesion measurements, as the lymph node returned to normal size (~1cm, Figure 2). For the purpose of additional exploratory analyses, two patients with target lesion reductions of 30% or better, but without documented follow-up scan data, were considered unconfirmed responders (Figure 2).
Figure 1.
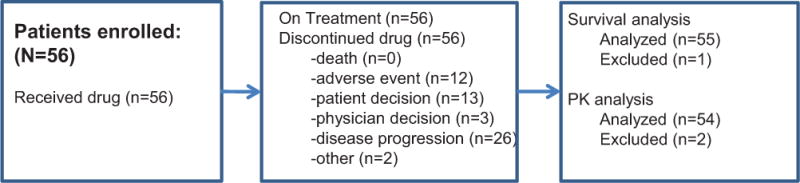
Consort diagram.
TABLE 1.
Patient demographics and efficacy
| Characteristics | No. of Patients (N=56) |
% |
|---|---|---|
| Age, years | ||
| Median | 65.5 | |
| Range | 30–81 | |
| Age Group | ||
| <65 | 27 | 48.2 |
| >=65 | 29 | 51.8 |
| Race | ||
| White | 45 | 80.4 |
| African American | 6 | 10.7 |
| Asian | 3 | 5.4 |
| American Indian | 2 | 3.6 |
| Baseline ECOG Performance Status | ||
| 0 | 28 | 50.0 |
| 1 | 27 | 48.2 |
| 2 | 1 | 1.8 |
| Histology | ||
| Endometrioid | 33 | 58.9 |
| Serous | 17 | 30.4 |
| Clear Cell | 2 | 3.6 |
| Other/Mixed | 4 | 7.1 |
| No. of Prior Chemotherapy Regimens | ||
| 0 | 1 | 1.8 |
| 1 | 29 | 51.8 |
| 2 | 26 | 46.4 |
| Prior Radiation Therapy | 30 | 53.6 |
| Confirmed Response | ||
| Complete Response | 2 | 3.6 |
| Partial Response | 1 | 1.8 |
| Stable Disease | 27 | 49.1 |
| Progressive Disease | 11 | 20.0 |
| Missing/Unevaluable | 14 | 25.5 |
| Progression-Free Survival (PFS) | ||
| Patients with PFS >6 months | 5 | 9.1 |
| Kaplan-Meier Estimate, PFS >6 months (%, 95% CI) | 20% (6.9%, 33.2%) | |
| Patients contributing a PFS event | 37 | 67.3 |
| Median PFS (months, 95% CI) | 3.5 (2.7, 3.7) | |
| Overall Survival (OS) | ||
| Patients contributing an OS event | 32 | 58.2 |
| Median OS (months, 95% CI) | 15.7 (9.2, 17.0) | |
Figure 2.
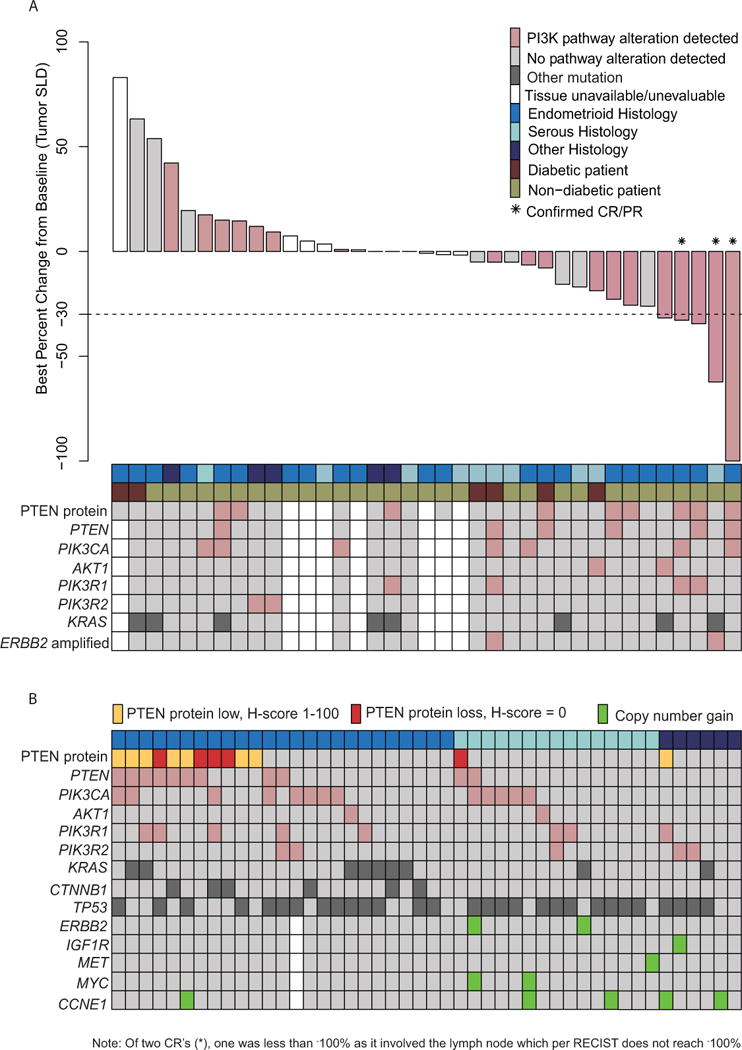
RECIST response and correlative biomarker data. (A) Best percent change from baseline. (B) Distribution of PI3K pathway alterations and other commonly altered genes across 46 endometrial cancer tumor samples sorted by histology. Clear cell and mixed histology tumor samples were categorized as “other.” PTEN mutations include frameshift and nonsense alterations only. PIK3CA mutations include missense alterations only.
Kaplan-Meier estimate of PFS at 6 months was 20% (95% CI: 7%, 33%) (Table 1, Figure 3). Median time to progression or death within 30 days of last study treatment was 5.7 months (95% CI: 1.1 months, 14.8 months). At the time of analysis, 58% of patients (32/55) experienced death while on study, including during survival follow-up, with median OS time of 15.7 months (95% CI: 9.2 months, 17.0 months; Table 1, Figure 3).
Figure 3.
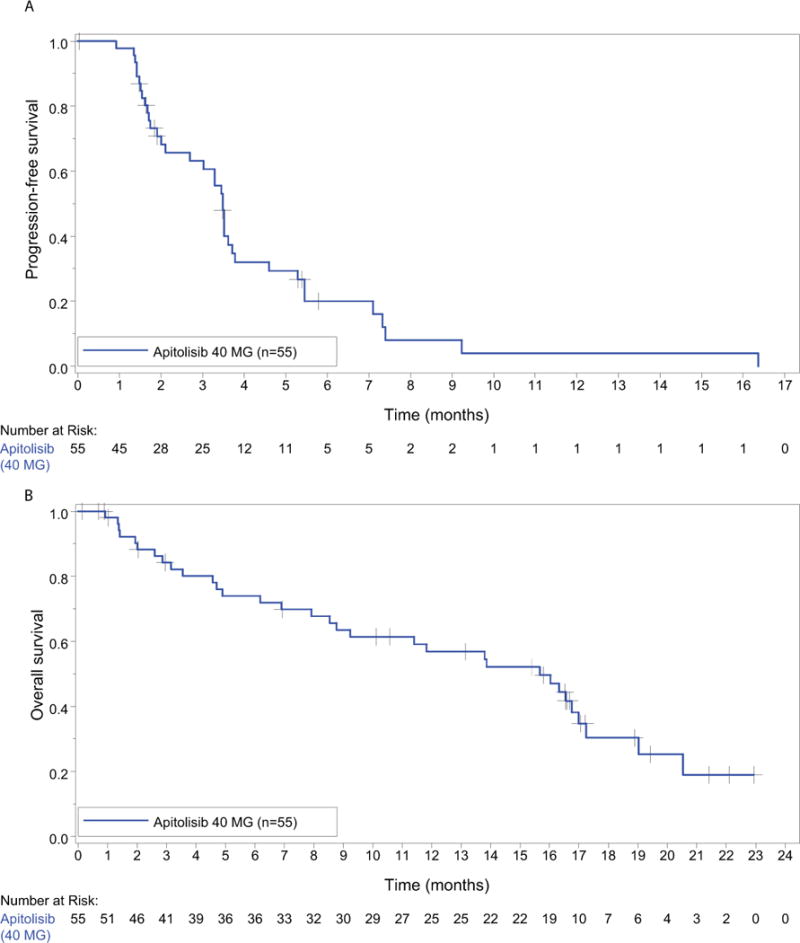
Kaplan-Meier graphs for progression-free survival and overall survival.
Treatment Exposure
A total of 54 patients were included in the pharmacokinetic-evaluable patient population. Comparison of median apitolisib Cmax suggested no difference between weeks 1, 5, and 9 values (0.469 μM, 0.447 μM, and 0.450 μM, respectively), with individual patient values ranging from 0.004 μM to 1.71 μM across all times. Generally, PK of apitolisib in the MAGGIE study was consistent with that in other apitolisib studies.19, 20
The median duration of treatment was 57 days (range 4–500). Median time on study was 69 (range 12–226) days for non-diabetic patients and 27 (range 4–84) days for diabetic patients. Nineteen patients discontinued prior to first tumor assessment; 8/13 diabetic patients discontinued prior to cycle 2 due to hyperglycemia (11/43 non-diabetic patients). Twenty-two of 56 (39%) patients required a dose reduction. All patients (56/56) discontinued study drug for reasons including disease progression (46%), withdrawal by the subject (23%), AEs (21%), physician’s decision (5%), and other reason (3%). Five of 13 (39%) diabetic patients and 7/43 (16%) non-diabetic patients discontinued study drug due to AEs. Two out of 13 (15%) diabetic patients and 24/43 (56%) non-diabetic patients discontinued study drug due to progression of disease.
Concomitant medications for diabetic patients verses non-diabetic patients included anti-diarrheal medications (23% vs. 42%), ≥1 treatment with biguanide metformin (23% vs. 21%), ≥1 treatment of oral hypoglycemic (acarbose, saxagliptin, or sitagliptin phosphate) (15% vs 2%), ≥1 treatment with sulfonylurea (23% vs. 14%), ≥1 treatment with insulin derivative (54% vs. 19%), and ≥1 treatment with steroid medications (23% vs. 63%).
Toxicity Profile and Safety
AEs were observed in all patients (56/56) of whom 94% (53/56) experienced treatment-related AEs. The most common treatment-related AEs reported in ≥10% of patients included hyperglycemia (32/56, 57%), fatigue (28/56, 50%), nausea (27/56, 48%), diarrhea (24/56, 43%), rash (21/56, 55%), decreased appetite (15/56, 27%), vomiting (14/56, 25%), and stomatitis and decreased weight each reported in 21% of patients (12/56) (Table 2). Treatment-related grade ≥3 AEs reported in 79% (44/56) of patients included hyperglycemia (23/56, 41%), diarrhea (11/56, 20%), and rash (11/56, 20%) (Table 2). A total of 59% (33/56) of patients experienced at least one serious AE (SAE) during the study, of which 32% (18/56) were treatment-related.
TABLE 2.
Treatment related adverse events in ≥ 10% patients by all grades and grade ≥ 3
| Adverse Event | All Grades | Grade ≥ 3 | ||
|---|---|---|---|---|
| n | % | n | % | |
| Hyperglycemia | 32 | 57.1 | 23 | 41.1 |
| Rash | 31 | 55.4 | 17 | 30.4 |
| Fatigue | 28 | 50.0 | 5 | 8.9 |
| Nausea | 27 | 48.2 | 1 | 1.8 |
| Diarrhea | 24 | 42.9 | 11 | 19.6 |
| Decreased Appetite | 15 | 26.8 | 2 | 3.6 |
| Vomiting | 14 | 25.0 | 0 | 0 |
| Stomatitis | 12 | 21.4 | 3 | 5.4 |
| Weight Decreased | 12 | 21.4 | 1 | 1.8 |
| Dysgeusia | 7 | 12.5 | 0 | 0 |
| Hypokalemia | 7 | 12.5 | 3 | 5.4 |
| Hypomagnesemia | 7 | 12.5 | 1 | 1.8 |
| Abdominal Pain | 6 | 10.7 | 1 | 1.8 |
| Anemia | 6 | 10.7 | 1 | 1.8 |
| Constipation | 6 | 10.7 | 0 | 0 |
| Dehydration | 6 | 10.7 | 1 | 1.8 |
| Dry Mouth | 6 | 10.7 | 0 | 0 |
| Mucosal Inflammation | 6 | 10.7 | 3 | 5.4 |
| Pyrexia | 6 | 10.7 | 0 | 0 |
AEs led to dose modification/interruption in 77% (43/56) and to withdrawal of study treatment in 30% (17/56). SAEs led to dose modification or interruption in 32% (18/56) of patients and to withdrawal of study treatment in 16% (9/56).
The incidence of patient deaths was 57% (32/56), inclusive of five patient deaths reported within 30 days of their last study treatment. The primary cause of death was disease progression in 25 patients, and multi-organ failure and kidney failure in one patient each, neither of which was attributed to the study drug. The primary cause of death was reported as unknown in five patients. In each of these five cases, the patient died 52 days or more after stopping apitolisib treatment.
Biomarker Analyses
In order to better understand molecular determinants of response to PI3K/mTOR inhibition, we performed comprehensive molecular profiling consisting of targeted Next Generation Sequencing (NGS), copy number analysis (CNA) for 40 cancer-related genes, and immunohistochemistry (IHC) for PTEN protein. PTEN protein loss by IHC was associated with frameshift or nonsense mutations as determined by the NGS assay (Figure 4). Composite analysis across the 46 patient samples with evaluable tissue showed that 67% of patients had at least one alteration in the PI3K pathway (Figure 2). Endometrioid patients had a higher frequency of PI3K pathway alterations compared to serous patients (76% vs 67%), whereas copy number gains were found almost exclusively in serous, clear cell, and mixed histology cancers (Figure 2). Missense mutations in PIK3CA or complete loss of PTEN were found in 28% and 11% of patients, respectively (Figure 2, Table 3), with higher prevalence in endometrioid tumors relative to other histological subtypes. Concomitant PI3K pathway activating alterations were found in 24% of patients. The three patients with confirmed response and the two with unconfirmed response all had an alteration in either PTEN, PIK3CA, AKT1, or ERBB2 (Figure 2). Among patients with PIK3CA, PTEN, AKT1, or ERBB2 alterations, the 5 patients who achieved response (3 confirmed and 2 unconfirmed) had higher drug exposures (AUCss) after two weeks of treatment compared to those whose best response was stable disease (Figure 5A). In addition, patients without these PI3K alterations had exposure levels similar to those who achieved responses, but exhibited either progressive or stable disease (Figure 5B).
Figure 4.
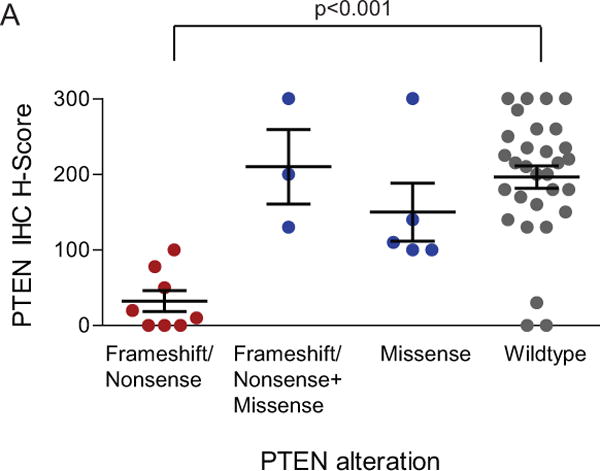
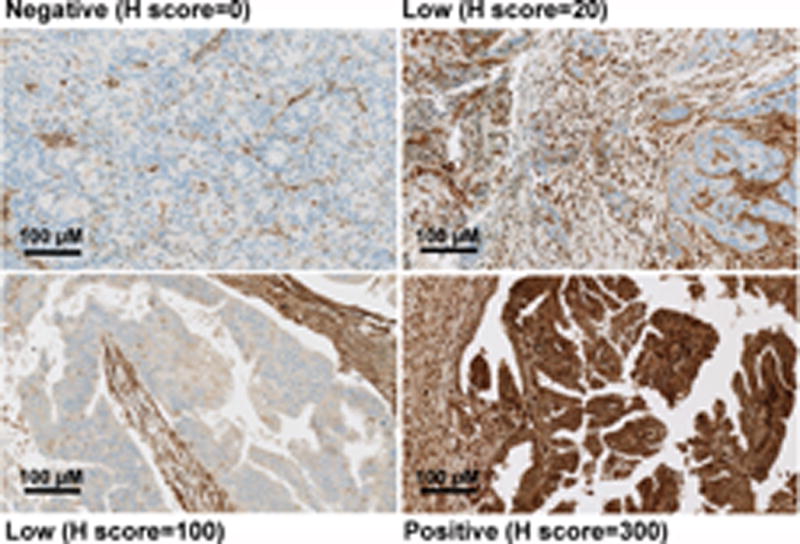
Correlation of PTEN protein expression and alteration type in MAGGIE study endometrial cancer tumor samples. (A) Scatter plot of PTEN H-scores grouped by alteration type. Each point represents the H-score from a single tumor sample. The horizontal line for each group represents the mean H-score +/− the standard error. Wilcoxon rank sum test P value shown for frameshift or nonsense vs wildtype. (B) Examples of PTEN IHC staining from representative endometrial cancer samples, ranging from total absence of PTEN in the tumor compartment (H-score 0), to PTEN expression in tumor cells equivalent to surrounding normal and stromal cells (H-score 300).
TABLE 3.
Prevalence of PI3K pathway alterations in MAGGIE study endometrial cancer tumor samples.
| Overall | Endometrioid | Serous | * Bourgon et al. | Cheung et al. | TCGA endometrioid | TCGA serous | |
|---|---|---|---|---|---|---|---|
| n = 46 | n = 25 | n = 15 | n = 73 | n = 243 | |||
| PTEN protein loss | 11% | 16% | 7% | 38% | 49% | NA | NA |
| PTEN mutation | 35% | 48% | 20% | 63% | 44% | 65% | 10% |
| PTEN mutation1 | 24% | 36% | 13% | ||||
| PIK3CA mutation | 35% | 32% | 47% | 58% | 40% | 56% | 42% |
| PIK3CA mutation2 | 28% | 32% | 33% | ||||
| AKT1 mutation | 4% | 4% | 7% | 11% | 0.8% | 9% | 4% |
| PIK3R1 mutation | 20% | 20% | 20% | 29% | 20% | 22% | 12% |
| PIK3R2 mutation | 11% | 8% | 7% | 19% | 5% | NA | NA |
96% of samples of endometrioid histology
PTEN frameshift and nonsense mutations only
PIK3CA missense mutations only
Figure 5.
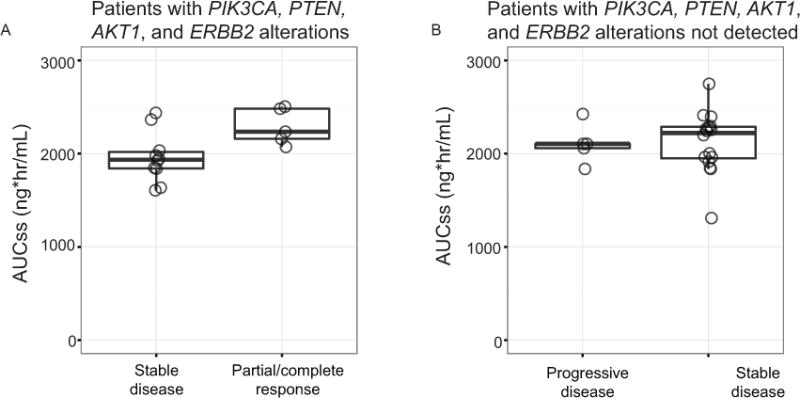
Apitolisib exposure and patient response. Scatter plot showing AUC levels (ng*hr/mL) as a measure of drug exposure for (A) patients with tumors harboring a PIK3CA, PTEN, AKT1, or ERBB2 alteration, or (B) patients without these alterations. Patients are grouped according to tumor response. Partial/complete response includes 3 confirmed and 2 unconfirmed patients.
DISCUSSION
In this study, tolerability of apitolisib at 40 mg QD was limited and resulted in either treatment discontinuation prior to first tumor assessment or limited dose intensity in a large proportion of patients. The short treatment times and reduced dose intensity posed a challenge in interpreting the lack of efficacy observed at this dose of apitolisib, and consequently provided only limited information regarding the therapeutic potential of PI3K/mTOR dual-target inhibitors in refractory endometrial cancer. Patients here had higher rates of grade 3 rash than anticipated from AEs reported in phase I patients treated with apitolisib 40 mg QD. Other common AEs included hyperglycemia, fatigue, nausea, and diarrhea. Despite appropriate management of these AEs, approximately one-third of the patient population discontinued protocol therapy prior to first tumor assessment, resulting in an inability to assess activity of apitolisib activity in this cohort. Given experience with other agents targeting this pathway, AEs such as rash and hyperglycemia likely represent specific on-target effects due to complete blockade of PI3K and mTOR signaling and may limit the therapeutic window of inhibitors targeting multiple nodes in the pathway.21–23 Indeed, phase I testing suggested that apitolisib showed effective pathway knockdown based on decreases in pS6 in serial biopsies at doses as low as 25 mg QD, suggesting that the current study evaluated a pharmacologically active dose.24
We performed comprehensive biomarker profiling for this advanced/recurrent endometrial cancer population. Our findings are consistent with previous reports suggesting endometrial carcinoma is a PI3K pathway driven disease, though the prevalence of alterations in key genes such as PIK3CA and PTEN was lower than has been described in some previous studies (Table 3).3, 4, 14 For instance, others3, 14 found PIK3CA mutations in 40% and 58% of patients, compared to 35% in this study. Similarly, complete PTEN protein loss was reported in 49% and 38% of cases in those studies, compared to 11% here. The PIK3CA mutation prevalence is similar to the 22% reported in a study of recurrent endometrial cancer patients enrolled in a phase II study of letrozole and everolimus.25 We also observed concomitant mutations in multiple PI3K pathway components at a lower frequency than the >40% rate reported.3 The differences may be attributable to prior studies surveying an overall cross section of endometrial cancers whereas this study focused on advanced/recurrent patient populations. Of note, TCGA Research Network reported that one-quarter of the grade 3 endometrioid cases profiled clustered with the serous cases, exhibiting similar copy number alterations and mutation spectra, including few mutations in PTEN (<15%) and high frequency of TP53 mutations (>90%). It is possible that the endometrioid cases in this study may have been enriched for this “serous-like” molecular phenotype, leading to the lower prevalence of PTEN mutations.4 An implication of this finding is that studies in advanced or recurrent endometrial cancer populations may benefit from enrichment for specific biomarkers in the PI3K pathway.
Caution is required in interpreting the correlative studies and relationship to clinical benefit, given the retrospective nature of the analysis and small number of patients. However, the results are consistent with an interpretation wherein patients with one or more alterations in PI3K signaling components may potentially show benefit from PI3K pathway inhibition if they receive sufficient drug exposure, but that apitolisib has a relatively narrow therapeutic index and efficacy is limited by poor tolerability. A phase II study of the PI3K inhibitor, pilarlisib, also showed limited activity, but similarly appeared to have benefit in patients with pathway alterations such as PI3R1 mutations and PTEN loss.26 While further development of single agent apitolisib in advanced/recurrent endometrial cancer is not planned, future studies of more selective inhibitors of specific nodes in PI3K/mTOR signaling might benefit from patient enrichment strategies.
Supplementary Material
Precis for use in table of contents.
Endometrial cancer patients with ≥1 alterations in PI3K signaling components may potentially benefit from single-agent dual PI3K/mTOR inhibitor, apitolisib, if sufficient drug exposure is received; apitolisib has a relatively narrow therapeutic index with poor tolerability and limited efficacy. Selective inhibitors of the PI3K/mTOR signaling may benefit from patient enrichment via biomarker data.
Acknowledgments
Grant: P30 CA009748.
Funding Source
This study was funded by Genentech, Inc., South San Francisco, CA.
Footnotes
Author contributions
Conception and design: Vicky Makker, Jennifer O. Lauchle, Mark R. Lackner, Carol Aghajanian
Collection and assembly of data: Vicky Makker, Fernando O. Recio, Ling Ma, Ursula Matulonis, Houston N. Gilbert, Joseph A. Ware, Rui Zhu, Shan Lu, Ling-Yuh Huw, Yulei Wang, Hartmut Koeppen, Jill M. Spoerke.
All authors contributed to data analysis and interpretation.
All authors contributed to manuscript writing.
All authors provided final approval of manuscript.
Disclosures
The following authors have no conflicts of interest to declare: V. Makker, F.O. Recio, and L. Ma. J.O. Lauchle, H. Parmar, H.N. Gilbert, J.A. Ware, R. Zhu, S. Lu, L.Y. Huw, Y. Wang, H. Koeppen, J.M. Spoerke, and M.R. Lackner are employees of Genentech, Inc., South San Francisco, CA, and stockholders of Roche.
The following authors have disclosures: U. Matulonis has served on the advisory board at Genentech and at Astrazeneca; C. Aghajanian has served on the advisory board at AstraZeneca and received travel expenses from Abbvie.
References
- 1.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 2.Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77–82. doi: 10.1097/01.cco.0000198021.99347.b9. [DOI] [PubMed] [Google Scholar]
- 3.Cheung LW, Hennessy BT, Li J, et al. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer Discov. 2011;1:170–185. doi: 10.1158/2159-8290.CD-11-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 6.Miller D, Filiaci V, Fleming G, et al. Late-Breaking Abstract 1: Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:771. [Google Scholar]
- 7.Slomovitz BM, Lu KH, Johnston T, et al. A phase 2 study of the oral mammalian target of rapamycin inhibitor, everolimus, in patients with recurrent endometrial carcinoma. Cancer. 2010;116:5415–5419. doi: 10.1002/cncr.25515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oza A, Poveda A, Clamp A, et al. A randomized phase II (RP2) trial of ridaforolimus (R) compared with progestin (P) or chemotheraphy (C) in female adult patients with advanced endometrial carcinoma. Journal of Clinical Oncology. 2011:29. (suppl; abstr 5009) [Google Scholar]
- 9.Colombo N, McMeekin DS, Schwartz PE, et al. Ridaforolimus as a single agent in advanced endometrial cancer: results of a single-arm, phase 2 trial. Br J Cancer. 2013;108:1021–1026. doi: 10.1038/bjc.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsoref D, Welch S, Lau S, et al. Phase II study of oral ridaforolimus in women with recurrent or metastatic endometrial cancer. Gynecol Oncol. 2014;135:184–189. doi: 10.1016/j.ygyno.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 11.Wallin JJ, Edgar KA, Guan J, et al. GDC-0980 is a novel class I PI3K/mTOR kinase inhibitor with robust activity in cancer models driven by the PI3K pathway. Mol Cancer Ther. 2011;10:2426–2436. doi: 10.1158/1535-7163.MCT-11-0446. [DOI] [PubMed] [Google Scholar]
- 12.Ding X, Li F, McKnight J, et al. A supported liquid extraction-LC-MS/MS method for determination of GDC-0980 (Apitolisib), a dual small-molecule inhibitor of class 1A phosphoinositide 3-kinase and mammalian target of rapamycin, in human plasma. J Pharm Biomed Anal. 2014;100:150–156. doi: 10.1016/j.jpba.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 14.Bourgon R, Lu S, Yan Y, et al. High-throughput detection of clinically relevant mutations in archived tumor samples by multiplexed PCR and next-generation sequencing. Clin Cancer Res. 2014;20:2080–2091. doi: 10.1158/1078-0432.CCR-13-3114. [DOI] [PubMed] [Google Scholar]
- 15.Spoerke JM, O’Brien C, Huw L, et al. Phosphoinositide 3-kinase (PI3K) pathway alterations are associated with histologic subtypes and are predictive of sensitivity to PI3K inhibitors in lung cancer preclinical models. Clin Cancer Res. 2012;18:6771–6783. doi: 10.1158/1078-0432.CCR-12-2347. [DOI] [PubMed] [Google Scholar]
- 16.Wilson TR, Xiao Y, Spoerke JM, et al. Development of a robust RNA-based classifier to accurately determine ER, PR, and HER2 status in breast cancer clinical samples. Breast Cancer Res Treat. 2014;148:315–325. doi: 10.1007/s10549-014-3163-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aghajanian C, Sill MW, Darcy KM, et al. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2011;29:2259–2265. doi: 10.1200/JCO.2010.32.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman RL, Sill MW, Lankes HA, et al. A phase II evaluation of aflibercept in the treatment of recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;127:538–543. doi: 10.1016/j.ygyno.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolly SO, Wagner AJ, Bendell JC, et al. Phase I Study of Apitolisib (GDC-0980), Dual Phosphatidylinositol-3-Kinase and Mammalian Target of Rapamycin Kinase Inhibitor, in Patients with Advanced Solid Tumors. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powles T, Lackner MR, Oudard S, et al. Randomized Open-Label Phase II Trial of Apitolisib (GDC-0980), a Novel Inhibitor of the PI3K/Mammalian Target of Rapamycin Pathway, Versus Everolimus in Patients With Metastatic Renal Cell Carcinoma. J Clin Oncol. 2016 doi: 10.1200/JCO.2015.64.8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schindler K, Abraham R, Shah P, et al. Clinical and histologic characterization of dermatologic adverse events from the pan-I3K inhibitor buparlisib (BKM-120) J Clin Oncol. 2014:32. (suppl; abstr e20639) [Google Scholar]
- 23.Bauer TM, Patel MR, Infante JR. Targeting PI3 kinase in cancer. Pharmacol Ther. 2015;146:53–60. doi: 10.1016/j.pharmthera.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Yan Y, Spoerke J, Wu J, et al. 495 The PI3K/mTOR Inhibitor GDC-0980 Demonstrates Target Engagement and Pathway Modulation in Tumor Tissue at Tolerated Doses. European Journal of Cancer. 2012;48:153. [Google Scholar]
- 25.Slomovitz BM, Jiang Y, Yates MS, et al. Phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma. J Clin Oncol. 2015;33:930–936. doi: 10.1200/JCO.2014.58.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matulonis U, Vergote I, Backes F, et al. Phase II study of the PI3K inhibitor pilaralisib (SAR245408; XL147) in patients with advanced or recurrent endometrial carcinoma. Gynecol Oncol. 2015;136:246–253. doi: 10.1016/j.ygyno.2014.12.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


