Abstract
Given both smoking and vitamin D are associated with coronary heart disease (CHD) via inflammation and smoking may interfere with the local antiinflammatory effects of vitamin D. We hypothesized that the relationship between smoking and severity of CHD may be modified by vitamin D.
A cross-sectional study was conducted. 25-OH vitamin D values were determined in 348 consecutive patients (mean age 62.4 ± 10.5 years; 56.3% male) undergoing coronary angiography at the Heart Center of Chaoyang Hospital affiliated to Capital Medical University between the period of September 2014 and May 2015. We categorized the patients into 2 groups based on 25-OH vitamin D levels, that is, severe hypovitaminosis D (25-OH vitamin D < 10 ng/mL) and higher vitamin D (25-OH vitamin D > = 10 ng/mL). Multivariable logistic regression models were used to estimate odds ratios (ORs) of severe coronary stenosis or higher Gensini score across three smoking status, that is, never smokers, former smokers, and current smokers in severe hypovitaminosis D and higher vitamin D groups, respectively.
Of these patients, we identified 212 (60.9%) cases of severe CHD and 161 (46.3%) cases of severe hypovitaminosis D. Multivariable logistic regression model showed the ORs of severe CHD were 1.94 (95% confidence interval [CI]: 0.47, 7.98) for former smokers and 2.62 (95% CI: 0.83, 8.24) for current smokers, compared with never smokers in group with severe hypovitaminosis D (P-trend = 0.005). In contrast, smoking was not found to be significantly associated with severe CHD in group with higher 25-OH vitamin D (P-trend = 0.115). We found a significant interaction between smoking status and vitamin D on presence of severe CHD (P-interaction = 0.015). In terms of Gensini score as a dependent variable, similar results were identified.
Our finding indicated the association between smoking and severity of CHD appeared to be substantially stronger among patients with severe hypovitaminosis D as compared with those with higher vitamin D levels. This suggests vitamin D sufficiency may have a protective effect against the damaging effects of smoking on coronary artery. Future cohort studies are warranted to confirm this finding.
Keywords: coronary angiography, coronary heart disease, interaction, smoking, vitamin D
1. Introduction
Smoking has been established to be associated with various disorders, including coronary heart disease (CHD).[1,2] Smoking cessation has also been advocated for decades in China; however, the smoking pattern in China has changed little in the last 3 decades. It is showed that the current smoking prevalence decreased mildly from 33.88% in 1984 to 28.1% in 2010 for adults aged 15 years or older in the 4 national smoking surveys in China.[3]
In practice, potential reasons, at least partially, for some smokers continuing smoking is that a lot of people who continue smoking keep healthy even in their late life, thus smoking seems to be not as harmful as advertised. Existing evidence suggests that the effect of smoking on public health may be modified by environmental factors as well as gene variation,[4–9] which mean a high-risk smoking population may exist. If that is the case, it may be helpful to quit smoking by finding these high-risk smokers and providing them with strengthened advocacy.
A large body of prior evidence has shown that vitamin D deficiency was associated with CHD.[10–13] One of the potential underlying mechanisms for this association may be the fact that vitamin D suppresses inflammation of coronary arteries and vitamin D deficiency may increase inflammation.[13–15] Vitro studies and animal models suggest that smoking may interfere with the local antiinflammatory effects of vitamin D.[16] A study in the urban elderly also showed smoking status was an effect modifier that changed the association between vitamin D deficiency and high-sensitivity C-reactive protein (hs-CRP).[17] Given these findings, we hypothesized that there was a synergistic effect of smoking with vitamin D on the risk of CHD. Thus, the aim of this study was to investigate whether there was effect modification by vitamin D on the association between smoking and severity of coronary artery stenosis assessed by coronary angiography. If the hypothesis was confirmed, it might be helpful to advocate smoke quitting, especially in the high-risk population. To our knowledge, no prior studies have explored an effect modification by vitamin D on the association between smoking and severity of coronary stenosis.
2. Materials and methods
2.1. Study population
In this cross-sectional study, we consecutively recruited 371 patients undergoing coronary angiography at the Heart Center of Chaoyang Hospital affiliated with Capital Medical University between the period of September 2014 and May 2015. Of these patients, 23 cases lacking vitamin D values were excluded. Thus, a total of 348 patients were left in present analysis. Relevant current diagnoses, comorbidities, smoking habits, and results of laboratory test were identified from inpatient medical files.
The inclusion criteria included patients who were suspected of suffering from CHD based on their typically paroxysmal symptoms of chest discomfort and/or ischemia evidence by a noninvasive test such as a dynamic change of electrocardiogram or myocardial enzymes. These patients also needed to consent to undergo a coronary angiogram and additional vitamin D tests at admission. We excluded the patients who suffered from severe liver or kidney diseases, acute or chronic inflammation, or malignancy in this study. Patients who were taking vitamin D at admission or refused to give an informed consent were also ruled out.
Blood pressure was measured after resting at least 10 minutes after admission by nurses. Measurements were performed twice with 10-minute intervals and averaged. Hypertension was defined as a systolic blood pressure of 140 mm Hg or more or a diastolic blood pressure of 90 mm Hg or more (or both) or current treatment for hypertension. Diabetes was defined as a fasting glucose of >7 or >11 mmol/L at any time within 1 month of their angiogram, or a patient was on either oral hypoglycemic agents or insulin. Body mass index was computed as weight in kilograms divided by height per square meter (kg/m2). Smoking was categorized as current-, former-, and never smokers. Current smokers were patients who reported current regular smoking and included those who had quit regular smoking <1 year before recording, and former smokers were those who had quit regular smoking ≥1 year before recording. The study was approved by the ethics committee at the Chaoyang Hospital and all patients provided written informed consent.
2.2. Angiographic analysis
To assess the severity of coronary stenosis for patients undergoing coronary angiography, 1 cardiologist independent of the study carried out the angiographic analysis with a percentage stenosis given to the major epicardial arteries and subbranches. Another cardiologist who was also independent of the study reviewed the results of angiographic analysis provided by the 1st cardiologist. They resolved any disagreements by discussion and consensus or by consulting a 3rd independent cardiologist. A severe stenosis, that is, severe CHD, was defined as a 50% or more in left main artery or a 70% or more in other major coronary artery or major branch. Given that Gensini score system is the most widely used system to quantify angiographic CHD burden according to the literature,[18] we also used it for the assessment of severity and extensiveness of CHD in present study. Gensini score was calculated by allocating a severity score to each coronary stenosis based on the location and degree of luminal narrowing.[19]
2.3. Routine laboratory examinations
Blood was obtained for routine laboratory examinations after about 12 hours of fasting before elective angiography. When requiring urgent coronary angiography, blood tests were performed following 12 hours of fasting after angiography except for myocardial enzyme and routing blood cell testing. Fasting plasma glucose was measured using the glucose oxidase method. Direct enzymatic methods were used to determine total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, and lipoprotein (a) cholesterol. Serum 25-OH vitamin D was measured by ELISA method.
2.4. Statistical analysis
Descriptive statistics were shown as mean ± standard deviation or median (interquartile range) for continuous variables, and count variables were shown as percent (%). Baseline characteristics were compared across the 4 subgroups using Mann–Whitney, Chi-square test, or Student t test. Given a value of 25-OH vitamin D < 10 ng/mL was considered as severe hypovitaminosis D and to have pathological implications for both the musculoskeletal and cardiovascular systems,[11,20,15] we categorized the participants to following 2 groups based on 25-OH vitamin D levels: 25-OH vitamin D < 10 ng/mL and 25-OH vitamin D > = 10 ng/mL. We also modeled 25-OH vitamin D as a continuous variable to explore whether a nonlinear relationship existed between 25-OH vitamin D and presence of severe CHD by using a logistic regression model with a multivariate fractional polynomial (MFP) approach.[21]
We used multivariable logistic regression models to estimate odds ratios (ORs) of the presence of severe CHD across 3 smoking subgroups with never smokers being considered as referent and presence of severe CHD as a dependent variable. Moreover, we also treated Gensini score as a dependent variable. Due to the fact that Gensini score and its transformation did not follow a normal distribution, we could not model it as a continuous variable by using a linear regression. Thus, we divided it into tertiles, that is, <6, 6–28, and > = 28, and used an ordered logistic regress model with the tertiles as a dependent variable to estimate ORs of the extent of CHD across the smoking subgroups. Covariates in multivariable models were chosen for their clinical relevance and significant univariate associations with severe CHD. The models were 1st adjusted for age and sex, and further adjusted for hypertension, diabetes, and high-density lipoprotein (HDL) cholesterol. We set a multiplicative interaction term of smoking status (current, former, and never) and 25-OH vitamin D levels (<10 and > = 10 ng/mL) in a logistic regression model and tested its effect on the risks of CHD, independent of smoking status, 25-OH vitamin D levels, and other confounding factors. Also, we set another multiplicative interaction term of smoking status (ever smoking including current and former smoking, and never smoking) and 25-OH vitamin D levels as a continuous variable in a logistic regression model. All P values were 2-tailed, and a significance of 0.05 was used. All statistical analyses were conducted using STATA 12.0 (StataCorp LP, College Station, TX).
3. Results
Of the total 348 patients, 56.3% were male and the mean age was 62.4 ± 10.5 (mean ± standard deviation) years (range: 28–86, median: 62). Severe CHD was identified in 212 patients (60.9%). Mean vitamin D of these patients was 11.9 ± 6.4 ng/mL. Of these patients, there were 161 (46.3%) cases with severe hypovitaminosis D, that is, 25-OH vitamin D < 10 ng/mL.
Table 1 shows baseline characteristics of the study population. More proportion of severe hypovitaminosis D was identified in group with severe CHD compared with those without severe CHD.
Table 1.
Characteristics of participants according to severity of coronary artery.

When the relation between 25-OH vitamin D and severe CHD was examined as vitamin D being a continuous variable by using a multivariable logistic regression model with a multivariate fractional polynomial, we found that 25-OH vitamin D was negatively associated with severe CHD, with no evidence of nonlinear association (P for nonlinear = 0.809). Additionally, the logistic regression model revealed the multivariable-adjusted OR for severe CHD in 25-OH vitamin D > = 10 ng/mL group versus 25-OH vitamin D < 10 ng/mL was 0.52 (95% confidence interval [CI]: 0.32, 0.85).
We found an interaction effect between smoking status (ever and never smoking) and 25-OH vitamin D as a continuous variable as well as a binary variable (25-OH vitamin D < 10 vs ≧10 ng/mL) on severe CHD (P for interaction = 0.049 and 0.045, respectively). In never smokers, 25-OH vitamin D was significantly negatively associated with severe CHD (OR: 0.94; 95% CI: 0.89, 0.99; P = 0.033). In contrast, in smokers, we did not identify such a significant association between them (OR: 0.97; 95% CI: 0.92, 1.02; P = 0.247). When grouping the patients based on vitamin D levels, we found multivariable-adjusted OR for severe CHD in ever-smokers versus never-smokers was 3.14 (95% CI: 1.41, 7.00) among patients with 25-OH vitamin D < 10 ng/mL; in contrast, it was 1.65 (95% CI: 0.74, 3.65) in those with 25-OH vitamin D ≧ 10 ng/mL.
Table 2 shows the OR for severe CHD according to smoking status and 25-OH vitamin D levels. In group with 25-OH vitamin D < 10 ng/mL, the ORs of severe CHD for former smokers and current smokers appeared to an increasing trend, compared with nonsmokers (P for trend = 0.005 in the multivariable-adjusted model). In contrast, we did not observe such a trend in the patients with 25-OH vitamin D > = 10 ng/mL. A significant interaction between smoking status and 25-OH vitamin D level on severe CHD was also found (Peffectmodification = 0.015).
Table 2.
ORs for presence of severe coronary stenosis according to 25-OH vitamin D/smoking status.

Table 3 shows, similar to the ORs of severe CHD, the trend to be higher Gensini score was statistically significant for former and current smokers, compared with nonsmokers in group with 25-OH vitamin D < 10 ng/mL (P for trend = 0.004 in the multivariable-adjusted model). In contrast, smoking was also not significantly associated with higher Gensini score in group with higher 25-OH vitamin D level. A significant interaction was also detected between smoking status and 25-OH vitamin D level on higher Gensini score (Peffectmodification = 0.008 in multivariable-adjusted model).
Table 3.
ORs for high Gensini score according to 25-OH vitamin D/smoking status.

Figure 1 presents the multivariate-adjusted ORs of smokers (as compared with never smokers) for severe CHD as well as higher Gensini score are smaller in patients with 25-OH vitamin D ≥ 10 ng/mL, compared to those with <10 ng/mL.
Figure 1.

ORs and 95% CIs (T-shaped bars) of smokers versus never smokers, for the presence of severe coronary heart disease (white columns) and higher Gensini score (dark columns) according to 25-OH vitamin D levels. Results were adjusted for age, sex, hypertension, diabetes, and HDL cholesterol. Smokers refer to former smokers or current smokers. Peffectmodification = 0.045 for severe CHD and = 0.023 for higher Gensini score. CHD = coronary heart disease, CI = confidence interval, HDL = high-density lipoprotein, OR = odds ratio.
The lowest prevalence of severe CHD was observed in nonsmokers with higher Vitamin D; therefore, we used this group as our reference group in Fig. 2 to assess ORs of severe CHD and higher Gensini score in the other 3 combination groups of smoking status and 25-OH vitamin D levels. Both ORs of severe CHD (Fig. 2A) and higher Gensini score (Fig. 2B) were higher in the other 3 combination groups, compared with reference group. Of note, smokers with severe hypovitaminosis D had a highest OR for both severe CHD and higher Gensini score.
Figure 2.
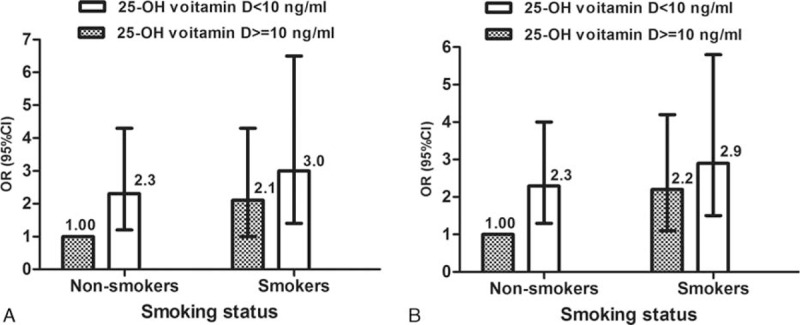
ORs and 95% CIs (T-shaped bars) for presence of severe CHD (A) and higher Gensini score (B) according to smoking status and 25-OH vitamin D levels. Dark columns, 25-OH vitamin D > = 10 ng/mL; white columns, 25-OH vitamin D < 10 ng/mL. Results were adjusted for age, sex, hypertension, diabetes, and HDL cholesterol. Nonsmokers with a higher 25-OH vitamin D level had the lowest probability of severe CHD or higher Gensini score and therefore were used as the reference group. Among the other 3 groups, smokers with lower 25-OH vitamin D level had a greatest and significantly risk of severe CHD or higher Gensini score (P < 0.01). Smokers refer to former smokers or current smokers. CHD = coronary heart disease, CI = confidence interval, HDL = high-density lipoprotein, OR = odds ratio.
4. Discussion
In this study in patients referred for coronary angiography, we found 25-OH vitamin D was independently associated with both presence of severe CHD and extent of coronary artery stenosis; after adjusting for potential confounders, the association between smoking status and extent of coronary stenosis as well as presence of severe CHD was much stronger among patients with severe hypovitaminosis D (25-OH vitamin D < 10 ng/mL) than those with higher vitamin D levels (25-OH vitamin D > = 10 ng/mL); smoking was not found to be associated with severe CHD or extent of coronary stenosis in patients with higher vitamin D levels; a significant interaction was detected between smoking status and 25-OH vitamin D level on severity of coronary stenosis. Our study is the 1st to examine the cumulative effect of 25-OH vitamin D and smoking status on severity of CHD.
Our finding regarding the association between 25-OH vitamin D and severity of CHD is in line with a growing body of existing evidence showing that 25-OH vitamin D deficiency is significantly associated with increased risk of CHD,[10–13,22–24] but is inconsistent with a few studies that failed to show this significant association.[25–27] The exact reasons for the differing results across these studies are uncertain. We reasoned effect modifications of some variables might, at least partly, account for it. In this study, there exited an interaction effect between smoking and 25-OH vitamin D on severity of coronary stenosis. In never smokers, 25-OH vitamin D was significantly negatively associated with severe CHD. In contrast, in smokers, we did not identify such a significant association between them. This means one may easily find a significant association between vitamin D and CHD in a study with a lower prevalence of smoking. In the majority of studies which show a significant association between vitamin D and CHD, such as aforementioned studies,[10–12,24] the smoking rate is relatively low, ranging from 18% to 55%. In contrast, in the study by Skaaby et al[27] which showed no association between vitamin D and CHD, the smoking rate was around 70%.
There have existed several explanations for the contribution of 25-OH vitamin D to cardiovascular health. As noted, 25-OH vitamin D deficiency is associated with increased vascular stiffness and inflammation,[13,28] impaired endothelial function,[28,29] increased coronary calcification,[30] and increased insulin resistance.[31] These may be the underlying mechanisms of vitamin D deficiency associated with higher risk of CHD.
The most novel and important finding in present study was that we found a significant interaction between 25-OH vitamin D and smoking status on the severity of coronary stenosis. This finding is similar to the results of a prior study on the interaction between vitamin D and smoking on lung function. In that longitudinal cohort study, Lange et al[32] found there was an effect modification by vitamin D status on the associated between smoking and lung function. A number of previous studies have also identified that the effect of smoking on public health may be modified by environmental factors as well as gene variation.[4–9] These findings suggest smoking may be more harmful to certain particular population, which, at least partly, support our finding on the effect modification of vitamin D on the association between smoking and severity of CHD.
There are several possible mechanisms whereby higher vitamin D levels could have a protective effect against damage caused by smoking on coronary artery. A variety of studies have shown that smoking induces oxidative stress, vascular inflammation, platelet aggregation, and vascular endothelial injury,[33–38] these alterations may be, at least partly, responsible for the increased risk of CHD in smokers compared with nonsmokers.[36] Of note, a few studies have demonstrated higher 25-OH vitamin D is associated with improved endothelial function.[28,39,40] Studies have also demonstrated that vitamin D has immunomodulatory and antiinflammatory effect via several pathways.[14,41,42] Thus, we speculate the antiinflammatory effect and the role of improving endothelial function of higher vitamin D values may be, at least partly, the mechanisms of its protective action against smoke. Additionally, there have been studies showing that vitamin D can act as an antioxidant[43] or induce production of antioxidants.[44] This effect could counteract the oxidative stress of smoking that leads to coronary endothelial dysfunction and atherosclerosis.[38]
In terms of the results in present study, there is another issue worth mentioning. Although the estimated ORs of former smokers were lower than that of current smokers when presence of severe CHD was treated as a dependent variable in the logistic regression model, that was not the case when Gensini score was considered as a dependent variable in the group with vitamin D <10 ng/mL. The potential reasons for this may be that the sample size in the smoking subgroup was relatively small, which resulted in insignificant point estimates of ORs with wide confidence intervals in most subgroups; however, the trends of ORs for former and current smokers were significant in groups with vitamin D < 10 ng/mL, regardless of whether the presence of severe CHD or Gensini score was treated as a dependent variable.
The strengths of this study that deserve mention are as follows. To our knowledge, it is the 1st study to examine the interaction between 25-OH vitamin D and smoking on the severity of coronary stenosis on coronary angiography. Additionally, we not only examined the interaction between 25-OH vitamin D and smoking status on the presence of severe CHD, but also explored their interaction effect on the extent of coronary stenosis assessed by Gensini score. And we found a consistent modification effect on them.
4.1. Limitations of the study
There are also some limitations that should be mentioned. First, the cross-sectional design of the study is a major limitation. Thus, a causality cannot be drawn from present study and the results should be treated conservatively. It needs to be confirmed in a future prospective study.
Second, the sample size in this study is relatively small, especially in each smoking subgroup, thus the confidence intervals of ORs in each smoking group were wide, which probably meant the models did not well fit. Nevertheless, when we used Hosmer–Lemeshow X2 test to assess the calibration of the models, we found that the models were all well calibrated with the observed data (all P > 0.05), indicating fitting degrees of the models in this study were acceptable.
Third, this is a single-center study and the participants were not sampled from the general population, limiting its generalization. Thus, a prospective cohort study in a general population is warranted to confirm our findings.
Fourth, we chose a 25 (OH) vitamin D cut-point of 10 ng/mL, based on literature not by justifying it, to investigate its effect modification. This seems to be arbitrary. However, a cutoff value of vitamin D as 10 ng/mL has been defined as severe hypovitaminosis D[11,20] and believed to have pathological implications for both the musculoskeletal and cardiovascular systems.[15] Moreover, we have also tried other cutoffs, such as 20 ng/mL, that is, a cutoff for vitamin D deficiency,[26,32,45] but failed to find a significant effect modification. These suggest the optimal dichotomized value of vitamin D to identify a modification effect of vitamin D on association between smoking and severity of CHD might be 10 ng/mL in this study.
Last, in this study, a potential for measure bias regarding data reliability should be mentioned. For instance, measure bias might exist when we measured vitamin D levels. Although the precise of the vitamin D measurement was satisfactory, with coefficient of variations of intra- and interassay being 7.1% and 9.7%, respectively, we only measured vitamin D levels one time. However, vitamin D levels vary over time. This may result in measure bias. Of note, in a study by Lange et al,[32] vitamin D was measured in a cross-sectional design and then 3 times in a longitudinal design; the finding on vitamin D measured one time is same as that from the longitudinal multivariable model using repeated vitamin D levels, that is, there were similar effect modifications by vitamin D status on the association between smoking and lung function. Additionally, Major et al[46] analyzed 25-OH vitamin D in a prospective, nationwide study at 2 time points within a 1-year period, most measured in different seasons and found the intraclass correlation coefficient (ICC) was 0.72, which suggest an individual's 25-OH vitamin D level is relatively stable over a 1-year period and a single blood sample may provide a reasonable average for 25-OH vitamin D over a 1-year period. Even so, we believe a future study measuring vitamin D levels repeatedly is needed to confirm our findings.
In conclusion, the findings in this study add to the existing evidence suggesting that serum vitamin D level is independently associated with the severity of coronary artery stenosis. Importantly, we found that the association between smoking and severity of coronary artery stenosis was modified by 25-OH vitamin D levels. This message may be of great importance from a public health perspective, because it is potentially helpful to recommend smoking cessation in the high-risk smokers, that is, those with 25-OH vitamin <10 ng/mL. Additionally, though higher vitamin D level attenuates the association between smoking status and severity of CHD, we are not recommending smoking in those with higher vitamin D level, given that it is nonsmokers, but not smokers, with higher vitamin D level that have the lowest OR of severe CHD.
Footnotes
Abbreviations: CHD = coronary heart disease, OR = odds ratio.
The authors have no funding conflicts of interest to disclose.
References
- 1.Peto R, Lopez AD, Boreham J, et al. Mortality from tobacco in developed countries: Indirect estimation from national vital statistics. Lancet 1992; 339:1268–1278. [DOI] [PubMed] [Google Scholar]
- 2.Stallones RA. The association between tobacco smoking and coronary heart disease. Int J Epidemiol 2015; 44:735–743. [DOI] [PubMed] [Google Scholar]
- 3.Hou L, Jiang J, Liu B, et al. Association between smoking and deaths due to colorectal malignant carcinoma: a national population-based case-control study in china. Br J Cancer 2014; 110:1351–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eshak ES, Iso H, Yamagishi K, et al. Modification of the excess risk of coronary heart disease due to smoking by seafood/fish intake. Am J Epidemiol 2014; 179:1173–1181. [DOI] [PubMed] [Google Scholar]
- 5.Hozawa A, Folsom AR, Sharrett AR, et al. Does the impact of smoking on coronary heart disease differ by low-density lipoprotein cholesterol level?: The atherosclerosis risk in communities (aric) study. Circ J 2006; 70:1105–1110. [DOI] [PubMed] [Google Scholar]
- 6.Merhi M, Demirdjian S, Hariri E, et al. Impact of inflammation, gene variants, and cigarette smoking on coronary artery disease risk. Inflamm Res 2015; 64:415–422. [DOI] [PubMed] [Google Scholar]
- 7.Han Y, Dorajoo R, Ke T, et al. Interaction effects between paraoxonase 1 variants and cigarette smoking on risk of coronary heart disease in a Singaporean Chinese population. Atherosclerosis 2015; 240:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatzis G, Tousoulis D, Papageorgiou N, et al. Combined effects of smoking and interleukin-6 and c-reactive protein genetic variants on endothelial function, inflammation, thrombosis and incidence of coronary artery disease. Int J Cardiol 2014; 176:254–257. [DOI] [PubMed] [Google Scholar]
- 9.Tomita LY, Roteli-Martins CM, Villa LL, et al. Associations of dietary dark-green and deep-yellow vegetables and fruits with cervical intraepithelial neoplasia: modification by smoking. Br J Nutr 2011; 105:928–937. [DOI] [PubMed] [Google Scholar]
- 10.Liew JY, Sasha SR, Ngu PJ, et al. Circulating vitamin d levels are associated with the presence and severity of coronary artery disease but not peripheral arterial disease in patients undergoing coronary angiography. Nutr Metab Cardiovasc Dis 2015; 25:274–279. [DOI] [PubMed] [Google Scholar]
- 11.Verdoia M, Schaffer A, Barbieri L, et al. Impact of gender difference on vitamin d status and its relationship with the extent of coronary artery disease. Nutr Metab Cardiovasc Dis 2015; 25:464–470. [DOI] [PubMed] [Google Scholar]
- 12.Chen WR, Chen YD, Shi Y, et al. Vitamin d, parathyroid hormone and risk factors for coronary artery disease in an elderly chinese population. J Cardiovasc Med (Hagerstown) 2015; 16:59–68. [DOI] [PubMed] [Google Scholar]
- 13.Kunadian V, Ford GA, Bawamia B, et al. Vitamin d deficiency and coronary artery disease: a review of the evidence. Am Heart J 2014; 167:283–291. [DOI] [PubMed] [Google Scholar]
- 14.Zittermann A, Schleithoff SS, Koerfer R. Putting cardiovascular disease and vitamin d insufficiency into perspective. Br J Nutr 2005; 94:483–492. [DOI] [PubMed] [Google Scholar]
- 15.Lavie CJ, Dinicolantonio JJ, Milani RV, et al. Vitamin d and cardiovascular health. Circulation 2013; 128:2404–2406. [DOI] [PubMed] [Google Scholar]
- 16.Hansdottir S, Monick MM, Lovan N, et al. Vitamin d decreases respiratory syncytial virus induction of nf-kappab-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J Immunol 2010; 184:965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee H, Kim KN, Lim YH, et al. Interaction of vitamin d and smoking on inflammatory markers in the urban elderly. J Prev Med Public Health 2015; 48:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neeland IJ, Patel RS, Eshtehardi P, et al. Coronary angiographic scoring systems: an evaluation of their equivalence and validity. Am Heart J 2012; 164:547.e1–552.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 1983; 51:606. [DOI] [PubMed] [Google Scholar]
- 20.Vierucci F, Del Pistoia M, Fanos M, et al. Vitamin d status and predictors of hypovitaminosis d in italian children and adolescents: a cross-sectional study. Eur J Pediatr 2013; 172:1607–1617. [DOI] [PubMed] [Google Scholar]
- 21.Royston P, Sauerbrei W. Building multivariable regression models with continuous covariates in clinical epidemiology – with an emphasis on fractional polynomials. Methods Inf Med 2005; 44:561–571. [PubMed] [Google Scholar]
- 22.Wang L, Song Y, Manson JE, et al. Circulating 25-hydroxy-vitamin d and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes 2012; 5:819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schottker B, Jorde R, Peasey A, et al. Vitamin d and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ 2014; 348:g3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen WR, Qian YA, Chen YD, et al. The effects of low vitamin d on coronary artery disease. Heart Lung Circ 2014; 23:314–319. [DOI] [PubMed] [Google Scholar]
- 25.Ho JS, Cannaday JJ, Barlow CE, et al. Low 25-oh vitamin d levels are not associated with coronary artery calcium or obstructive stenoses. Coron Artery Dis 2015; 26:521–525. [DOI] [PubMed] [Google Scholar]
- 26.Alsancak Y, Cengel A, Akyel A, et al. Relationship between serum vitamin d levels and angiographic severity and extent of coronary artery disease. Eur J Clin Invest 2015; 45:940–948. [DOI] [PubMed] [Google Scholar]
- 27.Skaaby T, Husemoen LL, Pisinger C, et al. Vitamin d status and incident cardiovascular disease and all-cause mortality: a general population study. Endocrine 2013; 43:618–625. [DOI] [PubMed] [Google Scholar]
- 28.Al Mheid I, Patel R, Murrow J, et al. Vitamin d status is associated with arterial stiffness and vascular dysfunction in healthy humans. J Am Coll Cardiol 2011; 58:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu ZM, Woo J, Wu SH, et al. The role of vitamin d in blood pressure, endothelial and renal function in postmenopausal women. Nutrients 2013; 5:2590–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malik R, Aneni EC, Roberson L, et al. Measuring coronary artery calcification: Is serum vitamin d relevant? Atherosclerosis 2014; 237:734–738. [DOI] [PubMed] [Google Scholar]
- 31.Esteghamati A, Aryan Z, Esteghamati A, et al. Vitamin d deficiency is associated with insulin resistance in nondiabetics and reduced insulin production in type 2 diabetics. Horm Metab Res 2015; 47:273–279. [DOI] [PubMed] [Google Scholar]
- 32.Lange NE, Sparrow D, Vokonas P, et al. Vitamin d deficiency, smoking, and lung function in the normative aging study. Am J Respir Crit Care Med 2012; 186:616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siasos G, Tsigkou V, Kokkou E, et al. Smoking and atherosclerosis: mechanisms of disease and new therapeutic approaches. Curr Med Chem 2014; 21:3936–3948. [DOI] [PubMed] [Google Scholar]
- 34.Frohlich M, Sund M, Lowel H, et al. Independent association of various smoking characteristics with markers of systemic inflammation in men. Results from a representative sample of the general population (Monica Augsburg survey 1994/95). Eur Heart J 2003; 24:1365–1372. [DOI] [PubMed] [Google Scholar]
- 35.Gasparyan AY, Ayvazyan L, Mikhailidis DP, et al. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des 2011; 17:47–58. [DOI] [PubMed] [Google Scholar]
- 36.Leone A. Smoking, haemostatic factors, and cardiovascular risk. Curr Pharm Des 2007; 13:1661–1667. [DOI] [PubMed] [Google Scholar]
- 37.Newby DE, Wright RA, Labinjoh C, et al. Endothelial dysfunction, impaired endogenous fibrinolysis, and cigarette smoking: a mechanism for arterial thrombosis and myocardial infarction. Circulation 1999; 99:1411–1415. [DOI] [PubMed] [Google Scholar]
- 38.Naya M, Morita K, Yoshinaga K, et al. Long-term smoking causes more advanced coronary endothelial dysfunction in middle-aged smokers compared to young smokers. Eur J Nucl Med Mol Imaging 2011; 38:491–498. [DOI] [PubMed] [Google Scholar]
- 39.Syal SK, Kapoor A, Bhatia E, et al. Vitamin d deficiency, coronary artery disease, and endothelial dysfunction: observations from a coronary angiographic study in Indian patients. J Invasive Cardiol 2012; 24:385–389. [PubMed] [Google Scholar]
- 40.Jablonski KL, Chonchol M, Pierce GL, et al. 25-hydroxyvitamin d deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension 2011; 57:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baeke F, Takiishi T, Korf H, et al. Vitamin D: modulator of the immune system. Curr Opin Pharmacol 2010; 10:482–496. [DOI] [PubMed] [Google Scholar]
- 42.Gurses KM, Tokgozoglu L, Yalcin MU, et al. Markers of subclinical atherosclerosis in premenopausal women with vitamin d deficiency and effect of vitamin d replacement. Atherosclerosis 2014; 237:784–789. [DOI] [PubMed] [Google Scholar]
- 43.Danilenko M, Wang Q, Wang X, et al. Carnosic acid potentiates the antioxidant and prodifferentiation effects of 1alpha,25-dihydroxyvitamin d3 in leukemia cells but does not promote elevation of basal levels of intracellular calcium. Cancer Res 2003; 63:1325–1332. [PubMed] [Google Scholar]
- 44.Hanada K, Sawamura D, Nakano H, et al. Possible role of 1,25-dihydroxyvitamin d3-induced metallothionein in photoprotection against uvb injury in mouse skin and cultured rat keratinocytes. J Dermatol Sci 1995; 9:203–208. [DOI] [PubMed] [Google Scholar]
- 45.Ross AC. The 2011 report on dietary reference intakes for calcium and vitamin d. Public Health Nutr 2011; 14:938–939. [DOI] [PubMed] [Google Scholar]
- 46.Major JM, Graubard BI, Dodd KW, et al. Variability and reproducibility of circulating vitamin d in a nationwide U.S. population. J Clin Endocrinol Metab 2013; 98:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]


