Abstract
Cryptosporidium is a significant cause of diarrhea worldwide especially in children. Infection may end fatally in immunocompromised patients. Multi-attribute analysis was used to determine the lab utility of 4 diagnostics; coproscopy of AF stained fecal smear, fecal immunoassays by ICT and ELISA and copro-nPCR assay targeting Hsp90 gene, for detection of Cryptosporidium in stool of 250 Egyptian children (150 diarrheic and 100 non-diarrhaeic children). Also, to determine Cryptosporidium molecular prevalence. Cryptosporidium was an important enteric pathogen among both diarrheic and non-diarrheic study children with a clearly high prevalence of 16.4 % (n = 41). Conventional methods had perfect specificity (100 %) but couldn`t be used as a consistent single detection method due to their lowered sensitivities. Multi-attribute analysis ranked nPCR the highest test for lab use. Being the test with the best diagnostic yield, nPCR is a reliable diagnostic test and is going to replace conventional methods for reliable detection of Cryptosporidium.
Keywords: Cryptosporidium, Hsp90, Copro-DNA, Nested PCR, Multi-attribute, Fecal immunoassay, ELISA, ICT, AF stain
Introduction
Cryptosporidium, the apicomplexan intracellular protozoan parasite, is a significant cause of diarrhea worldwide especially in children and immuno-compromised patients that may have devastating consequences and end fatally (Caccio et al. 2005). In Egypt, it is reported as a virulent agent of diarrhea especially in childhood with varied prevalence (Youssef et al. 2008). It can infect a wide range of many vertebrates, including humans (Chalmers et al. 2009).
Laboratory diagnosis of cryptosporidiosis is usually done by microscopic detection of Cryptosporidium oocyst in stained smears from stool specimens (Weitzel et al. 2006). Variety of fecal immunoassays has been developed to establish more sensitive and cost-effective methods to diagnose Cryptosporidium. These methods are based on the detection of parasite copro-antigens using conjugated anti-Cryptosporidium monoclonal antibodies (mAb). Three approaches have proven useful; (1) immunofluorescence assays, (2) enzyme linked immunosorbent assay (ELISA) and (3) immunochromatography (ICT) lateral-flow immunoassays, with available varieties of commercial kits. Each has a more or less similar level of sensitivity (Morgan et al. 1998). However, using conventional methods (coproscopy and copro immunoassays) had limitation as some Cryptosporidium infections remained undetected (Kehl et al. 1995).
Recently, PCR based methods for the laboratory diagnosis of cryptosporidiosis were developed and showed excellent specificity and sensitivity, compared with antigen detection, and microscopy (Salyer et al. 2012).
The aim of the present study is to compare between 4 of the used diagnostic methods; 3 conventional (microscopy of AF stained fecal smear and 2 fecal immunoassays; ICT and ELISA) and one molecular (nPCR targeting Hsp90 gene) for detection of cryptosporidiosis in human stool samples concerning their diagnostic yield and lab use. Also, is to determine the molecular prevalence of Cryptosporidium among study individuals.
Materials and methods
Study type and population
This cross sectional study was conducted over 250 children; 150 diarrheic patients (symptomatic group = GI) attending outpatient clinic in Abu El Rish hospital, Kasr Al-Ainy School of Medicine, Cairo University, Cairo, Egypt and 100 age matched non diarrheic, apparently healthy children (asymptomatic group = GII) from May 2012 to February 2013.
Specimen collection and processing
A single fecal sample was obtained from each child and part of the specimen was preserved in formalin saline fixative for parasitological coproscopic examination and AF staining and the rest of the specimen was divided into two parts and stored at −20 °C for immunoassays and molecular studies.
Coproscopy and immunoassays were carried out in the Diagnostic and Research Unit of Parasitic Diseases (DRUP) and copro-nPCR assay was held in Lab of Molecular Medical Parasitology (LMMP), Department of Medical Parasitology, Faculty of Medicine, Cairo University, Egypt. All fecal samples were examined as follow:-
Parasitological coproscopy and AF stain
Fecal samples were examined directly by wet mount and after using modified Ritchie’s biphasic concentration method (Garcia 2007), the samples were permanently stained using cold kinyoun’s AF stain (Biostain Ready Reagents Ltd Manchester, England) to detect Cryptosporidium copro-oocyst.
Copro–immunoassays
Part of fecal samples that were submitted frozen, were examined for detection of Cryptosporidium coproantigen using two immunoassays; ICT lateral flow dip strip test using RIDA®QUICK Cryptosporidium ICT dip strip (R-Biopharm AG, Germany) and ELISA using RIDASCREEN® Cryptosporidium ELISA Kit (R-Biopharm AG, Landwehrstr. 54, D-64293 Darmstadt, Germany) according to manufacturer`s instructions.
Copro-nPCR assays
The remained part of frozen fecal samples were subjected to thermal shock (5 cycles of deep freezing in liquid nitrogen and immediately transferred into water bath 95 °C each for 5 min.), and then extraction of genomic DNA was performed using Favor Prep stool DNA isolation Mini Kit (Favorgen Biotech corporation ping-Tung 908, Taiwan, Cat. No. FASTI001) with modification in the form of prolongation of incubation to 95 °C for one hour then the purified DNA was measured for concentration and purity. Amplification of 835–844 of Hsp90 using two sets of oligonuclutide primers: Hsp90-F3 (5′-CTA GTG AAA GCT ACG AGT TCC AA-3′) and Hsp90-R3 (5′-TCT ATTTCA CCT TCG GCG GAA AA 3′) for the primary reaction and a fragment of 676–685 for the secondary reaction using: Hsp90-F4 (5′-GGA TAT TAT TAT TAA CTC TCT CTA TTCTCA GAA-3′) and Hsp90-R4 (5′-CCA TAT TGC CTT TTC TAC ATT AAC-3′) (Fig. 1). The reaction mixture consisted of 12.5 µl master mix, 200 nM from each primer, 0.2 units of Taq polymerase (as an activator) and 3 µl of the template DNA for the primary reaction and 1 µl for the secondary reaction in a total volume of 25 µl. The cycling conditions were carried out according to Feng et al. (2009) with modification in the form of 50 °C annealing temperature for the primary and the secondary reactions. The amplified products were visualized with 1.5 % agarose gel electrophoresis after ethidium bromide staining.
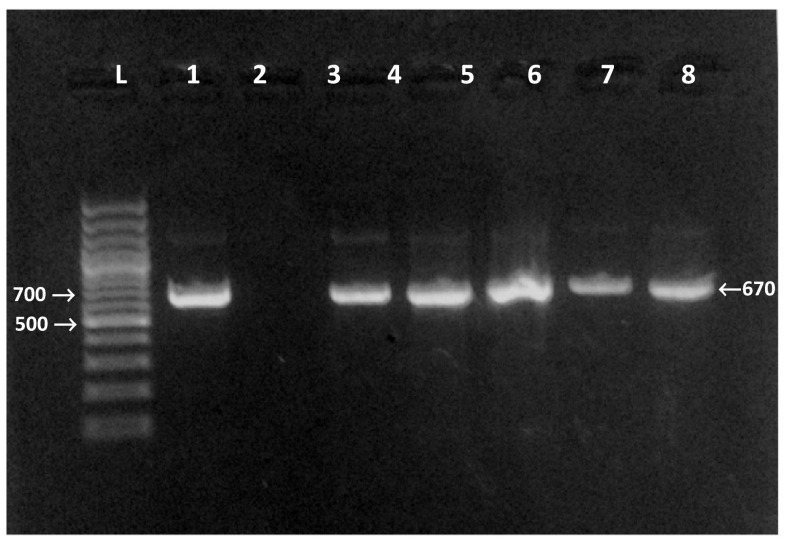
Fig. 1.
Agarose gel electrophoresis for the products of the nPCR targeting Hsp90 gene of Cryptosporidium at 676–685 bp. L 100 bp DNA molecular weight marker. Lane 1 positive control. Lanes 2 negative control. Lanes 3–7 positive samples
Statistical analysis
Data were coded and entered using the statistical package SPSS version 17 (Chicago, IL, USA) for statistical analysis. Comparisons between groups were done using Chi square test for qualitative variables and data were considered statistically significant if P values was <0.05.
Sensitivity, specificity, PPV, NPV, accuracy were calculated to test the diagnostic yield and kappa agreement was done to test the validity of conventional microscopy and immunoassays in relation to nPCR results considering it a nominated gold standard.
The Multi-attribute utility theory (Mac Pherson and Mc Queen 1993) and analytical hierarchy process (Dolan 1989) were used to evaluate the lab utility of the used diagnostic procedure. Six attributes (listed in Table 1) prioritized by assigning its importance over the other as per the laboratory’s infrastructure and, then were given a rank order from 1 to 4, with 1 being least desirable and 4 being most desirable. Subsequently attribute priority value (Table 1) was multiplied by the ranks given for each attribute for every diagnostic technique.
Table 1.
Ranks given for each attribute in Multi-attribute evaluation of the used different diagnostic techniques
| Evaluation item (attributes) | Totala | |||||||
|---|---|---|---|---|---|---|---|---|
| Performance | Costs | Ease of | Batch ability | Species identific-ation | ||||
| Sensitivity | Specificity | Use | Interpretation | |||||
| Priority value | 0.35 | 0.35 | 0.95 | 0.9 | 0.15 | 0.5 | 0.07 | – |
| Rank of method | ||||||||
| AF stain | 1 | 4 | 4 | 3 | 1 | 1 | 0 | 8.90 |
| ICT | 2 | 4 | 1 | 4 | 4 | 3 | 0 | 8.75 |
| ELISA | 3 | 4 | 2 | 2 | 2 | 2 | 1 | 7.52 |
| nPCR | 4 | 4 | 3 | 1 | 3 | 4 | 4 | 9.28 |
aIn order to obtain lab use, priority values were multiplied by the ranks given for each attribute for every diagnostic technique, sum up was given in total
Results
Multi-attribute analysis ranked nPCR the highest one for lab use followed by AF stain, ICT and lastly ELISA (Table 1).
Screening of the 250 samples revealed that n-PCR targeting Hsp90 gene was able to detect Cryptosporidium copro-DNA in 35(23.4 %) of GI and 6(6 %) in GII, followed by ELISA and ICT assays, they were able to detect Cryptosporidium copro-antigen in 16(10.7 %) and 15 (10 %) samples, respectively of GI and in 2(2 %) and one (1 %) samples, respectively of GII.
AF stained stool smears detected Cryptosporidium oocyst in 14(9.3 %) samples of the GI and no positive cases were detected among GII (Table 2).
Table 2.
Results of AF stain, ICT, ELISA and nPCR in detection of Cryptosporidium among both studied groups
| Symptomatic group (n = 150) | Asymptomatic group (n = 100) | All study groups (n = 250) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NPCR | nPCR | nPCR | ||||||||
| +ve | −ve | Total | +ve | −ve | Total | +ve | −ve | Total | ||
| AF stain | ||||||||||
| +ve | 14 | 0 | 14 | 0 | 0 | 0 | 14 | 0 | 14 | |
| −ve | 21 | 115 | 136 | 6 | 94 | 100 | 27 | 209 | 236 | |
| Total | 35 | 115 | 150 | 6 | 94 | 100 | 41 | 209 | 250 | |
| ICT | ||||||||||
| +ve | 15 | 0 | 15 | 1 | 0 | 1 | 16 | 0 | 16 | |
| −ve | 20 | 115 | 135 | 5 | 94 | 99 | 25 | 209 | 234 | |
| Total | 35 | 115 | 150 | 6 | 94 | 100 | 41 | 209 | 250 | |
| ELISA | ||||||||||
| +ve | 16 | 0 | 16 | 2 | 0 | 2 | 18 | 0 | 18 | |
| −ve | 19 | 115 | 134 | 4 | 94 | 89 | 23 | 209 | 232 | |
| Total | 35 | 115 | 150 | 6 | 94 | 100 | 41 | 209 | 250 | |
Data presented as n
Considering n-PCR as a nominated reference standard, ELISA showed the highest sensitivity followed by ICT, AF stain came lastly.
The diagnostic yield, accuracy and Kappa agreement of the used diagnostics were shown in Table 3. There were moderate agreements between the used tests.
Table 3.
Results of diagnostic yield, accuracy and Kappa agreement of the used diagnostics among all study individuals
| AF stain | ICT | ELISA | |
|---|---|---|---|
| Sensitivity | 34 % | 39 % | 43.9 % |
| Specificity | 100 % | 100 % | 100 % |
| PPV | 100 % | 100 % | 100 % |
| NPV | 88.5 % | 89.3 % | 90 % |
| Accuracy | 94.8 % | 96.4 % | 98 % |
| Kappaa | 0.46 | 0.51 | 0.56 |
aKey for Kappa: Poor agreement <0, Slight agreement 0.01–0.20, Fair agreement 0.21–0.40, Moderate agreement 0.41–0.60, Substantial agreement 0.61–0.8, Almost perfect agreement 0.81–1.00
Discussion
Evaluation of the true ranking of one diagnostic method over another for decision making, should be based on the laboratory utility instead of being limited to the diagnostic yields (sensitivity and specificity) (Tahira et al. 2012). The Multi-attribute utility theory (Mac Pherson and Mc Queen 1993) and analytical hierarchy process (Dolan 1989) that identify, characterize and combine different parameters that were used in the present study to evaluate the true ranking of the used diagnostics for detection of Cryptosporidium. Multi-attribute analysis proved that nPCR had the highest rank for lab use followed by AF stain then ICT and ELISA was the last one.
Similar findings were reported by El-Hamshary et al. (2008), El-Settawy and Fathy (2012). However, Elgun and Koltas (2011) reported that ELISA had the highest score followed by PCR and AF stain, while Kehl et al. (1995) ranked AF stain higher than ELISA.
Based on the obtained results Cryptosporidium was a prevalent public health problem among the studied Egyptian children (16.4 %, 41 cases) and was detected in both symptomatic and asymptomatic groups indicating the possibility of occurrence of asymptomatic carrier state for Cryptosporidium in non diarrheic immuno-competent children. Cryptosporidiosis is under diagnosed and laboratories should screen all stool specimens for Cryptosporidium.
Using PCR, different Cryptosporidium prevalence rates were reported in Egypt (Hassan et al. 2002; El-Mohamady et al. 2006; El-Shazly et al. 2007; El-Hamshary et al. 2008; El-Settawy and Fathy 2012) and worldwide (Morgan et al. 1998; Khurana et al. 2012; Salyer et al. 2012), in Egypt the prevalence ranged from 10.7 to 25.5 %. The difference in the reported prevalence may be attributed to differences in study population, demographic, behavioral, environmental and socioeconomic factors, diagnostic methods, time of the study (summer vs. winter), nutritional status of the children, and educational level of parents (AL-Hindi et al. 2007).
Due to its higher sensitivity, PCR-based methods were nominated by many authors (Jex et al. 2008; Salyer et al. 2012) and the present study as the gold standard test. Microscopy and copro immunoassays were of prefect specificity with 100 % specificity and PPV. However they were of limited sensitivity with many cases escaping diagnosis. Conflicting reports concerning the sensitivity of fecal immunoassays over microscopy were recorded (Kehl et al. 1995).
Different sensitivities of immunoassays for detection of Cryptosporidium coproantigen were reported (Morgan et al. 1998; Abdel-Baki et al. 2004; El-Hamshary et al. 2008; Elgun and Koltas 2011; El-Settawy and Fathy 2012), possible explanation was that commercially available coproantigen detection formats react to different sets of surface epitopes using mAbs which may react weakly or may not react with antigens of different Cryptosporidium species in clinical samples. Added to this, microscopy and fecal immunoassays had a detection limit that had been reported to be 50,000–500,000 oocysts/g of stool (Smith 2008).
The advantage of rapid detection using immunoassay was limited by the persistence of antigens for several days, after treatment or an abortive infection or by a cross-reaction with other antigens (Garcia and Shimizu 1997). It was reported that several mAbs against oocysts antigens of C.parvum cross react with other parasitic life cycle stages, other Cryptosporidium species or other coccidian parasites (Chrisp et al. 1991; McDonald et al. 1995; Nina et al. 1992; Ortega-Mora et al. 1992; Robert et al. 1994).
Beside its poor sensitivity, consuming time, the need of expertise and the technical experience, the reduced excretion of Cryptosporidium oocysts in non-diarrheic patients may explain failure of AF staining microscopy to detect oocysts in any of the present study formed stool samples (Current and Garcia 1991; Tzipori et al. 1995; Weitzel et al. 2006).
Challenge to incomparable sensitivity of PCR (Salyer et al. 2012) is the reliable extraction of Cryptosporidium copro-DNA from oocyst stage in stools. This requires a cell disruption; include thermal treatment; boiling, and freeze–thaw cycles, chemical and/or mechanical treatment. Also, the highly sensitive primer set specific to the Hsp90 gene together with the nPCR procedure further enhance the specificity in addition to sensitivity of PCR.
Being the test with highest diagnostic yield, performance and lab use, copro-nPCR amplification technique using nPCR makes it a more appropriate option financially particularly in developing countries and an obvious choice for improved detection of Cryptosporidium from stool that is going to replace conventional methods in near future.
Acknowledgments
Conflict of interest
The authors declare that they have no Conflict of interests.
References
- Abdel-Baki M, Younis T, Habib K, Ramadan NI, Metwally DM, Ismail KA, Saber MM. Molecular identification of coproantigen of Cryptosporidium parvum compared to conventional staining techniques. J Egypt Soc Parasitol. 2004;34:967–978. [PubMed] [Google Scholar]
- AL-Hindi AI, EL Manama AA, Elnabris KJ. Cryptosporidiosis among children attending Al-Nasser Pediatric Hospital, Gaza, Palestine. Turk J Med Sci. 2007;37:367–372. [Google Scholar]
- Caccio SM, Thompson RC, McLauchlin J, Smith HV. Unravelling Cryptosporidium and Giardia epidemiology. Trends Parasitol. 2005;21:430–437. doi: 10.1016/j.pt.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Chalmers RM, Robinson G, Elwin K, Hadfield SJ, Xiao L, Ryan U, Modha D, Mallaghan C. Cryptosporidium sp. rabbit genotype, a newly identified human pathogen. Emerg Infect Dis. 2009;15:829–830. doi: 10.3201/eid1505.081419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrisp CE, Suckow MA, Fayer R, Arrowood MJ, Healey MC, Sterling CR. Comparison of the host ranges and antigenicity of Cryptosporidium parvum and Cryptosporidium wairi from Guinea pigs. J Protozool. 1991;39:406–409. doi: 10.1111/j.1550-7408.1992.tb01471.x. [DOI] [PubMed] [Google Scholar]
- Current WL, Garcia LS. Cryptosporidiosis. Clin Microbiol Rev. 1991;4:325–328. doi: 10.1128/CMR.4.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan JG. Medical decision making using the analytic hierarchy process: choice of initial antimicrobial therapy for acute pyelonephritis. Med Decis Making. 1989;9:51–56. doi: 10.1177/0272989X8900900109. [DOI] [PubMed] [Google Scholar]
- Elgun G, Koltas IS. Cryptosporidium spp. antigen by ELISA method in stool specimens obtained from patients with diarrhea. Parasitol Res. 2011;108:395–397. doi: 10.1007/s00436-010-2079-4. [DOI] [PubMed] [Google Scholar]
- El-Hamshary EM, El-Sayed HF, Hussein EM, Rayan HZ, Rasha H, Soliman RH. Comparison of polymerase chain reaction, immunochromatographic assay and staining techniques in diagnosis of cryptosporidiosis. PUJ. 2008;1:77–86. [Google Scholar]
- El-Mohamady H, Abdel-Messih I, Youssef F, Said M, Farag H, Shaheen HI, Rockabrand DM, Luby SB, Hajjeh R, Sanders JW, Monteville MR, Klena JD, Frenck RW. Enteric pathogens associated with diarrhea in children in Fayoum, Egypt. Diag Microbiol Infect Dis. 2006;56:1–5. doi: 10.1016/j.diagmicrobio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- El-Settawy MA, Fathy GM. Evaluation and comparison of PCR, coproantigen ELISA and microscopy for diagnosis of Cryptosporidium in human diarrheic specimens. J Am Sci. 2012;8:1385. [Google Scholar]
- El-Shazly AM, Soltan DM, El-Sheikha HM, Sadek GS, Morsy AT. Correlation of ELISA coproantigen and oocysts count to the severity of cryptosporidiosis parvum in children. J Egypt Soc Parasitol. 2007;37:107–120. [PubMed] [Google Scholar]
- Feng Yaoyu, Dearen Theresa, Cama Vitaliano, Xiao Lihua. 90-Kilodalton Heat Shock Protein, Hsp90, as a Target for GenotypingCryptosporidiumspp. Known To Infect Humans. Eukaryotic Cell. 2009;8(4):478–482. doi: 10.1128/EC.00294-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia LS. Diagnostic Medical Parasitology. 5. Washington D. C: ASM Press; 2007. [Google Scholar]
- Garcia LS, Shimizu RY. Evaluation of nine immunoassay kits (enzyme immunoassay and direct fluorescence) for detection of Giardia lamblia and Cryptosporidium parvum in human fecal specimens. J Clin Microbiol. 1997;35:1526–1529. doi: 10.1128/jcm.35.6.1526-1529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan S, Sabry H, Amer N, Shalaby MA, Mohamed NA, Gaballah H. Incidence of cryptosporidiosis in immunodeficient cancer patients in Egypt. J Egypt Soc Parasitol. 2002;32:33–46. [PubMed] [Google Scholar]
- Jex AR, Smith HV, Monis PT, Campbell BE, Gasser RB. Cryptosporidium - biotechnological advances in the detection, diagnosis and analysis of genetic variation. Biotechnol Adv. 2008;26:304–317. doi: 10.1016/j.biotechadv.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Kehl KSC, Cicirello H, Havens PL. Comparison of four different methods for the detection of Cryptosporidium species. J Clin Microbiol. 1995;33:416–418. doi: 10.1128/jcm.33.2.416-418.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana S, Sharma P, Sharma A, Malla N. Evaluation of Ziehl-Neelsen staining, auramine phenol staining, antigen detection enzyme linked immunosorbent assay and polymerase chain reaction, for the diagnosis of intestinal cryptosporidiosis. Trop Parasitol. 2012;2:20–23. doi: 10.4103/2229-5070.97234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Pherson DW, Mc Queen R. Cryptosporidiosis: multi-attribute evaluation of six diagnostic methods. J Clin Microbiol. 1993;31:198–202. doi: 10.1128/jcm.31.2.198-202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald V, McCross MV, Petry F. Localisation of parasite antigens in Cryptosporidium parvum infected epithelial cells using monoclonal antibodies. Parasitology. 1995;110:259–268. doi: 10.1017/S0031182000080847. [DOI] [PubMed] [Google Scholar]
- Morgan UM, Pallant L, Dwyer BW, Forbes DA, Rich G, Thompson RCA. Comparison of PCR and microscopy for detection of Cryptosporidium parvum in human fecal specimens: clinical trial. J Clin Microbiol. 1998;36:995–998. doi: 10.1128/jcm.36.4.995-998.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nina JMS, McDonald V, Deer RMA, Wright SE, Dyson DA, Chiodini PL, McAdam KPWJ. Comparative study of the antigenic compositin of oocyst isolates of Cryptosporidium parvum from different hosts. Parasite Immunol. 1992;14:227–232. doi: 10.1111/j.1365-3024.1992.tb00463.x. [DOI] [PubMed] [Google Scholar]
- Ortega-Mora LM, Tronsco JM, Rojo-Vazquez FA, Gonzalez-Bautista M. Cross reactivity of polyclonal serum antibodies generated against Cryptosporidium parum oocysts. Infect Immun. 1992;60:3442–3445. doi: 10.1128/iai.60.8.3442-3445.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert B, Antoine H, Dreze F, Coppe P, Collard A. Characterization of a high molecular weight antigen of Cryptosporidium parvum micronemes possessing epitopes that are cross-reactive with all parasitic life cycle stages. Vet Res. 1994;25(4):384–398. [PubMed] [Google Scholar]
- Salyer S, Gillespie T, Rwego I, Chaman C, Goldberg T. Epidemiology and molecular relationships of Cryptosporidium spp. in people, primates, and livestock from Western Uganda. PLoS. 2012;6:1–6. doi: 10.1371/journal.pntd.0001597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HV. Diagnostics. In: Fayer R, Xiao L, editors. Cryptosporidiosis of man and animals. Boca Raton: CRC Press and IWA Publishing; 2008. pp. 173–208. [Google Scholar]
- Tahira F, Khan HM, Shukla I, Shujatullah F, Malik MA, Shahid M. Prevalence of Cryptosporidium in children with diarrhoea in north Indian tertiary care hospital. J Community Med Health Edu. 2012;2:1–3. [Google Scholar]
- Tzipori S, Rand W, Theodos C. Evaluation of a two-phase scid mouse model preconditioned with anti-interferon-g monoclonal antibody for drug testing against Cryptosporidium parvum. J Infect Dis. 1995;172:1160–1164. doi: 10.1093/infdis/172.4.1160. [DOI] [PubMed] [Google Scholar]
- Weitzel T, Dittrich S, Mo¨hl I, Adusu E, Jelinek T. Evaluation of seven commercial antigen detection tests for Giardia and Cryptosporidium in stool samples. Clin Microbiol Infect. 2006;12:656–659. doi: 10.1111/j.1469-0691.2006.01457.x. [DOI] [PubMed] [Google Scholar]
- Youssef FG, Adib I, Riddle MS, Schlett CDA. Review of cryptosporidiosis in Egypt. J Egypt Soc Parasitol. 2008;38:9–28. [PubMed] [Google Scholar]