Abstract
SNAP-25 is a neurotransmitter vesicular docking protein which has been associated with brain disorders such as attention deficit hyperactivity disorder, bipolar disorder and schizophrenia. In this project, we were interested if clinical factors are associated with differential SNAP-25 expression. We examined the SNAP-25 isoform mRNA and protein levels in postmortem cortex Brodmann's area 9 (BA9) and BA24 (n = 29). Subjects were divided by psychiatric diagnosis, clinical variables including mood state in the last week of life and lifetime impulsiveness. We found affected subjects with a diagnosis of alcohol use disorder (AUD) had a lower level of SNAP-25b BA24 protein compared to those without AUD. Hispanic subjects had lower levels of SNAP-25a, b and BA9 mRNA than Anglo-American subjects. Subjects who smoked had a total pan (total) SNAP-25 BA9/BA24 ratio. Subjects in the group with a low level of anxious-psychotic symptoms had higher SNAP-25a BA24 mRNA compared to normal controls, and both the high and low symptoms groups had higher pan (total) SNAP-25 BA9/BA24 ratios than normal controls. These data expand our understanding of clinical factors associated with SNAP-25. They suggest that SNAP-25 total and isoform levels may be useful biomarkers beyond limited neurological and psychiatric diagnostic categories.
Key Words: Alcohol dependence, Bipolar disorder, Molecular psychiatry, Prefrontal cortex, Schizophrenia, Synaptic neurobiology, SNAP-25, Exocytosis, Smoking, Ethnicity
Introduction
The 25-kDa synaptosomal-associated protein (SNAP-25) is a T-SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein) [1,2] which acts in concert with the integral membrane protein syntaxin [3], allowing neuronal vesicles to attach to the plasma membrane and release its content in response to calcium signaling. There are two SNAP-25 isoforms, and they are widely expressed with both regional and cellular compartment specificity [4,5]. The isoforms arise from alternative splicing of exon 5 and are developmentally regulated [6]. SNAP-25a is the dominant form prenatally, and SNAP-25b predominates after birth in humans. SNAP-25 is involved in most neurotransmissions, including acetylcholine, glutamate, GABA, dopamine and serotonin [7,8,9,10,11].
Changes in SNAP-25 can either be a reflection of altered neurotransmission, or it can directly change neurotransmission with downstream-altered behavior. For example, an amino acid substitution of the protein kinase C phosphorylation site on SNAP-25 produces anxiety behavior with a working memory deficit [7], and phosphorylated SNAP-25 is associated with acute stress [8]. Further supporting the idea of a critical role of SNAP-25 in human brain disorder are numerous studies which associate total, but not isoform, levels of SNAP-25 with bipolar disorder (BP), schizophrenia [12,13,14,15,16,17], attention deficit hyperactivity disorder, chronic pain [16,18,19], anxiety and epilepsy [20].
In this work, we wanted to determine if there were differential SNAP-25 isoform levels based on variables such as psychiatric diagnoses, medication use, alcohol use and the level of emotional symptoms at the end of life. To accomplish this, we studied two brain areas thought to be involved in the pathology of both neurological and psychiatric disorders, including BP and schizophrenia [21,22]: the dorsolateral prefrontal cortex [Brodmann's area (BA) 9] and the anterior cingulate cortex (ACC; BA24). In these areas, we measured SNAP-25 mRNA expression and protein levels of normal controls, individuals who had BP and those who had schizophrenia.
Materials and Methods
Subjects
This study examines SNAP-25 isoform mRNA expression and protein levels from the postmortem prefrontal cortex in control (n = 12), BP (n = 9) and schizophrenia (n = 8) subjects. A trained clinician interviews the next of kin (NOK) about the donor (psychological autopsy) to provide diagnostic information and information about mood state (lifetime and in the last week of life). To establish the clinical diagnosis, a DSM-IV-based Mini-International Neuropsychiatric Interview (MINI) [23] is administered to the NOK about the deceased. Information from these interviews, in addition to a review of all available medical records, is presented to the expert diagnostician consensus group, whose inter-rater reliability for the MINI was 0.8 for BP and schizophrenia. Medications listed are those that were prescribed at the time of death. Mood state in the last week of life is determined via semi-structured interviews. The Bipolar Inventory Symptoms Scale (BISS) [24] generates a total severity score and 5 symptom factor scores including mania, depression, anxiety, irritability and psychosis [25]. Lifetime impulsivity is measured using the Barratt Impulsiveness Scale [26].
Postmortem Tissue
The cerebrum was hemisected and cut into 1-cm-thick coronal blocks starting at the frontal pole, digitally photographed to document anatomical location, immediately frozen in isopentane (2-methylbutane, Fisher), chilled with dry ice to −60°C, and then stored at −80°C. A neuropathologist examined all brain samples for both gross and microscopic neuropathology. All subjects included in this study were free of confounding neuropathology. Toxicology results were obtained from the Bexar County Forensic Toxicology Laboratory. Psychotropic medication was identified in 13 of the 17 affected subjects (table 1). Control tissue was toxicology free of drugs of abuse. Table 1 lists the causes of death and toxicological results. For tissue identification of BA9 (dorsolateral prefrontal cortex) and BA24 (ACC), we used the criteria described by Rajkowska and Goldman-Rakic [27].
Table 1.
Cause of death and postmortem toxicology
| Clinical diagnosis | Cause of death | Toxicology |
|---|---|---|
| BP | pneumonia | diphenhydramine |
| BP | suicide – hanging | citalopram, papaverine, ethanol |
| BP | myocardial infarction | caffeine |
| BP | cardiomyopathy | naproxen, amitriptyline, nortriptyline, o-desmethylvenlafaxine, venlafaxine, diphenhydramine, metoprolol, metoclopramide, morphine |
| BP | suicide – overdose | atropine, citalopram, desmethylcitalopram, clonazepam, lamotrigine |
| BP | suicide – overdose | methadone, citalopram, trimethoprim |
| BP | accidental – toluene overdose | bupropion, tramadol, toluene |
| BP | suicide – overdose | atropine, olanzapine |
| BP | suicide – overdose | quetiapine, cotinine, ethanol, lamotrigine, nicotine, theobromine, caffeine |
| Schizophrenia | myocardial infraction | acetaminophen, lidocaine, monoethylglycinexylidide, midazolam |
| Schizophrenia | lung cancer | none |
| Schizophrenia | cardiomyopathy | metoprolol, venlafaxine, o-desmethylvenlafaxine, olanzapine |
| Schizophrenia | cardiomyopathy | bupropion, mirtazapine, nortriptyline, paroxetine, propranolol, clonazepam, norpropoxyphene, propoxyphene, carbamazepine, chlorpromazine, quetiapine, zolpidem, acetaminophen |
| Schizophrenia | coronary artery disease | haloperidol |
| Schizophrenia | suicide – overdose | citalopram/escitalopram, clonazepam, cotinine, lamotrigine, quetiapine |
| Schizophrenia | coronary artery disease | amitriptyline, nortriptyline, clozapine, ethanol |
| Schizophrenia | cardiomyopathy | mirtazapine, trazodone, diphenhydramine, oxycodone |
| Normal control | coronary artery disease | none |
| Normal control | arrhythmia | atropine |
| Normal control | gunshot – homicide | none |
| Normal control | coronary artery disease | none |
| Normal control | myocardial infarction | salicylic acid, acetaminophen, naproxen, chlorpheniramine, atropine |
| Normal control | coronary artery disease | atropine |
| Normal control | coronary artery disease | none |
| Normal control | coronary artery disease | caffeine |
| Normal control | coronary artery disease | caffeine |
| Normal control | coronary artery disease | none |
| Normal control | coronary artery disease | ethanol |
| Normal control | coronary artery disease | none |
Microfluidic High-Throughput RT-qPCR
Eighty milligrams of frozen tissue was dissected from BA9 and BA24, and total RNA was isolated using the RNeasy Lipid Tissue Mini Kit (Qiagen, Valencia, Calif., USA), per instructions; 100 μg of RNA was reverse transcribed utilizing the High-Capacity RNA-to-cDNA kit (Life Technologies, Grand Island, N.Y., USA). The cDNA was used as a template for microfluidic high-throughput RT-qPCR on the Fluidigm Biomark (San Francisco, Calif., USA) [28]. The cDNA was preamplified according to the Fluidigm Gene Expression Specific Target Amplification protocol using TaqMan PreAmp Master Mix and the following TaqMan gene expression assay primer/probes (Life Technologies/Applied Biosystems): pan SNAP-25 (primer spans exon 5 and encompasses both a and b isoforms) #Hs00268296_m1; SNAP-25b #Hs00938966_m1; SNAP-25a #Hs00938960_m1; glyceraldehyde 3-phosphate dehydrogenase (GAPDH) #4333764F.
The amplified product was diluted 1:5, mixed with TaqMan Universal PCR Master Mix (2×; Life Technologies, PN 4304437) and GE Sample Loading Reagent (20×; Fluidigm, PN 85000746), and loaded onto the sample wells of the 48.48 Dynamic Array integrated fluidic circuit (IFC). The TaqMan assay primer/probes were diluted to 10× concentration and loaded onto the assay wells of the IFC. Assay samples were run in duplicate, thus generating duplicates per sample in one IFC. qPCR was performed according to the Biomark protocol as follows: 2 min at 50°C, 10 min at 95°C, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. Data were processed by user detector threshold settings (each gene has independent thresholds) and linear baseline correction using Biomark Real-Time PCR Analysis software (v.3.0.4).
Relative quantification of the gene products was calculated using the ΔCt method [29] and transformed using 2-ΔCt, with GAPDH as the endogenous control. This gene was chosen as the reference gene due to general stability and minimal variation across diagnostic groups [30]. We found no significant differences in gene expression of GAPDH in BA9 [F(2, 16) = 0.96, p = 0.40] or in BA24 [F(2, 15) = 3.1, p = 0.08] across diagnostic groups in this cohort of subjects.
Western Blotting
In brief, 50 mg of tissue was homogenized in 5× volume of cold 0.05 m Tris-buffered saline (pH 7.4) with protease inhibitor cocktail (Sigma Chemical Company No. P8340) and 1% NP-40 at 4°C. The homogenate was centrifuged for 10 min at 1,000 g at 4°C. The pellet (P1) was resuspended in 100 μl of homogenization buffer and centrifuged for 15 min at 12,000 g at 4°C. The pellet (P2) was resuspended in 300 µl of homogenization buffer, and protein concentration was determined using the Bradford method. Samples were denatured with the addition of Laemmli buffer and β-mercaptoethanol and heated to 95°C for 5 min. All samples were standardized, and 5 µg of total protein was electrophoresed on a 4-15% SDS-PAGE gradient gel (Bio-Rad, Cat. No. 456-1086). The protein was transferred to PVDF membrane blots (Millipore Corp., Immobilon-P, Cat. No. IPVH07850) in 1× Towbin solution with 15% methanol at 100 V (250 mA) for 1 h at 24°C. Blots were rinsed in ddH2O and incubated in LI-COR Odyssey Blocking Buffer (Lincoln, Nebr., USA) overnight at 4°C.
SNAP-25a and b isoform-specific antibodies [4] were used for immunodetection. The blots were incubated for 1 h on a shaker at 24°C in either rabbit anti-SNAP-25a antibody (1:2,000) with mouse anti-β-actin antibody (Sigma, Cat. No. A1978; 1:10,000) in Odyssey Blocking Buffer or SNAP-25b (1:4,000) with mouse anti-β-actin antibody (Sigma, Cat. No. A1978; 1:10,000) in Odyssey Blocking Buffer. The blots were rinsed 3 times in Tris-buffered saline with 1% Tween 20 (TTBS) for 5 min each and then incubated in the secondary antibody for 1 h in the dark, at room temperature with constant shaking. Near-infrared (IR) dye-tagged secondary antibodies from Rockland Immunochemicals Inc. (Gilbertsville, Pa., USA) were utilized as follows: anti-rabbit IgG IRDye 700DX conjugated (1:4,000) was used to detect SNAP-25a and SNAP-25b, and anti-mouse IgG IRDye 800CW conjugated (1:10,000) was used to detect β-actin. The blots were rinsed again 3 times for 5 min in TTBS and visualized using an Odyssey Imaging System (LI-COR Biotechnology). SNAP-25a and b isoforms were measured at 700 nm and β-actin at 800 nm.
The samples were run in duplicate and values averaged. Data are expressed as the relative SNAP-25 level = immunointensity of SNAP-25 isoform/immunointensity of β-actin.
Statistics
SPSS Statistics version 20 (Chicago, Ill., USA), was used for the analysis. Normally distributed data were analyzed with the univariate analysis of covariance (ANCOVA) and post hoc correction by the Bonferroni method. When comparing groups according to psychiatric diagnoses (BP, schizophrenia, normal control) and with the alcohol use disorder (AUD) groups (AUD, no AUD, normal control), nonnormally distributed data [SNAP-25 pan (total) BA9 mRNA, SNAP-25 pan (total) BA24 mRNA, SNAP-25b/a BA9 protein ratio, symptom scales] were analyzed with the Mann-Whitney U test. Pairwise comparison was performed with the Kruskal-Wallis test. We used the Mann-Whitney U test to compare ethnicity (Hispanic, Anglo-American) and smoking (yes, no) groups. Categorical data were compared between groups by χ2 analysis. We used the Spearman correlation to look at pH and SNAP-25 along with comparing mRNA with protein levels. One subject in the normal control group and 1 subject in the BP SNAP-25b/a BA9 protein group were outliers by >2 standard deviations from the mean, and these measurements were not included in the analysis.
Results
SNAP-25 Protein Levels
Protein levels of SNAP-25a and SNAP-25b isoforms were examined in BA9 and BA24 of the prefrontal cortexes from bipolar, schizophrenia and normal control subjects. Figure 1 shows the detection of single bands for SNAP-25 isoform a and b proteins at the predicted molecular weight. We also looked at the ratio of SNAP isoforms within and the SNAP-25 pan (total) ratio between the anatomical areas BA9 and BA24.
Fig. 1.
Western blot of SNAP-25a and b protein isoforms BA9 and BA24. The two panels on the left are representative fluorescent scans of SNAP-25a protein blots, and the two panels on the right are representative scans of SNAP-25b blots. The top band is actin and the bottom band is SNAP-25. The upper two panels are samples from BA9, while the lower two panels are samples from BA24. Samples were run in duplicate, and their respective diagnosis is listed below each lane: BP, schizophrenia, control. Molecular weight markers (not shown) are labeled in the center, the 38-kDa band of the ladder appearing near the green sample bands (β-actin, molecular weight 42 kDa), and the 28-kDa band of the ladder appearing near the red sample bands (SNAP-25, molecular weight 25 kDa). Colors refer to the online version only.
All subjects were first separated into groups based on smoking status (yes, n = 21; no, n = 8), family psychiatric history (yes, n = 7; no, n = 19), handedness (right, n = 22; left, n = 5), gender (male, n = 21; female, n = 8) and ethnic heritage (Hispanic, n = 7; Anglo-American, n = 22). Those that smoked had a significantly lower pH (p = 0.04) and elevated SNAP-25a BA9 (p = 0.004) compared to nonsmokers (table 2; fig. 2a). No significant correlations were found between SNAP-25a BA9 protein and pH in smokers (p = 0.1) or nonsmokers (p = 0.3). There was no statistical difference between the numbers of male and female smokers (p = 0.85; male smokers, n = 15; male nonsmokers, n = 6; female smokers, n = 6; female nonsmokers, n = 2). As we were interested in gender differences, we subdivided the smoking and nonsmoking subjects according to gender. Figure 2b shows that the SNAP-25a BA9 protein differences were found only in men (p = 0.01). No other significant differences in protein were found between these groups. The symptom severity at the end of life was likewise similar across groups (data not shown), except that those with a family psychiatric history were younger and had more severe symptoms (table 3).
Table 2.
Demographic and symptom data by smoking status
| Nonsmoking |
Smoking |
p | |||
|---|---|---|---|---|---|
| n | mean ± SD | n | mean ± SD | ||
| Family psychiatric history (yes/no) | 1/5 | 6/14 | 1.0 | ||
| Handedness (left/right) | 0/6 | 5/16 | 0.2 | ||
| Ethnicity (Hispanic/Anglo-American) | 3/5 | 4/17 | 0.3 | ||
| Sex (male/female) | 6/2 | 15/6 | 0.8 | ||
| Suicide (yes/no) | 2/6 | 4/17 | 0.3 | ||
| AUD (yes/no) | 2/6 | 5/16 | 0.9 | ||
| Substance use disorder (yes/no) | 0/8 | 2/19 | 0.4 | ||
| Psychiatric diagnosis (yes/no) | 4/4 | 13/8 | 0.1 | ||
| Antidepressant use (yes/no) | 2/6 | 8/13 | 0.5 | ||
| Benzodiazepine use (yes/no) | 0/8 | 2/19 | 0.4 | ||
| Antiseizure mood stabilizer use (yes/no) | 0/8 | 4/17 | 0.2 | ||
| Neuroleptic use (yes/no) | 1/7 | 6/15 | 0.4 | ||
| Age, years | 8 | 48 ± 15 | 21 | 54 ± 10 | 0.3 |
| Postmortem interval, h | 8 | 27 ± 7 | 21 | 28 ± 5 | 0.8 |
| pH | 8 | 6.5 ± 0.2 | 21 | 6.3 ± 0.3 | 0.04 |
| RNA integrity number | 8 | 7.2 ± 0.6 | 21 | 6.8 ± 1.1 | 0.3 |
| Total BISS score | 5 | 34 ± 42 | 18 | 21 ± 21 | 0.9 |
| BISS mania factor score | 5 | 8 ± 12 | 18 | 8 ± 11 | 1.0 |
| BISS depression factor score | 5 | 4 ± 12 | 18 | 20 ± 9 | 0.04 |
| BISS irritability factor score | 5 | 5 ± 6 | 18 | 3.3 ± 5 | 1.0 |
| BISS anxiety factor score | 5 | 6 ± 8 | 18 | 2 ± 3 | 0.02 |
| BISS psychosis factor score | 5 | 1.4 ± 2 | 18 | 1.7 ± 3 | 1.0 |
| BPRS | 5 | 23 ± 14 | 18 | 38 ± 17 | 0.8 |
| Barratt Impulsiveness score | 5 | 30 ± 19 | 18 | 29 ± 15 | 0.2 |
| SNAP-25 pan (total) mRNA BA9/24 ratio | 8 | 1.2 ± 0.2 | 19 | 0.9 ± 0.3 | 0.03 |
BPRS = Brief Psychiatric Rating Scale. Medications are those that were prescribed at the time of death. Bold print indicates p < 0.05.
Fig. 2.
SNAP-25 pan (total) BA9/BA24 mRNA ratio and SNAP-25a BA9 protein level with smoking. a SNAP-25a BA9 protein relative immunointensity. b SNAP-25a BA9 protein relative immunointensity grouped by sex. c Pan (total) SNAP-25 BA9/BA24 mRNA ratio. d Pan (total) SNAP-25 BA9/BA24 mRNA ratio grouped by the affected (combined BP and schizophrenia) subjects and normal controls. Error bars = ±1 SD.
Table 3.
Demographic and symptom data by history of family psychiatric illness
| Without family psychiatric history |
With family psychiatric history |
p | |||
|---|---|---|---|---|---|
| n | mean ± SD | n | mean ± SD | ||
| Handedness (left/right) | 3/16 | 1/6 | 0.9 | ||
| Ethnicity (Hispanic/Anglo-American) | 2/17 | 3/4 | 0.3 | ||
| Sex (male/female) | 12/4 | 4/2 | 0.7 | ||
| Smoking (yes/no) | 12/4 | 5/1 | 0.7 | ||
| Suicide (yes/no) | 2/14 | 2/4 | 0.3 | ||
| AUD (yes/no) | 1/15 | 3/3 | 0.04 | ||
| Substance use disorder (yes/no) | 0/16 | 6/6 | <0.7 | ||
| Psychiatric diagnosis (yes/no) | 5/11 | 6/0 | 0.004 | ||
| Antidepressant use (yes/no) | 4/15 | 4/3 | 0.08 | ||
| Benzodiazepine use (yes/no) | 1/18 | 0/7 | 0.5 | ||
| Antiseizure mood stabilizer use (yes/no) | 2/17 | 1/6 | 0.8 | ||
| Neuroleptic use (yes/no) | 2/17 | 4/3 | 0.03 | ||
| Age, years | 16 | 57 ± 11 | 6 | 44 ± 10 | 0.04 |
| Postmortem interval, h | 16 | 27 ± 5 | 6 | 30 ± 4 | 0.5 |
| pH | 16 | 6.3 ± 0.3 | 6 | 6.2 ± 0.3 | 0.1 |
| RNA integrity number | 16 | 7.1 ± 09 | 6 | 7.1 ± 1.1 | 0.9 |
| Total BISS score | 13 | 14.3 ± 22 | 5 | 49 ± 32 | 0.007 |
| BISS mania factor score | 13 | 4 ± 1 | 5 | 12 ± 7 | 0.8 |
| BISS depression factor score | 13 | 4 ± 12 | 5 | 20 ± 9 | 0.01 |
| BISS irritability factor score | 13 | 2 ± 3.5 | 5 | 6.4 ± 7 | 0.1 |
| BISS anxiety factor score | 13 | 2 ± 5 | 5 | 6.6 ± 5 | 0.04 |
| BISS psychosis factor score | 13 | 0.8 ± 2 | 5 | 4.2 ± 4 | 0.04 |
| BPRS | 13 | 23 ± 14 | 5 | 38 ± 17 | 0.04 |
| Barratt Impulsiveness score | 13 | 54 ± 15 | 5 | 86 ± 20 | 0.007 |
BPRS = Brief Psychiatric Rating Scale. Medications are those that were prescribed at the time of death. Bold print indicates p < 0.05.
We then examined SNAP-25 protein within the affected groups (those with BP and schizophrenia), looking at prescribed psychotropic medication use (n = 6 without medication and n = 11 with medication), drug use (n = 15 without drugs and n = 2 with drugs) and suicide (n = 11 without suicide and n = 6 with suicide), which did not show any differences. From the combined BP and schizophrenia groups, we recategorized the samples into those affected with a diagnosis of AUD and those affected but without AUD diagnosis, and compared these two groups with normal controls (table 4). Using family history of psychiatric illness and handedness as covariates, we found that SNAP-25b isoform protein levels in BA24 were significantly lower in subjects with AUD compared to those with no AUD and normal controls [F(4, 18) = 3.1; p = 0.04]. Results of pairwise comparisons of AUD with no AUD (p = 0.03) and normal control with AUD groups (p = 0.1) are shown in figure 3a. There was no difference in symptom severity between affected groups (AUD and no AUD), and both the AUD and no AUD groups were significantly more symptomatic than normal controls (table 4; figure 3b).
Table 4.
Demographic and symptom data by the AUD diagnosis
| No AUD |
AUD |
Normal control |
Statistics | ||||
|---|---|---|---|---|---|---|---|
| n | mean ± SD | n | mean ± SD | n | mean ± SD | ||
| Subjects, n | 10 | 7 | 12 | ||||
| Family psychiatric history (yes/no) | 3/5 | 4/2 | 0/11 | p = 0.0111 | |||
| Handedness (left/right) | 4/5 | 0/6 | 1/11 | p = 0.053 | |||
| Ethnicity (Hispanic/Anglo-American) | 3/7 | 2/5 | 2/10 | p = 0.1 | |||
| Sex (male/female) | 6/4 | 6/1 | 9/3 | p = 0.8 | |||
| Smoking (yes/no) | 8/2 | 5/2 | 8/4 | p = 0.1 | |||
| Suicide (yes/no) | 2/8 | 4/3 | 0/12 | p = 0.2 | |||
| Substance use disorder (yes/no) | 2/8 | 0/7 | 0/12 | p = 0.1 | |||
| Psychiatric diagnosis (BP/schizophrenia) | 4/6 | 5/2 | 0/12 | p = 0.2 | |||
| Antidepressant use (yes/no) | 5/5 | 5/2 | 0/12 | p = 0.4 | |||
| Benzodiazepine use (yes/no) | 1/9 | 1/6 | 0/12 | p = 0.4 | |||
| Antiseizure mood stabilizer use (yes/no) | 2/8 | 2/5 | 0/12 | F(4, 14) = 0.5, p = 0.7 | |||
| Neuroleptic use (yes/no) | 4/6 | 3/4 | 0/12 | p = 1.0 | |||
| Age, years | 49 ± 10 | 47 ± 7 | 56 ± 16 | F(92, 26) = 1.5, p = 0.2 | |||
| Postmortem interval, h | 27 ± 7 | 31 ± 3 | 26 ± 4.7 | F(2, 26) = 2.1, p = 0.15 | |||
| pH | 6.3 ± 0.3 | 6.4 ± 0.3 | 6.3 ± 0.3 | F(2, 26) = 0.5, p = 0.6 | |||
| RNA integrity number | 6.6 ± 1.0 | 6.7 ± 1.2 | 7.4 ± 0.78 | F(2, 26) = 2.2, p = 0.1 | |||
| Total BISS score | 39 ± 30 | 34 ± 24 | 3.4 ± 3 | ||||
| BISS mania factor score | 11 ± 10 | 15 ± 15 | 1 ± 2 | p = 0.021 | |||
| BISS depression factor score | 15 ± 15 | 13 ± 8 | 2 ± 2 | p = 0.011 | |||
| BISS irritability factor score | 6 ± 6 | 7 ± 5 | 0 ± 0 | p = 0.0011 | |||
| BISS anxiety factor score | 5 ± 7 | 5 ± 4 | 0.2 ± 0.6 | p = 0.0031 | |||
| BISS psychosis factor score | 3 ± 4 | 2 ± 2 | 0 ± 0 | p = 0.021 | |||
| BPRS | 40 ± 18 | 31 ± 8 | 16 ± 6 | p = 0.0011 | |||
| Barratt Impulsiveness score | 80 ± 16 | 86 ± 19 | 46 ± 6 | p = 0.0011 | |||
| SNAP-25b protein BA24 immunointensity | 1.8 ± 0.2 | 1.1 ± 0.2 | 0.6 ± 0.2 | F(4, 18) = 3.1, p = 0.042 | |||
BPRS = Brief Psychiatric Rating Scale. Data on 3 subjects are missing. Medications are those that were prescribed at the time of death. Bold print indicates p < 0.05.
Significant difference (p < 0.05) between the normal control and AUD and between normal control and no AUD.
Significant difference (p < 0.05) between normal control and no AUD.
Significant difference (p < 0.05) between normal control and AUD.
Fig. 3.
SNAP-25b BA24 protein-relative immunointensity and symptom level in AUD. a SNAP-25b BA24 protein relative immunointensity. b Psychiatric symptom level at the end of life: Barratt (Barratt Impulsiveness Scale), BPRS (Brief Psychiatric Rating Scale), total BISS (total BISS score), depression (BISS depression factor score), mania (BISS factor score), irritability (BISS irritability factor score), anxiety (BISS anxiety factor score) and psychosis (BISS psychosis factor score). 1 = Significant difference (p < 0.05) between the normal control and AUD and between normal control and no AUD; 2 = significant difference (p < 0.05) between normal control and no AUD; 3 = significant difference (p < 0.05) between normal control and AUD. There were no significant differences in symptoms between the AUD and no AUD groups. Error bars = ±1 SD.
There were significant differences in symptom severity at the end of life when comparing controls with subjects with BP or schizophrenia (fig. 4a; table 5). Using RNA integrity number and family history of psychiatric illness as covariates, no significant SNAP-25 protein differences were identified (table 6; fig. 4b, c).
Fig. 4.
Psychiatric symptoms, SNAP-25 mRNA and protein level in BP, schizophrenia and normal controls. a Psychiatric symptom level at the end of life: Barratt (Barratt Impulsiveness Scale), BPRS (Brief Psychiatric Rating Scale), total BISS (total BISS score), depression (BISS depression factor score), mania (BISS factor score), irritability (BISS irritability factor score), anxiety (BISS anxiety factor score) and psychosis (BISS psychosis factor score). 1 = Significant (p < 0.05) difference between normal control and BP, and between normal control and schizophrenia groups; 2 = significant difference (p < 0.05) between normal control and schizophrenia, and nonsignificant (p = 0.07) difference between normal control and BP; 3 = significant difference between normal control and BP, and nonsignificant difference (p = 0.06) between normal control and schizophrenia groups. Error bars = ±1 SD. b SNAP-25 mRNA levels. c SNAP-25 protein levels. d SNAP-25b/a BA24 protein and mRNA levels; SNAP-25b/a BA24 ratio, and pan (total) SNAP-25 mRNA BA9/BA24 ratio.
Table 5.
Demographic and symptom data by psychiatric diagnosis
| BP |
Schizophrenia |
Normal control |
Statistics | ||||
|---|---|---|---|---|---|---|---|
| n | mean ± SD | n | mean ± SD | n | mean ± SD | ||
| Subjects, n | 9 | 8 | 12 | ||||
| Family psychiatric history (yes/no) | 3/41 | 4/32 | 0/122 | p = 0.014 | |||
| Handedness (left/right) | 1/61 | 3/5 | 0/12 | p = 0.2 | |||
| Ethnicity (Hispanic/Anglo-American) | 2/7 | 3/5 | 2/10 | p = 0.6 | |||
| Sex (male/female) | 6/3 | 6/2 | 9/3 | p = 0.9 | |||
| Smoking (yes/no) | 5/4 | 8/0 | 8/4 | p = 0.1 | |||
| Suicide (yes/no) | 5/4 | 1/7 | 0/12 | p = 0.06 | |||
| Substance use disorder (yes/no) | 1/8 | 1/7 | 0/12 | p = 0.9 | |||
| AUD (yes/no) | 5/4 | 2/6 | 0/12 | p = 0.2 | |||
| Antidepressant use (yes/no) | 5/4 | 3/5 | 0/12 | p = 0.8 | |||
| Benzodiazepine use (yes/no) | 1/8 | 1/7 | 0/12 | p = 0.9 | |||
| Antiseizure mood stabilizer use (yes/no) | 3/6 | 1/7 | 0/12 | p = 0.3 | |||
| Neuroleptic use (yes/no) | 3/6 | 4/4 | 0/12 | p = 0.5 | |||
| Age, years | 47 ± 6.2 | 50 ± 11 | 56 ± 16 | F(2, 26) = 1.6, p = 0.2 | |||
| Postmortem interval, h | 27 ± 6.7 | 30 ± 4.6 | 26 ± 4.7 | F(2, 26) = 1.6, p = 0.2 | |||
| pH | 6.4 ± 0.3 | 6.3 ± 0.3 | 6.3 ± 0.3 | F(2, 26) = 0.76, p = 0.5 | |||
| RNA integrity number | 7 ± 0.8 | 6.2 ± 1.2 | 7.4 ± 0.78 | F(2, 26) = 3.8, p = 0.045 | |||
| Total BISS score | 38 ± 291 | 35 ± 251 | 3 ± 3.43 | p = 0.0014 | |||
| BISS mania factor score | 8 ± 11 | 17 ± 13 | 1 ± 2 | p = 0.0094 | |||
| BISS depression factor score | 18 ± 14 | 11 ± 10 | 2 ± 2 | p = 0.0084 | |||
| BISS irritability factor score | 5 ± 5 | 7 ± 6 | 0 ± 0 | p = 0.0014 | |||
| BISS anxiety factor score | 6 ± 6 | 3 ± 4 | 0.2 ± 1 | p = 0.0034 | |||
| BISS psychosis factor score | 6 ± 6 | 3 ± 4 | 0 ± 0 | p = 0.0144 | |||
| BPRS | 31 ± 14 | 41 ± 15 | 16 ± 6 | p = 0.0014 | |||
| Barratt Impulsiveness score | 78 ± 18 | 86 ± 16 | 46 ± 9 | p = 0.0014 | |||
BPRS = Brief Psychiatric Rating Scale. Bold print indicates p < 0.05.
Data not available for 2 subjects.
Data not available for 1 subject.
Data not available for 3 subjects.
Significant (p < 0.05) difference between normal control and BP, and between normal control and schizophrenia groups.
Significant difference (p < 0.05) between normal control and schizophrenia groups.
Table 6.
SNAP-25 levels in BP and schizophrenia groups and normal controls
| BP | Schizophrenia Normal control | Statistics | ||
|---|---|---|---|---|
| SNAP-25 pan (total) mRNA 2−ΔCt BA9 | 2.6 ± 1.4 | 2.4 ± 0.6 | 2.3 ± 0.8 | F(4, 15) = 0.6, p = 0.6 |
| SNAP-25a mRNA 2−ΔCt BA9 | 0.3 ± 0.11 | 0.29 ± 0.08 | 0.3 ± 0.08 | F(4, 15) = 0.6, p = 0.7 |
| SNAP-25b mRNA 2−ΔCt BA9 | 0.71 ± 0.2 | 0.73 ± 0.17 | 0.77 ± 0.14 | F(4, 15) = 0.4, p = 0.8 |
| SNAP-25a/b mRNA 2−ΔCt BA9 | 2.6 ± 0.6 | 2.6 ± 0.4 | 2.6 ± 0.3 | F(4, 15) = 0.2, p = 0.9 |
| SNAP-25 pan (total) mRNA 2−ΔCt BA24 | 2.5 ± 1.2 | 3.8 ± 3 | 2.5 ± 1.5 | F(4, 15) = 0.3, p = 0.9 |
| SNAP-25a mRNA 2−ΔCt BA24 | 0.31 ± 0.13 | 0.37 ± 0.12 | 0.31 ± 0.11 | F(4, 15) = 0.2, p = 0.9 |
| SNAP-25b mRNA 2−ΔCt BA24 | 0.42 ± 0.19 | 0.52 ± 0.16 | 0.5 ± 0.2 | F(4, 15) = 1.2, p = 0.4 |
| SNAP-25a/b mRNA 2−ΔCt BA24 | 1.6 ± 0.6 | 1.8 ± 0.4 | 1.6 ± 0.7 | F(4, 15) = 0.1, p = 1.0 |
| SNAP-25 pan (total) BA9/BA24 ratio | 1.0 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.2 | F(4, 15) = 0.4, p = 0.8 |
| SNAP-25a protein BA9 immunointensity | 0.8 ± 0.3 | 0.54 ± 0.3 | 0.6 ± 0.2 | F(4, 15) = 2.3, p = 0.1 |
| SNAP-25b protein BA9 immunointensity | 1.8 ± 0.4 | 1.7 ± 0.3 | 1.6 ± 0.5 | F(4, 15) = 0.4, p = 0.8 |
| SNAP-25b/a protein ratio BA9 | 2.3 ± 1.3 | 3.1 ± 2.5 | 2.7 ± 0.8 | F(4, 15) = 4.3, p = 0.2 |
| SNAP-25a protein BA24 immunointensity | 0.63 ± 0.4 | 0.99 ± 0.5 | 0.9 ± 0.6 | F(4, 15) = 0.7, p = 0.6 |
| SNAP-25b protein BA24 immunointensity | 1.3 ± 0.5 | 1.5 ± 0.4 | 1.5 ± 0.4 | F(4, 14) = 0.5, p = 0.7 |
| SNAP-25b/a protein ratio BA24 | 3.2 ± 2.9 | 2.2 ± 1.1 | 2.2 ± 1.1 | F(4, 15) = 0.2, p = 0.9 |
Values are expressed as mean ± SD.
We then hypothesized that symptom type and severity, rather than clinical diagnosis, would have an effect on SNAP-25 levels. Using the BISS mood state assessment data from the last week and Barratt Impulsiveness Scale, we looked at lifetime impulsiveness, total BISS severity score, BISS depression, BISS mania subgroups and BISS irritableness. The BISS anxiety and BISS psychosis groups were merged to a single combined anxiety-psychosis group because the subjects substantially overlapped (85%). All symptom groups were in turn subdivided into high and low severity groups. Using family psychiatric history as a covariate, no significant protein differences were noted.
SNAP-25 mRNA Level
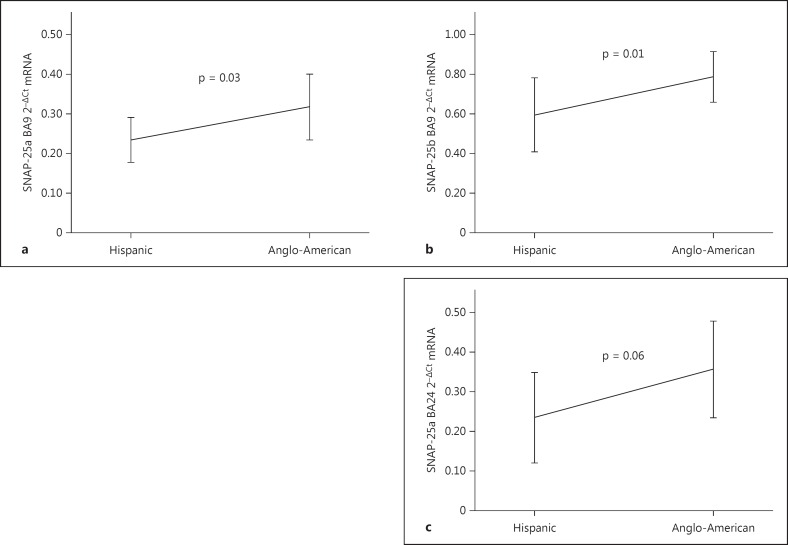
We then looked at mRNA levels. Using the category divisions described above, we found smokers had a higher pan (total) SNAP-25 BA9/BA24 mRNA ratio (p = 0.04; fig. 2c). We divided the smokers into affected (BP and schizophrenia) and nonaffected (normal control). Figure 2d shows that the SNAP-25 pan (total) mRNA BA9/BA24 smoking finding was only in the affected sample (p = 0.03). No significant correlations were found with pan (total) SNAP-25 BA9/BA24 ratio pH (smoking, p = 0.9; nonsmoking, p = 0.3). SNAP-25a and SNAP-25b isoforms in BA9 mRNA were significantly higher in the Anglo-American subjects compared to Hispanics (p = 0.03 and 0.01, respectively), and there was a trend for elevation in SNAP-25a BA24 (p = 0.06; fig. 5a-c).
Fig. 5.
SNAP-25 mRNA levels in subjects of Hispanic and Anglo-American origin. a SNAP-25a BA9. b SNAP-25b BA9. c SNAP-25a BA24. Error bars = ±1 SD.
There were no significant mRNA differences within affected groups (those with BP and schizophrenia), looking at prescribed psychotropic medication use, drug use and suicide. There was also no difference in SNAP-25 mRNA between the BP, schizophrenia and normal control groups (table 6; fig. 4b).
Figure 6a shows the pan (total) SNAP-25 BA9/BA24 ratio [F(5, 12) = 3.7; p = 0.03] was significantly elevated in both the high and low combined anxiety-psychosis groups compared to normal control (pairwise comparisons: low-normal control groups, p = 0.07; high-normal control, p = 0.01). There was no significant difference in the number of smokers between groups (no smoking low symptom, n = 0; no smoking high symptom, n = 2; no smoking normal control, n = 4; smoking low symptom, n = 6; smoking high symptom, n = 8; smoking normal control, n = 12), as demonstrated above. The nonsmoker pan (total) SNAP-25 BA9/BA24 ratio was greater than that of the smokers and represented a potential confounding factor. We reanalyzed the average combined anxiety-psychosis data without the nonsmokers [F(3, 10) = 4.9; p = 0.024] (fig. 6b). SNAP-25a BA24 was also significantly higher in the low symptom group compared to control [F(3, 19) = 4.7, p = 0.013; pairwise comparison between the low symptom and normal control groups: p = 0.004 (fig. 6c)].
Fig. 6.
SNAP-25 mRNA pan (total) BA9/BA24 ratio and SNAP-25a BA24 mRNA in the combined BISS factor anxiety and psychosis groups. a SNAP-25 mRNA pan (total) BA9/BA24 ratio with subjects grouped by level of combined BISS factors anxiety and psychosis and normal control. b Same as in a, with subjects subgrouped by smoking status. c SNAP-25a BA24 mRNA with subjects grouped by level of combined BISS factors anxiety and psychosis and normal control. Error bars = ±1 SD.
Finally, we looked at the correlations between SNAP-25 mRNA and protein isoforms in BP, schizophrenia and normal controls. We found that in the normal control group, SNAP-25b BA24 protein to mRNA had an R2 of −0.8 (p = 0.01). In the schizophrenia group, SNAP-25a BA9 had an R2 of −0.8 (p = 0.2).
Discussion
This report examining SNAP-25 isoform gene expression and protein levels in human postmortem tissue does not show differences in SNAP-25 isoforms across DSM diagnostic categories of BP and schizophrenia. Rather, we found changes related to ethnicity, substance exposure and mood state at the end of life. We had data on three substance group exposures: medications, alcohol and tobacco. Medications did not show any changes; however, both alcohol and tobacco did. The pathophysiology of ACC (BA24) SNAP-25b in AUD and smoking are unknown. However, there is evidence that alcohol's neurotoxicity can cause anterior cingulate atrophy [31] with a lower level of a noradrenalin marker [32]. SNAP-25 is involved with multiple neurotransmitters [11], including noradrenalin [33,34,35]. The lower level of ACC SNAP-25b implies that the noradrenergic deficits could be mediated through SNAP-25. Nicotinic acetylcholinergic receptors are widely distributed throughout the brain [36,37]. Smoking activates both the prefrontal cortex (BA9) and ACC (BA24) [38,39]. It is possible that the reduced pan (total) SNAP-25 BA9/BA24 ratio reflects smoking-induced alteration of the brain's acetylcholinergic distribution. These hypotheses can be directly evaluated with animal modeling.
The data show that the amount of SNAP-25b BA9 mRNA is lower in individuals of Hispanic origin compared to those of Anglo-American origin. There has been little to no work examining the molecular biological difference in psychiatric disorders between ethnicities. However, there is a growing literature studying differences in protective and genetic risk factors in substance use disorders across ethnicities. With some of these genetic differences located in neurotransmitter receptors, there may be associated differences in protein functioning [40,41]. This finding implies that postmortem molecular biology studies need to take into account the ethnicities of the subjects.
Our data showed that the individuals in the lower combined anxiety-psychosis symptom group had higher SNAP-25a B24 mRNA levels, which changed the isoform balance. This may be one mechanism where subtle changes in exocytotic proteins can cause or reflect human behavior. We feel the data support the idea that in postmortem research of SNAP-25, looking at the symptoms themselves rather than DSM diagnoses of BP and schizophrenia is important to understand the underlying biology.
Previous work has looked at pan (total) SNAP-25 in both BP and schizophrenia. For BP, there are only a few studies. They show either regional or no differences in pan (total) SNAP-25 [14,17,42], and our data support that pan (total) SNAP-25 is not differentially expressed in BP. Conversely, SNAP-25 and other exocytotic proteins have been extensively studied in schizophrenia [12,42,43,44,45,46,47,48]. These results are inconsistent and show a large variety of differential anatomical expression. One hypothesis explaining these results is that the findings are associated with the clinical syndrome of schizophrenia. An alternative hypothesis is that exocytotic proteins including SNAP-25 are sensitive to change based on a variety of nonspecific conditions including perinatal hypoxia and diabetes [49,50]. Our findings support the latter idea that variances in SNAP-25 levels across psychiatric groups may have more to do with the characteristics of the specific populations and not the clinical psychiatric diagnoses.
Another interesting possibility of SNAP-25's role in schizophrenia is seen in a study of SNARE complexes. The authors show that the individual SNARE components, including SNAP-25 levels, were not different between the schizophrenia and control subjects. Rather, they found greater SNARE complex formation in schizophrenia. The authors suggest it is not the total quantity of the SNARE components; instead, it is the binding affinities of the individual components that are different in schizophrenia [51].
To reduce confounding factors across diagnostic groups, we utilized tissue from only a single hemisphere [52,53,54], tissue of good RNA quality [52], and BP, schizophrenia and normal control groups matched for gender, handedness and smoking history [55]. We also looked at drugs of abuse and medication effects on SNAP-25 within the affected subjects. However, due to the great number of variables, it was not possible to have homogenous groups. To address this and multiple testing errors, we used statistical adjustments, or in the case of smoking we removed the nonsmoking subjects from the analysis and reanalyzed the data. We also subdivided the groups when the sample was large enough to provide meaningful data, i.e. symptom groups subdivided by smoking status. In the case of SNAP-25a BA24 mRNA, its amount was found to be higher in Anglo-American subjects and the low combined anxiety-psychosis symptom group, but we were unable to subdivide the sample due to the small number of Hispanic subjects. Future analysis with a greater sample size will be needed to have the power to answer whether the individual BISS factor groups of irritability, anxiety and psychosis have separate SNAP-25 differences or only as a group. The issue of medication exposure is difficult to address in postmortem research. While we demonstrate that there are no differences in SNAP-25 between the affected subjects taking medications and those not taking medications at the time of death, we cannot rule out that a lifetime expose could have had some effect. This issue can more fully be addressed with animal models.
There were only two significant correlations between mRNA and protein levels. The reasons for the lack of correlations are complex, ranging from experimental, i.e. sensitivity and measurement error, to biological, i.e. epigenetic control of gene expression [56]. Using animal modeling will be better at answering the reasons for limited protein and mRNA correlations.
In conclusion, this work looked at the influences on SNAP-25 total and isoform expression in the human brain. It appears that SNAP-25 can be a valuable biomarker for behavior and psychopathology, but less so to distinguish BP from schizophrenia. Our work, as with most postmortem research, should be used to help improve animal models of mental illness and normal behavior.
Statement of Ethics
All brain tissue was obtained with consent from the next of kin. The Southwest Brain Bank (SWBB) collection of postmortem tissue for research is conducted under the jurisdiction of the State of Texas Anatomical Review Board and under exempt review by the UTHSCSA Institutional Review Board. The next of kin psychological autopsy interview was approved by the UTHSCSA IRB, No. HSC20030197H.
Disclosure Statement
The University of New Mexico has a patent for the detection of SNAP-25, of which P.M. Thompson is the inventor. M. Takahashi, M. Itakura, E.A. Fucich, D.Y. Olukotun and D.A. Cruz declare no conflict of interest.
Acknowledgements
The authors wish to thank the donor families, without whom this work could not have been completed. P.M. Thompson has received funding from NIH and DOD. E.A. Fucich receives support from NIH T32 NS 082145.
References
- 1.Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 2.McMahon HT, Sudhof TC. Synapatic core complex of synaptobrevin, syntaxin and SNAP25 forms high affinity α-SNAP binding site. J Biol Chem. 1995;270:2213–2217. doi: 10.1074/jbc.270.5.2213. [DOI] [PubMed] [Google Scholar]
- 3.Rothman JE, Orci L. Molecular dissection of the secretory pathway. Nature. 1992;355:409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- 4.Yamamori S, Itakura M, Sugaya D, Katsumata O, Sakagami H, Takahashi M. Differential expression of SNAP-25 family proteins in the mouse brain. J Comp Neurol. 2011;519:916–932. doi: 10.1002/cne.22558. [DOI] [PubMed] [Google Scholar]
- 5.Prescott GR, Chamberlain LH. Regional and developmental brain expression patterns of SNAP25 splice variants. BMC Neurosci. 2011;12:35. doi: 10.1186/1471-2202-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bark IC. Structure of the chicken gene for SNAP-25 reveals duplicated exons encoding distinct isoforms of the protein. J Mol Biol. 1993;233:67–76. doi: 10.1006/jmbi.1993.1485. [DOI] [PubMed] [Google Scholar]
- 7.Kataoka M, Yamamori S, Suzuki E, Watanabe S, Sato T, Miyaoka H, Azuma S, Ikegami S, Kuwahara R, Suzuki-Migishima R, Nakahara Y, Nihonmatsu I, Inokuchi K, Katoh-Fukui Y, Yokoyama M, Takahashi M. A single amino acid mutation in SNAP-25 induces anxiety-related behavior in mouse. PLoS One. 2011;6:e25158. doi: 10.1371/journal.pone.0025158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamori S, Sugaya D, Iida Y, Kokubo H, Itakura M, Suzuki E, Kataoka M, Miyaoka H, Takahashi M. Stress-induced phosphorylation of SNAP-25. Neurosci Lett. 2014;561:182–187. doi: 10.1016/j.neulet.2013.12.044. [DOI] [PubMed] [Google Scholar]
- 9.Bragina L, Candiracci C, Barbaresi P, Giovedi S, Benfenati F, Conti F. Heterogeneity of glutamatergic and GABAergic release machinery in cerebral cortex. Neuroscience. 2007;146:1829–1840. doi: 10.1016/j.neuroscience.2007.02.060. [DOI] [PubMed] [Google Scholar]
- 10.Tafoya LC, Mameli M, Miyashita T, Guzowski JF, Valenzuela CF, Wilson MC. Expression and function of SNAP-25 as a universal SNARE component in GABAergic neurons. J Neurosci. 2006;26:7826–7838. doi: 10.1523/JNEUROSCI.1866-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tafoya LC, Shuttleworth CW, Yanagawa Y, Obata K, Wilson MC. The role of the t-SNARE SNAP-25 in action potential-dependent calcium signaling and expression in GABAergic and glutamatergic neurons. BMC Neurosci. 2008;9:105. doi: 10.1186/1471-2202-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karson CN, Mrak RE, Schluterman KO, Sturner WQ, Sheng JG, Griffin WS. Alterations in synaptic proteins and their encoding mRNAs in prefrontal cortex in schizophrenia: a possible neurochemical basis for ‘hypofrontality’. Mol Psychiatry. 1999;4:39–45. doi: 10.1038/sj.mp.4000459. [DOI] [PubMed] [Google Scholar]
- 13.Scarr E, Gray L, Keriakous D, Robinson PJ, Dean B. Increased levels of SNAP-25 and synaptophysin in the dorsolateral prefrontal cortex in bipolar I disorder. Bipolar Disord. 2006;8:133–143. doi: 10.1111/j.1399-5618.2006.00300.x. [DOI] [PubMed] [Google Scholar]
- 14.Gray LJ, Dean B, Kronsbein HC, Robinson PJ, Scarr E. Region and diagnosis-specific changes in synaptic proteins in schizophrenia and bipolar I disorder. Psychiatry Res. 2010;178:374–380. doi: 10.1016/j.psychres.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Carroll LS, Kendall K, O'Donovan MC, Owen MJ, Williams NM. Evidence that putative ADHD low risk alleles at SNAP25 may increase the risk of schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:893–899. doi: 10.1002/ajmg.b.30915. [DOI] [PubMed] [Google Scholar]
- 16.Balkarli A, Sengul C, Tepeli E, Balkarli H, Cobankara V. Synaptosomal-associated protein 25 (Snap-25) gene polymorphism frequency in fibromyalgia syndrome and relationship with clinical symptoms. BMC Musculoskelet Disord. 2014;15:191. doi: 10.1186/1471-2474-15-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fatemi SH, Earle JA, Stary JMLS, Sedgewick J. Altered levels of the synaptosomal associated protein SNAP-25 in hippocampus of subjects with mood disorders and schizophrenia. Neuroreport. 2001;12:3257–3262. doi: 10.1097/00001756-200110290-00023. [DOI] [PubMed] [Google Scholar]
- 18.Barr CL, Feng Y, Wigg K, Bloom S, Roberts W, Malone M, Schachar R, Tannock R, Kennedy JL. Identification of DNA variants in the SNAP-25 gene and linkage study of these polymorphisms and attention-deficit hyperactivity disorder. Mol Psychiatry. 2000;5:405–409. doi: 10.1038/sj.mp.4000733. [DOI] [PubMed] [Google Scholar]
- 19.Beeri MS, Haroutunian V, Schmeidler J, Sano M, Fam P, Kavanaugh A, Barr AM, Honer WG, Katsel P. Synaptic protein deficits are associated with dementia irrespective of extreme old age. Neurobiol Aging. 2012;33:1125–1128. doi: 10.1016/j.neurobiolaging.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohira K, Kobayashi K, Toyama K, Nakamura HK, Shoji H, Takao K, Takeuchi R, Yamaguchi S, Kataoka M, Otsuka S, Takahashi M, Miyakawa T. Synaptosomal-associated protein 25 mutation induces immaturity of the dentate granule cells of adult mice. Mol Brain. 2013;6:12. doi: 10.1186/1756-6606-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez-Larson MP, DelBello MP, Zimmerman ME, Schwiers ML, Strakowski SM. Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biol Psychiatry. 2002;52:93–100. doi: 10.1016/s0006-3223(02)01350-1. [DOI] [PubMed] [Google Scholar]
- 22.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 24.Bowden CL, Singh V, Thompson P, Gonzalez JM, Katz MM, Dahl M, Prihoda TJ, Chang X. Development of the bipolar inventory of symptoms scale. Acta Psychiatr Scand. 2007;116:189–194. doi: 10.1111/j.1600-0447.2006.00955.x. [DOI] [PubMed] [Google Scholar]
- 25.Thompson PM, Gonzalez JM, Singh V, Schoolfield JD, Katz MM, Bowden CL. Principal domains of behavioral psychopathology identified by the Bipolar Inventory of Signs and Symptoms Scale (BISS) Psychiatry Res. 2010;175:221–226. doi: 10.1016/j.psychres.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 26.Barratt ES. Anxiety and impulsiveness related to psychomotor efficiency. Percept Mot Skills. 1959;9:191–198. [Google Scholar]
- 27.Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex. II. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cereb Cortex. 1995;5:323–337. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- 28.Spurgeon SL, Jones RC, Ramakrishnan R. High throughput gene expression measurement with real time PCR in a microfluidic dynamic array. PLoS One. 2010;3:e1662. doi: 10.1371/journal.pone.0001662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 30.Abasolo N, Torrell H, Roig B, Moyano S, Vilella E, Martorell L. RT-qPCR study on post-mortem brain samples from patients with major psychiatric disorders: reference genes and specimen characteristics. J Psychiatr Res. 2011;45:1411–1418. doi: 10.1016/j.jpsychires.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Beck A, Wustenberg T, Genauck A, Wrase J, Schlagenhauf F, Smolka MN, Mann K, Heinz A. Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch Gen Psychiatry. 2012;69:842–852. doi: 10.1001/archgenpsychiatry.2011.2026. [DOI] [PubMed] [Google Scholar]
- 32.Casu MA, Dinucci D, Colombo G, Gessa GL, Pani L. Reduced DAT- and DBH-immunostaining in the limbic system of Sardinian alcohol-preferring rats. Brain Res. 2002;948:192–202. doi: 10.1016/s0006-8993(02)03220-1. [DOI] [PubMed] [Google Scholar]
- 33.Jones MD, Hess EJ. Norepinephrine regulates locomotor hyperactivity in the mouse mutant coloboma. Pharmacol Biochem Behav. 2003;75:209–216. doi: 10.1016/s0091-3057(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Y, Fang Q, Straub SG, Lindau M, Sharp GW. Noradrenaline inhibits exocytosis via the G protein betagamma subunit and refilling of the readily releasable granule pool via the αi1/2 subunit. J Physiol. 2010;588:3485–3498. doi: 10.1113/jphysiol.2010.190090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Kolli T, Hivley R, Jaber L, Zhao FI, Yan J, Herness S. Characterization of the expression pattern of adrenergic receptors in rat taste buds. Neuroscience. 2010;169:1421–1437. doi: 10.1016/j.neuroscience.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim MS, Pinto SM, Getnet D, Nirujogi RS, et al. A draft map of the human proteome. Nature. 2014;509:575–581. doi: 10.1038/nature13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paterson D, Nordberg A. Neuronal nicotinic receptors in the human brain. Prog Neurobiol. 2000;61:75–111. doi: 10.1016/s0301-0082(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 38.Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG, Hawkins M, Rao SM, Bandettini PA, Bloom AS. Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry. 1998;155:1009–1015. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]
- 39.Sutherland MT, Ray KL, Riedel MC, Yanes JA, Stein EA, Laird AR. Neurobiological impact of nicotinic acetylcholine receptor agonists: an activation likelihood estimation meta-analysis of pharmacologic neuroimaging studies. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2014.12.021. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo HR, Hou ZF, Wu J, Zhang YP, Wan YJ. Evolution of the DRD2 gene haplotype and its association with alcoholism in Mexican Americans. Alcohol. 2005;36:117–125. doi: 10.1016/j.alcohol.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Lou XY, Ma JZ, Payne TJ, Beuten J, Crew KM, Li MD. Gene-based analysis suggests association of the nicotinic acetylcholine receptor β1 subunit (CHRNB1) and M1 muscarinic acetylcholine receptor (CHRM1) with vulnerability for nicotine dependence. Hum Genet. 2006;120:381–389. doi: 10.1007/s00439-006-0229-7. [DOI] [PubMed] [Google Scholar]
- 42.Thompson PM, Egbufoama S, Vawter MP. SNAP-25 reduction in the hippocampus of patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:411–417. doi: 10.1016/S0278-5846(03)00027-7. [DOI] [PubMed] [Google Scholar]
- 43.Fatemi SH, Earle JA, Stary JMLS, Sedgewick J. Altered levels of the synaptosomal associated protein SNAP-25 in hippocampus of subjects with mood disorders and schizophrenia. Neuroreport. 2001;12:3257–3262. doi: 10.1097/00001756-200110290-00023. [DOI] [PubMed] [Google Scholar]
- 44.Honer WG, Falkai P, Bayer TA, Xie J, Hu L, Li H, Arango V, Mann JJ, Dwork AJ, Trimble WS. Abnormalities of SNARE mechanism proteins in anterior frontal cortex in severe mental illness. Cereb Cortex. 2002;12:349–356. doi: 10.1093/cercor/12.4.349. [DOI] [PubMed] [Google Scholar]
- 45.Thompson PM, Sower AC, Perrone-Bizzozero NI. Altered levels of the synaptosomal associated protein SNAP-25 in schizophrenia. Biol Psychiatry. 1998;43:239–243. doi: 10.1016/S0006-3223(97)00204-7. [DOI] [PubMed] [Google Scholar]
- 46.Young CE, Arima K, Xie J, Hu L, Beach TG, Falkai P, Honer WG. SNAP-25 deficit and hippocampal connectivity in schizophrenia. Cereb Cortex. 1998;8:261–268. doi: 10.1093/cercor/8.3.261. [DOI] [PubMed] [Google Scholar]
- 47.Eastwood SL, Cairns NJ, Harrison PJ. Synaptophysin gene expression in schizophrenia. Investigation of synaptic pathology in the cerebral cortex. Br J Psychiatry. 2000;176:236–242. doi: 10.1192/bjp.176.3.236. [DOI] [PubMed] [Google Scholar]
- 48.Barakauskas VE, Beasley CL, Barr AM, Ypsilanti AR, Li HY, Thornton AE, Wong H, Rosokilja G, Mann JJ, Mancevski B, Jakovski Z, Davceva N, Ilievski B, Dwork AJ, Falkai P, Honer WG. A novel mechanism and treatment target for presynaptic abnormalities in specific striatal regions in schizophrenia. Neuropsychopharmacology. 2010;35:1226–1238. doi: 10.1038/npp.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sommer JU, Schmitt A, Heck M, Schaeffer EL, Fendt M, Zink M, Nieselt K, Symons S, Petroianu G, Lex A, Herrera-Marschitz M, Spanagel R, Falkai P, Gebicke-Haerter PJ. Differential expression of presynaptic genes in a rat model of postnatal hypoxia: relevance to schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2010;260(suppl 2):S81–S89. doi: 10.1007/s00406-010-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.VanGuilder HD, Brucklacher RM, Patel K, Ellis RW, Freeman WM, Barber AJ. Diabetes downregulates presynaptic proteins and reduces basal synapsin I phosphorylation in rat retina. Eur J Neurosci. 2008;28:1–11. doi: 10.1111/j.1460-9568.2008.06322.x. [DOI] [PubMed] [Google Scholar]
- 51.Ramos-Miguel A, Beasley CL, Dwork AJ, Mann JJ, Rosoklija G, Barr AM, Honer WG. Increased snare protein-protein interactions in orbitofrontal and anterior cingulate cortices in schizophrenia. Biol Psychiatry. 2015;78:361–373. doi: 10.1016/j.biopsych.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Atz M, Walsh D, Cartagena P, Li J, Evans S, Choudary P, Overman K, Stein R, Tomita H, Potkin S, Myers R, Watson SJ, Jones EG, Akil H, Bunney WE Jr, Vawter MP. Methodological considerations for gene expression profiling of human brain. J Neurosci Methods. 2007;163:295–309. doi: 10.1016/j.jneumeth.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun T, Patoine C, Abu-Khalil A, Visvader J, Sum E, Cherry TJ, Orkin SH, Geschwind DH, Walsh CA. Early asymmetry of gene transcription in embryonic human left and right cerebral cortex. Science. 2005;308:1794–1798. doi: 10.1126/science.1110324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson MB, Kawasawa YI, Mason CE, Krsnik Z, Coppola G, Bogdanovic D, Geschwind DH, Mane SM, State MW, Sestan N. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron. 2009;62:494–509. doi: 10.1016/j.neuron.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li MD, Kane JK, Wang J, Ma JZ. Time-dependent changes in transcriptional profiles within five rat brain regions in response to nicotine treatment. Brain Res Mol Brain Res. 2004;132:168–180. doi: 10.1016/j.molbrainres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 56.Maier T, Guell M, Serrano L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009;583:3966–3973. doi: 10.1016/j.febslet.2009.10.036. [DOI] [PubMed] [Google Scholar]