Abstract
In contrast to other mammalian reservoirs, many bat species migrate long-distances and have the potential to introduce exotic pathogens to new areas. Bats have long been associated with blood-borne protozoal trypanosomes of the Schizotrypanum subgenus, which includes the zoonotic parasite Trypanosoma cruzi, agent of Chagas disease. Another member of the subgenus, Trypanosoma dionisii, infects bats of Europe and South America, and genetic similarities between strains from the two continents suggest transcontinental movement of this parasite via bats. Despite the known presence of diverse trypanosomes in bats of Central and South America, and the presence of T. cruzi-infected vectors and wildlife in the US, the role of bats in maintaining and dispersing trypanosomes in the US has not yet been reported. We collected hearts and blood from 8 species of insectivorous bats from 30 counties across Texas. Using PCR and DNA sequencing, we tested 593 bats for trypanosomes and found 1 bat positive for T. cruzi (0.17%), 9 for T. dionisii (1.5%), and 5 for Blastocrithidia spp. (0.8%), a group of insect trypanosomes. The T. cruzi-infected bat was carrying TcI, the strain type associated with human disease in the US. In the T. dionisii-infected bats, we detected three unique variants associated with the three infected bat species. These findings represent the first report of T. cruzi in a bat in the US, of T. dionisii in North America, and of Blastocrithidia spp. in mammals, and underscore the importance of bats in the maintenance of trypanosomes, including agents of human and animal disease, across broad geographic locales.
Keywords: Blastocrithidia, Chiroptera, Trypanosoma cruzi, Trypanosoma dionisii, Trypanosomes
1. Introduction
Bats are associated with a number of zoonotic pathogens (Calisher et al., 2006), and their reservoir potential may be heightened relative to other mammals due to their ability to fly, highly gregarious social structures, and long life spans (Luis et al., 2013). Long migration distances of some bat species may play a role in the circulation and spread of pathogens, as has been demonstrated for neotropical migratory birds (Cohen et al., 2015; Mukherjee et al., 2014).
The vector-borne protozoal parasite Trypanosoma cruzi, agent of Chagas disease, is of major public health importance and infects animals of virtually all mammalian orders (Gaunt and Miles, 2000). It is transmitted via the feces of hematophagous insects of the subfamily Triatominae (kissing bugs), and wildlife reservoirs play an important role in the maintenance and transmission of the parasite in sylvatic transmission cycles (Bern et al., 2011). T. cruzi is a genotypically heterogeneous species that has been divided into six discrete typing units (DTUs), TcI–TcVI (Zingales et al., 2012), and a seventh recently discovered bat-associated type TcBat (Lima et al., 2015a; Marcili et al., 2009a). The DTUs TcI and TcIV are enzootic in the southern United States. Evidence now suggests that T. cruzi and related parasites likely evolved originally from a bat trypanosome lineage, rather than evolving in isolation in mammals of South America, Antarctica, and Australia as previously theorized (Hamilton et al., 2012b; Lima et al., 2012, 2013).
The T. cruzi clade of trypanosomes is divided into two main sister phylogenetic lineages: the sugbenus Schizotrypanum and the T. rangeli/T. conorhini clades (Lima et al., 2015b). Bats have long been associated with trypanosomes of the Schizotrypanum subgenus, of which T. cruzi (sensu stricto) is the only member not restricted to bats (Barnabe et al., 2003; Molyneux, 1991). Other members of Schizotrypanum include T. dionisii in Old and New World bats, T. cruzi marinkellei in bats of Central and South America, and T. erneyi in African bats (Baker et al., 1978; Barnabe et al., 2003; Gardner and Molyneux, 1988; Lima et al., 2015a, 2012; Molyneux, 1991). Genetic similarities between strains of T. dionisii isolated from Europe and South America suggest the movement of this parasite via bats between the Old and New worlds (Hamilton et al., 2012a). Other species within the T. cruzi clade include: T. vespertilionis, T. conorhini, T. rangeli, T. livingstonei, and a number of others isolated from bats and other mammals or marsupials in Africa and Australia (Lima et al., 2015b).
The most common trypanosomes detected in neotropical bats are T. cruzi, T. c. marinkellei, T. dionisii, T. rangeli, and T. conorhini, with apparent prevalences ranging from 10 to 80% (Cottontail et al., 2009; García et al., 2012; Marcili et al., 2009a,b; Pinto et al., 2012; Ramírez et al., 2014). Despite the migration of some bat species between South, Central, and North America, and local presence of large numbers of T. cruzi-infected triatomine vectors across Mexico and the Southern US (Bern et al., 2011; Curtis-Robles et al., 2015; Ramsey et al., 2000), no study has reported the presence of T. cruzi or any trypanosome species in bats in North America. Our objective was to quantify the frequency at which bats were infected with trypanosomes and compare the genetic diversity of these parasites in bats from both peridomestic and sylvatic habitats across Texas.
2. Materials and methods
2.1. Peridomestic bats
Through collaboration with the Texas Department of State Health Services (DSHS), we acquired carcasses of bats previously submitted by the public and determined to be negative for rabies by state laboratories in Austin or El Paso. These bats were considered peridomestic because they were encountered directly by members of the public, often in homes or places of work. The majority (87%) of bats submitted for rabies testing in Texas are submitted because of concerns that they potentially exposed a person or domestic animal to rabies (Mayes et al., 2013). Bats were identified to species by personnel at the DSHS labs using morphological characteristics, including standard measurements such as antebrachium length (Ammerman et al., 2012). Bats had been stored in a freezer for up to three years prior to our study, but the majority (85%) were stored from 3 to 9 months. Each animal’s species, sex, and degree of autolysis were recorded, and the heart was collected and bisected in a biosafety level 2 cabinet. The apex of the heart was minced in preparation for DNA extraction.
2.2. Sylvatic bats
To represent sylvatic populations of bats that are less likely to be encountered directly by the public, bats were captured at three field sites in South Texas in Kenedy (27.174N, 97.864W), Jim Hogg (26.965N, 98.852W and 26.908N, 98.758W), Starr (26.737N, 98.774W), and Uvalde (29.435N, 99.685W) counties. In Kenedy, Jim Hogg, and Starr counties, bats were captured on large cattle ranches using mist nets set over low water tanks. In Uvalde county, bats were captured during emergence and return to a cave using hand-held mist nets (Waldien and Hayes, 1999). Bats were removed from mist nets, weighed, evaluated for species and sex identification, and manually restrained for blood collection. Species was determined without difficulty by morphologic features using a field guide of bats in Texas (Ammerman et al., 2012). A 25 g needle was used to puncture one of the interfemoral veins, and capillary tubes were used to collect a volume equal to no more than 1% of the animal’s body weight. Pressure was applied to the puncture site until bleeding had stopped and bats were then released directly or returned to a cloth bag to recover for up to 10 min then released. The capture of animals and all subsequent procedures were conducted according to the recommendations and approval of Texas A&M University IACUC (Institutional Animal Care and Use Committee) Animal Use Protocol 2015-0088 and Texas Parks and Wildlife Department scientific collections permit SPR-0512-917. Additionally, in collaboration with researchers performing a biodiversity study, we obtained hearts from bats collected as museum specimens from the ranch properties. These bats were captured in mist nets and euthanized via an overdose of halothane or isoflurane in accordance with IACUC permit 2015-0126 and Texas collections permit SPR-0409-082.
2.3. Trypanosome detection
DNA was extracted from blood and heart tissue using a commercial kit (E.Z.N.A Tissue DNA Kit; Omega Bio-Tek, Norcross, GA) following manufacturer’s instructions with an overnight lysis period. Extracted DNA was subjected to two separate PCR protocols for the detection of T. cruzi and other trypanosomes. First, a sensitive quantitative, real-time PCR for the specific detection of T. cruzi was performed using the Cruzi 1/2 primers and a 6-carboxyfluorescein (FAM)-labeled probe, Cruzi 3, as previously described (Piron et al., 2007; Ramírez et al., 2015), but with an initial denaturation time of 3 min. Based on internal laboratory validations, the cutoff for positive samples was determined to be a quantification cycle value of 33 or less. Next, all samples were subjected to a nested PCR targeting an 18S (SSU) rRNA-encoding gene fragment of trypanosomes, as previously described (Noyes et al., 1999; Pinto et al., 2015). Additionally, T. cruzi positive samples were subjected to a multiplex probe-based qPCR for determination of strain type (Cura et al., 2015). DNA extractions, primary and secondary amplifications, and product analyses were performed in separate dedicated laboratory areas. A negative control was included in each set of DNA extractions and a water negative control was used in PCR reactions as contamination controls. The DNA from T. cruzi Sylvio X10 clone4 (American Type Culture Collection, Manassas, VA) served as a positive control. Samples that gave positive results on the nested PCR were repeated on the same assay one or two more times for confirmation in consistency of results. Amplification products were separated on agarose gels, purified (ExoSAP-IT; Affymetrix, Santa Clara, CA), and sequenced in both forward and reverse at Eton Biosciences Inc. Resulting sequences were analyzed and aligned using MEGA7 software (Kumar et al., 2016), and compared to a national sequence database (GenBank) using the BLAST program (Altschul et al., 1990). We created alignments for each separate species group generated in this study (T. cruzi, T. dionisii, and Blastocrithidia) including representative reference sequences, as well as aligning all of the species together with additional reference sequences from other trypanosome species. Neighbor Joining trees were created in Mega7 with 1000 bootstrap replicates to compare sequences generated in the current study to representative sequences from GenBank.
2.4. Confirmatory PCRs
For the purpose of confirming our nested PCR findings, attempts were made to amplify and sequence additional genetic markers several months after the initial molecular work. The Blastocrithidia positive samples were subjected to a PCR targeting the 24Sα rRNA gene using primers D75 and D76 as described previously (Schijman et al., 2006; Souto et al., 1999). The remaining positive samples were subjected to a PCR previously used in the description of bat trypanosomes, targeting the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene (Maia da Silva et al., 2004).
Additionally, to assess the probability of T. cruzi detection from these mixed DNA samples in which the majority of DNA is from the bat host, 5% of the negative bat samples (n = 30), selected across a variety of autolysis scores and dates of extraction, were spiked with low concentration ( dilution, equivalent to <4 parasites) of T. cruzi positive control DNA and run on the nested PCR with a negative water control and positive control of the same concentration as that in the spiked samples but without the vertebrate DNA.
3. Results
3.1. Peridomestic bats
A total of 487 peridomestic bats were received from the Austin and El Paso DSHS labs, and we collected the heart from 474. Sampled bats originated from 29 counties across Texas (Fig. 1), and represented 8 insectivorous species of the family Vespertilionidae. The majority of bats sampled were Tadarida brasiliensis (82.5%, n = 391), followed by Nycticeius humeralis (7.8%, n = 37), Parastrellus hesperus (3.2%, n = 15), Lasiurus borealis (2.9%, n = 10), Antrozous pallidus (1.9%, n = 9), Lasiurus intermedius (1.5%, n = 7), Myotis velifer (0.8%, n = 4), and Perimyotis subflavus (0.8%, n = 1). There were 296 males (61.8%), 166 females (35%), and 15 for which sex could not be determined due to the condition of the carcass.
Fig. 1.

Map of Texas with sampled counties shaded according to sample size and shapes marking counties from which trypanosome-positive bats originated.
3.2. Sylvatic bats
We captured 105 bats in mist nets in 4 counties in south Texas (Fig. 1) and collected blood from 103. We obtained 16 hearts from animals collected for museum specimens. These sylvatic bats were of 3 species: T. brasiliensis (71%, n = 84), N. humeralis (29%, n = 34), and L. intermedius (0.8%, n = 1). There were 75 females (63%) and 44 males (37%).
3.3. Trypanosome detection
Samples from 593 bats were tested for trypanosomes using both nested PCR (for generic Trypanosoma detection) and qPCR (for specific T. cruzi detection). A single male peridomestic N. humeralis bat was positive for T. cruzi on both the qPCR and nested PCR; 9 peridomestic bats were positive for T. dionisii via nested PCR; and 4 peridomestic bats (3 T. brasiliensis, 1 N. humeralis) and 1 sylvatic bat (T. brasiliensis) were positive for Blastocrithidia spp. via nested PCR Tables 1 and 2). The T. cruzi positive sample was determined to be TcI on the multiplex qPCR. The T. cruzi positive bat was from Hidalgo county; T. dionisii positive bats were from Hidalgo, El Paso, and Webb counties; and Blastocrithidia spp. were from Hidalgo, Travis, Williamson, and Uvalde counties (Table 2; Fig. 1).
Table 1.
Species distribution and apparent prevalence of trypanosomes in bats tested.
| Species | # Tested |
T. cruzi
|
T. dionisii
|
Blastocrithidia spp.
|
|||
|---|---|---|---|---|---|---|---|
| # Positive | Apparent Prevalence | # Positive | Apparent Prevalence | # Positive | Apparent Prevalence | ||
| Tadarida brasiliensis | 476 | 0 | 0.0% | 5 | 1.1% | 4 | 0.8% |
| Nycticeius humeralis | 70 | 1 | 1.4% | 0 | 0.0% | 1 | 1.4% |
| Parastrellus hesperus | 15 | 0 | 0.0% | 2 | 13.3% | 0 | 0.0% |
| Antrozous pallidus | 9 | 0 | 0.0% | 2 | 22.2% | 0 | 0.0% |
| Othersa | 23 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| Total | 593 | 1 | 0.2% | 9 | 1.5% | 5 | 0.0% |
Other species include: Lasiurus borealis, Lasiurus intermedius, Myotis velifer, Perimyotis subflavus.
Table 2.
Demographic details of bats that tested positive for trypanosomes.
| Sample ID | Species | Sex | County | Trypanosome ID |
|---|---|---|---|---|
| A14-6132 | Nycticeius humeralis | M | Hidalgo | T. cruzi |
| A14-6383 | Tadarida brasiliensis | M | Hidalgo | T. dionisii |
| A15-1338 | Tadarida brasiliensis | F | Webb | T. dionisii |
| A15-1726 | Tadarida brasiliensis | M | Hidalgo | T. dionisii |
| A15-1920 | Tadarida brasiliensis | F | Webb | T. dionisii |
| R15-053 | Tadarida brasiliensis | M | El Paso | T. dionisii |
| R12-302 | Antrozous pallidus | M | El Paso | T. dionisii |
| R14-230 | Antrozous pallidus | M | El Paso | T. dionisii |
| R15-094 | Parastrellus hesperus | F | El Paso | T. dionisii |
| R15-092 | Parastrellus hesperus | F | El Paso | T. dionisii |
| A14-5860 | Tadarida brasiliensis | M | Williamson | Blastocrithidia spp. |
| A14-6260 | Tadarida brasiliensis | M | Travis | Blastocrithidia spp. |
| A14-6629 | Tadarida brasiliensis | F | Travis | Blastocrithidia spp. |
| FC1507-03 | Tadarida brasiliensis | F | Uvalde | Blastocrithidia spp. |
| 2015AU-3671 | Nycticeius humeralis | F | Hidalgo | Blastocrithidia spp. |
3.4. Confirmatory PCRs
For the PCR targeting the 24Sα region, 4/5 of the samples that were positive for Blastocrithidia on the nested PCR yielded a band on gel electrophoresis, and sequences were obtained from 3 of these. These sequences had 98–99% homology with a sequence of Blastocrithidia sp. from a Triatoma guasayana (AY820895). We obtained a partial GAPDH sequence with 96% homology to T. dionisii (GQ140363) from sample A14-1338. The remaining samples showed nonspecific amplification likely resulting from the mixed DNA template (i.e., not cultured parasite) that was studied. Of the subset of samples that were negative for trypanosomes in the nested PCR, none were determined to be positive in any other assay.
We detected T. cruzi in all 30 of the spiked samples with intensity of bands indistinguishable to that of the positive control which contained the same concentration of T. cruzi DNA but without bat host DNA.
3.5. Phylogenetic analysis
A phylogenetic tree was constructed including all of the 18S rRNA sequences generated in this study together with representative reference sequences from GenBank (Fig. 2). The T. cruzi-positive bat from the current study (GenBank accession KX227594) was grouped together with other TcI isolates, supporting the results from the strain typing qPCR.
Fig. 2.
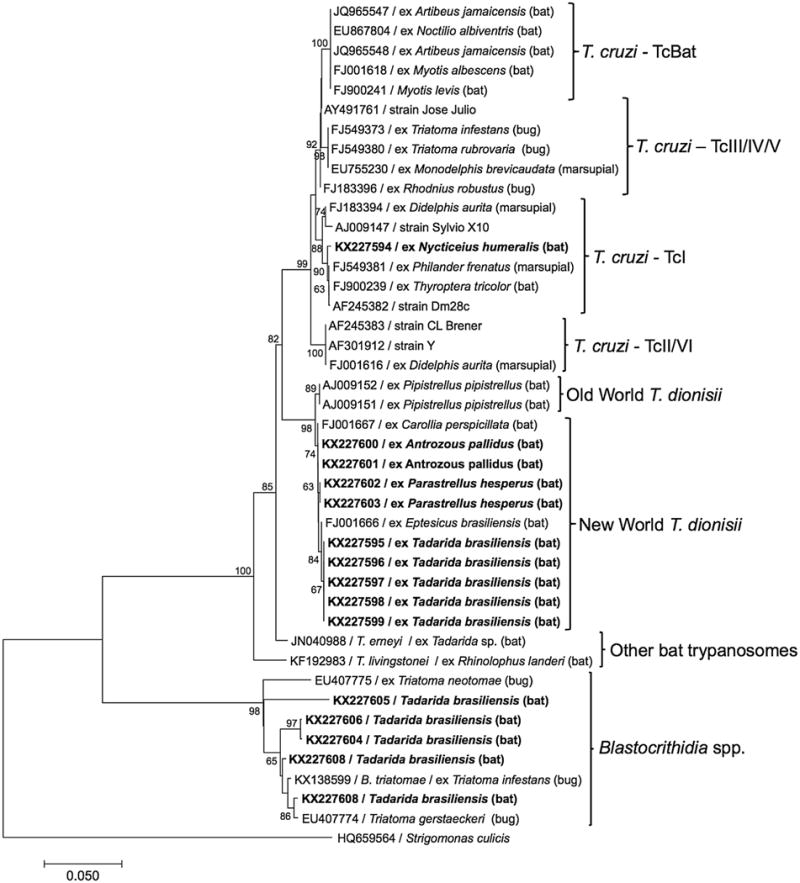
Phylogenetic tree comparing a 532 bp segment of the 18S rRNA gene of trypanosomes, constructed using the Neighbor-Joining method in Mega7. The sequences in bold were generated during this study.
The nine T. dionisii sequences from the current study represented three unique sequence variants, grouped within the clade of New World T. dionisii isolates. Within the 493 bp region of analysis, a total of 6 single nucleotide polymorphisms (SNPs) or indel events distinguished these variants from each other and from the most similar T. dionisii sequences in Genbank. All sequences obtained from the same bat species were identical to each other, yet differed among species, with the sequences obtained from A. pallidus (GenBank accessions KX227600-KX227601) and P. hesperus (KX227602-KX227603) being more similar to each other than to the sequences from T. brasiliensis (KX227595-KX227599; Fig. 2).
The five Blastocrithidia sequences from our study (KX227604-KX227608) all differed from each other and from previously published Blastocrithidia sequences, which have exclusively been reported from insects (Fig. 2). When compared to sequences in GenBank, the closest matches were with Blastocrithidia spp. isolated from true bugs (Hemiptera), including Blastocrithidia triatomae from a Triatoma protracta in Argentina (AF153037) and Blastocrithidia spp. isolated from Triatoma spp. in Texas (EU407774-EU407776). Among the 9 Blastocrithidia sequences of 592 base pairs included in the alignment, there were 38 indel events and 86 SNPs. One sequence we generated, from a Tadarida brasiliensis in Travis county (KX227605), was especially different from the others, forming a separate clade on the phylogenetic tree (Fig. 2).
4. Discussion
Trypanosomes were detected in 15 of 593 (2.6%) bats, with a single bat positive for T. cruzi. The overall level of trypanosome detection in bats across Texas is lower than that found in studies of bats from Brazil, Panama, Ecuador, and Colombia (Cavazzana et al., 2010; Cottontail et al., 2014; Pinto et al., 2015; Ramírez et al., 2014), which ranged from 11 to 37%. Meanwhile, a survey of bats in Mexico specifically for T. cruzi found that none of 116 were infected (Ramsey et al., 2012). However, the species of bats we sampled are different from those represented in previous studies, with the exception of a small number (2/6) of T. brasiliensis reported to be infected with uncharacterized trypanosomes in Brazil (Cavazzana et al., 2010).
Our findings likely represent a conservative estimate of the true prevalence, given the aged nature of some carcasses. Additionally, the degree to which cardiac tissue analysis reflects bat-level trypanosome infection status is not known. T. cruzi is well known to localize in heart muscle cells, but tissue tropism has not been established for T. dionisii or Blastocrithidia species. Previous surveys of trypanosomes in bats have almost exclusively used peripheral blood samples, and it is possible that the use of heart tissue is less sensitive for the detection of some species of trypanosomes. However, it should be noted that a small amount of cardiac blood was likely included with heart tissue of most samples subjected to PCR. Because our study design and use of predominantly bat carcasses did not allow for the use of hemoculture to isolate any of the detected trypanosomes in culture, we were unable to perform extensive genetic characterization.
4.1. Detection of T. cruzi and epidemiological importance
Assessing the epidemiological importance of T. cruzi in wild bats in the United States must consider many ecological factors. Although we detected T. cruzi in only a single evening bat (Nycticeius humeralis), this must be considered in the context of the overall population size of these bats and how they move across the landscape. Estimates of population sizes of most North American bats are challenging due to their small body size, nocturnalbehavior, and cryptic roost sites (Kunz, 2003), and population sizeof N. humeralis is estimated to be between 100,000 and 1 million (NatureServe, 2015). Given our observed frequency of infection of 1/72 (1.4%) in N. humeralis, extrapolation suggests between 1388 and 13,880 T. cruzi-infected evening bats across the US.
Although T. cruzi infection in bats was rare, bats may nonetheless serve as reservoirs if they are part of a community in which the pathogen can be permanently maintained and transmitted (Haydon et al., 2002). Texas is home to 32 species of bats and at least species of triatomines (Ammerman et al., 2012; Curtis-Robles et al., 2015). The two most common triatomine species encountered in Texas are Triatoma gerstaeckeri and T. sanguisuga and approximately 50–70% of these are infected with T. cruzi (Curtis-Robles et al., 2015; Kjos et al., 2009b). Bats have the opportunity to encounter triatomines during foraging and feeding at night when both are active, as well as potentially being fed upon by the bugs during the day when roosting in trees or caves. While there has been no specific research into whether North American bats feed on triatomines, a significant proportion of the diet of many species includes Hemipterans (Carter et al., 2004; McWilliams, 2005). An experimental trial documented infection of Phyllostomid bats with T. cruzi after feeding on infected triatomines (Thomas et al., 2007). Triatomines are notoriously found in nests and resting areas of terrestrial mammals (Lent and Wygodzinsky, 1979), and have been found in and around caves in Texas (Hamer et al., unpublished data) as well as under loose bark of trees (Lent and Wygodzinsky, 1979), sites similar to those where many species of bats roost during the day. Further, a blood-meal analysis study revealed the blood of an evening bat (N. humeralis) in a T. gerstaeckeri from Texas (Gorchakov et al., 2016), demonstrating triatomine-bat contact. The degree to which bats maintain parasitemia and thus are infectious to vectors has not been well-studied, however, the isolation via hemoculture of T. cruzi from blood of bats in Central and South America supports their status as a reservoir in those areas (Cavazzana et al., 2010; Lima et al., 2012; Pinto et al., 2015).
Further, assessing the epidemiological significance of T. cruzi-infected wildlife must also consider the parasite genetic strain and the degree to which it is infective to humans. Two main strain types of T. cruzi are endemic in the US, TcI and TcIV, with one report of TcII from rodents in Louisiana (Herrera et al., 2015). The T. cruzi-infected bat in our study harbored TcI, the only strain type associated with disease in humans in the US thus far (Roellig et al., 2008).
Finally, evaluating bats in the epidemiology of Chagas disease requires an understanding of the overall ecology of the wildlife reservoir system of T. cruzi in the US. Nearly all other wildlife species that have been evaluated and reported for infection with T. cruzi in the southern US are associated with a higher frequency of infection that what we found in bats. For example, reported apparent prevalence of T. cruzi infection is 75% in striped skunks (Mephitis mephitis), 60–70% in raccoons (Procyon lotor), 14% in bobcats (Lynx rufus), 14% in coyotes (Canis latrans), 14% in gray foxes (Urocyon cinereoargenteus), 34% in woodrats (Neotoma micropus), and 18% in other rodents (Charles et al., 2012; Curtis-Robles et al., 2016). Bats may play a unique role in this host community because of their ability to transport the pathogen over long distances during foraging or migration.
4.2. Detection of other trypanosomes
We detected T. dionisii in 9/593 bats (1.5%) of 3 species (T. brasiliensis, A. pallidus, P. hesperus). T. dionisii is a well-known trypanosome of bats in South America and Europe (Molyneux, 1991), but has not before been detected in North America. Although this parasite can enter cells and form pseudocysts in cardiac myocytes (Cavazzana et al., 2010; Gardner and Molyneux, 1988), there is no evidence that T. dionisii or other trypanosomes are pathogenic to bats. Based on the 18S rRNA gene fragment we sequenced, the Texas bat T. dionisii sequences all grouped with the New World isolates of T. dionisii. Further, we detected three unique variants that were uniform within each of the three infected bat species. Variants differed among species even within the same geographical area (Fig. 2). Additional genetic analyses may further characterize the ecological importance of these host-parasite associations.
As an unexpected finding, based on sequencing of two gene regions (18S rRNA and 24Sα rRNA), we detected bats infected with Blastocrithidia sp., a genus of trypanosome associated with the alimentary tract of insects of the order Heteroptera. They are considered monoxenous, restricted to a single host during their life cycle, and are closely related to other similar insect trypanosomes that infect other insect orders, and to the dixenous parasite Leishmania, a mammalian pathogen (Maslov et al., 2013). Blastocrithidia triatomae was isolated from a laboratory reared colony of Triatoma infestans (vectors of T. cruzi) in Argentina (Cerisola et al., 1971) and genetically similar organisms were isolated from several species of Triatoma in Texas (Kjos et al., 2009a). The PCR-based approach we used does not allow us to evaluate whether the parasite was alive or dead; further, the presence of this parasite in the heart tissue samples could reflect either infection of the cardiac myocytes or infection of the blood. It is possible that Blastocrithidia spp. are capable of travelling systemically within bats following the consumption of an infected insect, but the degree of transience and outcome of such an event is unknown.
4.3. Ecology of bat species infected with trypanosomes
T. brasiliensis is the most numerous species of bat in Texas, is commonly encountered by humans (Mayes et al., 2013), and was also the most well-represented species in our sample (80% of all bats). Both T. dionisii and Blastocrithidia spp. infected this species at a low frequency. T. brasiliensis are highly gregarious and migratory, and while the full range of the species extends from Argentina to the central US, the bats found in Texas in the warm months are thought to spend the winter in central Mexico (Villa and Cockrum, 1962). The county with the highest number of trypanosome-positive bats was El Paso, and two of the species in which T. dionisii was detected (A. pallidus and P. hesperus), were not collected from any other county. A. pallidus and P. hesperus are found throughout the western US down to Mexico and do not migrate (Ammerman et al., 2012). Due to the lack of migration, the trypanosome detections in these species likely reflect infection in the west Texas region of El Paso. N. humeralis, the only species in which T. cruzi was detected in our study, is found across the eastern US, west to central Texas and south to northern Mexico. Females are thought to be migratory, while males may remain in the southern part of the range through the summer (Ammerman et al., 2012). The single N. humeralis positive for T. cruzi was male, suggesting that infection was most likely acquired in south Texas. The T. cruzi and T. dionisii positive bats were all from counties along the Texas-Mexico border, a region of increasing concern for local transmission of the Chagas parasite to humans and dogs (Beard et al., 2002; Esteve-Gassent et al., 2014; Sarkar et al., 2010; Tenney et al., 2014).
4.4. Future directions
Future work to explore the trypanosomes of bats of the US should focus on acquiring a larger sample size of diverse species of bats, especially from counties along the US-Mexico border, and include sequencing of additional gene segments for more detailed phylogenetic analysis, as well as attempts to culture isolates. Through these efforts, advances could be made to expand the knowledge base of host associations, genetic diversity, and geographical range of bat-associated trypanosomes.
Acknowledgments
This is publication number 005 of the East Foundation. The authors would like to acknowledge Nicole Bertolini, Jessica Light, Aleyda Galán, Justin Henningsen, Edward Wozniak, and John Barnes for their assistance with conducting or organizing field efforts and East Foundation for access to ranches. We also acknowledge Lisa Auckland, Amani Bourji, and Faith Weeks for their assistance in the laboratory. Research support was provided in part by the National Center for Veterinary Parasitology (SAH). Student stipend support was provided by NIH T32 fellowship 2T32OD011083-06 (CLH), and NIH T35 2T35OD010991-11 (CCG).
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. http://dx.doi.org/10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Ammerman LK, Hice CL, Schmidly DJ. Bats of Texas Texas A&M. University Press; College Station, TX: 2012. [Google Scholar]
- Baker JR, Miles MA, Godfrey DG, Barrett TV. Biochemical characterization of some species of Trypansoma (Schizotrypanum) from bats (Microchiroptera) Am J Trop Med Hyg. 1978;27:483–491. doi: 10.4269/ajtmh.1978.27.483. [DOI] [PubMed] [Google Scholar]
- Barnabe C, Brisse S, Tibayrenc M. Phylogenetic diversity of bat trypanosomes of subgenus Schizotrypanum based on multilocus enzyme electrophoresis, random amplified polymorphic DNA, and cytochrome b nucleotide sequence analyses. Infect Genet Evol. 2003;2:201–208. doi: 10.1016/s1567-1348(02)00130-2. http://dx.doi.org/10.1016/S1567-1348(02)00130-2. [DOI] [PubMed] [Google Scholar]
- Beard CB, Pye G, Steurer FJ, Rodriguez R, Campman R, Peterson AT, Ramsey J, Wirtz RA, Robinson LE. Chagas disease in a domestic transmission cycle in southern Texas, USA. Emerg Infect Dis. 2002;9:103–105. doi: 10.3201/eid0901.020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bern C, Kjos S, Yabsley MJ, Montgomery SP. Trypanosoma cruzi and Chagas’ disease in the United States. Clin Microbiol Rev. 2011;24:655–681. doi: 10.1128/CMR.00005-11. http://dx.doi.org/10.1128/Cmr.00005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. Bats: important reservoir hosts of emerging viruses. Clin Microbiol Rev. 2006;19:531–545. doi: 10.1128/CMR.00017-06. http://dx.doi.org/10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter TC, Menzel MA, Chapman BR. Partitioning of food resources by syntopic eastern red (Lasiurus borealis), Seminole (L. seminolus) and evening (Nycticeius humeralis) bats. Am Midl Nat. 2004;151:186–191. http://dx.doi.org/10.1674/0003-0031(2004)151%5B0186:POFRBS%5D2.0.CO;2. [Google Scholar]
- Cavazzana M, Marcili A, Lima L, Maia da Silva F, Junqueira ÂCV, Veludo HH, Viola LB, Campaner M, Nunes VLB, Paiva F, Coura JR, Camargo EP, Teixeira MMG. Phylogeographical, ecological and biological patterns shown by nuclear (ssrRNA and gGAPDH) and mitochondrial (Cyt b) genes of trypanosomes of the subgenus Schizotrypanum parasitic in Brazilian bats. Int J Parasitol. 2010;40:345–355. doi: 10.1016/j.ijpara.2009.08.015. http://dx.doi.org/10.1016/j.ijpara.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Cerisola JA, Rohwedder R, Bozzini JP, Del Prado CE. Blastocrithidia triatomae n sp found in Triatoma infestans from Argentina. J Protozool. 1971;18:503–506. doi: 10.1111/j.1550-7408.1971.tb03362.x. [DOI] [PubMed] [Google Scholar]
- Charles RA, Kjos S, Ellis AE, Barnes JC, Yabsley MJ. Southern plains woodrats (Neotoma micropus) from Southern Texas are important reservoirs of two genotypes of Trypanosoma cruzi and host of a putative novel Trypanosoma species. Vector Borne Zoonotic Dis. 2012;13:22–30. doi: 10.1089/vbz.2011.0817. http://dx.doi.org/10.1089/vbz.2011.0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen EB, Auckland LD, Marra PP, Hamer SA. Avian migrants facilitate invasions of Neotropical ticks and tick-borne pathogens into the United States. Appl Environ Microbiol. 2015;81 doi: 10.1128/AEM.02656-15. http://dx.doi.org/10.1128/aem.02656-15, AEM. 02656-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottontail VM, Wellinghausen N, Kalko EKV. Habitat fragmentation and haemoparasites in the common fruit bat, Artibeus jamaicensis (Phyllostomidae) in a tropical lowland forest in Panamá. Parasitology. 2009;136:1133–1145. doi: 10.1017/S0031182009990485. http://dx.doi.org/10.1017/S0031182009990485. [DOI] [PubMed] [Google Scholar]
- Cottontail VM, Kalko EKV, Cottontail I, Wellinghausen N, Tschapka M, Perkins SL, Pinto CM. High local diversity of Trypanosoma in a common bat species, and implications for the biogeography and taxonomy of the T. cruzi clade. PLoS One. 2014;9:e108603. doi: 10.1371/journal.pone.0108603. http://dx.doi.org/10.1371/journal.pone.0108603.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cura CI, Duffy T, Lucero RH, Bisio M, Péneau J, Jimenez-Coello M, Calabuig E, Gimenez MJ, Valencia Ayala E, Kjos SA, Santalla J, Mahaney SM, Cayo NM, Nagel C, Barcán L, Málaga Machaca ES, Acosta Viana KY, Brutus L, Ocampo SB, Aznar C, Cuba CA, Gürtler RE, Ramsey JM, Ribeiro I, VandeBerg JL, Yadon ZE, Osuna A, Schijman AG. Multiplex real-time PCR assay using TaqMan probes for the identification of Trypanosoma cruzi DTUs in biological and clinical samples. PLoS Negl Trop Dis. 2015;9:e0003765. doi: 10.1371/journal.pntd.0003765. http://dx.doi.org/10.1371/journal.pntd.0003765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis-Robles R, Wozniak EJ, Auckland LD, Hamer GL, Hamer SA. Combining public health education and disease ecology research: using citizen science to assess chagas disease entomological risk in Texas. PLoS Negl Trop Dis. 2015;9:e0004235. doi: 10.1371/journal.pntd.0004235. http://dx.doi.org/10.1371/journal.pntd.0004235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis-Robles R, Lewis BC, Hamer SA. High Trypanosoma cruzi infection prevalence associated with rare cardiac pathology among wild carnivores in central Texas. Int J Parasitol Parasites Wildl. 2016:1–22. doi: 10.1016/j.ijppaw.2016.04.001. http://dx.doi.org/10.1016/j.ijppaw.2016.04.001. [DOI] [PMC free article] [PubMed]
- Esteve-Gassent MD, Pérez de León AA, Romero-Salas D, Feria-Arroyo TP, Patino R, Castro-Arellano I, Gordillo-Pérez G, Auclair A, Goolsby J, Rodriguez-Vivas RI, Estrada-Franco JG. Pathogenic Landscape of Transboundary Zoonotic Diseases in the Mexico-US Border along the Rio Grande. Front Public Health. 2014;2(6):177. doi: 10.3389/fpubh.2014.00177. http://dx.doi.org/10.3389/fpubh.2014.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García L, Ortiz S, Osorio G, Torrico MC, Torrico F, Solari A. Phylogenetic analysis of Bolivian bat trypanosomes of the subgenus schizotrypanum based on cytochrome B sequence and minicircle analyses. PLoS One. 2012;7:e36578. doi: 10.1371/journal.pone.0036578. http://dx.doi.org/10.1371/journal.pone.0036578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RA, Molyneux DH. Schizotrypanum in British bats. Parasitology. 1988;97(Pt. 1):43–50. doi: 10.1017/s0031182000066725. http://dx.doi.org/10.1017/s0031182000066725. [DOI] [PubMed] [Google Scholar]
- Gaunt M, Miles M. The ecotopes and evolution of triatomine bugs (triatominae) and their associated trypanosomes. Mem Inst Oswaldo Cruz. 2000;95:557–565. doi: 10.1590/s0074-02762000000400019. http://dx.doi.org/10.1590/S0074-02762000000400019. [DOI] [PubMed] [Google Scholar]
- Gorchakov R, Trosclair LP, Wozniak EJ, Feria-Arroyo PT, Garcia MN, Gunter SM, Murray KO. Trypanosoma cruzi infection prevalence and bloodmeal analysis in triatomine vectors of chagas disease from rural peridomestic locations in texas. J Med Entomol. 2016:2013–2014. doi: 10.1093/jme/tjw040. http://dx.doi.org/10.1093/jme/tjw040. [DOI] [PubMed]
- Hamilton PB, Cruickshank C, Stevens JR, Teixeira MMG, Mathews F. Parasites reveal movement of bats between the New and Old Worlds. Mol Phylogenet Evol. 2012a;63:521–526. doi: 10.1016/j.ympev.2012.01.007. http://dx.doi.org/10.1016/j.ympev.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton PB, Teixeira MMG, Stevens JR. The evolution of Trypanosoma cruzi: the bat seeding hypothesis. Trends Parasitol. 2012b;28:136–141. doi: 10.1016/j.pt.2012.01.006. http://dx.doi.org/10.1016/j.pt.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Haydon DT, Cleaveland S, Taylor LH, Laurenson MK. Identifying reservoirs of infection: a conceptual and practical challenge. Emerg Infect Dis. 2002;8:1468–1473. doi: 10.3201/eid0812.010317. http://dx.doi.org/10.3201/eid0812.010317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera CP, Licon MH, Nation CS, Jameson SB, Wesson DM. Genotype diversity of Trypanosoma cruzi in small rodents and Triatoma sanguisuga from a rural area in New Orleans, Louisiana. Parasites Vectors. 2015;8:123. doi: 10.1186/s13071-015-0730-8. http://dx.doi.org/10.1186/s13071-015-0730-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjos SA, Gillespie JJ, Olson JK, Snowden KF. Detection of Blastocrithidia spp. (Kinetoplastida: Trypanosomatidae) in chagas disease vectors from Texas, USA. Vector Borne Zoonotic Dis. 2009a;9:213–216. doi: 10.1089/vbz.2008.0027. http://dx.doi.org/10.1089/vbz.2008.0027. [DOI] [PubMed] [Google Scholar]
- Kjos SA, Snowden KF, Olson JK. Biogeography and Trypanosoma cruzi infection prevalence of chagas disease vectors in Texas, USA. Vector Borne Zoonotic Dis. 2009b;9:41–50. doi: 10.1089/vbz.2008.0026. http://dx.doi.org/10.1089/vbz.2008.0026. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016 doi: 10.1093/molbev/msw054. http://dx.doi.org/10.1093/molbev/msw054, msw054. [DOI] [PMC free article] [PubMed]
- Kunz TH. Monitoring Trends in Bat Populations of the United States and Territories: Problems and Prospects. 2003. Censusing bats: challenges, solutions, and sampling biases; pp. 9–19. (Publications of the US Geological Survey). [Google Scholar]
- Lent H, Wygodzinsky P. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas’ disease. Bull Am Mus Nat Hist. 1979;163(3):123–520. http://hdl.handle.net/2246/1282. [Google Scholar]
- Lima L, Maia da Silva F, Neves L, Attias M, Takata CSA, Campaner M, de Souza W, Hamilton PB, Teixeira MMG. Evolutionary insights from bat trypanosomes: morphological, developmental and phylogenetic evidence of a new species, Trypanosoma (Schizotrypanum) Erneyi sp nov., in African bats closely related to Trypanosoma (Schizotrypanum) cruzi and allied species. Ann Anat. 2012;163:856–872. doi: 10.1016/j.protis.2011.12.003. http://dx.doi.org/10.1016/j.protis.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Lima L, Espinosa-Álvarez O, Hamilton PB, Neves L, Takata CS, Campaner M, Attias MR, de Souza W, Camargo EP, Teixeira MM. Trypanosoma livingstonei: a new species from African bats supports the bat seeding hypothesis for the Trypanosoma cruzi clade. Parasites Vectors. 2013;6 doi: 10.1186/1756-3305-6-221. http://dx.doi.org/10.1186/1756-3305-6-221, 1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima L, Espinosa-Álvarez O, Ortiz PA, Trejo-Varón JA, Carranza JC, Pinto CM, Serrano MG, Buck GA, Camargo EP, Teixeira MMG. Genetic diversity of Trypanosoma cruzi in bats, and multilocus phylogenetic and phylogeographical analyses supporting Tcbat as an independent DTU (discrete typing unit) Acta Trop. 2015a;151:166–177. doi: 10.1016/j.actatropica.2015.07.015. http://dx.doi.org/10.1016/j.actatropica.2015.07.015. [DOI] [PubMed] [Google Scholar]
- Lima L, Espinosa-Álvarez O, Pinto CM, Cavazzana M, Pavan AC, Carranza JC, Lim BK, Campaner M, Takata CSA, Camargo EP, Hamilton PB, Teixeira MMG. New insights into the evolution of the Trypanosoma cruzi clade provided by a new trypanosome species tightly linked to Neotropical Pteronotus bats and related to an Australian lineage of trypanosomes. Parasites Vectors. 2015b;8:657. doi: 10.1186/s13071-015-1255-x. http://dx.doi.org/10.1186/s13071-015-1255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis AD, Hayman DTS, O’Shea TJ, Cryan PM, Gilbert AT, Pulliam JRC, Mills JN, Timonin ME, Willis CKR, Cunningham AA, Fooks AR, Rupprecht CE, Wood JLN, Webb CT. A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proc R Soc B: Biol Sci. 2013;280:20122753. doi: 10.1098/rspb.2012.2753. http://dx.doi.org/10.1098/rspb.2012.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia da Silva F, Noyes H, Campaner M, Junqueira ACV, Coura JR, Añez N, Shaw JJ, Stevens JR, Teixeira MMG. Phylogeny, taxonomy and grouping of Trypanosoma rangeli isolates from man, triatomines and sylvatic mammals from widespread geographical origin based on SSU and ITS ribosomal sequences. Parasitology. 2004;129:549–561. doi: 10.1017/s0031182004005931. http://dx.doi.org/10.1017/S0031182004005931. [DOI] [PubMed] [Google Scholar]
- Marcili A, Lima L, Cavazzana M, Junqueira ACV, Veludo HH, Maia da Silva F, Campaner M, Paiva F, Nunes VLB, Teixeira MMG. A new genotype of Trypanosoma cruzi associated with bats evidenced by phylogenetic analyses using SSU rDNA, cytochrome b and Histone H2B genes and genotyping based on ITS1 rDNA. Parasitology. 2009a;136:641. doi: 10.1017/S0031182009005861. http://dx.doi.org/10.1017/s0031182009005861. [DOI] [PubMed] [Google Scholar]
- Marcili A, Lima L, Valente VC, Valente SA, Batista JS, Junqueira ÂCV, Souza AI, da Rosa JA, Campaner M, Lewis MD, Llewellyn MS, Miles MA, Teixeira MMG. Comparative phylogeography of Trypanosoma cruzi TCIIc: new hosts, association with terrestrial ecotopes, and spatial clustering. Infect Genet Evol. 2009b;9:1265–1274. doi: 10.1016/j.meegid.2009.07.003. http://dx.doi.org/10.1016/j.meegid.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Maslov DA, Votýpka J, Yurchenko V, Lukeš J. Diversity and phylogeny of insect trypanosomatids: all that is hidden shall be revealed. Trends Parasitol. 2013;29:43–52. doi: 10.1016/j.pt.2012.11.001. http://dx.doi.org/10.1016/j.pt.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Mayes BC, Wilson PJ, Oertli EH, Hunt PR, Rohde RE. Epidemiology of rabies in bats in Texas (2001–2010) J Am Vet Med Assoc. 2013;243:1129–1137. doi: 10.2460/javma.243.8.1129. http://dx.doi.org/10.2460/javma.243.8.1129. [DOI] [PubMed] [Google Scholar]
- McWilliams LA. Variation in diet of the Mexican free-tailed bat (Tadarida Brasiliensis Mexicana) J Mammal. 2005;86:599–605. http://dx.doi.org/10.1644/1545-1542(2005)86[599:VIDOTM]2.0.CO;2. [Google Scholar]
- Molyneux DH. Trypanosomes of bats. In: Kreier JP, Baker, editors. Parasitic Protozoa. Academic Press; New York, NY: 1991. pp. 95–223. [Google Scholar]
- Mukherjee N, Beati L, Sellers M, Burton L, Adamson S, Robbins RG, Moore F, Karim S. Importation of exotic ticks and tick-borne spotted fever group rickettsiae into the United States by migrating songbirds. Ticks Tick Borne Dis. 2014;5:127–134. doi: 10.1016/j.ttbdis.2013.09.009. http://dx.doi.org/10.1016/j.ttbdis.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NatureServe. NatureServe Explorer: An Online Encyclopedia of Life [web application] [WWW Document] 2015 URL http://explorer.natureserve.org (accessed 09.01.16.)
- Noyes HA, Stevens JR, Teixeira M, Phelan J. A nested PCR for the ssrRNA gene detects Trypanosoma binneyi in the platypus and Trypanosoma sp in wombats and kangaroos in Australia. Int J Parasitol. 1999;29:331–339. doi: 10.1016/s0020-7519(98)00167-2. http://dx.doi.org/10.1016/S0020-7519(98)00167-2. [DOI] [PubMed] [Google Scholar]
- Pinto CM, Kalko E, Cottontail I, Wellinghausen N, Cottontail VM. TcBat a bat-exclusive lineage of Trypanosoma cruzi in the Panama Canal Zone, with comments on its classification and the use of the 18S rRNA gene for lineage identification. Infect Genet Evol. 2012;12:1328–1332. doi: 10.1016/j.meegid.2012.04.013. http://dx.doi.org/10.1016/j.meegid.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Pinto CM, Ocaña-Mayorga S, Tapia EE, Lobos SE, Zurita AP, Aguirre-Villacís F, MacDonald A, Villacís AG, Lima L, Teixeira MMG, Grijalva MJ, Perkins SL. Bats, trypanosomes, and triatomines in Ecuador: new insights into the diversity, transmission, and origins of Trypanosoma cruzi and chagas disease. PLoS One. 2015;10 doi: 10.1371/journal.pone.0139999. http://dx.doi.org/10.1371/journal.pone.0139999, e0139999-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piron M, Fisa R, Casamitjana N, López-Chejade P, Puig L, Vergés M, Gascón J, Prat JGI, Portús M, Sauleda S. Development of a real-time PCR assay for Trypanosoma cruzi detection in blood samples. Acta Trop. 2007;103:195–200. doi: 10.1016/j.actatropica.2007.05.019. http://dx.doi.org/10.1016/j.actatropica.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Ramírez JD, Tapia-Calle G, Muñoz-Cruz G, Poveda C, Rendón LM, Hincapié E, Guhl F. Trypanosome species in neo-tropical bats: biological, evolutionary and epidemiological implications. Infect Genet Evol. 2014;22:250–256. doi: 10.1016/j.meegid.2013.06.022. http://dx.doi.org/10.1016/j.meegid.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez JC, Cura CI, da Cruz Moreira O, Lages-Silva E, Juiz N, Velazquez E, Ramírez JD, Alberti A, Pavia P, Flores-Chávez MD, Muñoz-Calderon A, Pérez-Morales D, Santalla J, Marcos da Matta Guedes P, Péneau J, Marcet P, Padilla C, Cruz-Robles D, Valencia E, Crisante GE, Greif G, Zulantay I, Costales JA, Alvarez-Martínez M, Martínez NE, Villarroel R, Villarroel S, Sánchez Z, Bisio M, Parrado R, Maria da Cunha Galvão L, Jácome da Câmara AC, Espinoza B, Alarcón de Noya B, Puerta C, Riarte A, Diosque P, Sosa Estani S, Guhl F, Ribeiro I, Aznar C, Britto C, Yadón ZE, Schijman AG. Analytical validation of quantitative real-time PCR methods for quantification of Trypanosoma cruzi DNA in blood samples from chagas disease patients. J Mol Diagn. 2015;17:605–615. doi: 10.1016/j.jmoldx.2015.04.010. http://dx.doi.org/10.1016/j.jmoldx.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey JM, Ordoñez R, Cruz Celis A, Alvear AL, Chavez V, Lopez R, Pintor JR, Gama F, Carrillo S. Distribution of domestic Triatominae and stratification of Chagas disease transmission in Oaxaca, Mexico. Med Vet Entomol. 2000;14:19–30. doi: 10.1046/j.1365-2915.2000.00214.x. http://dx.doi.org/10.1046/j.1365-2915.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- Ramsey JM, Gutiérrez-Cabrera AE, Salgado-Ramírez L, Townsend Peterson A, Sánchez-Cordero V, Ibarra-Cerdeña CN. Ecological connectivity of Trypanosoma cruzi reservoirs and Triatoma pallidipennis hosts in an anthropogenic landscape with endemic Chagas disease. PLoS One. 2012;7:e46013. doi: 10.1371/journal.pone.0046013. http://dx.doi.org/10.1371/journal.pone.0046013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roellig DM, Brown EL, Barnabé C, Tibayrenc M, Steurer FJ, Yabsley MJ. Molecular typing of Trypanosoma cruzi isolates, United States. Emerg Infect Dis. 2008;14:1123–1125. doi: 10.3201/eid1407.080175. http://dx.doi.org/10.3201/eid1407.080175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Strutz SE, Frank DM, Rivaldi CL, Sissel B, Sanchez-Cordero V. Chagas disease risk in Texas. PLoS Negl Trop Dis. 2010;4:e836. doi: 10.1371/journal.pntd.0000836. http://dx.doi.org/10.1371/journal.pntd.0000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schijman AG, Lauricella MA, Marcet PL, Duffy T, Cardinal MV, Bisio M, Levin MJ, Kitron U, Gurtler RE. Differential detection of Blastocrithidia triatomae and Trypanosoma cruzi by amplification of 24sα ribosomal RNA genes in faeces of sylvatic triatomine species from rural northwestern Argentina. Acta Trop. 2006;99:50–54. doi: 10.1016/j.actatropica.2006.06.010. http://dx.doi.org/10.1016/j.actatropica.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Souto RP, Vargas N, Zingales B. Trypanosoma rangeli: discrimination from Trypanosoma cruzi based on a variable domain from the large subunit ribosomal RNA gene. Exp Parasitol. 1999;91:306–314. doi: 10.1006/expr.1998.4380. http://dx.doi.org/10.1006expr.1998.4380. [DOI] [PubMed] [Google Scholar]
- Tenney TD, Curtis-Robles R, Snowden KF, Hamer SA. Shelter dogs as sentinels for Trypanosoma cruzi transmission across Texas. Emerg Infect Dis. 2014;20:1323–1326. doi: 10.3201/eid2008.131843. http://dx.doi.org/10.3201/eid2008.131843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas ME, Rasweiler JJ, Iv, D’Alessandro A. Experimental transmission of the parasitic flagellates Trypanosoma cruzi and Trypanosoma rangeli between triatomine bugs or mice and captive neotropical bats. Mem Inst Oswaldo Cruz. 2007;102:559–565. doi: 10.1590/s0074-02762007005000068. [DOI] [PubMed] [Google Scholar]
- Villa BR, Cockrum EL. Migration in the guano bat Tadarida brasiliensis mexicana (Saussure) J Mammal. 1962;43:43–64. http://dx.doi.org/10.2307/1376879. [Google Scholar]
- Waldien DL, Hayes JP. A technique to capture bats using hand-held mist nets. Wildl Soc Bull. 1999 http://dx.doi.org/10.2307/3783959.
- Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM, Teixeira MMG, Schijman AG, Llewellyn MS, Lages-Silva E, Machado CR, Andrade SG, Sturm NR. The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect Genet Evol. 2012;12:240–253. doi: 10.1016/j.meegid.2011.12.009. http://dx.doi.org/10.1016/j.meegid.2011.12.009. [DOI] [PubMed] [Google Scholar]


