Abstract
The most important aspect of a preclinical study seeking to develop a novel therapy for neurological diseases is whether the therapy produces any clinically relevant functional recovery. For this purpose, neurobehavioral tests are commonly used to evaluate the neuroprotective efficacy of treatments in a wide array of cerebrovascular diseases and neurotrauma. Their use, however, has been limited in experimental subarachnoid hemorrhage studies. After several randomized, double-blinded, controlled clinical trials repeatedly failed to produce a benefit in functional outcome despite some improvement in angiographic vasospasm, more rigorous methods of neurobehavioral testing became critical to provide a more comprehensive evaluation of the functional efficacy of proposed treatments. While several subarachnoid hemorrhage studies have incorporated an array of neurobehavioral assays, a standardized methodology has not been agreed upon. Here, we review neurobehavioral tests for rodents and their potential application to subarachnoid hemorrhage studies. Developing a standardized neurobehavioral testing regimen in rodent studies of subarachnoid hemorrhage would allow for better comparison of results between laboratories and a better prediction of what interventions would produce functional benefits in humans.
Keywords: Animal models, behavior (rodent), cognitive impairment, subarachnoid hemorrhage, experimental
Introduction
Subarachnoid hemorrhage (SAH) resulting from rupture of saccular intracranial aneurysms is associated with severe morbidity and mortality.1 The incidence of aneurysmal SAH (aSAH) in the United States is estimated to be 9.7–14.5 per 100,000 person-year.1–3 Several clinical trials over the past decade have been conducted to improve neurological outcomes in patients afflicted with cerebral vasospasm after aneurysmal SAH.4–7 These studies were formulated on the basis of preclinical and clinical studies that showed efficacy in the reduction of arterial vasospasm and possible neuroprotective properties.8 Despite signs of some improvement in angiographic vasospasm, none of these trials showed a significant clinical benefit in neurological outcomes.9,10 These disappointing findings have led to calls for an evaluation of the translational experimental designs in the hopes of enhancing the potential for clinical success. Among these initiatives, the use of neurobehavioral testing as an objective outcome measure constitutes an exciting opportunity. Preclinical studies directed toward demonstrating enhanced behavioral function will improve the predictive value of animal models of SAH for clinical efficacy of novel therapeutic agents.
Neurobehavioral tests have long been used to evaluate treatments for multiple diseases including neonatal hypoxic brain injury, ischemic stroke and traumatic brain injury in preclinical animal studies.11–22 Functional recovery is the key end-point in clinical studies because morphological changes do not always correspond well with functional deficits. However, until recently similar testing was not widespread in experimental SAH studies. Outcomes in animal studies of SAH have typically relied on histological measurements and neurological scoring.23–26 The severity and resolution of arterial vasospasm was typically measured on histological specimens by digital morphometry or vessel caliber was calculated from subtraction angiography images.27,28 After randomized, double-blinded, controlled clinical trials such as those for the endothelin receptor antagonist Clazosentan and Tirilazad showed improvement in angiographic vasospasm but failed to produce a benefit in neurological function,6,10,29 consideration for more rigorous methods of neurobehavioral testing became of critical importance to evaluate the benefit of the proposed treatments. While many recent preclinical studies of SAH have incorporated neurobehavioral testing, standardized methodology applicable to SAH is yet to be developed. This is in contrast to well-established outcome measures that are used in animal models of neurotrauma.30 The goal of this review is to discuss different types of neurobehavioral tests and their relevance to rodent models of SAH.
General considerations
The cumulative injury caused by SAH is characterized by two distinct stages: early brain injury (EBI) and delayed cerebral ischemia (DCI). EBI refers to the acute brain injury following aneurysmal rupture.31 When blood is released into the subarachnoid space, intracranial pressure raises sharply, which results in decreased cerebral blood flow (CBF) and transient global ischemia associated with temporary loss of consciousness. Transient ischemia is also thought to cause endothelial cell death and blood–brain barrier (BBB) disruption, cerebral edema development, and neuronal apoptosis in the hippocampus, brain stem and cerebellum.31 At the molecular level, decreased NO and ATP, increased potassium, elevated endothelin-1 levels, persistent inflammation as observed by increased endothelial expression of cytokines and cell adhesion molecules, and activation of platelets, thrombin, matrix metalloproteinases (MMPs), and pro-apoptotic pathways are seen during the EBI phase of SAH.31,32
Shortly after the EBI period concludes, DCI ensues. DCI encompasses angiographic vasospasm, cortical spreading ischemia, microthrombosis, and microcirculation constriction, among other mechanisms.31 Increasing evidence suggests that inflammation and, more specifically, leukocyte-endothelial cell interactions as well as peri-vascular and parenchymal inflammation, depleted NO, and increased endothelin-1 play a critical role in the pathogenesis of vasospasm after SAH.33 Glutamate excitotoxicity and waves of cortical spreading depression are also implicated in seizure activity after SAH.34 These injuries are thought to be responsible for the long-term sequelae of SAH.
SAH survivors often present with symptoms of depression, anxiety, and impaired cognitive function in addition to motor weakness, sensory changes, or speech difficulties.35,36 Neurocognitive deficits studied in patients recovering from SAH also include attention and working memory deficits.37,38 These types of neuropsychological deficits have been correlated with atrophy of temporomesial structures as well as atrophic enlargement of ventricles and sulci with voxel-based morphological studies using MRI.39–41 Moreover, changes in hippocampal volume was very significantly associated with neuropsychiatric deficits one year after SAH.41 Although a multitude of scoring systems including Hunt & Hess (H&H), World Federation of Neurological Societies (WFNS), and Fisher grade have been used to predict patient outcomes, the impact of SAH on neurocognitive function is seldom rigorously assessed in routine clinical practice.
Although grading systems similar to the Fisher scale are being developed in animal models to evaluate the bleeding scale of induced SAH in vivo and ex vivo,26,42,43 evaluating neurobehavioral deficits post-SAH induction with scales similar to H&H or WFNS is not easily accomplished. Extrapolating from human subjects, it can be proposed that animals with induced SAH dying within 24 h of injury likely represent the high grade SAH subjects and could be comparable to H&H 4–5 grade patients; animals surviving beyond 24 h after injury likely represent less severe hemorrhages and could be comparable to H&H Grade 1–3 patients. These animals with longer survival constitute an ideal target for neurobehavioral testing.
Better characterization of functional outcomes after SAH may lead to studies with a higher potential for translation into clinical settings. Neurobehavioral tests can provide more precise, accurate, and objective results that, in combination with traditionally used outcome measures, would enhance the quality of the preclinical data used to design further clinical trials.
Handling and behavioral testing methodology in animal models of SAH
Handling can drastically affect neurobehavioral testing results; therefore, proper handling is mandatory for accurate results.44 Handling methods for mice and rats are similar, but there are subtle differences between the species, and methodology should be tailored to the species.45 Several methods can be used to hold or restrain animals, all of which can have different effects on their anxiety levels.44 The most commonly used method to capture or restrain a rodent is holding the animal by the tail. This method involves holding the base of the tail between the thumb and forefinger and lifting the animal while using the opposite gloved hand to support the body. Animals may also be restrained by holding the loose skin of the scruff.35,44 Two other methods previously described involve the use of a tunnel apparatus or cupping of the hands. Tunnel handling involves guiding the animal into a plastic tube and then using the plastic tube to lift the animal out of the cage or testing apparatus.35,44 With cupping, an animal is scooped up from the cage using one or both hands and allowed to sit or explore the palms of the hands for a period of time. Cupping is more appropriate for mice.44 To prevent jumping behavior, an animal can be cupped loosely with both hands to habituate it to human touch for the first few handling sessions. Similarly, hands can be used to close both ends of the tunnel to prevent the animal from escaping during the initial handling period. Detailed description of housing, husbandry and handling can be found elsewhere.45
Naïve laboratory animals generally seek to avoid human contact and are stressed by restraint. Several recent studies have shown that picking up by the tail is associated with increased aversion and stress levels compared to using a tunnel or cupping by open hand.35,44 These latter modes result in lower stress levels, better acceptance and willingness of the animal to interact with the experimenter.35,44 Therefore, handling the animals by more gentle methods is crucial to minimize stress levels and avoid its confounding effects. These methods are particularly important for cognitive tests.45 Tests that measure anxiety levels such as the elevated plus maze have been shown to be directly affected by handling.35 Although there are no reported data for the optimum duration of handling before the start of the experiment to achieve maximal acclimatization to the experimenter, minimum of 15 min of handling and 45 min of apparatus habituation and pretraining over the span of 2–3 days is considered acceptable.45 It is also important to keep in mind that different strains of rodents respond differently to restraint and exhibit variable anxiety levels towards humans. Different strains also exhibit variable motivation and learning ability to perform behavioral tests.45 In addition to proper handling methodology, gloves (and laboratory gowns/coats) may be rubbed with the soiled bedding of the animals to further reduce stress and help to habituate the subjects before each handling session.44 Manual sanitation of the equipment using 70% ethanol or other disinfectant agents followed by either air drying or drying with paper towels can be used to clean the apparatus before each animal is introduced to the testing space to avoid distraction by the pheromones or excretions left by previous subjects.
Neurological severity scoring
Several neurological severity scales involve grading animals on points scored during spontaneous activity or after induction of cranial nerve reflexes by an examiner blinded to group identity. One of the most commonly used grading scales, developed by Garcia and colleagues for ischemic stroke in 1995, evaluates spontaneous activity and motor responses to stimulation.46 It consists of a six-part test with a maximum score of 18 and its modified version for SAH can be used to evaluate neurobehavioral outcomes in rats and mice.47 Components of the tests are listed in Table 1. We have reviewed the literature using the keywords “subarachnoid hemorrhage,” “neurological scoring,” “neuroscore,” and “neurological severity,” for mice and rats separately (Supplementary Tables 1 and 2).
Table 1.
Modified Garcia score.
| Test | 0 Points | 1 Point | 2 Points | 3 Points |
|---|---|---|---|---|
| Spontaneous activity (in cage for 5 min) | No movement | Barely moves | Moves but does not approach 3 walls of cage | Moves and approaches ≥3 walls of cage |
| Spontaneous movement of all limbs | No movement | Slight limb movements | Moves all limbs slowly | Moves all limbs same as pre-SAH |
| Movement of forelimbs (outstretching when held by tail) | No outreaching | Slight outreaching | Outreach is limited and less than pre-SAH | Outreach same as pre-SAH |
| Climbing wall of wire cage | Falls from slope | Fails to climb | Climbs weakly | Normal climbing |
| Reaction to touch on both sides of trunk | No response | Weak response | Normal response | |
| Response to vibrissae touch | No response | Weak response | Normal response |
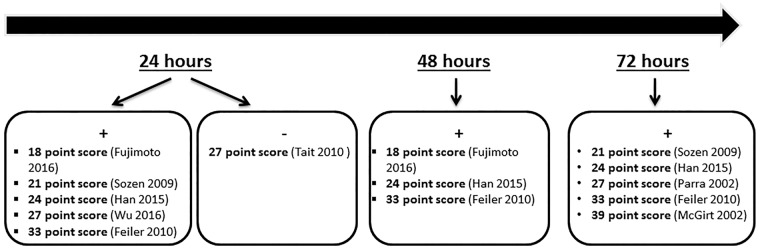
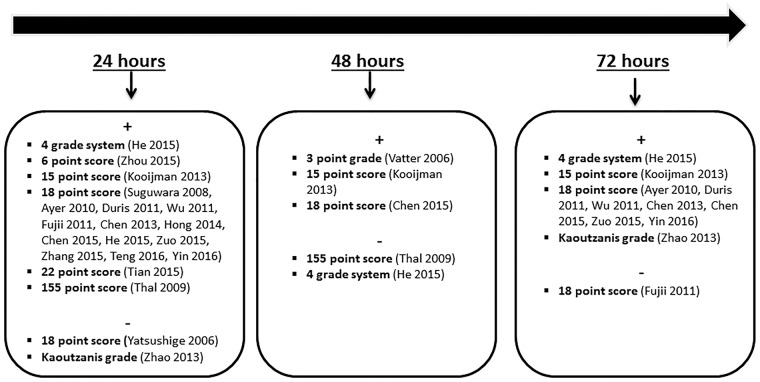
Neurological scores are thought to be most useful in the early EBI phase of SAH.47 In our literature review, we found that endovascular perforation model was the most commonly used in mouse studies which used neurological scoring; however, the wide variety of point systems used makes the comparison between the efficacy of each point score test challenging (Figure 1). At 1 and 3 days after SAH, the 18-point score was the most widely used neurological score scale in rats with positive results in majority of the studies (Figure 2). Although neurological severity grading scales such as the modified Garcia score have proven useful as complementary tools when testing differences among treated groups in translational studies, inter-observer reproducibility has not been rigorously assessed, the extent of examiner bias is unknown, and the lack of objective, precisely measurable variables, pose significant questions about the validity of the scale.
Figure 1.
Results of SAH studies using neurological scoring within 72 h in mice. (+ sign denotes list of studies with positive results, − sign denotes list of studies with negative results).
Figure 2.
Results of SAH studies using neurological scoring within 72 h in rats. (+ sign denotes list of studies with positive results, − sign denotes list of studies with negative results).
Vestibulomotor function tests
Rotarod
The Rotarod test was first described by Dunham and Miya in 1957.48 It provides quantitative assessment of motor function and has been used in experimental SAH mice by Mesis et al.49 The apparatus consists of a rotating cylinder with larger disks spaced so as to create compartments for individual animals. The device is typically set to accelerate at a constant speed. Often the rotation speed is set to increase from 4 rotations per minute (rpm) to 40 rpm in 300 s. This setting can be adjusted, depending on the severity of the injury, to make the task harder or easier. Rotarod latency is defined as the time until the animal falls off the cylinder or clings to the rotating rod for two consecutive rotations. The latency of response is recorded before SAH injury as baseline performance and at discrete time points after injury. Up to three trials are usually performed. Absolute time spent on the rotarod or percent change from baseline performance, using the average of trial scores or best score, can be used for analysis. Factors such as the diameter of the rotating rod, compartment spacing, pre-training variables (such as frequency and duration of pre-training), testing environments (under light or dark conditions) and testing times in relation to the daylight cycle of the animal, testing dates and associated “learning effects” due to increased frequency of testing procedures, pose a challenge for comparing data across different studies. Because it is a less time-consuming procedure, the Rotarod has gained popularity and its use has been more frequently reported in the SAH literature.49–57 Results of all the studies using the Rotarod in SAH-induced rodents are reviewed in Supplementary Table 3.
A lack of comparison between sham and SAH animals was found in a multitude of studies that utilized rotarod testing.49–52,55,58 In other studies where sham-operated and SAH animals were compared, significant differences were found in both short- and long-term survival. For example, in a short-term study using an endovascular perforation model, Wu et al. showed a significant difference in rotarod perforamance between sham and SAH mice at 24 h.59 Using the same model, Sherchan et al. found significant differences in rotarod latency between sham and SAH mice 21 days after SAH in a long-term study.53 Similar results were found at similar testing points for rats. Rats subjected to the rotating rod 14 days after SAH did significantly worse compared to shams using the endovascular perforation model.54 In another study in which both prechiasmatic blood injection and double cisternal blood injection models were compared, the authors showed significant differences between sham and SAH animals at 24 h after SAH, but failed to show any differences 7 and 35 days post-SAH.57 Moreover, they found no significant difference in rotarod latencies in SAH animals between the two models.57 Siler et al. also failed to find significant differences between sham and SAH animals 4–6 days post-injury.60 These findings suggest that in both rats and mice, significant differences in Rotarod performance was documented in the acute (24 h) and long terms (e.g., 14 and 21 days) but not in the mid-term (4–7 days).
Open field test
The open field test is used to assess locomotor exploratory and anxiety-like behavior in animals that are exposed to a novel environment. Using a video camera or infrared beams, an animal’s spontaneous locomotor activity is recorded typically for 5 min. Distance travelled, resting time, stereotypic time, ambulatory time, and time spent in the center of the box can be analyzed with accompanying software. Thigmotaxis, the natural tendency of animals to stay close to the periphery and avoid the center of novel environments, can be used as a measure of anxiety, in addition to spontaneous locomotion.61
Open field testing has been used in relatively few studies and provides an exciting opportunity for behavioral testing in SAH. Our literature search using the keywords “subarachnoid hemorrhage” and “open field test” revealed only two studies using rats and one study using mice. Although Hollig et al. failed to show significant differences in distance travelled and ambulatory time in SAH-induced rats 24 h after SAH induction using the endovascular perforation model,62 Boyko and colleagues reported significantly decreased locomotor activity, mean velocity, time and distance travelled in the central part of the field after a double-blood injection model in rats 3 weeks after SAH induction.63 However, in the same study, no statistical difference in open field test between control and SAH animals was found using in the single-blood injection model of SAH 3 weeks after blood injection.63 In an endovascular perforation model of SAH in mice, significant reduction in travelled distance was found in SAH-induced compared to sham-operated mice 96 h after SAH.56 Future studies can help further establish the behavioral profile of SAH animals in open field testing in various testing points using different models.
Beam balance and beam walking test
The beam balance test quantifies motor and vestibular functioning in animals by scoring their balancing and/or traversing performance.64 The animal is placed in the center of a wooden beam with its long axis parallel to the beam. The beam is elevated above a table, and a foam pad is positioned to protect the animals when they fall off the beam. Dimensions of the beam may vary. Scoring of performance is typically based on whether the animal can maintain balance and traverse the beam.65 A scoring system of 0 to 2 can be used if both tasks are evaluated: 0 when the animal cannot maintain balance on the beam at all; 1 when the animal can maintain balance but not traverse the beam; 2 when the animal can maintain balance and traverse the beam.66 To score the animals purely on balancing performance, scores are based on how long the animal is able to maintain balance, with points assigned to ranges of time.65
Rats undergoing SAH via a cisternal blood injection model showed poor performance on the beam balance test on the first day after surgery before their motor performance improved.64 In other studies, deficits in beam balance score and times were seen after cisternal blood injection on days 1 and 2.67–69 Beam traversing times were shown to be higher on days 1 to 4 after SAH induction in rats.67–69 There were no significant differences in the beam walking test in an endovascular perforation model of SAH 3, 7, 14, and 21 days after SAH in rats; however, the parameter used (% impaired steps) was different from that used in the previous studies.70 The beam balance test offers flexibility in measurements that can make experiments conducted in different laboratories difficult to compare.
Other potential vestibulomotor tests
Grip strength and CatWalk gait analysis are other two well-established tests that can be utilized for assessment of vestibulomotor function in SAH-induced animals. Forelimb and hindlimb grip strength measurement is a technique first described in 197971 and measures the maximal force an animal applies on a specially designed pull bar attached to a sensor. The dual sensor model allows the animal to grasp the pull bar with its paws and measures the maximum force attained when the animal is gently tugged back along a straight line leading away from the sensor until it releases the bar.19 This type of device has been used to assess forelimb neuromuscular function and integrity in experimental models of neuromuscular disorders including amyotrophic lateral sclerosis,72 transient ischemic attack,73 permanent ischemic stroke19 and TBI74 with variable results. The utility of this test in SAH models is not well established.
The CatWalk system is used to collect detailed gait analysis data rodents.75 As the animal traverses a walkway, a video camera records light deflection by the paws. Only the points of contact on the walkway are illuminated and the intensity of illumination reflects pressure of the applied paws.76 CatWalk software can further analyze up to 50+ parameters of gait including stride length, paw print intensity, paw print size, and swing phase (time between consecutive paw prints).77 SAH animal models have not been thoroughly studied using the CatWalk gait analysis system. However, it has been used in various animal models of neurological disease such as neuropathic pain, spinal cord contusion injury, sciatic nerve injury, Parkinson’s disease, and ischemic stroke.76,78–82 The CatWalk can provide valuable insights on altered gait in SAH animals and should be pursued further in SAH rodent studies. For blood injection models in which the femoral artery is used as a source of arterial blood, manipulation of the femoral nerve and possible hind limb ischemia induced with the blood withdrawal procedure should be kept in mind when evaluating hind limb deficits.
Cognitive tests
Morris water maze
Spatial learning, reference memory and working memory can be evaluated with a variety of tasks in rodents with SAH using the Morris Water Maze (MWM). This technique uses a circular pool of opaque water with a hidden platform available for escape. The platform is hidden by placing it just below the water surface. Opacity of the water is controlled with powdered milk or non-toxic white paint.83 The hidden platform and opaque water provide no local visual cues for escape to the platform; thus the MWM specifically assesses navigation and working memory skills.84 Hippocampal lesions impair initial post-operative navigation, further confirming the role of working memory in the task.85
Latency to escape, velocity, and distance swum are the most common measures of performance in the MWM.86 Animals are tested with repeated trials to track spatial learning over time. Travelling patterns can also be recorded by labeling the pool with North, South, East, and West markers and/or using a video camera placed above the pool. The platform can be moved between quadrants and may also be removed entirely to track free-swim patterns in what is known as a “probe trial.” Animals who perform well in the probe trial spend most of their time in the platform’s quadrant or swimming through or around the platform’s original location.83
Spatial learning is best assessed with four trials daily with random start locations, while the hidden platform remains in the same location within the tank.86 This task is known as spatial acquisition. To assess reference memory after spatial learning, a probe trial is given 24 h after the final spatial acquisition trial. As mentioned previously, no platform is present in the tank and the amount of time spent in each quadrant of the tank is the most important measure during this phase of testing. Changing the platform location each day and allowing two trials starting from the same location in the tank evaluates spatial working memory. The escape latency difference between the two trials is the main outcome in this type of testing.86
Several studies report varying results in MWM performance following the endovascular perforation model of SAH. For example, Hu et al. showed significant deficits in SAH rats 17–21 days after injury, although a treatment effect with hyperbaric oxygen was not seen in MWM performance.87 Using the same model, Sherchan et al. documented deficits between SAH and sham rats 21–25 days after injury.53 In this same study, a treatment effect was seen with minocycline, which improved the outcome.53 Similarly, using the same model, escape latency and distance swum was found to be increased for SAH-induced rats when the platform was moved to another location.88 Although deficits in MWM performance are reported with rats in the endovascular perforation model, Milner et al. found no significant difference between sham and SAH mice 17–21 days after SAH induction.89 It is not known whether the same model may produce deficits at different time points in mice. Moreover, no hemispherical or hippocampal volume changes were detected in SAH mice in a study by Atangana et al. using MRI, suggesting that current models of SAH may not induce significant radiological and functional deficits in hippocampus volume and neurocognitive tests respectively in SAH mice.90 Further research is needed to improve surgical SAH induction methods and assessment of neurocognitive deficits at different time points.
Autologous cisternal blood injection induced significant deficits on escape latencies 4–5 days after SAH in rats, and this effect was significantly alleviated by dimethylfumarate. However, the same blood injection did not produce changes in cued learning tasks.91 Using the cisternal double injection model in rats, Feng and colleagues demonstrated significant cognitive deficits in rats 5 days after SAH induction, and ceftriaxone improved these deficits.92 Using the same model, Takata et al. showed increased latencies and swimming distances in SAH rats at later time points, specifically days 29–35.93 A treatment effect was described with long-term simvastatin administration.51 In a double hemorrhage SAH model, simvastatin given for five weeks after surgery significantly improved escape latency and swimming distance despite a decrease in swim speed.94 MWM performance has not been studied in cisternal blood injection models of SAH in mice.
A prechiasmatic injection model of SAH in rats significantly reduced MWM performance in two different studies on postoperative days 4–5 and only day 5, respectively.95,96 Similarly, prechiasmatic injection to induce SAH did not induce deficits in cued learning, although deficits in spatial learning were evident 4–5 days after SAH.97–99 A treatment effect with tamoxifen, tert-butylhydroquinone (tBHQ), an Nrf2 activator, and YC-1, a hypoxia-inducible factor 1 (HIF-1) inhibitor, were also observed on those days.97–99 Sasaki et al. found a similar trend between the prechiasmatic injection model of SAH and the double blood injection model of SAH, reporting that in both experimental models, swimming speed was significantly increased and escape latency was decreased over time in rats tested 29–32 days after SAH induction.57
Elevated T maze and elevated plus maze tests
The elevated T-maze is a three-armed apparatus used to analyze memory and anxiety in laboratory animals. In the apparatus, two arms are open, and 40-cm high walls enclose the third; the closed arm is connected perpendicularly to the open arms. The apparatus is elevated above the ground by 50 cm.100 The elevated plus maze, on the other hand, has two open arms and two closed arms all perpendicular to each other.101 In both tests, the animals are placed in the center of the maze and allowed to explore it freely for a predetermined period of time. The animals can then be timed for their withdrawals from the ends of both open and closed arms and number of arm entries.101 The elevated T-maze presents an aversive experience to animals exploring the open arms because rats inherently fear openness and height.102 Inhibitory avoidance, representing conditioned fear, is measured when animals are placed at the end of the enclosed arm. Over trials, the animals learn inhibitory avoidance after repeatedly reaching the intersection of the maze and seeing the two open arms.103 Animals placed at the ends of open arms escape to the closed arm. Unconditioned fear is assessed in the second task. The elevated T-maze differs from the elevated plus-maze because it distinctly separates two types of fear.104 In healthy animals, withdrawal times from the closed arm is expected to increase over trials, while escape times from the open arms are expected to decrease over trials.
Inhibitory avoidance performance is reduced with the administration of the anxiolytics benzodiazepam, buspirone, ipsapirone, and ritanserin. The anxiogenic agents yohimbine, TFPP and mCPP facilitate inhibitory avoidance.103 In a study using the endovascular perforation model of SAH in rats, animals were placed in the closed arm and timed on their choice of one arm. On day 21 post-surgery, intracranial pressure correlated with delayed decision in the elevated T-maze.105 In another study by Sasaki et al., rats with SAH induced by prechiasmatic blood injection, showed decreased anxiety behavior on the elevated plus maze, whereas anxiety was increased in SAH rats undergoing cisternal double blood injection, suggesting that different models of SAH may induce different anxiety behavior in animals.57 The elevated T-maze and elevated plus maze are often used to research psychiatric disorder treatments; however, they can also reliably evaluate anxiety and memory in animal models of SAH.
Other potential neurocognitive tests
The radial arm maze (RAM) is another neurocognitive test that can be used to assess neurocognitive function in experimental SAH. Olton and Samuelson developed the RAM test in 1976 as a method for assessing memory and spatial learning in rats.106 With this device, both working and reference memory can be tested simultaneously with careful observation of rodent behavior during testing. To date, no studies have used the RAM to study the cognitive changes in rodent models of SAH. Briefly, the RAM consists of eight horizontal arms oriented around a central platform above the floor. Automated doors are located at the entrance of each arm and the rats or mice are placed on the central platform, from which they have to collect hidden food placed at the end of the arms. In the standard version of the RAM, animals are habituated to the environment by being placed on the central platform and allowed to explore the maze for 15 min each day. Food is scattered on the end of selected radial arms. On the last day of habituation (typically day 3), the number of food reinforcements is reduced to half, and the session ends when all eight arms have been visited. After this habituation period, the rats or mice are trained once daily for eight consecutive days. One piece of food is hidden at the end of each arm in a well, and the animal is allowed to freely explore the maze. Each session lasts until (a) all eight arms have been entered, (b) 10 min have passed since the start of the test, or (c) 2 min have passed since the animal’s last arm entrance. Arm entries are recorded for later analysis.
Sensory tests
Adhesive removal test
The Adhesive Removal Test (ART) was developed to assess sensorimotor asymmetry in rats with unilateral nigrostriatal damage.107 Schallert et al. found that the animals quickly removed any mildly adhesive paper from their skin and that that latency to removal of the adhesive materials could be measured. They placed sticky tape bilaterally on the snout, forelimbs, and hind limbs of animals and measured latencies to contact the tape and to remove it. Bilateral comparison showed significant differences between contralateral and ipsilateral responses after unilateral 6-OHDA microinfusions.107 The test has been used to investigate sensorimotor function in TBI and cerebral ischemia.108,109 For ART testing, a testing box can be used to contain the animal. The animal is acclimated to the testing box for 60 s before applying the sticky tabs. The same experimenter should apply the adhesive each time to ensure that pressure during application does not vary. The animal is given 120 s to remove the tape and should be timed for latency to contact the adhesive and latency to remove it.110 The ART allows for observations to be recorded, with or without the use of video capture during testing, and also removes subjectivity from data collection with the latency measurement.
The literature search using the keywords “subarachnoid hemorrhage” and “adhesive removal test” has revealed a single study. In the study by Kooijman et al., sensorimotor behavior was significantly affected 21 days after induction of SAH via endovascular puncture in rats.87
Possible applications of neurobehavioral tests in human clinical trials
The neurobehavioral tests described here have shown utility in different animal models and play a role in the preclinical research designs for functional outcome assessment for therapeutic drugs, especially for ischemic stroke and TBI. However, the methods of functional assessment for efficacy of a therapeutic agent in humans are far more simplistic and less precise than these strongly quantitative testing modalities in animals. The growing concern surrounding the failure of most of the clinical trials in fields of neurological diseases including SAH, calls for more precise outcome measures in humans.111 A study from the SAHIT investigators discusses potential reasons for failure of randomized clinical trials in SAH, some of which could be related to problems in outcome measures and the multifactorial nature of delayed cerebral ischemia.111 Other possible causes for failures in SAH trials are thought to stem from either the functional ineffectiveness of the tested therapies or the timing and dose of the treatment, inadequate sample size, insensitive or inappropriate outcome measures, the confounding effect of rescue therapies in placebo groups, treatment-associated side effects, and variations in practice across different centers.111 Similarly, a recent review of the phase III progesterone trials suggests possible weaknesses in the testing parameters used to evaluate the efficacy of progesterone therapy following TBI, suggesting that outcome measures used in humans for neurological diseases need to improved.112
The Glasgow Outcome Scale (GOS), extended GOS (GOS-E) and Modified Rankin Scale (mRS) as well as incidence of delayed ischemic deficits and duration/severity of vasospasm are the most commonly used outcome measures in SAH.111 However, many reviews question the clinical sensitivity and utility of the GOS, Disability Rating Scale (DRS), and the Functional Independence Measure (FIM).113–115 Similarly, a ceiling effect is thought to occur with mRS, where most patients are in the favorable outcome group, thus making it challenging to show any improvement with treatment.111
In the future, more sensitive tests for the evaluation of cognitive or neuropsychiatric outcomes as well as subtle testing tools for indices such as gait analysis, grip strength, and balance measures in humans should be used to better evaluate the efficacy of new therapies for debilitating neurological conditions such as SAH. Currently, gait analysis technology similar to that used in rodent models has been applied to human studies evaluating stroke patients.116 Grip strength and pinch strength have both been extensively evaluated in the follow-up care of stroke patients, and hand coordination testing has been applied to long-term follow-up of TBI patients.117,118 Although not directly applicable at this time, correlates to many of the tests performed with animal models may help in the delineation of subtle benefits to new clinical therapies in human patients.
Although clinical trials are conducted for both male and female patient populations, preclinical studies are based overwhelmingly on male animals. Sex differences are known to affect the incidence, mortality and possibly also functional outcome after SAH in humans119 as well as the pathophysiology of SAH in rats.120,121 However, there are very few studies the current literature investigating sex differences in functional outcome in experimental studies in SAH rodents using the neurobehavioral tests reviewed in this study.43
Conclusions
Neurobehavioral tests can offer valuable information about sensorimotor and cognitive changes in experimental SAH models in rodents. These tests can also be used to evaluate the efficacy of therapeutic agents. The failure of the randomized clinical trials testing an endothelin receptor antagonist as well as tirilazad5,29,122,123 supports the hypothesis that improvement in vasospasm alone, as an outcome measure, cannot predict functional improvement in patients.124 Until recently, complex neurobehavioral tests to evaluate the effect of therapies on functional outcome have not been widely used in SAH. Studies to evaluate neurobehavioral profiles in different rodent models and implementation of these neurobehavioral tests in SAH preclinical research can improve the probability of translating functionally effective drugs to the bedside and help to ensure the success of future clinical trials.
Supplementary Material
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
All authors certify that they have participated sufficiently in the work to take responsibility for the content, including participation in the concept, design, analysis, writing, revision or final approval of the manuscript. Study conception and design: Nefize Turan, Gustavo Pradilla; acquisition of data: Nefize Turan, Brandon A. Miller, Robert A. Heider, Maheen Nadeem; analysis and interpretation of data: Nefize Turan, Brandon A. Miller, Robert A. Heider, Maheen Nadeem; drafting of manuscript: Nefize Turan, Brandon A. Miller, Robert A Heider, Maheen Nadeem; critical revision: Brandon A. Miller, Iqbal Sayeed, Donald G. Stein, Gustavo Pradilla; final approval of the version to be published: Nefize Turan, Brandon A. Miller, Robert A. Heider, Maheen Nadeem, Iqbal Sayeed, Donald G. Stein, Gustavo Pradilla.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Connolly ES, Jr, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 2012; 43: 171137. [DOI] [PubMed] [Google Scholar]
- 2.Shea AM, Reed SD, Curtis LH, Alexander MJ, Villani JJ, Schulman KA. Characteristics of nontraumatic subarachnoid hemorrhage in the United States in 2003. Neurosurgery 2007; 61: 1131–7. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 3.Labovitz DL, Halim AX, Brent B, et al. Subarachnoid hemorrhage incidence among Whites, Blacks and Caribbean Hispanics: the Northern Manhattan Study. Neuroepidemiology 2006; 26: 147–150. [DOI] [PubMed] [Google Scholar]
- 4.Kirkpatrick PJ, Turner CL, Smith C, et al. Simvastatin in aneurysmal subarachnoid haemorrhage (STASH): a multicentre randomised phase 3 trial. Lancet Neurol 2014; 13: 666–675. [DOI] [PubMed] [Google Scholar]
- 5.Macdonald RL, Higashida RT, Keller E, et al. Randomised trial of clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid hemorrhage undergoing surgical clipping (CONSCIOUS-2). Acta Neurochir Suppl 2013; 115: 27– a31. [DOI] [PubMed] [Google Scholar]
- 6.Macdonald RL, Kassell NF, Mayer S, et al. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke 2008; 39: 3015–21. [DOI] [PubMed] [Google Scholar]
- 7.Leijenaar JF, Dorhout Mees SM, Algra A, et al. Effect of magnesium treatment and glucose levels on delayed cerebral ischemia in patients with subarachnoid hemorrhage: a substudy of the Magnesium in Aneurysmal Subarachnoid Haemorrhage trial (MASH-II). Int J Stroke 20151 Suppl A100): 108–12. [DOI] [PubMed] [Google Scholar]
- 8.Macdonald RL, Higashida RT, Keller E, et al. Preventing vasospasm improves outcome after aneurysmal subarachnoid hemorrhage: rationale and design of CONSCIOUS-2 and CONSCIOUS-3 trials. Neurocrit Care 2010; 13: 416–424. [DOI] [PubMed] [Google Scholar]
- 9.Loch Macdonald R. Vasospasm: my first 25 years-what worked? what didn't? what next? Acta Neurochir Suppl 2015; 120: 1–10. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S, Wang L, Liu M, et al. Tirilazad for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2010; 2: CD006778. [DOI] [PubMed] [Google Scholar]
- 11.Wali B, Ishrat T, Stein DG, et al. Progesterone improves long-term functional and histological outcomes after permanent stroke in older rats. Behav Brain Res 2016; 305: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wali B, Sayeed I, Stein DG. Improved behavioral outcomes after progesterone administration in aged male rats with traumatic brain injury. Restor Neurol Neurosci 2011; 29: 61–71. [DOI] [PubMed] [Google Scholar]
- 13.Sayeed I, Wali B, Stein DG. Progesterone inhibits ischemic brain injury in a rat model of permanent middle cerebral artery occlusion. Restor Neurol Neurosci 2007; 25: 151–159. [PubMed] [Google Scholar]
- 14.Hamm RJ. Neurobehavioral assessment of outcome following traumatic brain injury in rats: an evaluation of selected measures. J Neurotrauma 2001; 18: 1207–1216. [DOI] [PubMed] [Google Scholar]
- 15.Scheff SW, Baldwin SA, Brown RW, Kraemer PJ. Morris water maze deficits in rats following traumatic brain injury: lateral controlled cortical impact. J Neurotrauma 1997; 14: 615–27. [DOI] [PubMed] [Google Scholar]
- 16.Green EJ, Dietrich WD, van Dijk F, et al. Protective effects of brain hypothermia on behavior and histopathology following global cerebral ischemia in rats. Brain Res 1992; 580: 197–204. [DOI] [PubMed] [Google Scholar]
- 17.Markgraf CG, Green EJ, Hurwitz BE, et al. Sensorimotor and cognitive consequences of middle cerebral artery occlusion in rats. Brain Res 1992; 575: 238–246. [DOI] [PubMed] [Google Scholar]
- 18.Peterson BL, Won S, Geddes RI, et al. Sex-related differences in effects of progesterone following neonatal hypoxic brain injury. Behav Brain Res 2015; 286: 152–165. [DOI] [PubMed] [Google Scholar]
- 19.Wali B, Ishrat T, Won S, et al. Progesterone in experimental permanent stroke: a dose-response and therapeutic time-window study. Brain 2014; 137: 486–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yousuf S, Sayeed I, Atif F, et al. Delayed progesterone treatment reduces brain infarction and improves functional outcomes after ischemic stroke: a time-window study in middle-aged rats. J Cereb Blood Flow Metab 2014; 34: 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang H, Hua F, Wang J, et al. Progesterone and vitamin D: Improvement after traumatic brain injury in middle-aged rats. Horm Behav 2013; 64: 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geddes RI, Peterson BL, Stein DG, et al. Progesterone treatment shows benefit in female rats in a pediatric model of controlled cortical impact injury. PLoS One 2016; 11: e0146419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sozen T, Tsuchiyama R, Hasegawa Y, et al. Role of interleukin-1beta in early brain injury after subarachnoid hemorrhage in mice. Stroke 2009; 40: 2519–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostrowski RP, Tang J, Zhang JH. Hyperbaric oxygen suppresses NADPH oxidase in a rat subarachnoid hemorrhage model. Stroke 2006; 37: 1314–1318. [DOI] [PubMed] [Google Scholar]
- 25.McGirt MJ, Lynch JR, Parra A, et al. Simvastatin increases endothelial nitric oxide synthase and ameliorates cerebral vasospasm resulting from subarachnoid hemorrhage. Stroke 2002; 33: 2950–2956. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki H, Hasegawa Y, Kanamaru K, et al. Mechanisms of osteopontin-induced stabilization of blood-brain barrier disruption after subarachnoid hemorrhage in rats. Stroke 2010; 41: 1783–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pradilla G, Garzon-Muvdi T, Ruzevick JJ, et al. Systemic L-citrulline prevents cerebral vasospasm in haptoglobin 2-2 transgenic mice after subarachnoid hemorrhage. Neurosurgery 2012; 70: 747–756; discussion 56–57. [DOI] [PubMed] [Google Scholar]
- 28.Cuevas P, Carceller F, Nieto I, et al. Spasmolytic effect of acidic fibroblast growth factor in early cerebral vasospasm in the rat. Surg Neurol 1998; 49: 176–179; discussion 9–80. [DOI] [PubMed] [Google Scholar]
- 29.Jang YG, Ilodigwe D, Macdonald RL. Metaanalysis of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care 2009; 10: 141–147. [DOI] [PubMed] [Google Scholar]
- 30.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 1995; 12: 1–21. [DOI] [PubMed] [Google Scholar]
- 31.Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol 2014; 10: 44–58. [DOI] [PubMed] [Google Scholar]
- 32.Miller BA, Turan N, Chau M, et al. Inflammation, vasospasm, and brain injury after subarachnoid hemorrhage. Biomed Res Int 2014; 2014: 384342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaichana KL, Pradilla G, Huang J, et al. Role of inflammation (leukocyte-endothelial cell interactions) in vasospasm after subarachnoid hemorrhage. World Neurosurg 2010; 73: 22–41. [DOI] [PubMed] [Google Scholar]
- 34.Bell JD, Thomas TC, Lass E, et al. Platelet-mediated changes to neuronal glutamate receptor expression at sites of microthrombosis following experimental subarachnoid hemorrhage. J Neurosurg 2014, pp. 6: 1424–1431. [DOI] [PubMed] [Google Scholar]
- 35.Gouveia K, Hurst JL. Reducing mouse anxiety during handling: effect of experience with handling tunnels. PLoS One 2013; 8: e66401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeo SS, Choi BY, Chang CH, et al. Evidence of corticospinal tract injury at midbrain in patients with subarachnoid hemorrhage. Stroke 2012; 43: 223–941. [DOI] [PubMed] [Google Scholar]
- 37.Wallmark S, Lundstrom E, Wikstrom J, Ronne-Engstrom E. Attention deficits after aneurysmal subarachnoid hemorrhage measured using the test of variables of attention. Stroke 2015; 46: 1374–1376. [DOI] [PubMed] [Google Scholar]
- 38.Ellmore TM, Rohlffs F, Khursheed F. FMRI of working memory impairment after recovery from subarachnoid hemorrhage. Front Neurol 2013; 4: 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bendel P, Koivisto T, Niskanen E, et al. Brain atrophy and neuropsychological outcome after treatment of ruptured anterior cerebral artery aneurysms: a voxel-based morphometric study. Neuroradiology 2009; 51: 711–722. [DOI] [PubMed] [Google Scholar]
- 40.Bendel P, Koivisto T, Aikia M, et al. Atrophic enlargement of CSF volume after subarachnoid hemorrhage: correlation with neuropsychological outcome. AJNR Am J Neuroradiol 2010; 31: 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bendel P, Koivisto T, Hanninen T, et al. Subarachnoid hemorrhage is followed by temporomesial volume loss: MRI volumetric study. Neurology 2006; 67: 575–582. [DOI] [PubMed] [Google Scholar]
- 42.Sugawara T, Ayer R, Jadhav V, et al. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods 2008; 167: 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shishido H, Egashira Y, Okubo S, et al. A magnetic resonance imaging grading system for subarachnoid hemorrhage severity in a rat model. J Neurosci Meth 2015; 243: 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hurst JL, West RS. Taming anxiety in laboratory mice. Nat Meth 2010; 7: 825–856. [DOI] [PubMed] [Google Scholar]
- 45.Deacon RM. Housing, husbandry and handling of rodents for behavioral experiments. Nat Protoc 2006; 1: 936–946. [DOI] [PubMed] [Google Scholar]
- 46.Garcia JH, Wagner S, Liu KF, et al. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke 1995; 26: 627–634. discussion 35. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki H and Zhang JH. Neurobehavioral assessments of subarachnoid hemorrhage. In: Chen J, Xu X-M, Xu CZ, et al. (eds) Animal Models of Acute Neurological Injuries II: Injury and Mechanistic Assessments. vol 1. Totowa, NJ: Humana Press, 2012, pp. 435–440.
- 48.Dunham NW, Miya TS. A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc Am Pharm Assoc (Baltim) 1957; 46: 208–209. [DOI] [PubMed] [Google Scholar]
- 49.Mesis RG, Wang H, Lombard FW, et al. Dissociation between vasospasm and functional improvement in a murine model of subarachnoid hemorrhage. Neurosurg Focus 2006; 21: E4. [DOI] [PubMed] [Google Scholar]
- 50.Thal SC, Mebmer K, Schmid-Elsaesser R, et al. Neurological impairment in rats after subarachnoid hemorrhage – a comparison of functional tests. J Neurol Sci 2008; 268: 150–159. [DOI] [PubMed] [Google Scholar]
- 51.Takata K, Sheng H, Borel CO, et al. Simvastatin treatment duration and cognitive preservation in experimental subarachnoid hemorrhage. J Neurosurg Anesthesiol 2009; 21: 326–333. [DOI] [PubMed] [Google Scholar]
- 52.Sheng H, Reynolds JD, Auten RL, et al. Pharmacologically augmented S-nitrosylated hemoglobin improves recovery from murine subarachnoid hemorrhage. Stroke 2011; 42: 471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sherchan P, Lekic T, Suzuki H, et al. Minocycline improves functional outcomes, memory deficits, and histopathology after endovascular perforation-induced subarachnoid hemorrhage in rats. J Neurotrauma 2011; 28: 2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu Q, Vakhmjanin A, Li B, Tang J, Zhang JH. Hyperbaric oxygen therapy fails to reduce hydrocephalus formation following subarachnoid hemorrhage in rats. Med Gas Res 2014; 4: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang H, James ML, Venkatraman TN, et al. pH-sensitive NMDA inhibitors improve outcome in a murine model of SAH. Neurocrit Care 2014; 20: 119–131. [DOI] [PubMed] [Google Scholar]
- 56.Siler DA, Berlow YA, Kukino A, et al. Soluble epoxide hydrolase in hydrocephalus, cerebral edema, and vascular inflammation after subarachnoid hemorrhage. Stroke 2015; 7: 1916–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sasaki T, Hoffmann U, Kobayashi M, et al. Neurocrit Care. Epub ahead of print 19 February 2016. DOI: 10.1007/s12028-016-0250-1.
- 58.Mutoh T, Sasaki K, Yamamoto Y, et al. Isoflurane postconditioning with cardiac support promotes recovery from early brain injury in mice after severe subarachnoid hemorrhage. Life Sci 2016; 153: 35–40. [DOI] [PubMed] [Google Scholar]
- 59.Wu Y, Pang J, Peng J, et al. An apoE-derived mimic peptide, COG1410, alleviates early brain injury via reducing apoptosis and neuroinflammation in a mouse model of subarachnoid hemorrhage. Neurosci Lett 2016; 627: 92–99. [DOI] [PubMed] [Google Scholar]
- 60.Siler DA, Berlow YA, Kukino A, et al. Soluble epoxide hydrolase in hydrocephalus, cerebral edema, and vascular inflammation after subarachnoid hemorrhage. Stroke 2015; 46: 1916–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simon P, Dupuis R, Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav Brain Res 1994; 61: 59–64. [DOI] [PubMed] [Google Scholar]
- 62.Hollig A, Weinandy A, Liu J, et al. beneficial properties of argon after experimental subarachnoid hemorrhage: early treatment reduces mortality and influences hippocampal protein expression. Crit Care Med 2016; 44: e520–e529. [DOI] [PubMed] [Google Scholar]
- 63.Boyko M, Azab AN, Kuts R, et al. The neuro-behavioral profile in rats after subarachnoid hemorrhage. Brain Res 2013; 1491: 109–116. [DOI] [PubMed] [Google Scholar]
- 64.Germano AF, Dixon CE, d'Avella D, et al. Behavioral deficits following experimental subarachnoid hemorrhage in the rat. J Neurotrauma 1994; 11: 345–353. [DOI] [PubMed] [Google Scholar]
- 65.Combs DJ, D'Alecy LG. Motor performance in rats exposed to severe forebrain ischemia: effect of fasting and 1,3-butanediol. Stroke 1987; 18: 503–511. [DOI] [PubMed] [Google Scholar]
- 66.Modianos DT, Pfaff DW. Brain stem and cerebellar lesions in female rats. I. Tests of posture and movement. Brain Res 1976; 106: 31–46. [DOI] [PubMed] [Google Scholar]
- 67.Germano A, Imperatore C, d'Avella D, et al. Antivasospastic and brain-protective effects of a hydroxyl radical scavenger (AVS) after experimental subarachnoid hemorrhage. J Neurosurgery 1998; 88: 1075–1081. [DOI] [PubMed] [Google Scholar]
- 68.Merlo L, Cimino F, Scibilia A, et al. Simvastatin administration ameliorates neurobehavioral consequences of subarachnoid hemorrhage in the rat. J Neurotrauma 2011; 28: 2493–2501. [DOI] [PubMed] [Google Scholar]
- 69.Germano A, Caffo M, Angileri FF, et al. NMDA receptor antagonist felbamate reduces behavioral deficits and blood-brain barrier permeability changes after experimental subarachnoid hemorrhage in the rat. J Neurotrauma 2007; 24: 732–744. [DOI] [PubMed] [Google Scholar]
- 70.Silasi G, Colbourne F. Long-term assessment of motor and cognitive behaviours in the intraluminal perforation model of subarachnoid hemorrhage in rats. Behav Brain Res 2009; 198: 380–387. [DOI] [PubMed] [Google Scholar]
- 71.Meyer OA, Tilson HA, Byrd WC, et al. A method for the routine assessment of fore- and hindlimb grip strength of rats and mice. Neurobehav Toxicol 1979; 1: 233–236. [PubMed] [Google Scholar]
- 72.Ari C, Poff AM, Held HE, et al. Metabolic therapy with Deanna Protocol supplementation delays disease progression and extends survival in amyotrophic lateral sclerosis (ALS) mouse model. PLoS One 2014; 9: e103526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferrara A, El Bejaoui S, Seyen S, Tirelli E, Plumier JC. The usefulness of operant conditioning procedures to assess long-lasting deficits following transient focal ischemia in mice. Behav Brain Res 2009; 205: 525–534. [DOI] [PubMed] [Google Scholar]
- 74.Tsai YD, Liliang PC, Cho CL, et al. Delayed neurovascular inflammation after mild traumatic brain injury in rats. Brain Inj 2013; 27: 361–365. [DOI] [PubMed] [Google Scholar]
- 75.Deacon RM. Measuring motor coordination in mice. J Visual Exp 2013; 75: e2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vrinten DH, Hamers FF. ‘CatWalk' automated quantitative gait analysis as a novel method to assess mechanical allodynia in the rat; a comparison with von Frey testing. Pain 2003; 102: 203–209. [DOI] [PubMed] [Google Scholar]
- 77.Deumens R, Marinangeli C, Bozkurt A, Brook GA. Assessing motor outcome and functional recovery following nerve injury. Meth Mol Biol 2014; 1162: 179–188. [DOI] [PubMed] [Google Scholar]
- 78.Van Meeteren NL, Eggers R, Lankhorst AJ, et al. Locomotor recovery after spinal cord contusion injury in rats is improved by spontaneous exercise. J Neurotrauma 2003; 20: 1029–1037. [DOI] [PubMed] [Google Scholar]
- 79.Bozkurt A, Deumens R, Scheffel J, et al. CatWalk gait analysis in assessment of functional recovery after sciatic nerve injury. J Neurosci Meth 2008; 173: 91–98. [DOI] [PubMed] [Google Scholar]
- 80.Vlamings R, Visser-Vandewalle V, Koopmans G, et al. High frequency stimulation of the subthalamic nucleus improves speed of locomotion but impairs forelimb movement in Parkinsonian rats. Neuroscience 2007; 148: 815–823. [DOI] [PubMed] [Google Scholar]
- 81.Zhou M, Zhang W, Chang J, et al. Gait analysis in three different 6-hydroxydopamine rat models of Parkinson's disease. Neurosci Lett 2014; 584C: 184–189. [DOI] [PubMed] [Google Scholar]
- 82.Encarnacion A, Horie N, Keren-Gill H, et al. Long-term behavioral assessment of function in an experimental model for ischemic stroke. J Neurosci Meth 2011; 196: 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Meth 1984; 11: 47–60. [DOI] [PubMed] [Google Scholar]
- 84.Rapp PR, Rosenberg RA, Gallagher M. An evaluation of spatial information processing in aged rats. Behav Neurosci 1987; 101: 3–12. [DOI] [PubMed] [Google Scholar]
- 85.Morris RG, Schenk F, Tweedie F, et al. Ibotenate lesions of hippocampus and/or subiculum: dissociating components of allocentric spatial learning. Eur J Neurosci 1990; 2: 1016–1028. [DOI] [PubMed] [Google Scholar]
- 86.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 2006; 1: 848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kooijman E, Nijboer CH, van Velthoven CT, et al. Long-term functional consequences and ongoing cerebral inflammation after subarachnoid hemorrhage in the rat. PLoS One 2014; 9: e90584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Silasi G, Colbourne F. Long-term assessment of motor and cognitive behaviours in the intraluminal perforation model of subarachnoid hemorrhage in rats. Behav Brain Res 2009; 198: 380–387. [DOI] [PubMed] [Google Scholar]
- 89.Milner E, Holtzman JC, Friess S, et al. Endovascular perforation subarachnoid hemorrhage fails to cause Morris water maze deficits in the mouse. J Cereb Blood Flow Metab 2014; 34: 1571–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Atangana EN, Homburg D, Vajkoczy P, et al. Mouse cerebral magnetic resonance imaging fails to visualize brain volume changes after experimental subarachnoid hemorrhage. Acta Neurochir (Wien) 2015; 157: 37–42. [DOI] [PubMed] [Google Scholar]
- 91.Liu Y, Qiu J, Wang Z, et al. Dimethylfumarate alleviates early brain injury and secondary cognitive deficits after experimental subarachnoid hemorrhage via activation of Keap1-Nrf2-ARE system. J Neurosurg 2015; 123: 915–923. [DOI] [PubMed] [Google Scholar]
- 92.Feng D, Wang W, Dong Y, et al. Ceftriaxone alleviates early brain injury after subarachnoid hemorrhage by increasing excitatory amino acid transporter 2 expression via the PI3K/Akt/NF-kappaB signaling pathway. Neuroscience 2014; 268: 21–32. [DOI] [PubMed] [Google Scholar]
- 93.Takata K, Sheng H, Borel CO, et al. Long-term cognitive dysfunction following experimental subarachnoid hemorrhage: new perspectives. Exp Neurol 2008; 213: 336–344. [DOI] [PubMed] [Google Scholar]
- 94.Takata K, Sheng H, Borel CO, et al. Simvastatin treatment duration and cognitive preservation in experimental subarachnoid hemorrhage. J Neurosurg Anesth 2009; 21: 326–333. [DOI] [PubMed] [Google Scholar]
- 95.Jeon H, Ai J, Sabri M, et al. Learning deficits after experimental subarachnoid hemorrhage in rats. Neuroscience 2010; 169: 1805–1814. [DOI] [PubMed] [Google Scholar]
- 96.Chen G, Li Q, Feng D, et al. Expression of NR2B in different brain regions and effect of NR2B antagonism on learning deficits after experimental subarachnoid hemorrhage. Neuroscience 2013; 231: 136–144. [DOI] [PubMed] [Google Scholar]
- 97.Sun X, Ji C, Hu T, Wang Z, et al. Tamoxifen as an effective neuroprotectant against early brain injury and learning deficits induced by subarachnoid hemorrhage: possible involvement of inflammatory signaling. J Neuroinflammation 2013; 10: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Z, Ji C, Wu L, et al. Tert-butylhydroquinone alleviates early brain injury and cognitive dysfunction after experimental subarachnoid hemorrhage: role of Keap1/Nrf2/ARE pathway. PLoS One 2014; 9: e97685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dong Y, Li Y, Feng D, et al. Protective effect of HIF-1alpha against hippocampal apoptosis and cognitive dysfunction in an experimental rat model of subarachnoid hemorrhage. Brain Res 2013; 1517: 114–121. [DOI] [PubMed] [Google Scholar]
- 100.Viana MB, Tomaz C, Graeff FG. The elevated T-maze: a new animal model of anxiety and memory. Pharmacol Biochem Behav 1994; 49: 549–554. [DOI] [PubMed] [Google Scholar]
- 101.Korte SM, De Boer SF. A robust animal model of state anxiety: fear-potentiated behaviour in the elevated plus-maze. Eur J Pharmacol 2003; 463: 163–175. [DOI] [PubMed] [Google Scholar]
- 102.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Meth 1985; 14: 149–167. [DOI] [PubMed] [Google Scholar]
- 103.Graeff FG, Netto CF, Zangrossi H., Jr The elevated T-maze as an experimental model of anxiety. Neurosci Biobehav Rev 1998; 23: 237–246. [DOI] [PubMed] [Google Scholar]
- 104.Zangrossi H, Jr, Graeff FG. Behavioral validation of the elevated T-maze, a new animal model of anxiety. Brain Res Bull 1997; 44: 1–5. [DOI] [PubMed] [Google Scholar]
- 105.Lackner P, Vahmjanin A, Hu Q, et al. Chronic hydrocephalus after experimental subarachnoid hemorrhage. PloS One 2013; 8: e69571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schallert T, Upchurch M, Lobaugh N, et al. Tactile extinction: distinguishing between sensorimotor and motor asymmetries in rats with unilateral nigrostriatal damage. Pharmacol Biochem Behav 1982; 16: 455–462. [DOI] [PubMed] [Google Scholar]
- 107.Chen SF, Hung TH, Chen CC, et al. Lovastatin improves histological and functional outcomes and reduces inflammation after experimental traumatic brain injury. Life sciences 2007; 81: 288–98. [DOI] [PubMed] [Google Scholar]
- 108.Esneault E, Pacary E, Eddi D, et al. Combined therapeutic strategy using erythropoietin and mesenchymal stem cells potentiates neurogenesis after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab 2008; 28: 1552–1563. [DOI] [PubMed] [Google Scholar]
- 109.Bouet V, Boulouard M, Toutain J, et al. The adhesive removal test: a sensitive method to assess sensorimotor deficits in mice. Nat Protoc 2009; 4: 1560–1564. [DOI] [PubMed] [Google Scholar]
- 110.Macdonald RL, Jaja B, Cusimano MD, et al. SAHIT Investigators – on the outcome of some subarachnoid hemorrhage clinical trials. Transl Stroke Res 2013; 4: 286–296. [DOI] [PubMed] [Google Scholar]
- 111.Stein DG. Embracing failure: WHAT the Phase III progesterone studies can teach about TBI clinical trials. Brain Inj 2015; 29: 1259–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Howard RB, Sayeed I, Stein D. Suboptimal dosing parameters as possible factors in the negative Phase III clinical trials of progesterone in TBI. J Neurotrauma. Epub ahead of print 15 September 2015. DOI: 10.1089/neu.2015.4179 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Poon W, Vos P, Muresanu D, et al. Cerebrolysin Asian Pacific trial in acute brain injury and neurorecovery: design and methods. J Neurotrauma 2015; 32: 571–580. [DOI] [PubMed] [Google Scholar]
- 114.Maas AI, Menon DK, Lingsma HF, et al. Re-orientation of clinical research in traumatic brain injury: report of an international workshop on comparative effectiveness research. J Neurotrauma 2012; 29: 32–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wikstrom J, Georgoulas G, Moutsopoulos T, Seferiadis A. Intelligent data analysis of instrumented gait data in stroke patients-a systematic review. Comput Biol Med 2014; 51: 61–72. [DOI] [PubMed] [Google Scholar]
- 116. Olton DS and Samuelson RJ. Remembrance of places passed: Spatial memory in rats. J Exp Psychol Anim B 1976; 2: 97–116.
- 117.Bae JH, Kang SH, Seo KM, et al. Relationship between grip and pinch strength and activities of daily living in stroke patients. Ann Rehabil Med 2015; 39: 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ahlander AC, Persson M, Emanuelson I. Fifteen-year follow-up of upper limb function in children with moderate to severe traumatic brain injury. J Rehabil Med 2013; 45: 815–819. [DOI] [PubMed] [Google Scholar]
- 119.Turan N, Heider RA, Zaharieva D, et al. Sex differences in the formation of intracranial aneurysms and incidence and outcome of subarachnoid hemorrhage: review of experimental and human studies. Transl Stroke Res 2016; 7: 12–19. [DOI] [PubMed] [Google Scholar]
- 120.Friedrich V, Bederson JB, Sehba FA. Gender influences the initial impact of subarachnoid hemorrhage: an experimental investigation. PLoS One 2013; 8: e80101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Friedrich V, Bi W, Sehba FA. Sexual dimorphism in gene expression after aneurysmal subarachnoid hemorrhage. Neurol Res 2015; 37: 1054–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Macdonald RL, Higashida RT, Keller E, et al. Randomized trial of clazosentan in patients with aneurysmal subarachnoid hemorrhage undergoing endovascular coiling. Stroke 2012; 43: 1463–1469. [DOI] [PubMed] [Google Scholar]
- 123.Macdonald RL, Higashida RT, Keller E, et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol 2011; 10: 618–625. [DOI] [PubMed] [Google Scholar]
- 124.Young AM, Karri SK, Helmy A, et al. Pharmacologic Management of Subarachnoid Hemorrhage. World Neurosurg 2015; 84: 28–35. [DOI] [PubMed] [Google Scholar]
- 125.Parra A, McGirt MJ, Sheng H, et al. Mouse model of subarachnoid hemorrhage associated cerebral vasospasm: methodological analysis. Neurol Res 2002; 24: 510–516. [DOI] [PubMed] [Google Scholar]
- 126.McGirt MJ, Parra A, Sheng H, et al. Attenuation of cerebral vasospasm after subarachnoid hemorrhage in mice overexpressing extracellular superoxide dismutase. Stroke 2002; 33: 2317–23. [DOI] [PubMed] [Google Scholar]
- 127.Feiler S, Friedrich B, Scholler K, Thal SC, Plesnila N. Standardized induction of subarachnoid hemorrhage in mice by intracranial pressure monitoring. J Neurosci Meth 2010; 190: 164–170. [DOI] [PubMed] [Google Scholar]
- 128.Tait MJ, Saadoun S, Bell BA, Verkman AS, Papadopoulos MC. Increased brain edema in aqp4-null mice in an experimental model of subarachnoid hemorrhage. Neuroscience 2010; 167: 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Han BH, Vellimana AK, Zhou ML, et al. Phosphodiesterase 5 inhibition attenuates cerebral vasospasm and improves functional recovery after experimental subarachnoid hemorrhage. Neurosurgery 2012; 70: 178–86. discussion 86–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Terpolilli NA, Feiler S, Dienel A, et al. Nitric oxide inhalation reduces brain damage, prevents mortality, and improves neurological outcome after subarachnoid hemorrhage by resolving early pial microvasospasms. J Cereb Blood Flow Metab. Epub ahead of print 2 November 2015. DOI: 10.1177/0271678X15605848 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Egashira Y, Shishido H, Hua Y, Keep RF, Xi G. New grading system based on magnetic resonance imaging in a mouse model of subarachnoid hemorrhage. Stroke 2015; 46: 582–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mutoh T, Sasaki K, Nakamura K, et al. Value of three-dimensional maximum intensity projection display to assist in magnetic resonance imaging (MRI)-based grading in a mouse model of subarachnoid hemorrhage. Med Sci Monit 2016; 22: 2050–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fujimoto M, Shiba M, Kawakita F, et al. Deficiency of tenascin-C and attenuation of blood-brain barrier disruption following experimental subarachnoid hemorrhage in mice. J Neurosurg 2016; 124: 1693–1702. [DOI] [PubMed] [Google Scholar]
- 134.Vatter H, Weidauer S, Konczalla J, et al. Time course in the development of cerebral vasospasm after experimental subarachnoid hemorrhage: clinical and neuroradiological assessment of the rat double hemorrhage model. Neurosurgery 2006; 58: 1190–1197. discussion 1190–1197. [DOI] [PubMed] [Google Scholar]
- 135.Yatsushige H, Calvert JW, Cahill J, et al. Limited role of inducible nitric oxide synthase in blood-brain barrier function after experimental subarachnoid hemorrhage. J Neurotrauma 2006; 23: 1874–1882. [DOI] [PubMed] [Google Scholar]
- 136.Thal SC, Sporer S, Klopotowski M, et al. Brain edema formation and neurological impairment after subarachnoid hemorrhage in rats. Laboratory investigation. J Neurosurg 2009; 111: 988–994. [DOI] [PubMed] [Google Scholar]
- 137.Ayer R, Chen W, Sugawara T, et al. Role of gap junctions in early brain injury following subarachnoid hemorrhage. Brain Res 2010; 1315: 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Duris K, Manaenko A, Suzuki H, et al. alpha7 nicotinic acetylcholine receptor agonist PNU-282987 attenuates early brain injury in a perforation model of subarachnoid hemorrhage in rats. Stroke 2011; 42: 3530–3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu CT, Wen LL, Wong CS, et al. Temporal changes in glutamate, glutamate transporters, basilar arteries wall thickness, and neuronal variability in an experimental rat model of subarachnoid hemorrhage. Anesth Analg 2011; 112: 666–673. [DOI] [PubMed] [Google Scholar]
- 140.Khalili MA, Anvari M, Hekmati-Moghadam SH, et al. Therapeutic benefit of intravenous transplantation of mesenchymal stem cells after experimental subarachnoid hemorrhage in rats. J Stroke Cerebrovasc Dis 2012; 21: 445–451. [DOI] [PubMed] [Google Scholar]
- 141.Fujii M, Duris K, Altay O, et al. Inhibition of Rho kinase by hydroxyfasudil attenuates brain edema after subarachnoid hemorrhage in rats. Neurochem Int 2012; 60: 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hockel K, Scholler K, Trabold R, et al. Vasopressin V(1a) receptors mediate posthemorrhagic systemic hypertension thereby determining rebleeding rate and outcome after experimental subarachnoid hemorrhage. Stroke 2012; 43: 227–232. [DOI] [PubMed] [Google Scholar]
- 143.Chen S, Ma Q, Krafft PR, et al. P2X7R/cryopyrin inflammasome axis inhibition reduces neuroinflammation after SAH. Neurobiol Dis 2013; 58: 296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hong Y, Shao A, Wang J, et al. Neuroprotective effect of hydrogen-rich saline against neurologic damage and apoptosis in early brain injury following subarachnoid hemorrhage: possible role of the Akt/GSK3beta signaling pathway. PLoS One 2014; 9: e96212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhao D, Liu Q, Ji Y, et al. Effect of 18beta-glycyrrhetinic acid on cerebral vasospasm caused by asymmetric dimethylarginine after experimental subarachnoid hemorrhage in rats. Neurol Res 2015; 37: 476–483. [DOI] [PubMed] [Google Scholar]
- 146.Chen Y, Luo C, Zhao M, et al. Administration of a PTEN inhibitor BPV(pic) attenuates early brain injury via modulating AMPA receptor subunits after subarachnoid hemorrhage in rats. Neurosci Lett 2015; 588: 131–136. [DOI] [PubMed] [Google Scholar]
- 147.He Y, Xu L, Li B, et al. Macrophage-inducible c-type lectin/spleen tyrosine kinase signaling pathway contributes to neuroinflammation after subarachnoid hemorrhage in rats. Stroke 2015; 46: 2277–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.He J, Liu M, Liu Z, et al. Recombinant osteopontin attenuates experimental cerebral vasospasm following subarachnoid hemorrhage in rats through an anti-apoptotic mechanism. Brain Res 2015; 1611: 74–83. [DOI] [PubMed] [Google Scholar]
- 149.Zuo S, Li W, Li Q, et al. Protective effects of Ephedra sinica extract on blood-brain barrier integrity and neurological function correlate with complement C3 reduction after subarachnoid hemorrhage in rats. Neurosci Lett 2015; 609: 216–222. [DOI] [PubMed] [Google Scholar]
- 150.Zhou C, Xie G, Wang C, et al. Decreased progranulin levels in patients and rats with subarachnoid hemorrhage: a potential role in inhibiting inflammation by suppressing neutrophil recruitment. J Neuroinflammation 2015; 12: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang T, Su J, Guo B, et al. Apigenin protects blood-brain barrier and ameliorates early brain injury by inhibiting TLR4-mediated inflammatory pathway in subarachnoid hemorrhage rats. Int Immunopharmacol 2015; 28: 79–87. [DOI] [PubMed] [Google Scholar]
- 152.Tian Y, Guo SX, Li JR, et al. Topiramate attenuates early brain injury following subarachnoid haemorrhage in rats via duplex protection against inflammation and neuronal cell death. Brain Res 2015; 1622: 174–185. [DOI] [PubMed] [Google Scholar]
- 153.Teng Z, Jiang L, Hu Q, et al. Peroxisome proliferator-activated receptor beta/delta alleviates early brain injury after subarachnoid hemorrhage in rats. Stroke 2016; 47: 196–205. [DOI] [PubMed] [Google Scholar]
- 154.Yin C, Huang GF, Sun XC, et al. Tozasertib attenuates neuronal apoptosis via DLK/JIP3/MA2K7/JNK pathway in early brain injury after SAH in rats. Neuropharmacology 2016; 108: 316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.