Abstract
Silk patterns in a film of amorphous water-soluble fibroin are created by tailored exposure to femtosecond-laser pulses (1030 nm/230 fs) without the use of photo-initiators. This shows that amorphous silk can be used as a negative tone photo-resist. It is also shown that water insoluble crystalline silk films can be precisely ablated from a glass substrate achieving the patterns of crystalline silk gratings on a glass substrate. Bio-compatible/degradable silk can be laser structured to achieve conformational transformations as demonstrated by infrared spectroscopy.
I. INTRODUCTION
There is a growing interest in a 3D material processing by direct writing via tailored light exposure conditions in transparent resists, plastic, ceramic, and glassy materials, rather than using doping additives to facilitate structural modifications guided by the locus of the scanned laser beam. Ultra-short subpicosecond laser pulses are well suited for a high precision energy delivery by the direct or non-linear absorption and creation of optimized thermal conditions for required structural change.1,2 3D scaffolds for bio-medical applications made out of a polymeric matrix using photo-polymerization are one clear example where toxic photo-initiators with aromatic-ring additives, typically used to enhance absorption, have to be abolished. Without photo-initiators, spectral requirements for the resonant excitation wavelength are relaxed and energy delivery is tailored via intensity controlled avalanche absorption as it was demonstrated in the case of 3D writing in pure silicone3 and SZ2080 resist.4
Currently, silk fibroin arouses a lot of interest as a material for bio-scaffolding because of its bio-compatible and bio-degradable properties.5,6 The ability to form films and scaffolds also makes it a promising substrate for microfluidic devices.7 It was demonstrated that a water-based silk fibroin solution can be used as a positive tone resist in electron beam lithography (EBL).8,9 Furthermore, optical properties of silk fibroin can be modified by high MeV energy electron exposure and crystallization.8,10 A spin-coated film of silk fibroin was first crystallized by methanol-ethanol treatment and then electron beam exposure caused unzipping of the β-sheet network, typical for the secondary structure of crystallized silk, rendering the exposed regions water-soluble.9 Recently, it was demonstrated by using the on-chip calorimetry that amorphous water-soluble phase of silk can be recovered after fast 2000 K/s thermal quenching of molten silk.11 However, rapid thermal quenching is hampered by a markedly low temperature diffusivity of silk αT ≈ 1.5 × 10−7 m2/s.9 It was shown that direct laser ablation of thick silk films by using direct absorption of UV photons gave rise to 3D foams with large surface area, whereas ultra-short femtosecond laser pulses at near-IR wavelength enabled the high precision laser cutting of silk.12
Through the use of photo-initiators, silk can be crystallized by direct fs-laser writing.13 The application of ultra-short laser pulses for control of the crystallinity of silk without photo-initiator was the aim of this study. Earlier attempts to polymerize silk on glass using high 82 MHz repetition rate fs-laser pulses were not successful9 and only on a nanotextured black Si14 surface coated by gold a water insoluble silk island was found after prolonged exposure. Alternatively, very high electron exposure doses or MeV energies have to be used to induce crystallization.8,10
Here, printing of a spin-coated silk fibroin film using femtosecond laser exposure is demonstrated at the onset of glass surface ablation. This shows a realization of a silk negative tone resist. Crystallized silk films were laser ablated to fabricate similar grating patterns.
II. SAMPLES AND PROCEDURES
Silk fibroin was extracted from Bombyx mori cocoons according to a previously described method.9 Aqueous 10% silk solutions were used to make thin films on the surface of glass slides and CaF2 windows. Fibroin solutions were spin-coated at a speed of 3000 rpm for 40 s preceded by a low-speed spreading step for 10 s at 500 rpm. Subsequently, silk layers were dried at 90 °C for 1 min. The resultant silk film thickness was 250 ± 20 nm. The roughness of amorphous silk films was about 20 nm according to the atomic force microscopy (AFM) measurements. Thin films of an amorphous water-soluble silk fibroin were used for further laser-induce crystallization experiments. Preparation of water-insoluble crystallized silk fibroin layers amorphous silk films was carried out by soaking amorphous silk films in a methanol:ethanol (1:1 v/v) mixture for 10 min followed by water vapor annealing at 80 °C for 2 h.
Fibroin harvested from Antheraea pernyi living in the wild is structurally different from that of domesticated B. mori and was also used for laser crystallization. Solubility of fibroin from A. pernyi in water was slightly lower, however, spin coating and film thickness were very similar to B. mori silk. The presence of the tripeptide sequence Arg-Gly-Asp is a signature of A. pernyi fibroin and is in turn responsible for a special set of interactions with mammalian cells which leads to the promotion of cell adhesion.15 Crystallinity and β-sheet content in A. pernyi fibroin are lower as compared to B. mori.16 This makes the mechanical strength of A. pernyi fiber lower than B. mori fibers, however, has superior elasticity and toughness.17,18
Laser writing was carried out using λ = 1030 nm wavelength, tp = 230 fs duration pulses (Pharos, Light Conversion) operating at a frequency of 100 kHz. Focusing was carried out by an objective lens with a numerical aperture NA = 0.26 (Mitutoyo) and 0.5 as indicated where applies. Damage threshold of the cover glass (sample had a 250 nm film of silk on top) was Eth = 15.7 nJ/pulse for the 100 pulses per 1 μm writing speed for NA = 0.26. The irradiance/intensity was Ith = Eth/(πω02tp) ≈ 0.37 TW/cm2 corresponding to a fluence of Fth = Eth/(πω02) ≈ 86 mJ/cm2, where the beam waist was determined as ω0 = 0.61λ/NA, with 20 pulses overlapping. These values are well below single pulse damage threshold of a glass. It was confirmed by AFM that at a pulse energy of 0.95Eth only a trace of silk polymerization/crystallization occurred. At a slightly higher pulse energy Eth = 20 nJ/pulse glass ablation occurred as can be recognized by ripple formation, however, at those conditions, silk printing was also enhanced and was used for experiments. Polarization of the laser beam E was perpendicular (and parallel) to the scanning direction, which corresponded to reduced (enhanced) electronic thermal conductivity.19 However, at the employed low-NA focusing and repetition rate <0.2 MHz, anisotropy in heat flow was negligible and there was no measurable difference in the width of silk patterned silk lines.
Fourier transform infrared (FTIR) spectroscopy measurements were performed on a Vertex70 (Bruker) IR spectrometer in a microscope mode. Spectra were recorded from 500 × 500 μm silk patterns of closely packed lines on CaF2 windows in a transmission mode. Spectral range was 4000–900 cm−1 with a spectral resolution of 2 cm−1. The AFM investigations were performed on a Bruker Dimension iCon instrument in a contact mode. Surface mapping was done on a Bruker Contour Elite 3D optical microscope.
III. RESULTS AND DISCUSSION
The films of amorphous silk were irradiated by a fs-laser beam. After the laser exposure at the pulse density of 10 or 100 pulses/μm, the grating structures were developed in water. During the development, the untreated water-soluble silk fibroin was removed, while the laser-exposed silk remained forming 3-μm-wide at full width half maximum (FWHM) and ∼20-nm-high lines (Fig. 1(a)).
FIG. 1.
Optical profilometer surface mapping of an amorphous silk fibroin film after the laser exposure of 10 μm period grating pattern followed by the water development: top (a) and cross-sectional (b) views. Laser exposure conditions: pulse energy Ep = 20 nJ, overlap of N = 105 pulses/mm at repetition rate of 100 kHz, NA = 0.26. Inset in (b) shows a typical AFM 3D surface profile; E is orientation of the linear polarization.
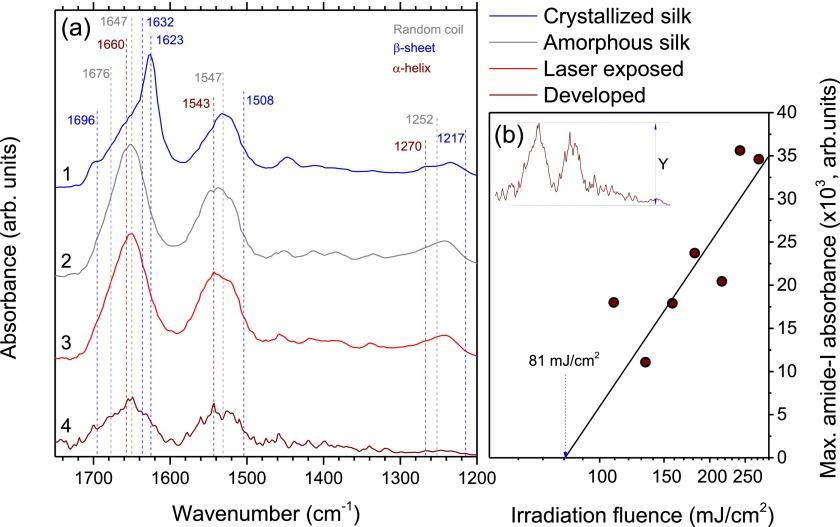
The structure of the protein can be revealed through the amide-I, amide-II, and amide-III bands at the FTIR spectra and its second derivative (not shown) to better resolve overlapping peaks (Fig. 2(a)). In general, in polypeptides, the amide-I band (1600–1700 cm−1) is mainly due to stretching vibrations in C=O and, to a lesser extent, of C-N groups. The more complex amide-II band (1510–1580 cm−1) is given rise by N-H in-plane bending as well as stretching vibrations of C-N and C-C. Finally, the amide-III band (1200–1280 cm−1) is governed by complex interactions with side chains and hydrogen bonding, hence, is difficult to relate to the secondary structure of a protein.
FIG. 2.
(a) FTIR absorbance spectra of silk fibroin films: (1) crystallized after methanol-ethanol treatment, (2) amorphous water-soluble, (3) after laser irradiation, and (4) after laser irradiation and development in water. Color of the wavenumber values that mark spectral features denote which secondary structure element they are associated with. (b) Dependence of the intensity of amide-I band after laser irradiation and water development from the irradiation fluence. Laser exposure conditions: base irradiation fluence Fth = 81 mJ/cm2, pulse density 100 pulses/μm (20 pulses overlapping) at a repetition rate of 100 kHz, using a NA = 0.5 lens. FTIR spectra recorded from 500 × 500 μm silk patterns on a CaF2 substrate on a Vertex70 Bruker FTIR microscope in the transmission mode.
In the spectrum of amorphous water-soluble regenerated silk fibroin, a strong peaks centered at 1647, 1547, and 1252 cm−1 are present in the aforementioned bands, which correspond to the random coil conformation of the protein.20 The discrete shoulder in the vicinity of 1676 cm−1 is associated with the bends and turns of the polypeptide chain.21,22 Laser irradiation of the amorphous silk fibroin diminishes the intensity of amide bands and at the same time induces spectral alternations at 1660 and 1543 cm−1 wavenumbers, associated with the α-helix conformation.23 This is consistent with observation of changes in amorphous silk at high electron beam doses, where water radiolysis process gives rise to amorphous-to-helix folding and cross-linking of silk fibroin, resulting in insolubility in water.8 After the development of a line pattern in water, the overall intensity of the amide bands decreases by roughly a factor of ∼4 with higher intensities observed after the irradiation at higher laser fluences (Fig. 2(b)). From a spectral point of view, the main decrease in intensity is at the expense of random coil associated signatures while β-sheets-related contributions being more resilient, hence giving amide bands their broadened appearance. Furthermore, additional sharp lines appeared that could be tied to various products of laser-induced bond breaking or residual water released due to the hydrophobicity of the α-helices-enriched silk. The most significant lines are at related to α-helix 1660, and 1543 cm−1 bands, however, their uncharacteristically small width prevents definite assignment. In contrast to results observed for the exposure pattern of parallel lines, in the case of raster-scanned area pattern, the intensity of amide spectral bands rapidly diminishes with increased fluence. This observation indicates that conformational transitions of silk fibroin resulting in its insolubility in water occur because of the effects arising at the periphery of the laser beam.
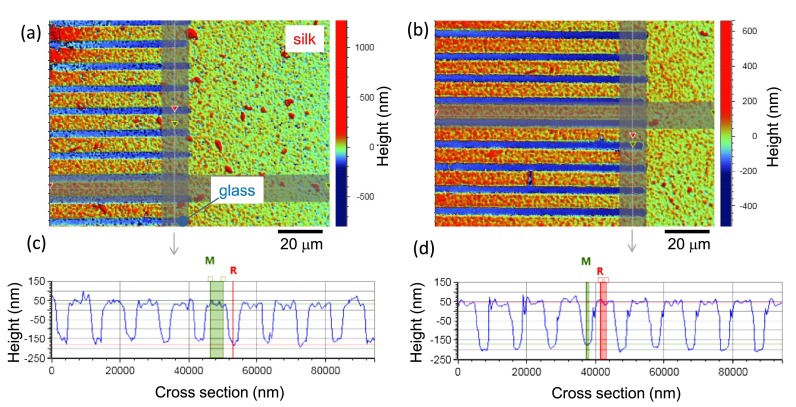
A typical AFM image of a laser printed crystalline silk after development in water is shown in Fig. 3. Formation of ripple patterns on glass was also recognizable (see the inset in Fig. 1(b)). Surface bulging and ablation of a borosilicate glass (cover glass) under high repetition rate irradiation by fs-laser pulses takes place via interplay of strongly localized energy absorption, low heat conductance, ablation, and surface tension effects even at low pulse energy as used in this study.24 Here, it was found that such structural modification of glass surface was required to obtain water insoluble crystalline silk. Since silk conformational transformation has a chemical origin, e.g., radiolysis of water and free radicals generation, establishing of cross-links, and a thermal activation has only secondary effect, a possible explanation can be found in the onset of ablation at which silk printing occurred. Ablation starts via strong ionization and electron ejection from the surface. This obviously favored silk crystallization since thermal annealing alone was not effective. This conjecture is additionally supported by experimental observation of only 20-nm-thick crystalline silk pattern out of the initial ∼250-nm amorphous film as well as by the stronger spectral signature of parallel lines exposed patterns comparing to raster-scanned areas.
FIG. 3.
AFM image of a laser crystallized silk and a cross sectional height profile. Laser exposure conditions: pulse energy Ep = 20 nJ, pulse density 100 pulses/μm (20 pulses overlapping) at a repetition rate of 100 kHz, NA = 0.26 lens. Conditions are for the onset of glass ablation.
Crystallized β-sheets-enriched water-insoluble silk fibroin films obtained through the methanol-ethanol treatment followed by the water vapor annealing were exposed to the fs-laser operating at 100 kHz repetition rate. Laser patterning results in a precise removal and stripping silk from substrate (Fig. 4). Following water development practically did not change the depth of the lines. The spectra do not change after the laser irradiation either. The direct ablation of silk has no damage to the protein structure of the remaining film. Very similar results on laser crystallization and ablation of the crystalline film were observed for A. pernyi fibroin.
FIG. 4.
Topology of the crystalline silk film laser ablated (a) and subsequently water washed (b). Laser exposure conditions: pulse energy Ep = 22 nJ, pulse density 100 pulses/μm (20 pulses overlapping) at a repetition rate of 100 kHz, NA = 0.26 lens.
Inherent constraint in laser printing or ablation of thin <λ/4 films is due to the light intensity maximum being at λ/4 distance above the surface (for light incident from the air onto sample). This limits efficiency of light energy delivery by linear or nonlinear absorption to the interface region, silk in this case. Using a polarization grating, interference effects, non-paraxiality and a longitudinal E-field component, or back-side illumination, it is possible to bring a higher light intensity to the interface.25,26 Follow-up experiments are planned to explore high irradiance delivery exactly at the interface between the glass substrate and silk.
IV. CONCLUSIONS
Laser printing of micrometer-wide of crystalline silk is demonstrated using fs-laser exposure of pure amorphous fibroin films of ∼250 nm in thickness. This is realization of a negative tone photo-resist without use of any photo-initiator. Very similar patterns can be created by fs-laser ablation of crystallized water-insoluble silk films. It is shown that laser exposure induces the local conformational transformation from random coils to α-helices of silk-I with a fraction of β-sheets. These changes lead to the modification of solubility in water. It opens the possibility for the laser printing of protein-based water-insoluble structures starting from regenerated silk fibroin. Laser writing/printing of silk patterns for functionalization of sensor regions with metal nanoparticles inside micro-sensor chips is expected to open a range of new capabilities in bio-medical and micro-fluidic fields.
ACKNOWLEDGMENTS
K.M. was supported by the Australian Research Council's Endeavour Research Fellowship grant. S.J. is grateful for partial support via the Australian Research Council DP130101205 Discovery project and to WoP—Workshop of Photonics, Ltd. for a technology transfer project. This work was part of Melbourne synchrotron beamtime proposal 10457 in 2016 and the nanotechnology ambassador fellowship program at the Melbourne Centre for Nanofabrication (MCN) in the Victorian Node of the Australian National Fabrication Facility (ANFF).
References
- 1. Malinauskas M., Žukauskas A., Hasegawa S., Hayasaki Y., Mizeikis V., Buividas R., and Juodkazis S., Light: Sci. Appl. , e16133 (2016). 10.1038/lsa.2016.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Malinauskas M., Farsari M., Piskarskas A., and Juodkazis S., Phys. Rep. , 1 (2013). 10.1016/j.physrep.2013.07.005 [DOI] [Google Scholar]
- 3. Rekštytė S., Malinauskas M., and Juodkazis S., Opt. Express , 17028 (2013). 10.1364/OE.21.017028 [DOI] [PubMed] [Google Scholar]
- 4. Malinauskas M., Žukauskas A., Bičkauskaitė G., Gadonas R., and Juodkazis S., Opt. Express , 10209 (2010). 10.1364/OE.18.010209 [DOI] [PubMed] [Google Scholar]
- 5. Hu Y., Zhang Q., You R., Wang L., and Li M., Adv. Mat. Sci. Eng. , 185905 (2012). 10.1155/2012/185905 [DOI] [Google Scholar]
- 6. Li G., Li Y., Chen G., He J., Han Y., Wang X., and Kaplan D. L., Adv. Healthcare. Mater. , 1134 (2015). 10.1002/adhm.201500002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tsioris K., Tilburey G. E., Murphy A. R., Domachuk P., Kaplan D. L., and Omenetto F. G., Adv. Funct. Mater. , 1083 (2010). 10.1002/adfm.200902050 [DOI] [Google Scholar]
- 8. Kim S., Marelli B., Brenckle M. A., Mitropoulos A. N., Gil E.-S., Tsioris K., Tao H., Kaplan D. L., and Omenetto F. G., Nat. Nanotechnol. , 306 (2014). 10.1038/nnano.2014.47 [DOI] [PubMed] [Google Scholar]
- 9. Morikawa J., Ryu M., Maximova K., Balcytis A., Senuitinas G., Fan L., Mizeikis V., Li J., Wang X., Zamengo M., Wang X., and Juodkazis S., RSC Adv. , 11863 (2016). 10.1039/C5RA20201A [DOI] [Google Scholar]
- 10. Asha S., Sangappa Y., and Ganesh S., J. Spectrosc. , 879296 (2015). 10.1155/2015/879296 [DOI] [Google Scholar]
- 11. Cebe P., Hu X., Kaplan D. L., Zhuravlev E., Wurm A., Arbeiter D., and Schick C., Sci. Rep. , 1130 (2013). 10.1038/srep01130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lazare S., Sionkowska A., Zaborowicz M., Planecka A., Lopez J., Dijoux M., Louména C., and Hernandez M. C., Appl. Phys. A , 67 (2012). 10.1007/s00339-011-6639-y [DOI] [Google Scholar]
- 13. Sun Y.-L., Li Q., Sun S.-M., Huang J.-C., Zheng B.-Y., Chen Q.-D., Shao Z.-Z., and Sun H.-B., Nat. Commun. , 8612 (2015). 10.1038/ncomms9612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Žukauskas A., Malinauskas M., Kadys A., Gervinskas G., Seniutinas G., Kandasamy S., and Juodkazis S., Opt. Express , 6901 (2013). 10.1364/OE.21.006901 [DOI] [PubMed] [Google Scholar]
- 15. Yan S., Zhao C., Wu X., Zhang Q., and Li M., Sci. China Chem. , 535 (2010). 10.1007/s11426-010-0093-0 [DOI] [Google Scholar]
- 16. Ling S., Qi Z., Knight D. P., Huang Y., Huang L., Zhou H., Shao Z., and Chen X., Biomacromolecules , 1885 (2013). 10.1021/bm400267m [DOI] [PubMed] [Google Scholar]
- 17. Du S., Li J., Zhang J., and Wang X., Mater. Des. , 766 (2015). 10.1016/j.matdes.2014.09.066 [DOI] [Google Scholar]
- 18. Tao H., Kaplan D. L., and Omenetto F. G., Adv. Mater. , 2824 (2012). 10.1002/adma.201104477 [DOI] [PubMed] [Google Scholar]
- 19. Rekštytė S., Gailevičius T. J. D., Malinauskas M., Mizeikis V., Gamaly E. G., and Juodkazis S., Adv. Opt. Mater. , 1209 (2016). 10.1002/adom.201600155 [DOI] [Google Scholar]
- 20. Boulet-Audet M., Vollrath F., and Holland C., J. Exp. Biol. , 3138 (2015). 10.1242/jeb.128306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu X., Shmelev K., Sun L., Gil E.-S., Park S.-H., Cebe P., and Kaplan D. L., Biomacromolecules , 1686 (2011). 10.1021/bm200062a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Handbook of Fiber Chemistry, edited Lewin M. ( Taylor & Francis Group, Boca Raton, FL, 2007). [Google Scholar]
- 23. Teramoto H. and Miyazawa M., Biomacromolecules , 2049 (2005). 10.1021/bm0500547 [DOI] [PubMed] [Google Scholar]
- 24. Vanagas E., Kudryashov I., Tuzhilin D., Juodkazis S., Matsuo S., and Misawa H., Appl. Phys. Lett. , 2901 (2003). 10.1063/1.1570514 [DOI] [Google Scholar]
- 25. Iwase H., Kokubo S., Juodkazis S., and Misawa H., Opt. Express , 4388 (2009). 10.1364/OE.17.004388 [DOI] [PubMed] [Google Scholar]
- 26. Jayawardhana S., Rosa L., Juodkazis S., and Stoddart P. R., Sci. Rep. , 2335 (2013). 10.1038/srep02335 [DOI] [PMC free article] [PubMed] [Google Scholar]