Abstract
Brain imaging has revealed alterations in dopamine uptake, release and receptor levels in patients with schizophrenia that have been resolved at the scale of striatal subregions. However, the underlying synaptic mechanisms are at a finer scale. Dopamine neuron synaptic actions vary across the striatum, involving not only variations in dopamine release, but also in dopamine neuron connectivity, cotransmission, modulation and activity. Optogenetic studies have revealed that dopamine neurons release dopamine in a synaptic signal mode, and that the neurons also release glutamate and GABA as cotransmitters, with striking regional variation. Fast glutamate and GABA cotransmission convey discrete patterns of dopamine neuron activity to striatal neurons. Glutamate may function not only in a signaling role at a subset of dopamine neuron synapses, but also in mediating vesicular synergy, contributing to regional differences in loading of dopamine into synaptic vesicles. Regional differences in dopamine neuron signaling are likely to be differentially involved in the schizophrenia disease process, and likely determine the subregional specificity of the action of psychostimulants that exacerbate the disorder, and antipsychotics that ameliorate the disorder. Elucidating dopamine neuron synaptic signaling offers the potential for achieving greater pharmacological specificity through intersectional pharmacological actions targeting subsets of dopamine neuron synapses.
Keywords: glutamate, GABA, corelease, cotransmission, vesicular synergy, optogenetics, nucleus accumbens
Dopamine (DA) dysfunction is central to the pathophysiology of schizophrenia (1), with regional variations that likely shape schizophrenia symptoms. This idea arose with the early view that mesolimbic DA signaling mediated positive (psychotic) symptoms that could be treated with DA D2 receptor (D2R) antagonists, while blockade of nigrostriatal DA transmission accounted for extrapyramidal side effects, and possible worsening of negative (deficit) symptoms. Observations made in rats (2), and extended to non-human primates (3), showed that propsychotic psychostimulants, such as amphetamine, elicit the greatest DA release in the limbic or ventral striatum (vStr). With the impetus of adding serotonin 2A receptor blockade to antipsychotics, atypical antipsychotics emerged (4); they appeared to have fewer side effects due to more selective targeting of the vStr (5). However, vStr selectivity was challenged by more recent observations that DA release is increased in schizophrenia in the associative dorsal Str (dStr) where it correlates with positive symptoms, and diminished in the vStr where it correlates with negative symptoms (6, 7). These findings — although apparently contradictory — point to heterogeneity in dopamine function across the Str, and the importance of understanding regional differences in DA neuron synaptic actions. Mechanisms underlying regional differences in clinical imaging of DA release in schizophrenia can be investigated in rodents under similar baseline and pharmacological challenge conditions.
DA neurons, identified by the presence of the DA synthetic enzyme tyrosine hydroxylase (8), show significant functional heterogeneity. This heterogeneity is evident in differences in gene expression (9–11), electrophysiological properties (11, 12), projection-specific functions (13, 14), drug sensitivity (15), and vulnerability in neurodegenerative disorders (16, 17). Such heterogeneity is likely to be fundamental to understanding differential vulnerability in schizophrenia, as well as therapeutics. While electrochemical techniques have provided considerable insight into DA release and its variation (18), the synaptic actions of DA neurons have been harder to discern. Optogenetics has enabled selective targeting of DA neurons (19) to enable functional connectivity analyses (20, 21) that have now made the synaptic actions of these neurons accessible to study. In this review, we describe multiple dimensions of heterogeneity in DA neuron synaptic signaling in the Str and functional implications.
Striatal cytoarchitecture
The invariant Str cytoarchitecture — with about 95% GABAergic spiny projection neurons (SPNs), 5% GABAergic and cholinergic interneurons (ChIs), and prominent input from midbrain DA neurons and cortical and thalamic glutamatergic neurons (22–24) — engendered the early idea that the function of striatal circuits was homogenous across striatal regions. However, this view has been increasingly supplanted by findings of striatal heterogeneity.
Different striatal regions receive distinctly different excitatory inputs defining broad divisions into associative, sensorimotor and limbic domains (25, 26). The pattern of cortical inputs helps to define the correspondence between striatal regions in humans and rodents (Figure 1). The sensorimotor Str receives inputs from primary motor and premotor cortices and comprises in the primate the dorsolateral putamen and dorsolateral caudate (25, 27, 28), which corresponds to the lateral portion of the dorsal Str in rodents (26, 27, 29). The associative Str receives inputs from association areas of the cortex (dorsolateral prefrontal cortex) and comprises in the primate large parts of the rostral putamen and most of the head, body and tail of the caudate (25, 27, 28), and corresponds to the medial portion of the dorsal Str in rodents (27, 30, 31). The vStr receives inputs from the hippocampus and amygdala, and from orbitofrontal and anterior cingulate cortices and comprises in the primate the nucleus accumbens (NAc) and ventral parts of the caudate and putamen (25, 27, 28, 32). In rodents, the vStr corresponds to the NAc and the striatal component of the olfactory tubercle (OT) (27, 30, 33). While the rodent NAc is subdivided into core and shell regions with different connectivity and function (34, 35), this division is not so clear in primates, although diffusion tractography identifies putative core (lateral-rostral NAc) and shell (medial-caudal NAc) divisions (36).
Fig 1. Functional subdivisions of the striatum in humans and rodents.
Functionally, the striatum (Str) can be divided into corresponding limbic (magenta), associative (green) and sensorimotor (blue) regions, in both human (left) and rodent (right), determined by cortical inputs mediating each function. The schematics shown are midway along the anterior-posterior axis; there are substantial phylogenetic differences both anteriorly and posteriorly (25, 73). The NAc, which makes up the vStr is indicated by the dashed lines. In mouse, a second dashed line indicates the border between the accumbens core and shell. Orientation of sections is indicated by arrows. Abbreviations; Cd: caudate, Pu: putamen, NAc: nucleus accumbens, dStr: dorsal striatum, OT: olfactory tubercle. Striatal outlines are modified from atlases (135, 136).
DA neurons project from the ventral midbrain to the Str topographically (Figure 2). Medial DA neurons in the ventral tegmental area (VTA) project predominantly to vStr. More lateral DA neurons in the substantia nigra (SN) project predominantly to associative and sensorimotor dStr domains (33, 37–39). Individual DA neurons target compact striatal domains that may subtend as much as 5% of the total striatal volume (40). The density of DAergic input to the striatal subregions is highest in the dStr and lowest in the NAc shell region of the vStr (41). Str SPNs project back to the ventral midbrain in a matching topology, predominantly targeting GABAergic neurons (21, 42), but also making connections to DA neurons (43, 44), with the exception of DA neurons projecting to the posterior Str that receive a wider range of inputs, from the globus pallidus, subthalamic nucleus and zona incerta (45).
Fig 2. Topography of midbrain dopamine neuron projections to the striatum in rodents.
DA neurons project topographically (indicated by color spectrum) along the medial-lateral axis. More medially located DA neurons in the VTA project to the ventral Str, the NAc medial shell and medial OT. More laterally located DA neurons project to more dorsolateral Str (33). The topography extends to primates (137).
Synaptic dopamine signaling in the striatum
DA signaling in the Str has been thought to be mediated mainly by diffusion of DA to extrasynaptic G-protein coupled DA receptors, that is by volume transmission (46, 47), engendering a range of modulatory effects, including the regulation of the excitability of SPNs and interneurons, and presynaptic regulation of excitatory input (46, 48–51). Modulatory actions of DA mediate longer time-scale control of motivational salience, vigor and social behavior (52 Gunaydin, 2014, 1535–1551, 53). However, DA neurons mediate faster, discrete DA synaptic responses, in addition to volume transmission.
In ventral midbrain slices, DA neurons produce sub-second D2R-mediated dendrodendritic inhibitory postsynaptic responses in neighboring DA neurons (54). Optogenetic studies have revealed subsecond DA synaptic responses in dStr brain slices (55) seen as a pause in the firing of ChIs and associated with a sub-second hyperpolarization (55, 56), mediated by a D2R coupling to G-protein coupled inward rectifier K+ channels (GIRKs) (55). D2R-mediated inhibitory postsynaptic currents (IPSCs) in Str ChIs have a latency of about 8 msec (55). Considering that the latency involves the time for channelrhodopsin 2 (ChR2) mediated depolarization (57), the activation of transmitter release machinery and G-protein coupled receptor transduction (slower than that of ionotropic receptors), the D2-IPSC is mediated monosynaptically. Although a D2-IPSC has not been reported in D2-SPNs, with GIRK2 transfection D2-IPSCs become detectable (58), indicating that DA neurons can elicit synaptic DA signals in projection areas, so long as D2Rs are proximate to DA release sites.
Dopamine neurons release multiple neurotransmitters
The possibility that DA neurons might exert fast actions via cotransmission was first suggested by the report of excitatory responses in the dStr evoked by stimulation of the nigrostriatal pathway (59). This observation was supported by detection of the glutamate synthetic enzyme glutaminase in DA neurons by immunocytochemistry (60). DA neuron glutamate cotransmission was demonstrated directly in microcultures of single identified rat VTA DA neurons (61), and monosynaptic DA neuron excitatory connections were subsequently detected in mouse brain slices (62), and shown to depend on the expression of vesicular glutamate transporter 2 (VGLUT2) (63). Then, with conditional expression of channelrhodopsin 2 (ChR2) in DA neurons (19), optogenetic stimulation of DA neuron terminals in the NAc showed that DA neurons mediate glutamatergic responses in the intact circuitry of the brain slice (55, 57, 64–66).
DA neurons also corelease GABA (67), derived in part from the action of glutamic acid decarboxylase (GAD), in part from uptake from the extracellular milieu via the plasma membrane GABA transporter 1 (mGAT1) (68), but mainly via synthesis by aldehyde dehydrogenase 1a1 (69). DA neurons do not express vesicular GABA transporter (VGAT); instead they load GABA into vesicles apparently mediated by the vesicular monoamine transporter 2 (VMAT2) (67, 68). In contrast to glutamate cotransmission, which is seen in a subset of DA neurons, most DA neurons appear capable of GABA cotransmission (68, 69). However, GABA IPSC amplitude declines rapidly with repeated stimulation at the in vivo firing frequency of DA neurons (56), so its role in fast DA neuron signaling remains to be demonstrated.
Regional heterogeneity in dopamine neuron synaptic actions
The three transmitters released by DA neurons — DA, glutamate and GABA — have different synaptic actions in different postsynaptic target neurons in different striatal subregions. Studies in brain slices from transgenic mice have so far focused on DA neuron connections to SPNs and ChIs in three Str regions, the NAc medial shell, NAc core and the dStr (Figure 3). DA neurons make the most prominent glutamatergic connections in the most medial, anterior part of the vStr, the medial shell (66). DA neuron glutamatergic connections to ChIs are the strongest, strong enough to drive firing without coincident excitatory inputs, while those to SPNs are weaker (55). GABA cotransmission is not seen in medial shell ChIs (55), which receive input from VTA GABA neurons (70). In the medial shell, following the burst in ChIs driven by DA neuron burst firing, ChIs show a post-burst hyperpolarization mediated by a sub-second D2R-mediated hyperpolarization, involving small conductance Ca2+ dependent K+ channels (SK channels), but not GABA (55). Interestingly, the olfactory tubercle (OT), which shares functions with the NAc medial shell (33), shows almost the same pattern of DA neuron transmission (65), differing only in the additional late contribution of a D5R-mediated burst (65).
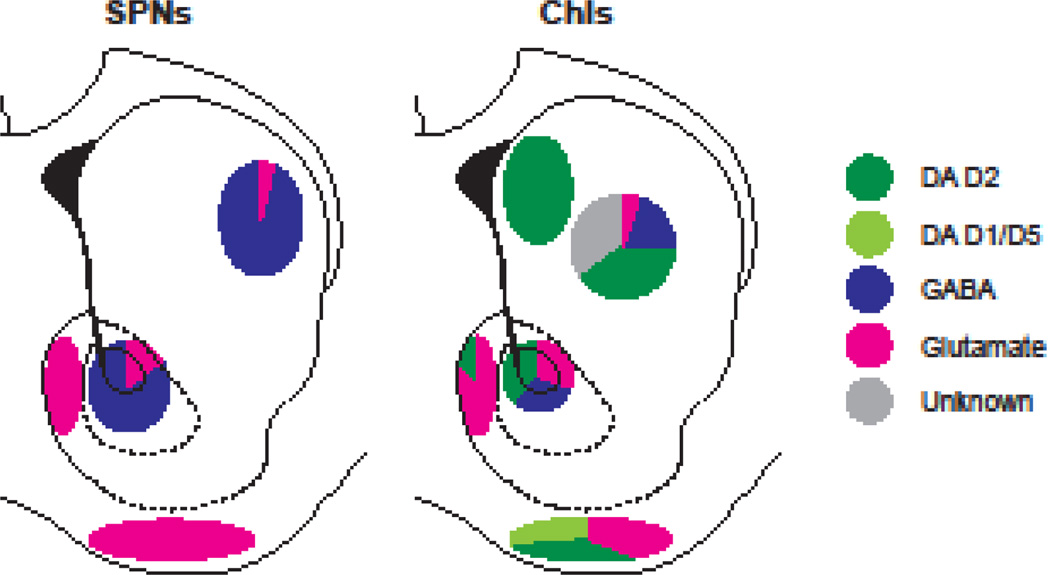
Fig 3. Heterogeneity in DA neuron synaptic actions in the mouse striatum.
Optogenetic stimulation of DA neuron terminals elicits fast synaptic responses in principal Str neurons, spiny projection neurons (SPNs) and cholinergic interneurons (ChIs). The relative strength of each transmitter response in different Str regions is shown in pie charts. For reports without details of recording locations, the pie chart is located at the center of the region (e.g., dStr, Ref. 56). Responses seen in each area are schematized in pie charts by color: DA D2 receptor (dark green), DA D1 receptor (light green), GABAA receptor (dark blue), ionotropic glutamate receptor (magenta), and unknown excitatory transmitter (gray). Striatal outlines are the same as used in Figure 1. The schemes are a compendium of the several optogenetic studies (56, 62, 65–68).
In the NAc core, weak glutamate, GABA and fast D2R responses are observed in ChIs (55), but overall, DA neuron phasic firing drives a reduction in ChI firing (55) mediated by GABA cotransmission. Weak DA neuron GABA-mediated IPSCs are seen in SPNs (68, 69). In the dStr, DA neurons drive a D2R-mediated pause in the firing of ChIs in the most medial anterior dStr (55). In one report, no clear glutamate or GABA cotransmission was seen (55), while in another GABA cotransmission and a slow excitatory synaptic current, mediated by an unidentified transmitter, were seen (56). Glutamate cotransmission was not observed in SPNs in the medial dStr in two studies in which VTA DA neurons were transfected with ChR2 (64, 66), while weak glutamate cotransmission was seen in two others in which lateral SNc DA neurons were transfected with ChR2 and recordings likely made in the more lateral dStr (67, 69). Thus, DA neuron glutamate cotransmission to SPNs appears to extend beyond the NAc to the lateral dStr, but not to the medial dStr; strong GABA cotransmission is observed in dStr SPNs, independent of glutamate cotransmission (56, 67, 68).
DA neuron glutamatergic responses are strong and invariably seen in NAc shell and olfactory tubercle (OT), and this correlates with DA neuron VGLUT2 expression in medial VTA DA neurons projecting to the NAc, while SN DA neurons projecting to the dStr do not express VGLUT2 (71, 72). This topography does not, however, align with recording of DA neuron glutamate cotransmission in lateral dStr SPNs; this could be mediated by the non-topographical DA neuron projection of medial dorsal VTA DA neurons to the lateral dStr, seen in rats (73, 74), and presumably also in mice. Thus, DA neuron synaptic connections to ChIs appear to encode DA neuron phasic firing positively in the medial shell and OT in the vStr, via glutamatergic signaling. In contrast, DA neuron synaptic connections to both ChIs and SPNs in the dStr encode DA neuron phasic firing negatively in the form of pauses mediated by inhibitory D2R and GABA signaling.
Regional heterogeneity in dopamine neuron synaptic terminals
Regional differences in DA release in schizophrenia could be due to alterations in DA neuron terminals. The match in increased F-DOPA uptake (7) and amphetamine-induced DA release in schizophrenia (6, 7) points to pathological presynaptic modulation of DA release (75). DA neuron transmission in the Str is modulated locally by presynaptic receptors (47, 76–78) that contribute significantly to regional heterogeneity. D2R potently inhibit DA release (47), but there is no evidence for heterogeneity in autoreceptor function within the Str.
In contrast, presynaptic acetylcholine (ACh) receptors, which potently drive DA release (79), show significant regional heterogeneity in their function, and have been extensively implicated in the pathogenesis of schizophrenia. Evidence for the extends from linkage to the α7 nicotinic receptor (80), extensive use of nicotine by patients (81), therapeutic potential of nicotinic cholinergic agonists (82), etiologic evidence for early developmental alterations in cholinergic balance (83), and postmortem evidence for altered cholinergic signaling in schizophrenia (84, 85). Nicotine-mediated desensitization shapes the balance between tonic and phasic firing on DA release, which may contribute to regional heterogeneity (86). Accentuation of DA neuron burst firing in the dStr may falsely emphasize less salient cue signals in patients with schizophrenia (87), while lower phasic DA release in the vStr, which correlates with negative symptoms, possibly contributes to disturbance in salience signals.
Synchronized ChI activity drives DA release in the dStr via nACh receptors on DA neuron terminals (88–90), while in the NAc ChI activity drives DA release through concerted activation of nicotinic and glutamate receptors. ChI terminals express auto-inhibitory muscarinic ACh receptors (mAChR), which reduce ACh release and temper nicotinic stimulation of DA release (91). Activation of nACh on DA neuron terminals or inhibition of mACh receptors on ChIs increases the DA release generated with single action potentials and limits DA release by subsequent action potentials in a burst (91). In contrast to the modest difference between single pulse-evoked and train-evoked DA release in the dStr (91, 92), DA release in the NAc shows robust frequency dependence, where nACh inhibition or mACh activation enhance frequency responsiveness (92–94). Reducing ACh synthesis in ChIs (95) or blockade of nACh receptors (86, 96) in the dStr enhances frequency responsiveness, showing the crucial contribution of nACh receptors to regional heterogeneity. Differences in nACh receptor subunit composition in DA neuron terminals projecting to the dStr and NAc, as well as mACh receptors in ChIs, likely contribute further to heterogeneity (91, 93, 94).
DA release is determined by vesicular loading, mediated by the vesicular monoamine transporter 2 (VMAT2) and modulated by VGLUT2, vesicular release, and reuptake, mediated by the DA transporter (DAT), all of which contribute to heterogeneity in DA release (78). Modulation of any of these steps can control DA release. VGLUT2 — which mediates glutamate cotransmission — also impacts vesicular DA loading (63) through vesicular synergy (97). VGLUT2-mediated glutamate uptake dissipates the vesicular electrical gradient enabling further activity of the vesicular proton pump, vesicular acidification and DA packing (47, 63, 98). Considering the prominent expression of VGLUT2 in VTA DA neurons, the contribution of vesicular synergy to DA release is likely to be most prominent in the NAc. Although there is clear evidence for the anatomical segregation of dopamine and glutamate release sites (99), this may not extend to all release sites, and estimates of segregation may be limited by the sensitivity of immunocytochemical detection.
Variation in presynaptic Ca2+ channels controlling release contribute to heterogeneity, with more channel types in dStr DA neuron terminals than in NAc terminals, as well as differences in Ca2+ buffering (100), conferring heterogeneous, complex frequency dynamics. In the non-human primate, release mechanisms per se show heterogeneity, with higher DA release probability in the dStr and lower probability in the vStr, estimated from differences in Ca2+ availability (101). DAT expression is higher in SNc compared to VTA DA neurons (102–104) so released DA is subject to more robust reuptake in the dStr than the NAc (105). Thus, heterogeneity in DA neuron presynaptic terminal function contributes strongly to regional variation in DA neuron signaling across the Str.
Regional heterogeneity in dopamine neuron activity
Heterogeneity in DA neuron signaling is driven by regional differences in the in vivo patterns of DA neuron firing. DA neuron firing depends on both intrinsic membrane properties and synaptic input (106). In brain slices, where most synaptic inputs to DA neurons are truncated, DA neurons show pacemaker firing at about 3–5 Hz (106). DA neurons in vivo alternate between tonic and phasic firing, with bursts of about 5 spikes at 15–20 Hz, determined by patterns of synaptic input. Differences in intrinsic conductances determine the regularity and frequency of pacemaker firing (104). The pacemaker firing of DA neurons is driven by slow membrane potential oscillations (106, 107). The responsible intrinsic conductances differ between SNc and VTA DA neurons (107). In SNc DA neurons, slow oscillations are generated by low-threshold voltage-gated Ca2+ channels and SK channels (108, 109), while in VTA DA neurons, in addition to the contribution of SK channels, the depolarizing phase of the oscillation is shaped by two types of Na+ channels; non-voltage dependent leak currents and voltage-dependent tetrodotoxin-sensitive persistent currents (110).
Burst-firing shows regional heterogeneity with a gradient of decreasing burst firing from the VTA to the lateral SNc (111). Burst firing of DA neurons is driven by synchronous excitatory glutamate or nicotinic acetylcholine (nAChR) synaptic input; the medial-lateral gradient in burst firing is likely driven by regional differences in excitatory inputs. NMDA receptors are crucial for naturalistic burst firing in DA neurons (112), but their expression level shows a mediolateral gradient, opposite to the gradient in firing (113), and so does not account for the greater burst firing seen in VTA compared to SNc DA neurons (111). Modulation of intrinsic conductances can affect burst firing of DA neurons by changing overall excitability. Indeed, when the expression of SK3, the most prominent subtype of SK channels in DA neurons (108), is reduced in VTA DA neurons, in vivo firing of DA neurons increases and more bursting is observed (114). SK3 expression also shows a mediolateral gradient, with higher expression in lateral midbrain DA neurons (108, 115), and so correlates with the gradient in excitability. Thus heterogeneity in several intrinsic currents contributes to heterogeneity in the tonic and phasic firing of DA neurons, and thus to heterogeneity in their synaptic actions in the Str.
Regional increases in DA release in schizophrenia could be due to altered patterns of DA neuron activity. PET imaging of DA receptor ligand displacement reveals DA release (116) extending to the regional level in patients with schizophrenia (6), but has limited spatial as well as temporal resolution. Specifically, the relative contribution of tonic and phasic DA release to the increased DA signal seen in schizophrenia cannot be resolved due to limited temporal resolution. In the vStr of rodents, larger increases in DA release are driven by DA neuron bursting (117). Real-time electrochemical measurements in awake rats reveal further that time-averaged extracellular DA arises mainly from phasic release (118, 119). Thus, in the vStr, brain imaging of DA release likely reflects phasic DA neuron activity. In the dStr where DA release caused by single spike and burst firing are similar, and DA release does not show frequency potentiation (92, 94), the increased DA release in the associative Str in patients with schizophrenia (6) may reflect both higher tonic and phasic DA release. Possibly different dynamics in DA release in dStr versus vStr contribute to the greater increase seen in schizophrenia in the associative Str.
Regional differences in psychotropic drug action at striatal dopamine neuron terminals
Extrinsic neuromodulators, such as psychostimulants, act preferentially in vStr domains, in rodents as shown by microdialysis (2) and in non-human primates as shown by ligand displacement (3). In humans, the greatest amphetamine-induced ligand displacement is seen in the vStr and also in the sensorimotor Str (putamen) (120, 121). Consistent with the greater DA release in vStr in rodents, amphetamine and cocaine at lower doses appear to act preferentially in the vStr to drive hyperlocomotion, while at higher doses they act in the dStr to drive stereotypy (122–124).
A single exposure to amphetamine can significantly perturb Str DA neuron connections in a regionally and synaptically selective fashion (55). Low-dose amphetamine appears to affect glutamatergic connections in the NAc medial shell preferentially, while high-dose affects DA transmission across the Str. The relative sensitivity of NAc medial shell glutamate connections may impact DA release. Indeed, mice with a DA neuron conditional VGLUT2 deletion show an attenuated response to psychostimulants (63, 125). While this may be due to the dependence of DA neuron development on VGLUT2 expression (126), it may be due also to vesicular synergy. VGLUT2 mediates vesicular uptake of glutamate, which acting as a counter ion may enhance dopamine packaging, and confer increased sensitivity to amphetamine action. A further possibility is that the selective impact of amphetamine on DA neuron glutamate connections significantly alters their functional connectivity to ChIs in a manner commensurate with increased propensity to drug use. Interestingly, glutamate corelease in Str ChIs — mediated by VGLUT3 — also modulates psychostimulant sensitivity (127), but whether VGLUT3 is differentially distributed in ChIs has yet to be resolved. Conceivably, cotransmission preferentially sensitizes a subset of Str synapses to psychostimulants.
Antipsychotic drugs may exert regionally selective synaptic actions in the Str due to dynamics of vesicular uptake, exocytic release, and reuptake. As lipophilic weak-bases, most antipsychotic drugs accumulate in acidic organelles, including synaptic vesicles, where they are trapped by intraluminal protonation (128–130). This mechanism would lead to the concentration of antipsychotics at synaptic sites, as the drugs would accumulate in synaptic vesicles, undergo activity dependent release (130, 131), and then be taken back up, in an intrasynaptic recycling process. However, acidotropic uptake depends on vesicular acidity, and not on VMAT2 expression (130), so the accumulation is not specific to DA neurons (132–134). However, amphetamine, which acts selectively at DA neuron terminals, preferentially releases antipsychotic drugs from DA neuron synapses (130). And, DA neuron terminals in the vStr with more prominent expression of VGLUT2 are likely to show more robust acidification, as mentioned above. Antipsychotic drugs may preferentially — although not specifically — accumulate in DA neuron terminals in the vStr, and this may be important in determining their regional and dose-dependent actions in the Str. Determining the distribution of VGLUT2 in primate DA neurons will thus be important for evaluating the contribution of cotransmission to regional drug effects.
Implications of regional heterogeneity in dopamine neuron synaptic function for brain imaging and psychotropic drug action
The range of rodent studies we have discussed reveal striking regional heterogeneity in DA neuron synaptic actions at multiple levels that could underlie regional differences seen in brain imaging of dopamine function in schizophrenia. The finer detail in DA neuron synaptic transmission revealed by optogenetics indicates that DA neuron transmission involves a differential blend of three neurotransmitters and functions in Str subregions. The cotransmitters not only provide temporally precise information of DA neuron firing, particularly that of phasic firing, but also modulate striatal microcircuits by controlling ChI activity, which in turn feeds back on DA terminals, regulating DA neuron transmission. Heterogeneity in the influence of DA neuron activity on ChI activity likely plays a major role in regional differences in the dynamics of DA neuron signaling, shaping schizophrenia symptoms. Increased understanding of the cellular basis for regional drug action should inform interpretation of the brain imaging signal in schizophrenia, as well as enabling intersectional targeting of drugs to achieve better therapeutic effects with regional selectivity.
Acknowledgments
We thank Anissa Abi-Dargham, Peter Balsam, Jonathan Javitch, Christoph Kellendonk, Holly Moore and Eleanor Simpson for discussion. Our research has been supported by NIH P50 MH086404 (SR), R01 DA038966 (SR), NARSAD (NC, SM, AK) and the FQRS (LY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–786. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drevets WC, Price JC, Kupfer DJ, Kinahan PE, Lopresti B, Holt D, et al. PET measures of amphetamine-induced dopamine release in ventral versus dorsal striatum. Neuropsychopharmacology. 1999;21:694–709. doi: 10.1016/S0893-133X(99)00079-2. [DOI] [PubMed] [Google Scholar]
- 4.Colpaert FC. Discovering risperidone: the LSD model of psychopathology. Nat Rev Drug Discov. 2003;2:315–320. doi: 10.1038/nrd1062. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman JA, Bymaster FP, Meltzer HY, Deutch AY, Duncan GE, Marx CE, et al. Antipsychotic drugs: Comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol Rev. 2008;60:358–403. doi: 10.1124/pr.107.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67:231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- 7.Egerton A, Chaddock CA, Winton-Brown TT, Bloomfield MA, Bhattacharyya S, Allen P, et al. Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: findings in a second cohort. Biol Psychiatry. 2013;74:106–112. doi: 10.1016/j.biopsych.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Margolis EB, Coker AR, Driscoll JR, Lemaître A-I, Fields HL. Reliability in the identification of midbrain dopamine neurons. PLoS ONE. 2010;5:e15222. doi: 10.1371/journal.pone.0015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pupe S, Wallén-Mackenzie A. Cre-driven optogenetics in the heterogeneous genetic panorama of the VTA. Trends Neurosci. 2015;38:375–386. doi: 10.1016/j.tins.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Anderegg A, Poulin J-F, Awatramani R. Molecular heterogeneity of midbrain dopaminergic neurons - Moving toward single cell resolution. FEBS Lett. 2015;589:3714–3726. doi: 10.1016/j.febslet.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roeper J. Dissecting the diversity of midbrain dopamine neurons. Trends Neurosci. 2013;36:336–342. doi: 10.1016/j.tins.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Liss B, Roeper J. Individual dopamine midbrain neurons: functional diversity and flexibility in health and disease. Brain Res Rev. 2008;58:314–321. doi: 10.1016/j.brainresrev.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Lammel S, Lim BK, Malenka RC. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 2014;76:351–359. doi: 10.1016/j.neuropharm.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, Fields H. Midbrain dopamine neurons: Projection target determines action potential duration and dopamine D2 receptor inhibition. J Neurosci. 2008;28:8908–8913. doi: 10.1523/JNEUROSCI.1526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mrejeru A, Marti-Prats L, Avegno EM, Harrison NL, Sulzer D. A subset of ventral tegmental area dopamine neurons responds to acute ethanol. Neuroscience. 2015;290:649–658. doi: 10.1016/j.neuroscience.2014.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sulzer D, Surmeier DJ. Neuronal vulnerability, pathogenesis, and Parkinson's disease. Mov Disord. 2013;28:715–724. doi: 10.1002/mds.25187. [DOI] [PubMed] [Google Scholar]
- 17.Brichta L, Greengard P. Molecular determinants of selective dopaminergic vulnerability in Parkinson's disease: an update. Front Neuroanat. 2014;8:152. doi: 10.3389/fnana.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wickham RJ, Addy NA, Wightman M. Advances in studying phasic dopamine signaling in brain reward mechanisms. Frontiers in bioscience (Elite edition) 2013:1–26. doi: 10.2741/e678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci. 2007;10:663–668. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- 21.Chuhma N, Tanaka KF, Hen R, Rayport S. Functional connectome of the striatal medium spiny neuron. J Neurosci. 2011;31:1183–1192. doi: 10.1523/JNEUROSCI.3833-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith AD, Bolam JP. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci. 1990;13:259–265. doi: 10.1016/0166-2236(90)90106-k. [DOI] [PubMed] [Google Scholar]
- 23.Tepper JM, Koos T, Wilson CJ. GABAergic microcircuits in the neostriatum. Trends Neurosci. 2004;27:662–669. doi: 10.1016/j.tins.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Kawaguchi Y. Local Circuit Neurons in the Frontal Cortico-Striatal System. Boston, MA: Springer US; 2003. pp. 125–148. [Google Scholar]
- 25.Haber SN, McFarland NR. The concept of the ventral striatum in nonhuman primates. Ann N Y Acad Sci. 1999;877:33–48. doi: 10.1111/j.1749-6632.1999.tb09259.x. [DOI] [PubMed] [Google Scholar]
- 26.Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Joel D, Weiner I. The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience. 2000;96:451–474. doi: 10.1016/s0306-4522(99)00575-8. [DOI] [PubMed] [Google Scholar]
- 28.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- 29.Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- 31.Groenewegen HJ, Berendse HW, Wolters JG, Lohman AH. The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: evidence for a parallel organization. Prog Brain Res. 1990;85:95–116. doi: 10.1016/s0079-6123(08)62677-1. [DOI] [PubMed] [Google Scholar]
- 32.Parent A. Extrinsic connections of the basal ganglia. Trends Neurosci. 1990;13:254–258. doi: 10.1016/0166-2236(90)90105-j. [DOI] [PubMed] [Google Scholar]
- 33.Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zahm DS. Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Ann N Y Acad Sci. 1999;877:113–128. doi: 10.1111/j.1749-6632.1999.tb09264.x. [DOI] [PubMed] [Google Scholar]
- 35.Floresco SB. The nucleus accumbens: an interface between cognition, emotion, and action. Annu Rev Psychol. 2015;66:25–52. doi: 10.1146/annurev-psych-010213-115159. [DOI] [PubMed] [Google Scholar]
- 36.Baliki MN, Mansour A, Baria AT, Huang L, Berger SE, Fields HL, et al. Parceling human accumbens into putative core and shell dissociates encoding of values for reward and pain. J Neurosci. 2013;33:16383–16393. doi: 10.1523/JNEUROSCI.1731-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 39.Yetnikoff L, Lavezzi HN, Reichard RA, Zahm DS. An update on the connections of the ventral mesencephalic dopaminergic complex. Neuroscience. 2014;282:23–48. doi: 10.1016/j.neuroscience.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, Arai R, et al. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J Neurosci. 2009;29:444–453. doi: 10.1523/JNEUROSCI.4029-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doucet G, Descarries L, Garcia S. Quantification of the dopamine innervation in adult rat neostriatum. Neuroscience. 1986;19:427–445. doi: 10.1016/0306-4522(86)90272-1. [DOI] [PubMed] [Google Scholar]
- 42.Xia Y, Driscoll JR, Wilbrecht L, Margolis EB, Fields HL, Hjelmstad GO. Nucleus accumbens medium spiny neurons target non-dopaminergic neurons in the ventral tegmental area. J Neurosci. 2011;31:7811–7816. doi: 10.1523/JNEUROSCI.1504-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 44.Lerner TN, Shilyansky C, Davidson TJ, Evans KE, Beier KT, Zalocusky KA, et al. Intact-brain analyses reveal distinct information carried by SNc dopamine subcircuits. Cell. 2015;162:635–647. doi: 10.1016/j.cell.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menegas W, Bergan JF, Ogawa SK, Isogai Y, Umadevi Venkataraju K, Osten P, et al. Dopamine neurons projecting to the posterior striatum form an anatomically distinct subclass. Elife. 2015;4:e10032. doi: 10.7554/eLife.10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76:33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sulzer D, Cragg SJ, Rice ME. Striatal dopamine neurotransmission: regulation of release and uptake. Basal ganglia. 2016;6:123–148. doi: 10.1016/j.baga.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, et al. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42:653–663. doi: 10.1016/s0896-6273(04)00265-x. [DOI] [PubMed] [Google Scholar]
- 49.Kreitzer AC. Physiology and pharmacology of striatal neurons. Annu Rev Neurosci. 2009;32:127–147. doi: 10.1146/annurev.neuro.051508.135422. [DOI] [PubMed] [Google Scholar]
- 50.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schultz W. Updating dopamine reward signals. Curr Opin Neurobiol. 2013;23:229–238. doi: 10.1016/j.conb.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Panigrahi B, Martin KA, Li Y, Graves AR, Vollmer A, Olson L, et al. Dopamine is required for the neural representation and control of movement vigor. Cell. 2015;162:1418–1430. doi: 10.1016/j.cell.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 54.Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 55.Chuhma N, Mingote S, Moore H, Rayport S. Dopamine neurons control striatal cholinergic neurons via regionally heterogeneous dopamine and glutamate signaling. Neuron. 2014;81:901–912. doi: 10.1016/j.neuron.2013.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Straub C, Tritsch NX, Hagan NA, Gu C, Sabatini BL. Multiphasic modulation of cholinergic interneurons by nigrostriatal afferents. J Neurosci. 2014;34:8557–8569. doi: 10.1523/JNEUROSCI.0589-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, et al. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci. 2010;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marcott PF, Mamaligas AA, Ford CP. Phasic dopamine release drives rapid activation of striatal D2-receptors. Neuron. 2014;84:164–176. doi: 10.1016/j.neuron.2014.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kitai ST, Sugimori M, Kocsis JD. Excitatory nature of dopamine in the nigro-caudate pathway. Exp Brain Res. 1976;24:351–363. doi: 10.1007/BF00235003. [DOI] [PubMed] [Google Scholar]
- 60.Kaneko T, Akiyama H, Nagatsu I, Mizuno N. Immunohistochemical demonstration of glutaminase in catecholaminergic and serotoninergic neurons of rat brain. Brain Res. 1990;507:151–154. doi: 10.1016/0006-8993(90)90535-j. [DOI] [PubMed] [Google Scholar]
- 61.Sulzer D, Joyce MP, Lin L, Geldwert D, Haber SN, Hattori T, et al. Dopamine neurons make glutamatergic synapses in vitro. J Neurosci. 1998;18:4588–4602. doi: 10.1523/JNEUROSCI.18-12-04588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chuhma N, Zhang H, Masson J, Zhuang X, Sulzer D, Hen R, et al. Dopamine neurons mediate a fast excitatory signal via their glutamatergic synapses. J Neurosci. 2004;24:972–981. doi: 10.1523/JNEUROSCI.4317-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hnasko TS, Chuhma N, Zhang H, Goh GA, Sulzer D, Palmiter RD, et al. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65:643–656. doi: 10.1016/j.neuron.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wieland S, Du D, Oswald MJ, Parlato R, Kohr G, Kelsch W. Phasic dopaminergic activity exerts fast control of cholinergic interneuron firing via sequential NMDA, D2, and D1 receptor activation. J Neurosci. 2014;34:11549–11559. doi: 10.1523/JNEUROSCI.1175-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mingote S, Chuhma N, Kusnoor SV, Field B, Deutch AY, Rayport S. Functional Connectome Analysis of Dopamine Neuron Glutamatergic Connections in Forebrain Regions. J Neurosci. 2015;35:16259–16271. doi: 10.1523/JNEUROSCI.1674-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tritsch NX, Ding JB, Sabatini BL. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490:262–266. doi: 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tritsch NX, Oh WJ, Gu C, Sabatini BL. Midbrain dopamine neurons sustain inhibitory transmission using plasma membrane uptake of GABA, not synthesis. Elife. 2014;3:e01936. doi: 10.7554/eLife.01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim JI, Ganesan S, Luo SX, Wu YW, Park E, Huang EJ, et al. Aldehyde dehydrogenase 1a1 mediates a GABA synthesis pathway in midbrain dopaminergic neurons. Science. 2015;350:102–106. doi: 10.1126/science.aac4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown MTC, Tan KR, O’Connor EC, Nikonenko I, Muller D, Lüscher C. Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature. 2012;492:452–456. doi: 10.1038/nature11657. [DOI] [PubMed] [Google Scholar]
- 71.Taylor SR, Badurek S, Dileone RJ, Nashmi R, Minichiello L, Picciotto MR. GABAergic and glutamatergic efferents of the mouse ventral tegmental area. J Comp Neurol. 2014;522:3308–3334. doi: 10.1002/cne.23603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morales M, Root DH. Glutamate neurons within the midbrain dopamine regions. Neuroscience. 2014;282C:60–68. doi: 10.1016/j.neuroscience.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pennartz CM, Berke JD, Graybiel AM, Ito R, Lansink CS, van der Meer M, et al. Corticostriatal interactions during learning, memory processing, and decision making. J Neurosci. 2009;29:12831–12838. doi: 10.1523/JNEUROSCI.3177-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maurin Y, Banrezes B, Menetrey A, Mailly P, Deniau JM. Three-dimensional distribution of nigrostriatal neurons in the rat: relation to the topography of striatonigral projections. Neuroscience. 1999;91:891–909. doi: 10.1016/s0306-4522(98)00681-2. [DOI] [PubMed] [Google Scholar]
- 75.Lyon GJ, Abi-Dargham A, Moore H, Lieberman JA, Javitch JA, Sulzer D. Presynaptic regulation of dopamine transmission in schizophrenia. Schizophr Bull. 2011;37:108–117. doi: 10.1093/schbul/sbp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rice ME, Patel JC, Cragg SJ. Dopamine release in the basal ganglia. Neuroscience. 2011;198:112–137. doi: 10.1016/j.neuroscience.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brimblecombe KR, Cragg SJ. Substance P Weights Striatal Dopamine Transmission Differently within the Striosome-Matrix Axis. J Neurosci. 2015;35:9017–9023. doi: 10.1523/JNEUROSCI.0870-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cachope R, Cheer JF. Local control of striatal dopamine release. Front Behav Neurosci. 2014;8:188. doi: 10.3389/fnbeh.2014.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marenco S, Carson RE, Berman KF, Herscovitch P, Weinberger DR. Nicotine-induced dopamine release in primates measured with [11C]raclopride PET. Neuropsychopharmacology. 2004;29:259–268. doi: 10.1038/sj.npp.1300287. [DOI] [PubMed] [Google Scholar]
- 80.Olincy A, Freedman R. Nicotinic mechanisms in the treatment of psychotic disorders: a focus on the α7 nicotinic receptor. Handb Exp Pharmacol. 2012:211–232. doi: 10.1007/978-3-642-25758-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leonard S, Mexal S, Freedman R. Smoking, Genetics and Schizophrenia: Evidence for Self Medication. J Dual Diagn. 2007;3:43–59. doi: 10.1300/J374v03n03_05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Freedman R. alpha7-nicotinic acetylcholine receptor agonists for cognitive enhancement in schizophrenia. Annu Rev Med. 2014;65:245–261. doi: 10.1146/annurev-med-092112-142937. [DOI] [PubMed] [Google Scholar]
- 83.Ross RG, Hunter SK, McCarthy L, Beuler J, Hutchison AK, Wagner BD, et al. Perinatal choline effects on neonatal pathophysiology related to later schizophrenia risk. Am J Psychiatry. 2013;170:290–298. doi: 10.1176/appi.ajp.2012.12070940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Holt DJ, Bachus SE, Hyde TM, Wittie M, Herman MM, Vangel M, et al. Reduced density of cholinergic interneurons in the ventral striatum in schizophrenia: an in situ hybridization study. Biol Psychiatry. 2005;58:408–416. doi: 10.1016/j.biopsych.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 85.Scarr E, Gibbons AS, Neo J, Udawela M, Dean B. Cholinergic connectivity: it's implications for psychiatric disorders. Front Cell Neurosci. 2013;7:55. doi: 10.3389/fncel.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- 87.Kapur S, Mizrahi R, Li M. From dopamine to salience to psychosis--linking biology, pharmacology and phenomenology of psychosis. Schizophr Res. 2005;79:59–68. doi: 10.1016/j.schres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 88.Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M, et al. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep. 2012;2:33–41. doi: 10.1016/j.celrep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75:58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 90.Wang L, Zhang X, Xu H, Zhou L, Jiao R, Liu W, et al. Temporal components of cholinergic terminal to dopaminergic terminal transmission in dorsal striatum slices of mice. J Physiol. 2014;592:3559–3576. doi: 10.1113/jphysiol.2014.271825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Threlfell S, Cragg SJ. Dopamine signaling in dorsal versus ventral striatum: the dynamic role of cholinergic interneurons. Front Syst Neurosci. 2011;5:11. doi: 10.3389/fnsys.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Patel JC, Rice ME. Monitoring axonal and somatodendritic dopamine release using fast-scan cyclic voltammetry in brain slices. Methods Mol Biol. 2013;964:243–273. doi: 10.1007/978-1-62703-251-3_15. [DOI] [PubMed] [Google Scholar]
- 93.Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ. Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology. 2008;33:2158–2166. doi: 10.1038/sj.npp.1301617. [DOI] [PubMed] [Google Scholar]
- 94.Threlfell S, Clements MA, Khodai T, Pienaar IS, Exley R, Wess J, et al. Striatal muscarinic receptors promote activity dependence of dopamine transmission via distinct receptor subtypes on cholinergic interneurons in ventral versus dorsal striatum. J Neurosci. 2010;30:3398–3408. doi: 10.1523/JNEUROSCI.5620-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Patel JC, Rossignol E, Rice ME, Machold RP. Opposing regulation of dopaminergic activity and exploratory motor behavior by forebrain and brainstem cholinergic circuits. Nature Commun. 2012;3:1172. doi: 10.1038/ncomms2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- 97.El Mestikawy S, Wallen-Mackenzie A, Fortin GM, Descarries L, Trudeau LE. From glutamate co-release to vesicular synergy: vesicular glutamate transporters. Nat Rev Neurosci. 2011;12:204–216. doi: 10.1038/nrn2969. [DOI] [PubMed] [Google Scholar]
- 98.Hnasko TS, Edwards RH. Neurotransmitter corelease: mechanism and physiological role. Annu Rev Physiol. 2012;74:225–243. doi: 10.1146/annurev-physiol-020911-153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang S, Qi J, Li X, Wang H-L, Britt JP, Hoffman AF, et al. Dopaminergic and glutamatergic microdomains in a subset of rodent mesoaccumbens axons. Nat Neurosci. 2015;18:386–392. doi: 10.1038/nn.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brimblecombe KR, Gracie CJ, Platt NJ, Cragg SJ. Gating of dopamine transmission by calcium and axonal N-, Q-, T- and L-type voltage-gated calcium channels differs between striatal domains. J Physiol. 2015;593:929–946. doi: 10.1113/jphysiol.2014.285890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cragg SJ. Variable dopamine release probability and short-term plasticity between functional domains of the primate striatum. J Neurosci. 2003;23:4378–4385. doi: 10.1523/JNEUROSCI.23-10-04378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Blanchard V, Raisman-Vozari R, Vyas S, Michel PP, Javoy-Agid F, Uhl G, et al. Differential expression of tyrosine hydroxylase and membrane dopamine transporter genes in subpopulations of dopaminergic neurons of the rat mesencephalon. Brain Res Mol Brain Res. 1994;22:29–38. doi: 10.1016/0169-328x(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 103.Hurd YL, Pristupa ZB, Herman MM, Niznik HB, Kleinman JE. The dopamine transporter and dopamine D2 receptor messenger RNAs are differentially expressed in limbic- and motor-related subpopulations of human mesencephalic neurons. Neuroscience. 1994;63:357–362. doi: 10.1016/0306-4522(94)90535-5. [DOI] [PubMed] [Google Scholar]
- 104.Lammel S, Hetzel A, Häckel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 105.Cragg SJ, Hille CJ, Greenfield SA. Functional domains in dorsal striatum of the nonhuman primate are defined by the dynamic behavior of dopamine. J Neurosci. 2002;22:5705–5712. doi: 10.1523/JNEUROSCI.22-13-05705.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Paladini CA, Roeper J. Generating bursts (and pauses) in the dopamine midbrain neurons. Neuroscience. 2014;282C:109–121. doi: 10.1016/j.neuroscience.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 107.Morikawa H, Paladini CA. Dynamic regulation of midbrain dopamine neuron activity: intrinsic, synaptic, and plasticity mechanisms. Neuroscience. 2011;198:95–111. doi: 10.1016/j.neuroscience.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wolfart J, Neuhoff H, Franz O, Roeper J. Differential expression of the small-conductance, calcium-activated potassium channel SK3 is critical for pacemaker control in dopaminergic midbrain neurons. J Neurosci. 2001;21:3443–3456. doi: 10.1523/JNEUROSCI.21-10-03443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mercuri NB, Bonci A, Calabresi P, Stratta F, Stefani A, Bernardi G. Effects of dihydropyridine calcium antagonists on rat midbrain dopaminergic neurones. Br J Pharmacol. 1994;113:831–838. doi: 10.1111/j.1476-5381.1994.tb17068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Khaliq ZM, Bean BP. Pacemaking in dopaminergic ventral tegmental area neurons: depolarizing drive from background and voltage-dependent sodium conductances. J Neurosci. 2010;30:7401–7413. doi: 10.1523/JNEUROSCI.0143-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schiemann J, Schlaudraff F, Klose V, Bingmer M, Seino S, Magill PJ, et al. K-ATP channels in dopamine substantia nigra neurons control bursting and novelty-induced exploration. Nat Neurosci. 2012;15:1272–1280. doi: 10.1038/nn.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, et al. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci U S A. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Krabbe S, Duda J, Schiemann J, Poetschke C, Schneider G, Kandel ER, et al. Increased dopamine D2 receptor activity in the striatum alters the firing pattern of dopamine neurons in the ventral tegmental area. Proc Natl Acad Sci U S A. 2015;112:E1498–E1506. doi: 10.1073/pnas.1500450112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Soden ME, Jones GL, Sanford CA, Chung AS, Guler AD, Chavkin C, et al. Disruption of dopamine neuron activity pattern regulation through selective expression of a human KCNN3 mutation. Neuron. 2013;80:997–1009. doi: 10.1016/j.neuron.2013.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sarpal D, Koenig J, Adelman JP, Brady D, Prendeville L, Shepard P. Regional distribution of SK3 mRNA-containing neurons in the adult and adolescent rat ventral midbrain and their relationship to dopamine-containing cells. Synapse. 2004;53:104–113. doi: 10.1002/syn.20042. [DOI] [PubMed] [Google Scholar]
- 116.Laruelle M. Imaging neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 117.Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- 118.Owesson-White CA, Roitman MF, Sombers LA, Belle AM, Keithley RB, Peele JL, et al. Sources contributing to the average extracellular concentration of dopamine in the nucleus accumbens. J Neurochem. 2012;121:252–262. doi: 10.1111/j.1471-4159.2012.07677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sombers LA, Beyene M, Carelli RM, Wightman RM. Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J Neurosci. 2009;29:1735–1742. doi: 10.1523/JNEUROSCI.5562-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- 121.Riccardi P, Li R, Ansari MS, Zald D, Park S, Dawant B, et al. Amphetamine-induced displacement of [18F] fallypride in striatum and extrastriatal regions in humans. Neuropsychopharmacology. 2006;31:1016–1026. doi: 10.1038/sj.npp.1300916. [DOI] [PubMed] [Google Scholar]
- 122.Yates JW, Meij JTA, Sullivan JR, Richtand NM, Yu L. Bimodal effect of amphetamine on motor behaviors in C57BL/6 mice. Neurosci Lett. 2007;427:66–70. doi: 10.1016/j.neulet.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- 124.Michel A, Tirelli E. Conditioned hyperkinesia induced by cocaine in mice is dose-dependent but not correlated with the unconditioned response or the contextually-sensitized response. Behav Pharmacol. 2002;13:59–71. doi: 10.1097/00008877-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 125.Birgner C, Nordenankar K, Lundblad M, Mendez JA, Smith C, le Greves M, et al. VGLUT2 in dopamine neurons is required for psychostimulant-induced behavioral activation. Proc Natl Acad Sci U S A. 2010;107:389–394. doi: 10.1073/pnas.0910986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fortin GM, Bourque MJ, Mendez JA, Leo D, Nordenankar K, Birgner C, et al. Glutamate corelease promotes growth and survival of midbrain dopamine neurons. J Neurosci. 2012;32:17477–17491. doi: 10.1523/JNEUROSCI.1939-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sakae DY, Marti F, Lecca S, Vorspan F, Martin-Garcia E, Morel LJ, et al. The absence of VGLUT3 predisposes to cocaine abuse by increasing dopamine and glutamate signaling in the nucleus accumbens. Mol Psychiatry. 2015;20:1448–1459. doi: 10.1038/mp.2015.104. [DOI] [PubMed] [Google Scholar]
- 128.De Duve C, De Barsy T, Poole B, Troulet A, Tulkens P, Van Hoof F. Lysosomotrophic agents. Biochem Pharmacol. 1974;23:2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]
- 129.Rayport S, Sulzer D. Visualization of antipsychotic binding to living mesolimbic neurons reveals D2 receptor mediated, acidotropic and lipophilic components. J Neurochem. 1995;65:691–703. doi: 10.1046/j.1471-4159.1995.65020691.x. [DOI] [PubMed] [Google Scholar]
- 130.Tucker KR, Block ER, Levitan ES. Action potentials and amphetamine release antipsychotic drug from dopamine neuron synaptic VMAT vesicles. Proc Natl Acad Sci U S A. 2015;112:E4485–E4494. doi: 10.1073/pnas.1503766112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schmalzing G. The role of a transmembrane pH gradient in uptake and release of imipramine and haloperidol in synaptosomes. Mol Pharmacol. 1988;34:888–895. [PubMed] [Google Scholar]
- 132.Tischbirek CH, Wenzel EM, Zheng F, Huth T, Amato D, Trapp S, et al. Use-dependent inhibition of synaptic transmission by the secretion of intravesicularly accumulated antipsychotic drugs. Neuron. 2012;74:830–844. doi: 10.1016/j.neuron.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 133.Kornhuber J, Schultz A, Wiltfang J, Meineke I, Gleiter CH, Zochling R, et al. Persistence of haloperidol in human brain tissue. Am J Psychiatry. 1999;156:885–890. doi: 10.1176/ajp.156.6.885. [DOI] [PubMed] [Google Scholar]
- 134.Gemperle AY, Enz A, Pozza MF, Luthi A, Olpe HR. Effects of clozapine, haloperidol and iloperidone on neurotransmission and synaptic plasticity in prefrontal cortex and their accumulation in brain tissue: an in vitro study. Neuroscience. 2003;117:681–695. doi: 10.1016/s0306-4522(02)00769-8. [DOI] [PubMed] [Google Scholar]
- 135.Mai JK, Assheuer J, Paxinos G. Atlas of the human brain. 2nd. Amsterdam; Boston: Elsevier Academic Press; 2004. [Google Scholar]
- 136.Paxinos G, Franklin KBJ. Paxinos and Franklin's the Mouse Brain in Stereotaxic Coordinates. 4th. Amsterdam: Academic Press; 2013. [Google Scholar]
- 137.Haber SN, Behrens TEJ. The neural network underlying incentive-based learning: implications for interpreting circuit disruptions in psychiatric disorders. Neuron. 2014;83:1019–1039. doi: 10.1016/j.neuron.2014.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]