ABSTRACT
The ELT-2 GATA factor is the predominant transcription factor regulating gene expression in the C. elegans intestine, following endoderm specification. We comment on our previous study (Wiesenfahrt et al., 2016) that investigated how the elt-2 gene is controlled by END-1, END-3 and ELT-7, the 3 endoderm specific GATA factors that lie upstream in the regulatory hierarchy. We also discuss the unexpected result that ELT-2, if expressed sufficiently early and at sufficiently high levels, can specify the C. elegans endoderm, replacing the normal functions of END-1 and END-3.
KEYWORDS: C. elegans, ELT-2, ELT-7, END-1, END-3, GATA factor, gene regulation, intestine, transcription
The C. elegans endoderm
The intestine (endoderm or E lineage) of the small free-living nematode Caenorhabditis elegans is clonally derived from a single cell (the E blastomere) of the 8 cell embryo1,2 (Fig. 1). The maternal effect genes that pattern the early embryo and that distinguish the fate of the E cell from the fate of its sister and cousin lineages have been studied intensely (for reviews, please see references2,3). In the present commentary, we concern ourselves with events that lie downstream of this early embryonic patterning, namely the zygotic transcription factor network that first specifies the E cell and then drives endoderm differentiation, maintenance and function for the remainder of the animal's lifetime.2,4
Figure 1.
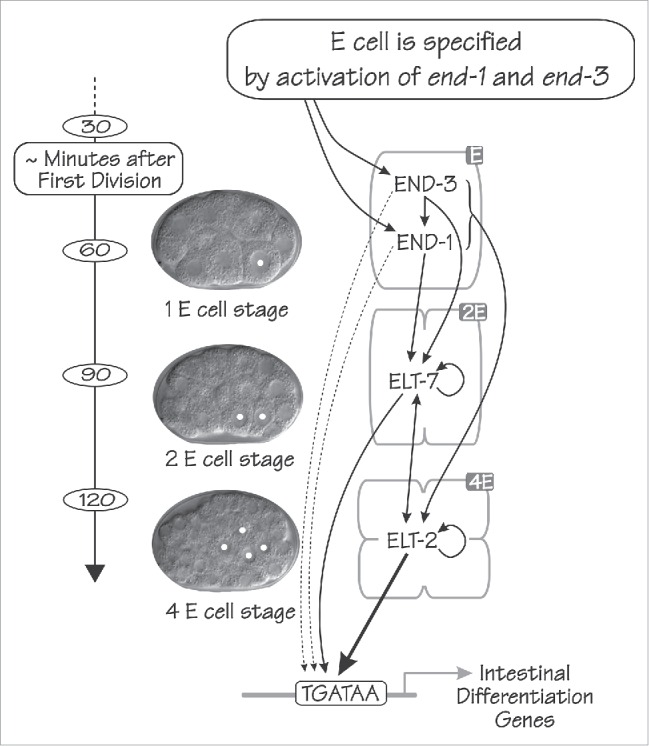
The regulatory network that specifies and differentiates the C. elegans endoderm consists of a cascade of 4 GATA-type transcription factors: END-3, END-1, ELT-7 and ELT-2 in order of appearance. The time scale of early C. elegans embryogenesis is shown at left (minutes at 20°C after the first cell division). In the center are shown 3 differential interference contrast images of early C. elegans embryos, at the 1E, 2E and 4E cell stage from top to bottom; E cell nuclei are marked with white dots. Arrows indicate both proposed and established regulatory relations between the 4 GATA factors, as well as between the 4 GATA factors and genes expressed in the differentiated intestine. (From Wiesenfahrt et al.4).
The major regulators driving the development of the C. elegans endoderm are all GATA-type transcription factors. GATA factors are key transcriptional regulators associated with endoderm throughout the animal kingdom.5 Expression of the C. elegans endodermal GATA factors is completely restricted to the E lineage, with the order of appearance as shown in Figure 1. The fate of the E cell is specified by the expression of the 2 small GATA factors END-1 and END-3.6,7 In the absence of both END-1 and END-3, the E cell is not specified and can adopt the fate of its cousin blastomere C.6,7 Expression of end-1 and end-3 is transient and transcripts disappear within 1–3 cell cycles.8-10 The current model (Fig. 1) is that END-1/3 activate expression of the genes encoding 2 further GATA-type transcription factors: ELT-7 and ELT-2. The elt-7 gene is activated at the 2E cell stage; the elt-2 gene is activated at the 2E cell stage in a few embryos but at the 4E cell stage in most embryos.4,8-11 Both ELT-2 and ELT-7 are expressed throughout the remaining several weeks of the worm's lifespan.4,11,12 ELT-4 is an additional GATA factor expressed in the endoderm but appears to be a truncated upstream duplication of ELT-2 and has been suggested to be an evolutionary relic without detectable function.13
To a first approximation, end-1 and end-3 are redundant6,14 and loss of elt-7 has no obvious effect.12,15 However, closer examination of mutant embryos reveals subtle phenotypes that reflect the inner workings of the endoderm regulatory network and that reveal the reasons for the network's robustness or, under different circumstances, its fragility In contrast to the redundancies seen with end-1 and end-3 mutations and the lack of effect seen with mutation of elt-7, loss of elt-2 causes complete larval arrest shortly after hatching; the brush-border microvilli are stunted and distorted, the intestine lumen is blocked and larvae arrest presumably because of starvation.11 Many intestinal genes respond to both over-expression and under-expression of ELT-2.15 Furthermore, a preferred ELT-2 binding site is highly enriched in intestinal promoters; alteration of these cis-acting sites invariably has a strong effect on target gene expression.15,16 Together, these considerations led us to propose a model in which ELT-2 is directly involved in every act of transcription in the differentiating and mature intestine (with the likely exception of ribosomal genes) at all stages of the life cycle.15,16 This model of course does not rule out roles for other intestine transcription factors (significant roles for ELT-7 have already been described12,17) and an area of current interest is how ELT-2 acts together with the several hundred other transcription factors that have been identified in the intestinal transcriptome, presumably to allow the maturing and mature intestine to meet physiological demands and environmental challenges (as one example, Anderson and Leibold18 describe how ELT-2 might participate in the complex control of iron metabolism genes).
In Wiesenfahrt et al.,4 we were concerned primarily with the role of ELT-2 within the transcriptional network that produces the early embryonic endoderm. We approached the following questions:
How is transcription of the elt-2 gene controlled, both at the level of cis-acting regulatory sites and trans-acting regulatory factors?
Can we obtain any measure for the robustness of the endoderm transcription network? In particular, can the levels of ELT-2 re-establish themselves if perturbed?
Can ELT-2 specify the endoderm lineage, replacing the normal function of END-1 and END-3?
Transcriptional regulation of the elt-2 gene
Ectopic expression of END-1, END-3, ELT-7 or ELT-2 itself can drive expression of an elt-2 promoter::reporter construct throughout the embryo.6,11,12,17,19,20 Such observations are consistent with the model shown in Figure 1 in which END-1 and/or END-3 can “initiate” elt-2 expression in the early embryo, ELT-2 can “maintain” its own expression at later stages and ELT-7 could be involved in both elt-2 initiation and maintenance.12 However, this simple 2-phase initiation-maintenance model must only be a first approximation to the real in vivo situation. For example, the ectopic ELT-2 expression experiment suggests that ELT-2 exerts a positive influence on its own expression but at some point there must be either a delicately balanced ELT-2 degradation pathway or a countervailing transcriptional repression in order to avoid a runaway positive feedback loop. Indeed, we certainly expect that other non-GATA type transcription factors will be involved in maintaining ELT-2 levels after early embryogenesis, responding to physiological or environmental needs.
To define the cis-acting regions that control elt-2 expression, we first compared 5′-flanking regions from the C. elegans elt-2 genes with those of its homologs in other nematodes. In Wiesenfahrt et al.4, we showed a dot-matrix comparison with C. briggsae. Figure 2 shows additional dot-matrix comparisons with the elt-2 genes of C. remanei and C. japonicum.21 In the comparison with C. briggsae,4 3 conserved regions (CR I, CR II and CRIII) could be detected spanning ∼5 kb upstream of the elt-2 genes. These same 3 regions can be seen in the comparison with C. remanei. With the more distant relative C. japonicum, the upstream CRIII region appears to be most highly conserved even in the reduced stringency comparison shown in Figure 2. We performed chromatin immunoprecipitation and sequencing (ChIP-seq) experiments and detected high ELT-2 occupancy in vivo at each of the 3 CR regions of the elt-2 promoter,4 suggesting that these regions contribute to elt-2 maintenance (Fig. 2). The ChIP-Seq data presented in Wiesenfahrt et al.4 and shown in Figure 2 derived from L3 larvae but the same 3 ELT-2 occupancy peaks can also be detected in embryos (EON and JDM, unpublished results).
Figure 2.
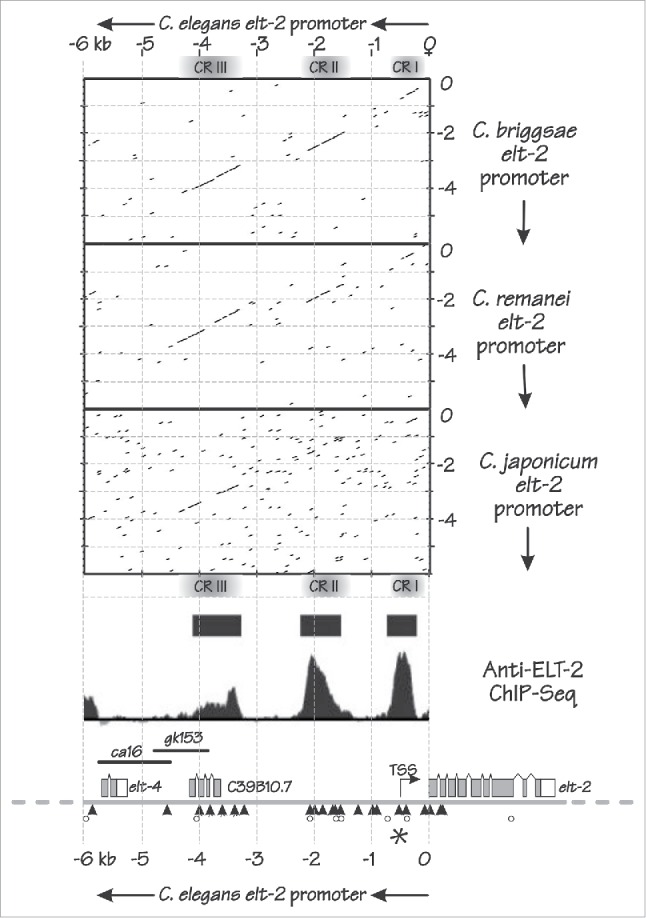
Features of the C. elegans elt-2 promoter. Dot matrix comparisons (EMBOSS dotmatcher) between 6 kb upstream of the C. elegans elt-2 ATG initiation codon and the corresponding 6 kb upstream of the elt-2 homologs in C. briggsae (upper), C. remanei (middle) and C. japonicum (lower). The dot matrix diagonals observed in the C. briggsae and C. remanei comparisons indicate 3 conserved regions (CR I, CR II and CR III); in the (reduced stringency) comparison with the more distantly related C. japonicum, CR III appears the most highly conserved region. The dot matrix plots are aligned with the genomic region containing the C. elegans elt-2 gene, showing the upstream elt-4 gene, the uncharacterized ORF C39B10.7 that appears to overlap with CR III, 2 genomic deletions (ca16 and gk153) that have no obvious effects on elt-2 expression, the elt-2 transcription start site (TSS) and the elt-2 coding region itself. TGATAA sites are indicated by black triangles, WGATAR sites that are not TGATAA sites are indicated by open circles and the unique TGATAA “A” site identified by Du et al.26 is indicated by an asterisk. ELT-2 ChIP-seq reveals 3 regions of high ELT-2 occupancy that align with the 3 conserved regions: solid bars represent peaks called as significant, defined in 4.
Having determined key features of the ELT-2 promoter architecture using sequence conservation and ChIP-seq, we were interested in further dissecting the ELT-2 promoter functionally. To determine the length of the upstream region that influences elt-2 expression, we began with our previous finding that 4.3 kb of the elt-2 5′-flanking region, when fused to the elt-2 coding region tagged with GFP and with an unc-54 3′UTR (integrated into the genome as a multicopy array) is able to rescue the lethality of the elt-2(ca15) mutation (strain JM168; our unpublished results). We also showed that a slightly longer region driving a GFP transcriptional reporter is able to reconstitute the endogenous ELT-2 expression pattern as revealed by a specific anti-ELT-2 antibody.4 Thus a 5 kb 5′-flanking region, which includes all 3 conserved regions, seemed like an appropriate construct with which to begin the promoter analysis. Our analysis proceeded by standard methods that produce extrachromosomal concatenated arrays containing “dozens” to possibly “hundreds” of copies of the plasmid being tested22. Although such multicopy transgenic arrays reproduce the endogenous expression patterns strongly and accurately, they suffer from a major disadvantage: each array is unique for a particular transgenic strain, the copy numbers can differ, and hence expression levels produced by different transgenic constructs can only be compared semi-quantitatively.
We first investigated constructs in which each of the 3 conserved regions was separately fused to a GFP reporter. Reporter expression was correctly restricted to the E lineage but varied significantly in overall activity depending on the tested region. We ranked the activity of the 3 individual conserved regions in the order CR III > CR I >> CR II. CR III could drive reporter expression in the early embryo (∼4E cell stage) continuing into post-hatching larval stages; CR I produced activity that initiated later (16 E cell stage) but was not expressed after hatching; CR II produced no detectable activity on its own. However, when conserved regions were fused pair-wise, it was clear that regions could “synergize,” i.e. the activity of a tandem pair of regions was greater than would be expected judging from the activity of the individual regions. In particular, CR II, which had no activity on its own, could augment the activity of both CR I and CR III. The activities of CR II and CR III were augmented by the presence of CR I, either resulting from its own activity as an enhancer or as a basal promoter. We made no attempt to separate these 2 activities because basal promoter activity in C. elegans seems flexible and, in some cases, even unnecessary.23
In our transgenic assay, CR III appears to be the major enhancer driving expression of the elt-2 gene and, in fact, can function effectively when fused to a naïve basal promoter (the C. elegans heat shock promoter with all heat shock elements removed) in place of the native CR I. We thus chose the CR III region to investigate in more detail. There are 30 potential GATA factor binding sites (WGATAR) within the 5 kb elt-2 5′-flanking region, of which 22 are TGATAA, the site that is highly enriched in intestinal promoters.15,16,24 Four TGATAA sites can be identified in CR III and counterparts of each site can be identified in the CR III sequence of C. briggsae. Ablation of all 4 of these TGATAA sites abolishes enhancer activity of CR III, from which we conclude that CR III contains no other non-TGATAA site whose activity is sufficient for CR III enhancer activity. In fact, even the 3 GATA sites that are not TGATAA do not appear to contribute to CR III enhancer activity. None of the 4 TGATAA sites appear to be “special” because constructs in which TGATAA sites were ablated individually, pairwise or even 3 at a time all retained enhancer activity. Thus, we were led to a model that features parallel, perhaps independent, contributions of each TGATAA site to the overall enhancer activity of CR III. As has been investigated in much more detail in certain yeast promoters,25 these are the properties associated with a robust, graded (i.e., not all-or-none) response of gene activity and might be exactly what is needed to maintain precise quantitative control over ELT-2 levels through the various developmental stages and other vicissitudes of the normal worm's life. In support of such a distributed input model for the enhancer activity of CR III, we showed that END-1, ELT-7 and ELT-2 protein (produced in baculovirus infected insect cells) can bind in vitro to each of the 4 TGATAA sites found in CR III. We have not yet assessed whether the different proteins have different affinities to a particular site; however, we can infer that the different CR III TGATAA sites are bound with only modest differences in affinity (say, 5-10-fold) by each individual protein.
Du et al.26 recently reported that mutation of an ACTGATAAGA site at 527 bps upstream of the ELT-2 ATG (referred to as the “A” site) essentially abolishes elt-2 expression driven by a proximal region of the elt-2 promoter, roughly equivalent to our CR I. The “A” site retained its unique properties within a longer promoter fragment that included CR I and the proximal portion of CR II. However, longer fragments of the elt-2 regulatory region, especially those including CR III, were not investigated. The transgenic technique used by Du et al.26 within the early embryo was commendably quantitative, involving single copy insertions into a standard chromosomal site and then detecting absolute transcript levels by oligonucleotide-based in situ hybridization. Our analysis did not identify this particular site as special if only because we did not look: we showed that the enhancer activity of CR III was not enhanced by fusion to a version of CR I in which 3 conserved TGATAA sites (including the “A” site) as well as a conserved AGATAA site were abolished. That is, our results with CR I are consistent with those of Du et al.26 but our assay was insensitive and designed for a different purpose. Du et al.26 mutated groups of other TGATAA sites in CR I and the proximal portion of CR II, all with the ‘A” site intact but, by and large, saw relatively minor effects; mutations were weakly repressing or weakly activating, depending on context (and also food). Thus it becomes an interesting question exactly what is the basis for the unique behavior of the −527 bp TGATAA site. Perhaps the importance of this site is related to its proximity (∼20 bp) to the initiation site for transcription.27 The importance of this site should not reflect the binding of ELT-2 (which should not have been produced yet) nor the binding of ELT-7 (which is not necessary), leaving END-1 and/or END-3 as possible culprits, and Du et al.26 showed that both proteins can indeed bind in vitro.
Ablation of the unique “A” site within the endogenous elt-2 promoter is now feasible using CRISPR.28 If Du et al.26 are correct, the “A” site knockout should be lethal and the target strain would have to be pre-balanced to survive. Alternatively, if the “A” site knockout is not lethal, perhaps the site is crucial only within the short promoter fragments assayed by Du et al.26 but is not necessary within the broader context of the full promoter. We have recently reported just such a situation: transcriptional control of the C. elegans vit-2 vitellogenin gene has an enhancer in which several factors/sites are crucial when the enhancer is assayed in isolation but the same factors/sites provide only a fraction of the activity of the overall vit-2 promoter.29 At the moment, there are 2 pieces of evidence that the “A” site might not be necessary within the overall elt-2 promoter: (i) We mentioned above the result that CR III can drive endoderm transcription when fused to a naïve basal promoter (i.e. without the natural CR I site and, in fact, without GATA sites)4, and; (ii) a series of unidirectional deletions, beginning from a restriction site immediately downstream of the “A” site and proceeding upstream (i.e., removing the “A” site) is still able to drive reporter expression in the embryonic endoderm (see Supplementary Figure 4 of4). However, we noted several points at which the results from the uni-directional deletions appeared to disagree with results obtained using individual conserved regions, possibly because of the different reporters used. Overall, the CRISPR-based “A” site knockout would still seem the best experiment.
C.elegans promoters are short (an average of only several kb) but the promoters of transcription factors tend to be longer, presumably because they house more complex regulatory circuits.30 It is certainly possible that the 5 kb of elt-2 5′-flanking region investigated by Wiesenfahrt et al.4 could still only be a fraction of the overall elt-2 regulatory region. Immediately upstream of the elt-4 duplication in the C. elegans elt-2 promoter, there is a suspiciously gene-free 5 kb region that should be investigated to see if it too holds sequences that influence elt-2 transcription. Finally, we note that one of the curiousities of conserved region CR III is that it overlaps with a predicted but otherwise unannotated open reading frame (C39B10.7 on Fig. 2). We could detect no obvious phenotype by ablating possible function of this region, either with the chromosomal deletion gk153 (Fig. 2) or by performing RNAi. A low level of transcripts associated with this region can indeed be detected4 but transcripts are often associated with active transcriptional enhancers.31
ELT-2 can specify the C. elegans endoderm
It is a common occurrence in animal development that related transcription factors are expressed in different spatial or temporal patterns. The question naturally arises whether the in vivo functions of these transcription factors differ because of the different expression patterns or because of differences in the intrinsic properties of the 2 proteins. Experimentally, can transcription factor #1 replace transcription factor #2 if expressed under the #2 control region? An early example of such an experiment was to replace the mouse Engrailed En-1 gene with the Engrailed En-2 gene, expressed under the En-1 gene's regulatory sequences.32 In this case, replacement was successful, arguing that, at the protein level, both transcription factors are interchangeable. In other cases, the situation is more complicated. Interchange of GATA factors during mouse hematopoiesis may only give partial rescue but both the results and the interpretations depend on the detailed experimental design.33,34 There have been numerous similar experiments performed over the years with other gene pairs but each situation appears to have its own solution.
We wished to determine if ELT-2, expressed early under the end-1 promoter, can replace all other GATA factors expressed in the endoderm. END-1 and END-3 are quite different from ELT-2 (Fig. 3); within the 25 amino acids of the zinc finger DNA binding domain, 52–56 % of the residues are identical (52–64 % similar); however, the degree of identity/similarity is much lower in other parts of the proteins. Our initial expectation was that END-1 and/or END-3, besides activating the elt-7 and elt-2 genes (Fig. 1), would control a set of genes uniquely associated with endoderm specification; it seemed unlikely that such specification genes could also be controlled by ELT-2, whose normal occupation is to control differentiation genes. To test this expectation, we expressed elt-2 cDNA under control of the end-1 promoter and tested whether this construct could rescue animals that were mutated in end-1, end-3, elt-7 (and, for completeness, elt-4). Owraghi et al.7 had already shown that animals homozygous for end-1 end-3 null mutations were unable to specify the intestine and, as might have been expected, things did not get better when animals were also made homozygous for mutations in elt-7 and elt-4. These animals were initially kept alive by virtue of an extrachromosomal transgenic array expressing the end-1 gene (and a red marker). Into these rescued transgenic worms, we introduced a second multicopy extrachromosomal transgenic array containing the end-1 promoter::elt-2 cDNA + a green marker. Progeny were then examined to see if the red marker (and hence the rescuing end-1 gene) could be lost, leaving behind only the array with the green marker (and the “rescuing” elt-2 gene). In other words, we tested if the end-1 promoter::elt-2 gene could rescue the quadruple elt-7 end-1 end-3; elt-4 mutation. To our surprise, the end-1 promoter::elt-2 could indeed rescue the quadruple mutation. Roughly 85 % of the transgenic animals were rescued and even those that were not rescued did not arrest with an unspecified or mis-specified E blastomere (the phenotype of an end-1 end-3 double) but rather with a distinct identifiable intestine. Any defective phenotypes of the rescued embryos were mild, especially in the early embryo when such defects might have been expected to be greatest; for example, the cell division from 2E cells to 4E cells was slightly precocious, as if transcription of the wee1.1 gene35 has not been activated sufficiently early. Rather curiously, more severe phenotypes (slower hatching times and lower brood sizes) were observed later in development, when it might have been expected that the endogenous elt-2 gene had now taken over and the rescued animal had only smooth sailing ahead. Overall, however, any remaining phenotypes appear remarkably minor. We conclude that there are no genes necessary for C. elegans endoderm specification that cannot be appropriately regulated by ELT-2.
Figure 3.
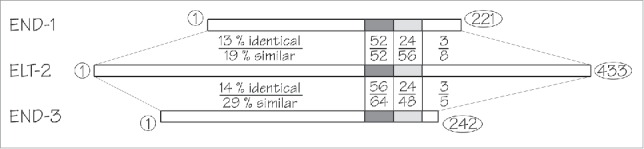
Schematic alignment between the ELT-2 protein and the END-1 and END-3 proteins. The highest degree of conservation (52–56% identical residues) is found in the zinc finger DNA binding domain (dark gray). The adjacent 25 amino acid basic region (light gray) shows lower levels of conservation (24% identical residues). Remaining regions of the proteins (unshaded) show low levels of conservation. The number of amino acid residues in each protein is shown beside their respective C-termini.
We also showed that elt-7 cDNA expressed under control of the end-1 promoter can, like the counterpart construct with elt-2 cDNA, also rescue the lethality associated with end-1 end-3 mutations. However, ELT-7 cannot maintain endoderm differentiation in the long run and requires ELT-2. On the other hand, not just any GATA factor can rescue the mutations: when the same type of rescue experiments were attempted with the C. elegans hypodermal GATA factor ELT-336 or the mouse endodermal GATA factor mGATA4, both expressed under control of the end-1 promoter, no rescue was observed.
Although rescue of the quadruple elt-7 end-1 end-3; elt-4 mutant is efficient when the end-1 promoter::elt-2 cDNA construct is present as a multicopy array (either extra-chromosomal or integrated into the genome), a single copy inserted into the genome37 is unable to rescue. Perhaps ELT-2 regulation of endoderm specification genes is less efficient than their normal regulation under control of END-1/END-3 and such inefficiency might require higher ELT-2 levels to compensate. Antibody staining shows that ELT-2 protein in the rescued embryos can always be detected at the 2E cell stage, at roughly the level it can ordinarily be detected at the 8E cell stage of a non-transgenic wildtype embryo, i.e. ELT-2 is over-expressed but not massively so. A further interesting feature of the rescue is that the antibody cannot detect ELT-2 protein at the 1E cell stage, suggesting that perhaps the rescued endoderm is specified at the 2E cell stage under these conditions, not at the 1E cell stage as in wildtype embryos. (We note that ELT-2 protein could be detected at the 1E cell stage before the end-1 promoter::elt-2 multicopy array had been integrated into the genome; such decrease in expression upon array integration is not uncommon).
Possible insights into the evolution and robustness of the endoderm gene regulatory network
We had previously speculated on possible reasons why the C. elegans endoderm regulatory pathway involves multiple GATA factors (END-1, END-3, ELT-7 and ELT-2, not counting ELT-4) when ELT-2 can apparently perform all the necessary functions by itself, at least when expressed sufficiently early and at sufficiently high levels. One potential explanation is that redundant pathways are capable of producing greater fidelity in development;38 in the present case, perhaps the redundancies demonstrated between end-1 and end-36 14 and between elt-2 and elt-712,15 lower the endoderm specification failure rate sufficiently for a multi-GATA-factor endoderm to be selected. As an aside, because END-3 can activate the end-1 gene, END-1 and END-3 can activate both elt-7 and elt-2 genes, ELT-2 and ELT-7 can also activate the elt-2 gene, and because all 4 factors can probably contribute to the activation of terminally differentiated genes, the network should be described as illustrating “distributed robustness” rather than simply redundancy;39,40 for example, compare the network topology of Figure 1 with that shown schematically in Figure 2 of Felix and Wagner.40 A second possible explanation was to free the elt-2 gene from control by maternal SKN-1 and POP-1, 2 major transcription factors that each show a maternal and zygotic expression phase. A third explanation may only be a molecularly oriented view of distributed robustness and developmental fidelity. Perhaps during the rapid early phases of cell division that characterize the C. elegans embryo, 2 smaller proteins such as END-1 and END-3 are able to specify endoderm more efficiently and more definitively than could the larger ELT-2 protein. An interesting implication of such a mechanism can be considered. Maduro41 has cited his unpublished analysis of nematode endodermal GATA factors and reports that the small end-1/3 like genes (and the even smaller upstream med-1/2 genes) are not found outside of the elegans supergroup of Caenorhabditis species, presumably all of which feature rapid embryonic development. In Ascaris suum, the early embryonic cell divisions require most of a day,42 compared to the 15–20 minutes found in C. elegans.1 It would be an interesting experiment to determine if slower developing nematodes such as Ascaris actually use ELT-2 to specify their intestine, in addition to using ELT-2 for its conserved role in intestinal differentiation. Results in the literature are mildly encouraging for such a suggestion. Embryogenesis in the sheep parasite Haemonchus contortus lasts 5-10-fold longer than does embryogenesis in C. elegans43 and presumably so do the early embryonic cell divisions. In C. elegans, ELT-2 is first detected in the 4E cell stage embryo in most embryos but in H. contortus embryos, the ELT-2 homolog first makes its appearance at the 2E cell stage44 (but apparently not at the 1E cell stage.)
What does the ability of ELT-2 to both specify and differentiate the C. elegans endoderm reveal about network stability or fragility? As noted earlier, we were surprised that the ELT-2 rescue of the quadruple elt-7 end-1 end-3; elt-4 mutant seemed to be so robust. Even the 15% of embryos that arrested still had obviously specified and apparently well-differentiated intestines. We did not observe any obvious switch of E-to-C cell fate, as had been reported for the end-1 end-3 double mutant.6,7 And yet, there is extensive evidence that defects in the normal specification process, such as mutations in end-3,6 or engineering end-1 and end-3 to be insensitive to med-1/2 activation,3 or combining end-1 or end-3 mutations with defects in upstream signaling pathways,3,45,46 can result in significant defects in endoderm formation, whether measured as penetrance (i.e., the % of embryos that do not express a particular differentiation marker such as gut granules) or as expressivity (e.g. the variation in the number of endoderm cells, both above and below the normal number). The endoderm pathway seems most fragile during its earliest phases and it is perhaps here that both redundancy and rapidity are most important. Under conditions where a fraction of the embryos do not produce endoderm, do the remaining fraction of embryos still produce “perfect endoderm” nonetheless; in other words, can endoderm formation be represented as “all or none”? Strictly no (see, for example, Maduro et al.3 and Robertson et al.35) and yet it remains an impressive approximation, testifying to the robustness of a developmental lineage and to some stalwart inner property of the gene regulatory network. Much like Monte Python's Black Knight progressively diminishing by the actions of King Arthur's sword, the progressive removal of the input pathways to endoderm specification still seems to leave a defiant remnant. Indeed, even an imperfect endoderm can be shown to correct itself. For example, it had been previously shown that ELT-2 levels in an end-3 mutant embryo, in the middle of embryogenesis, were reduced by ∼50%.14 In Wiesenfahrt et al.,4 we showed that ELT-2 levels in end-3 embryos have re-established themselves to wildtype levels by the time the mutant animals hatch several hours later. Other defects remain and perhaps these can be ascribed, not to an imperfect ability to re-establish the normal state of the gene regulatory network but to irreversible cellular defects that occur early during the most fragile periods of endoderm formation.
Stability and robustness of regulatory hierarchies are important phenomena both to define theoretically and to analyze experimentally. The same considerations, writ much larger, must also contribute to birth defects in humans.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The majority of the work discussed in this article was supported by an operating grant (MOP 67135) from the Canadian Institutes of Health Research to JDM; the Chip-Seq/RNA-Seq experiments were funded by NIH grant 5R01GM104050. EON was supported by a Damon Runyon Cancer Research Foundation Postdoctoral Fellowship Award (2083-11). JDM gratefully acknowledges salary support from the Alberta Heritage Foundation for Medical Research (now AIHS) and from the Canada Research Chairs Program.
References
- [1].Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol 1983; 100:64-119; PMID:6684600; http://dx.doi.org/ 10.1016/0012-1606(83)90201-4 [DOI] [PubMed] [Google Scholar]
- [2].McGhee JD. The Caenorhabditis elegans intestine. Wiley Interdiscip Rev 2013; 2:347-67; PMID:23799580; http://dx.doi.org/ 10.1002/wdev.93 [DOI] [PubMed] [Google Scholar]
- [3].Maduro MF, Broitman-Maduro G, Choi H, Carranza F, Wu AC, Rifkin SA. MED GATA factors promote robust development of the C. elegans endoderm. Dev Biol 2015; 404:66-79; PMID:25959238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wiesenfahrt T, Berg JY, Osborne Nishimura E, Robinson AG, Goszczynski B, Lieb JD, McGhee JD. The function and regulation of the GATA factor ELT-2 in the C. elegans endoderm. Development 2016; 143:483-91; PMID:26700680; http://dx.doi.org/ 10.1242/dev.130914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Patient RK, McGhee JD. The GATA family (vertebrates and invertebrates). Curr Opin Genet Dev 2002; 12:416-22; PMID:12100886; http://dx.doi.org/ 10.1016/S0959-437X(02)00319-2 [DOI] [PubMed] [Google Scholar]
- [6].Maduro MF, Hill RJ, Heid PJ, Newman-Smith ED, Zhu J, Priess JR, Rothman JH. Genetic redundancy in endoderm specification within the genus Caenorhabditis. Dev Biol 2005; 284:509-22; PMID:15979606; http://dx.doi.org/ 10.1016/j.ydbio.2005.05.016 [DOI] [PubMed] [Google Scholar]
- [7].Owraghi M, Broitman-Maduro G, Luu T, Roberson H, Maduro MF. Roles of the Wnt effector POP-1/TCF in the C. elegans endomesoderm specification gene network. Dev Biol 2009; PMID:19818340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Baugh LR, Hill AA, Claggett JM, Hill-Harfe K, Wen JC, Slonim DK, Brown EL, Hunter CP. The homeodomain protein PAL-1 specifies a lineage-specific regulatory network in the C. elegans embryo. Development 2005; 132:1843-54; PMID:15772128; http://dx.doi.org/ 10.1242/dev.01782 [DOI] [PubMed] [Google Scholar]
- [9].Raj A, Rifkin SA, Andersen E, van Oudenaarden A. Variability in gene expression underlies incomplete penetrance. Nature 2010; 463:913-8; PMID:20164922; http://dx.doi.org/ 10.1038/nature08781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nair G, Walton T, Murray JI, Raj A. Gene transcription is coordinated with, but not dependent on, cell divisions during C. elegans embryonic fate specification. Development 2013; 140:3385-94; PMID:23863485; http://dx.doi.org/ 10.1242/dev.098012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fukushige T, Hawkins MG, McGhee JD. The GATA-factor elt-2 is essential for formation of the Caenorhabditis elegans intestine. Dev Biol 1998; 198:286-302; PMID:9659934 [PubMed] [Google Scholar]
- [12].Sommermann EM, Strohmaier KR, Maduro MF, Rothman JH. Endoderm development in Caenorhabditis elegans: the synergistic action of ELT-2 and -7 mediates the specification–>differentiation transition. Dev Biol 2010; 347:154-66; PMID:20807527; http://dx.doi.org/ 10.1016/j.ydbio.2010.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fukushige T, Goszczynski B, Tian H, McGhee JD. The evolutionary duplication and probable demise of an endodermal GATA factor in Caenorhabditis elegans. Genetics 2003; 165:575-88; PMID:14573471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Boeck ME, Boyle T, Bao Z, Murray J, Mericle B, Waterston R. Specific roles for the GATA transcription factors end-1 and end-3 during C. elegans E-lineage development. Dev Biol 2011; 358:345-55; PMID:21854766; http://dx.doi.org/ 10.1016/j.ydbio.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].McGhee JD, Sleumer MC, Bilenky M, Wong K, McKay SJ, Goszczynski B, Tian H, Krich ND, Khattra J, Holt RA, et al.. The ELT-2 GATA-factor and the global regulation of transcription in the C. elegans intestine. Dev Biol 2007; 302:627-45; PMID:17113066; http://dx.doi.org/ 10.1016/j.ydbio.2006.10.024 [DOI] [PubMed] [Google Scholar]
- [16].McGhee JD, Fukushige T, Krause MW, Minnema SE, Goszczynski B, Gaudet J, Kohara Y, Bossinger O, Zhao Y, Khattra J, et al.. ELT-2 is the predominant transcription factor controlling differentiation and function of the C. elegans intestine, from embryo to adult. Dev Biol 2009; 327:551-65; PMID:19111532; http://dx.doi.org/ 10.1016/j.ydbio.2008.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Riddle MR, Weintraub A, Nguyen KC, Hall DH, Rothman JH. Transdifferentiation and remodeling of post-embryonic C. elegans cells by a single transcription factor. Development 2013; 140:4844-9; PMID:24257624; http://dx.doi.org/ 10.1242/dev.103010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Anderson CP, Leibold EA. Mechanisms of iron metabolism in Caenorhabditis elegans. Front Pharmacol 2014; 5:113; PMID:24904417; http://dx.doi.org/ 10.3389/fphar.2014.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhu J, Fukushige T, McGhee JD, Rothman JH. Reprogramming of early embryonic blastomeres into endodermal progenitors by a Caenorhabditis elegans GATA factor. Genes Dev 1998; 12:3809-14; PMID:9869634; http://dx.doi.org/ 10.1101/gad.12.24.3809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fukushige T, Hendzel MJ, Bazett-Jones DP, McGhee JD. Direct visualization of the elt-2 gut-specific GATA factor binding to a target promoter inside the living Caenorhabditis elegans embryo. Proc Natl Acad Sci U S A 1999; 96:11883-8; PMID:10518545; http://dx.doi.org/ 10.1073/pnas.96.21.11883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kiontke KC, Felix MA, Ailion M, Rockman MV, Braendle C, Penigault JB, Fitch DH. A phylogeny and molecular barcodes for Caenorhabditis, with numerous new species from rotting fruits. BMC Evol Biol 2011; 11:339; PMID:22103856; http://dx.doi.org/ 10.1186/1471-2148-11-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO Journal 1991; 10:3959-70; PMID:1935914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wenick AS, Hobert O. Genomic cis-regulatory architecture and trans-acting regulators of a single interneuron-specific gene battery in C. elegans. Dev Cell 2004; 6:757-70; PMID:15177025; http://dx.doi.org/ 10.1016/j.devcel.2004.05.004 [DOI] [PubMed] [Google Scholar]
- [24].Pauli F, Liu Y, Kim YA, Chen PJ, Kim SK. Chromosomal clustering and GATA transcriptional regulation of intestine-expressed genes in C. elegans. Development 2006; 133:287-95; PMID:16354718; http://dx.doi.org/ 10.1242/dev.02185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Stewart-Ornstein J, Nelson C, DeRisi J, Weissman JS, El-Samad H. Msn2 coordinates a stoichiometric gene expression program. Curr Biol 2013; 23:2336-45; PMID:24210615; http://dx.doi.org/ 10.1016/j.cub.2013.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Du L, Tracy S, Rifkin SA. Mutagenesis of GATA motifs controlling the endoderm regulator elt-2 reveals distinct dominant and secondary cis-regulatory elements. Dev Biol 2016; 412:160-70; PMID:26896592; http://dx.doi.org/ 10.1016/j.ydbio.2016.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kruesi WS, Core LJ, Waters CT, Lis JT, Meyer BJ. Condensin controls recruitment of RNA polymerase II to achieve nematode X-chromosome dosage compensation. eLife 2013; 2:e00808; PMID:23795297; http://dx.doi.org/ 10.7554/eLife.00808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Xu S. The application of CRISPR-Cas9 genome editing in Caenorhabditis elegans. J Genet Genomics 2015; 42:413-21; PMID:26336798; http://dx.doi.org/ 10.1016/j.jgg.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Goszczynski B, Captan VV, Danielson AM, Lancaster BR, McGhee JD. A 44 bp intestine-specific hermaphrodite-specific enhancer from the C. elegans vit-2 vitellogenin gene is directly regulated by ELT-2, MAB-3, FKH-9 and DAF-16 and indirectly regulated by the germline, by daf-2/insulin signaling and by the TGF-beta/Sma/Mab pathway. Dev Biol 2016; 413:112-27; PMID:26963674; http://dx.doi.org/ 10.1016/j.ydbio.2016.02.031 [DOI] [PubMed] [Google Scholar]
- [30].Nelson CE, Hersh BM, Carroll SB. The regulatory content of intergenic DNA shapes genome architecture. Genome Biol 2004; 5:R25; PMID:15059258; http://dx.doi.org/ 10.1186/gb-2004-5-4-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Arner E, Daub CO, Vitting-Seerup K, Andersson R, Lilje B, Drablos F, Lennartsson A, Ronnerblad M, Hrydziuszko O, Vitezic M, et al.. Gene regulation. Transcribed enhancers lead waves of coordinated transcription in transitioning mammalian cells. Science 2015; 347:1010-4; PMID:25678556; http://dx.doi.org/ 10.1126/science.1259418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hanks M, Wurst W, Anson-Cartwright L, Auerbach AB, Joyner AL. Rescue of the En-1 mutant phenotype by replacement of En-1 with En-2 [see comments]. Science 1995; 269:679-82; PMID:7624797; http://dx.doi.org/ 10.1126/science.7624797 [DOI] [PubMed] [Google Scholar]
- [33].May G, Soneji S, Tipping AJ, Teles J, McGowan SJ, Wu M, Guo Y, Fugazza C, Brown J, Karlsson G, et al.. Dynamic analysis of gene expression and genome-wide transcription factor binding during lineage specification of multipotent progenitors. Cell stem cell 2013; 13:754-68; PMID:24120743; http://dx.doi.org/ 10.1016/j.stem.2013.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ferreira R, Wai A, Shimizu R, Gillemans N, Rottier R, von Lindern M, Ohneda K, Grosveld F, Yamamoto M, Philipsen S. Dynamic regulation of Gata factor levels is more important than their identity. Blood 2007; 109:5481-90; PMID:17327407; http://dx.doi.org/ 10.1182/blood-2006-11-060491 [DOI] [PubMed] [Google Scholar]
- [35].Robertson SM, Medina J, Lin R. Uncoupling different characteristics of the C. elegans E lineage from differentiation of intestinal markers. PLoS One 2014; 9:e106309; PMID:25181289; http://dx.doi.org/ 10.1371/journal.pone.0106309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gilleard JS, McGhee JD. Activation of hypodermal differentiation in the Caenorhabditis elegans embryo by GATA transcription factors ELT-1 and ELT-3. Mol Cell Biol 2001; 21:2533-44; PMID:11259601; http://dx.doi.org/ 10.1128/MCB.21.7.2533-2544.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Frokjaer-Jensen C, Davis MW, Sarov M, Taylor J, Flibotte S, LaBella M, Pozniakovsky A, Moerman DG, Jorgensen EM. Random and targeted transgene insertion in Caenorhabditis elegans using a modified Mos1 transposon. Nat Methods 2014; 11:529-34; PMID:24820376; http://dx.doi.org/ 10.1038/nmeth.2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nowak MA, Boerlijst MC, Cooke J, Smith JM. Evolution of genetic redundancy. Nature 1997; 388:167-71; PMID:9217155; http://dx.doi.org/ 10.1038/40618 [DOI] [PubMed] [Google Scholar]
- [39].Wagner A. Robustness and Evolvability in Living Systems. Princeton, NJ: Princeton University Press, 2005. [Google Scholar]
- [40].Felix MA, Wagner A. Robustness and evolution: concepts, insights and challenges from a developmental model system. Heredity 2008; 100:132-40; PMID:17167519; http://dx.doi.org/ 10.1038/sj.hdy.6800915 [DOI] [PubMed] [Google Scholar]
- [41].Maduro MF. Developmental robustness in the Caenorhabditis elegans embryo. Mol Reprod Dev 2015; 82:918-31; PMID:26382067; http://dx.doi.org/ 10.1002/mrd.22582 [DOI] [PubMed] [Google Scholar]
- [42].Azzaria M, McGhee JD. DNA synthesis in the early embryo of the nematode Ascaris suum. Dev Biol 1992; 152:89-93; PMID:1628758; http://dx.doi.org/ 10.1016/0012-1606(92)90158-D [DOI] [PubMed] [Google Scholar]
- [43].Khatun F, Begum N, Akter S, Mondal M. in vitro study of environmental and nutritional factors on the hatching and development of eggs of Haemonchus contortus. Banglasesh Vet 2013; 30:1-9. [Google Scholar]
- [44].Couthier A, Smith J, McGarr P, Craig B, Gilleard JS. Ectopic expression of a Haemonchus contortus GATA transcription factor in Caenorhabditis elegans reveals conserved function in spite of extensive sequence divergence. Mol Biochem Parasitol 2004; 133:241-53; PMID:14698436; http://dx.doi.org/ 10.1016/j.molbiopara.2003.10.012 [DOI] [PubMed] [Google Scholar]
- [45].Maduro MF, Kasmir JJ, Zhu J, Rothman JH. The Wnt effector POP-1 and the PAL-1/Caudal homeoprotein collaborate with SKN-1 to activate C. elegans endoderm development. Dev Biol 2005; 285:510-23; PMID:16084508; http://dx.doi.org/ 10.1016/j.ydbio.2005.06.022 [DOI] [PubMed] [Google Scholar]
- [46].Maduro MF, Broitman-Maduro G, Mengarelli I, Rothman JH. Maternal deployment of the embryonic SKN-1–>MED-1,2 cell specification pathway in C. elegans. Dev Biol 2007; 301:590-601; PMID:16979152; http://dx.doi.org/ 10.1016/j.ydbio.2006.08.029 [DOI] [PubMed] [Google Scholar]


