Abstract
Optic neuritis (ON) is an acquired disorder of the optic nerve due to inflammation, demyelination or degeneration. We report a child who presented with acute onset bilateral visual loss who, following a diagnosis of ON, was treated and had excellent visual recovery. Paediatric ON is considered to be different clinical entity to adult ON. Although in children ON is usually parainfectious or postinfectious, it can be the first presenting feature of multiple sclerosis or neuromyelitis optica spectrum disease. In this paper, we discuss the literature on treatment of ON and prediction of risk of recurrence.
Background
Optic neuritis (ON) is an important cause of unilateral or bilateral acute onset visual loss in children. It can occur in isolation or in association with systemic autoimmune conditions. ON has an incidence of two per million children per year.1 Although ON in children is usually parainfectious or postinfectious, it can be the first presenting feature of multiple sclerosis (MS) or neuromyelitis optica spectrum disease (NMOSD). Unlike adults there is no established treatment consensus in children though treatment with corticosteroids is recommended.2
Case presentation
A 7-year-old girl presented with acute severe deterioration of her vision over the course of 3 days. She was born in the UK to Egyptian parents and prior to this, she had no visual symptoms. She had noticed slight blurring of her vision 3 days previously but had not mentioned this to her parents. On the day before presentation, she told her dad that she could not watch TV properly ‘as everything was blurry’. On the day of her presentation, her teacher called the parents from school as the girl could not read or write and was unable to see the lines in her exercise book. Parents had brought samples of her writing from that week (figure 1), showing progressive deterioration of her handwriting over the course of 3 days. There was no history of preceding illness. There was no family history of autoimmunity and there was no relevant social history.
Figure 1.
Sample of patient's handwriting showing deterioration over 3 days.
On examination, right visual acuity was perception of light only, and on the left counting fingers at 20 cm with minimally preserved colour vision. Acuity on formal testing was 1/60 bilaterally (log MAR 1.0). Conjugate eye movements were full range and pain free.
No conjunctival congestion or lid swelling was noted. Funduscopy demonstrated bilateral optic disc swelling (figure 2). A relative afferent pupillary defect (RAPD) was noted in the right eye. Colour vision (Ishihara) at presentation was right 0/17 and left 1/17 (ie, test plate only). At presentation, near visual acuity was right: not possible, left N48.
Figure 2.
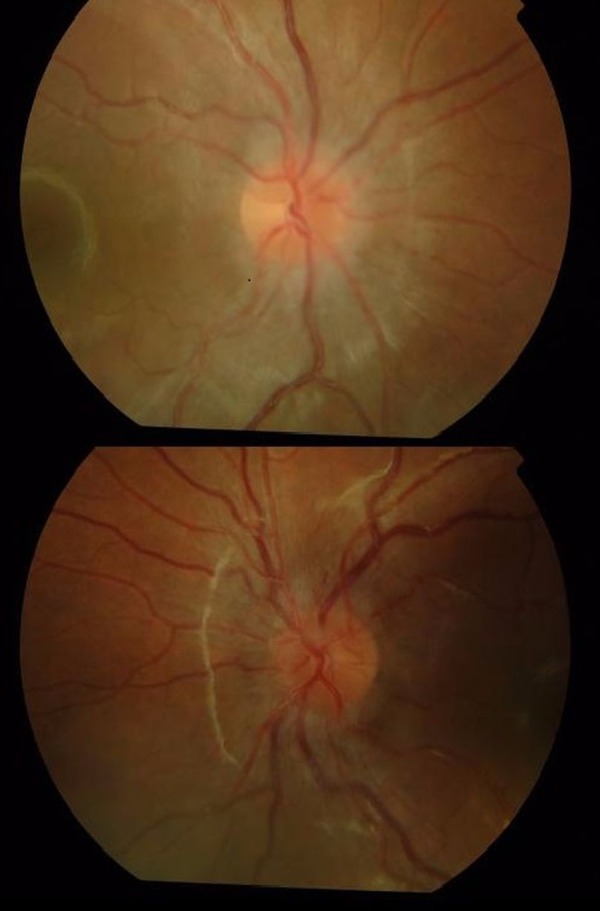
Right and left retinal photographs of posterior pole of the eye, 2 days after presentation. The optic disc is central in the image. There is mild disc swelling and loss of the clear disc margins with softening of the edges.
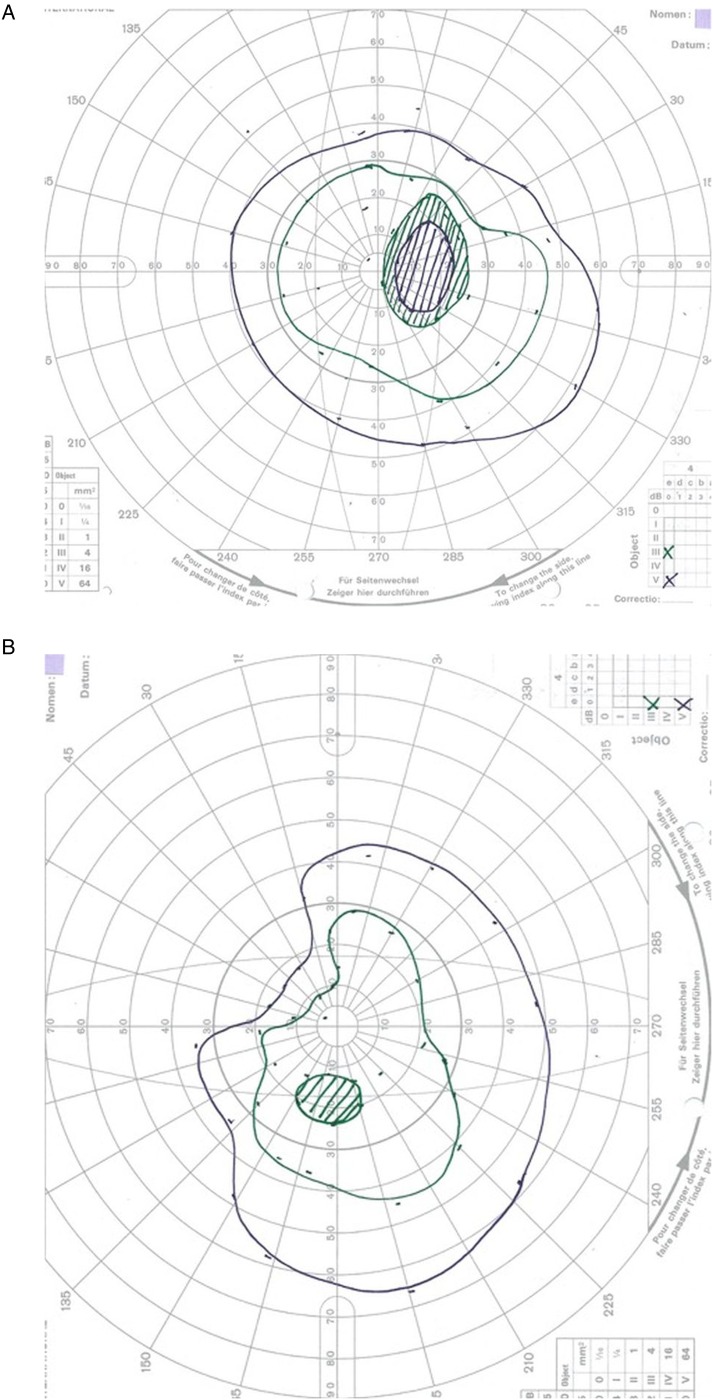
Goldmann visual fields on day 2 of admission shows enlarged blind spot, plus constriction in both eyes (figure 3A, B).
Figure 3.
(A and B) Goldmann visual fields in the acute phase showing enlarged blind spot plus constriction in both eyes. The green isopter is III4e, and the purple is V4e.
Rest of the neurological examination was normal. Coordination and gait could not be adequately tested due to her marked visual impairment, but no significant cerebellar signs were observed.
Investigations
Blood inflammatory markers were normal. Blood analysis was also negative for viruses, mycoplasma and Lyme serology. Autoantibodies and aquaporin antibodies were negative. Cerebrospinal fluid (CSF) analysis showed normal cell count and protein, negative microscopy and virology. Oligoclonal bands were negative in the CSF.
A gadolinium-enhanced, fat suppressed MRI (figure 4) showed bilaterally abnormal optic nerve enhancement in keeping with acute bilateral ON.
Figure 4.
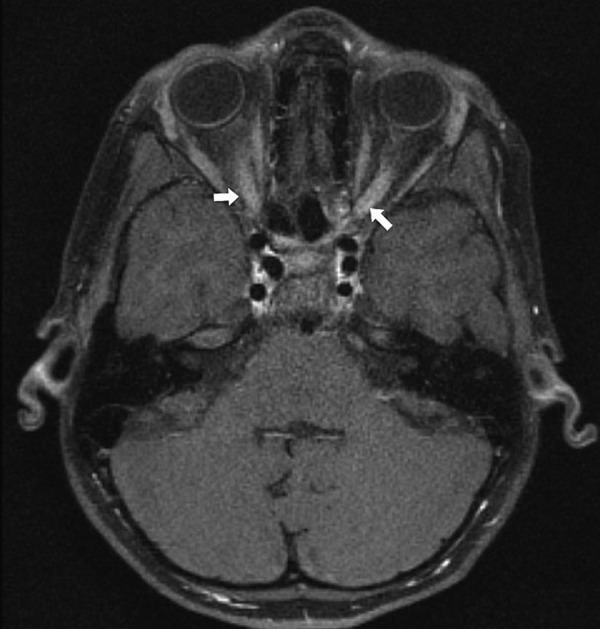
MRI brain with gadolinium showing bilateral postcontrast enhancement of optic nerves.
Differential diagnosis
A number of conditions can present with acute onset visual loss and these must be considered in the differential diagnosis. Table 1 lists the differential diagnosis and appropriate investigations to be considered.
Table 1.
Differential diagnosis of ON in children (adapted from Shams and Plant3)
| Diagnostic aids | ||
|---|---|---|
| Corticosteroid-responsive optic neuropathy | Sarcoidosis, systemic lupus erythematosus, Behçet's syndrome NMOSD |
Serum ACE ANA, dsDNA, lupus anticoagulant, anticardiolipin antibody Aquaporin 4 antibody |
| Other inflammatory conditions | Postinfection, postvaccination, neuroretinitis, acute disseminated encephalomyelitis | History Blood/CSF analysis MRI brain |
| Compressive optic neuropathies | Primary optic nerve tumours, gliomas, meningioma, craniopharyngioma, arterial aneurysms | MRI brain and orbits MRA/angiogram |
| Infections | Tuberculosis, Lyme disease, toxocariasis or helminthitis (usually visible retinal/optic head lesion) | Serology |
| Infiltrative | Leukaemia Lymphoma |
MRI brain Blood/CSF analysis |
| Nutritional | Vitamin B12 deficiency | Vitamin B12 level |
| Toxic | Ethambutol toxicity | History |
| Genetic | Leber hereditary optic neuropathy Biotinidase deficiency |
DNA analysis Serum biotinidase |
| Factitious visual loss | Diagnosis of exclusion |
NMOSD, neuromyelitis optica spectrum disease; ON, optic neuritis.
Treatment
She was treated with a standard regime of intravenous methylprednisolone, followed by oral prednisolone that was weaned over 4 weeks.
Outcome and follow-up
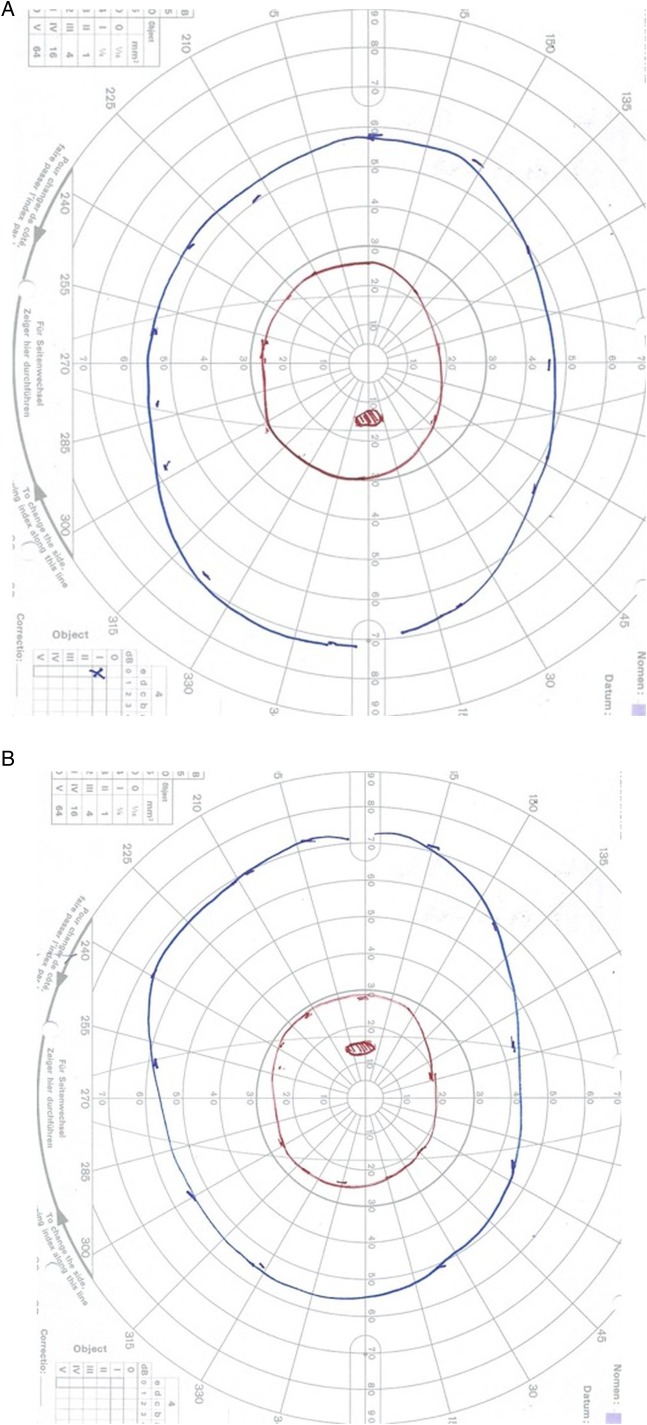
Her visual acuity after 10 days was 6/60 bilaterally, and it gradually improved with time. At follow-up, near visual acuity was right N5, left N5 (ie, normal). Colour vision (Ishihara) at follow-up (5 months later) was right 13/13, left 13/13. She had normal Goldman visual fields at 18 months follow-up in both eyes (figure 5A, B).
Figure 5.
(A and B) Normal goldmann visual fields in both eyes at 18 months follow-up. The blue isopter is I4e, and the red is I2e. The red is the smaller of the two targets.
Her neurological examination remained normal.
In summary, our patient had idiopathic ON from which she had made a full recovery with no relapses at 5-year follow-up.
Discussion
Clinical presentation
The common presentation is acute or subacute onset bilateral or unilateral visual loss. The diagnostic criteria for ON are acute or subacute loss of vision and ≥1 of: RAPD (unilateral cases), visual field deficit or scotoma, impaired colour vision, optic disc oedema or abnormal visual evoked potentials. MRI is not necessary for diagnosis. Visual acuity at presentation is usually severely affected, 6/60 or worse in 70–100%.4–7 In addition to decreased visual acuity, children can have orbital pain/painful eye movements, decreased colour vision and contrast sensitivity, photopsia and a spectrum of visual field defects.
Average age of presentation is 8.6–11.8 years.4 8–13 Bilateral involvement may occur simultaneously or sequentially and is seen in 40–70% of cases.4 10–14 Younger children (<10 years of age) are more likely to have bilateral ON.14 A history of preceding upper respiratory tract infection is reported in 30–70% of cases7 15–17 and there may also have preceding headache/supraorbital pain or painful eye movements.10 18
Investigation
MRI brain and orbit with contrast should be performed in all cases not only to diagnose ON but also to exclude any other intracranial pathology and look for any other signs of demyelination. Blood and CSF analysis must be performed to look for infection and inflammation. Visual evoked potential can be useful in very young children. Optical coherence tomography can provide specific diagnostic clues to differentiate MS–ON and NMOSD–ON as discussed below.19 20
Treatment
Unlike the ONTT (Optic Neuritis Treatment Trial) study in adults, there have been no clinical trials in children looking into treatment of ON. Current clinical practice vary from a wait and watch approach for unilateral and mild bilateral cases to treatment with corticosteroids in severe ON.8 9 21 The regime commonly described in literature is a course of intravenous methylpredisolone 30 mg/kg/day for 3–5 days followed by oral prednisolone at 1 mg/kg/day with slow wean over 4–6 weeks. It is important to recognise that the response to treatment can vary due to underlying aetiology and these must be investigated appropriately.
Prognosis
Children with idiopathic ON, even those with severe visual loss, have very good recovery of visual function. The visual recovery spontaneously begins at 2–3 weeks and can continue up to 2 years. Visual recovery to 6/12 or better is seen in 70–85%.4 6 22 23 A single series5 reported that patients with unilateral disease had an excellent prognosis (100% had better than 6/12) compared to those with bilateral disease (only 50% ≥6/12 and 35% >6/60). Colour vision defects commonly persist.4 Younger children (<6 years) have better visual prognosis than older children.22 After an episode of ON, mild pallor may persist in the temporal optic disc along with RAPD and perception of Uhtoffs phenomenon.7
Long-term risk prediction
In children with first episode of ON, it is important to be able to distinguish whether an episode of ON is due to MS or NMOSD as the therapeutic options differ and in fact interferons can worsen NMOSD.24–27
MS versus NMOSD
The two consistent risk factors for MS following an episode of ON include positive MRI brain lesions at presentation of ON7 15 and presence of CSF oligoclonal bands.18 28 29 It remains unclear if the age of onset of ON influences the future risk of MS.8 14
Risk for NMOSD is significant if the child has positive Aquaporin 4 IgG antibodies at presentation of ON.24 30 Optic nerve involvement in NMOSD in comparison to MS is more likely to be recurrent, often simultaneously bilateral, involves the chiasm and cause altitudinal visual field defects with severe residual visual loss.31–33
A review by Bennett et al has shown that the retinal nerve fibre layer (RNFL) is significantly reduced in patients with NMOSD with ON compared to healthy controls and that RNFL thinning may be an early phenomenon.34 NMO–ON affects the entire peripapillary RNFL with particular involvement of the superior and inferior quadrant in comparison to MS–ON where temporal quadrant is more affected.34 Macular thinning is more severe in NMO–ON than in MS–ON, in keeping with the poorer visual recovery observed in ON with NMOSD. Patients with NMOSD are also more likely to develop microcystic macular oedema by ocular coherence tomography in affected eyes.34 35
More recently, MOG antibodies have also been identified in children with ON, recurrent ON and NMOSD syndrome who are Aquaporin 4 antibody negative.36 37 Although the precise predictive value of these antibodies remains to be determined, these children appear to have a non-MS course of demyelination.37
Learning points.
Optic neuritis is rare in children but early recognition is important for diagnosis and prompt treatment.
Most children have very good recovery of visual function.
Optic neuritis could be the first presentation of serious underlying disorder like multiple sclerosis or neuromyelitis optica spectrum disease; so, detailed investigations of the first presentation and appropriate follow-up are important.
Footnotes
Contributors: SR and ML were responsible for conception of the case report. SR drafted the initial manuscript. DM, MA and ML revised the manuscript critically.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Banwell B, Kennedy J, Sadovnick D et al. Incidence of acquired demyelination of the CNS in Canadian children. Neurology 2009;72:232–9. 10.1212/01.wnl.0000339482.84392.bd [DOI] [PubMed] [Google Scholar]
- 2.Waldman AT, Gorman MP, Rensel MR et al. Management of pediatric central nervous system demyelinating disease: consensus of United States neurologists. J Child Neurol 2011;26:675–82. 10.1177/0883073810395141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shams PN, Plant GT. Optic neuritis: a review. Int MS J 2009;16:82–9. [PubMed] [Google Scholar]
- 4.Absoud M, Cummins C, Desai N et al. Childhood optic neuritis: clinical features and outcome. Arch Dis Child 2011;96:860–2. 10.1136/adc.2009.175422 [DOI] [PubMed] [Google Scholar]
- 5.Morales DS, Siatkowski RM, Howard CW et al. Optic neuritis in children. J Pediatr Ophthalmol Strabismus 2000;37:254–9. [PubMed] [Google Scholar]
- 6.El-Dairi MA, Ghasia F, Bhatti MT. Pediatric optic neuritis. Int Ophthalmol Clin 2012;52:29–49. 10.1097/IIO.0b013e318259dec6 [DOI] [PubMed] [Google Scholar]
- 7.Pérez-Cambrodí RJ, Gómez-Hurtado Cubillana A, Merino-Suárez ML et al. Optic neuritis in pediatric population: a review in current tendencies of diagnosis and management. J Optom 2014;7:125–30. 10.1016/j.optom.2013.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boomer JA, Siatkowski RM. Optic neuritis in adults and children. Semin Ophthalmol 2003;18:174–80. 10.1080/08820530390895172 [DOI] [PubMed] [Google Scholar]
- 9.Hickman SJ, Dalton CM, Miller DH et al. Management of acute optic neuritis. Lancet 2002;360:1953–62. 10.1016/S0140-6736(02)11919-2 [DOI] [PubMed] [Google Scholar]
- 10.Riikonen R, Donner M, Erkkliä H. Optic neuritis in children and its relationship to multiple sclerosis: a clinical study of 21 children. Dev Med Child Neurol 1988;30:349–59. 10.1111/j.1469-8749.1988.tb14560.x [DOI] [PubMed] [Google Scholar]
- 11.Banwell B, Ghezzi A, Bar-Or A et al. Multiple sclerosis in children: clinical diagnosis, therapeutic strategies, and future directions. Lancet Neurol 2007;6:887–902. 10.1016/S1474-4422(07)70242-9 [DOI] [PubMed] [Google Scholar]
- 12.Chabas D, Ness J, Belman A et al. Younger children with MS have a distinct CSF inflammatory profile at disease onset. Neurology 2010;74:399–405. 10.1212/WNL.0b013e3181ce5db0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Absoud M, Lim MJ, Chong WK et al. Paediatric acquired demyelinating syndrome: incidence, clinical and magnetic resonance imaging features. Mult Scler 2013;19:76–86. 10.1177/1352458512445944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waldman AT, Stull LB, Galetta SL et al. Pediatric optic neuritis and risk of multiple sclerosis: meta-analysis of observational studies. J AAPOS 2011;15:441–6. 10.1016/j.jaapos.2011.05.020 [DOI] [PubMed] [Google Scholar]
- 15.Lucchinetti CF, Kiers L, O'Duffy A et al. Risk factors for developing multiple sclerosis after childhood optic neuritis. Neurology 1997;49:1413–18. 10.1212/WNL.49.5.1413 [DOI] [PubMed] [Google Scholar]
- 16.Kriss A, Francis DA, Cuendet F et al. Recovery after optic neuritis in childhood. J Neurol Neurosurg Psychiatr 1988;51:1253–8. 10.1136/jnnp.51.10.1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cakmakli G, Kurne A, Güven A et al. Childhood optic neuritis: the pediatric neurologist's perspective. Eur J Paediatr Neurol 2009;13:452–7. 10.1016/j.ejpn.2008.09.003 [DOI] [PubMed] [Google Scholar]
- 18.Alper G, Wang L. Demyelinating optic neuritis in children. J Child Neurol 2009;24:45 10.1177/0883073808321052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett JL, de Seze J, Lana-Peixoto M et al. Neuromyelitis optica and multiple sclerosis: seeing differences through optical coherence tomography. Mult Scler 2015;21:678–88. 10.1177/1352458514567216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelfand JM, Nolan R, Schwartz DM et al. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain 2012;135:1786–93. 10.1093/brain/aws098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonhomme GR, Mitchell EB. Treatment of pediatric optic neuritis. Curr Treat Options Neurol 2012;14:93–102. 10.1007/s11940-011-0159-0 [DOI] [PubMed] [Google Scholar]
- 22.Brady KM, Brar AS, Lee AG et al. Optic neuritis in children: clinical features and outcome. J AAPOS 1999;3:98–103. 10.1016/S1091-8531(99)70078-9 [DOI] [PubMed] [Google Scholar]
- 23.Wilejto M, Shroff M, Buncic JR et al. The clinical features, MRI findings, and outcome of optic neuritis in children. Neurology 2006;67:258–62. 10.1212/01.wnl.0000224757.69746.fb [DOI] [PubMed] [Google Scholar]
- 24.de Sèze J, Kremer L, Collongues N. Neuromyelitis optica spectrum disorder (NMOSD): a new concept. Rev Neurol (Paris) 2016;172:256–62. 10.1016/j.neurol.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 25.Kim SH, Kim W, Li XF et al. Does interferon beta treatment exacerbate neuromyelitis optica spectrum disorder? Mult Scler 2012;18:1480–3. 10.1177/1352458512439439 [DOI] [PubMed] [Google Scholar]
- 26.Palace J, Leite MI, Nairne A et al. Interferon beta treatment in neuromyelitis optica: increase in relapses and aquaporin 4 antibody titers. Arch Neurol 2010;67:1016–17. 10.1001/archneurol.2010.188 [DOI] [PubMed] [Google Scholar]
- 27.Papeix C, Vidal JS, de Seze J et al. Immunosuppressive therapy is more effective than interferon in neuromyelitis optica. Mult Scler 2007;13:256–9. 10.1177/1352458506070732 [DOI] [PubMed] [Google Scholar]
- 28.Alper G, Heyman R, Wang L. Multiple sclerosis and acute disseminated encephalomyelitis diagnosed in children after long-term follow-up: comparison of presenting features. Dev Med Child Neurol 2009;51:480–6. 10.1111/j.1469-8749.2008.03136.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dale RC, Pillai SC. Early relapse risk after a first CNS inflammatory demyelination episode: examining international consensus definitions. Dev Med Child Neurol 2007;49:887–93. 10.1111/j.1469-8749.2007.00887.x [DOI] [PubMed] [Google Scholar]
- 30.Wingerchuk DM, Banwell B, Bennett JL et al. International Panel for NMO Diagnosis. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–89. 10.1212/WNL.0000000000001729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merle H, Olindo S, Bonnan M et al. Natural history of the visual impairment of relapsing neuromyelitis optica. Ophthalmology 2007;114:810–15. 10.1016/j.ophtha.2006.06.060 [DOI] [PubMed] [Google Scholar]
- 32.Merle H, Olindo S, Jeannin S et al. Visual field characteristics in neuromyelitis optica in absence of and after one episode of optic neuritis. Clin Ophthalmol 2013;7:1145–53. 10.2147/OPTH.S43894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wingerchuk DM, Weinshenker BG. Neuromyelitis optica. Handb Clin Neurol 2014;122:581–99. 10.1016/B978-0-444-52001-2.00025-X [DOI] [PubMed] [Google Scholar]
- 34.Mealy MA, Whetstone A, Orman G et al. Longitudinally extensive optic neuritis as an MRI biomarker distinguishes neuromyelitis optica from multiple sclerosis. J Neurol Sci 2015;355:59–63. 10.1016/j.jns.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khanna S, Sharma A, Huecker J et al. Magnetic resonance imaging of optic neuritis in patients with neuromyelitis optica versus multiple sclerosis. J Neuroophthalmol 2012;32:216–20. 10.1097/WNO.0b013e318254c62d [DOI] [PubMed] [Google Scholar]
- 36.Reindl M, Di Pauli F, Rostásy K et al. The spectrum of MOG autoantibody-associated demyelinating diseases. Nat Rev Neurol 2013;9:455–61. 10.1038/nrneurol.2013.118 [DOI] [PubMed] [Google Scholar]
- 37.Hacohen Y, Absoud M, Deiva K et al. Myelin oligodendrocyte glycoprotein antibodies are associated with a non-MS course in children. Neurol Neuroimmunol Neuroinflamm 2015;2:e81 10.1212/NXI.0000000000000081 [DOI] [PMC free article] [PubMed] [Google Scholar]