Abstract
Adrenal hemangioma is an uncommon benign vascular tumor that is often discovered incidentally. It has never been reported in association with familial adenomatous polyposis. We report a case of a 60-year old man with a history of familial adenomatous polyposis, in whom a huge retroperitoneal cyst of 18x17 cm was discovered during routine radiologic evaluation. Because of the impossibility of ruling out the presence of malignancy, surgical cystectomy was performed, associated to a scheduled total colectomy. Pathological examination revealed that the cyst corresponded to an adrenal cavernous hemangioma. Colonic adenomas did not show signs of degeneration. Screening for adenomatous polyposis coli (APC) gene mutation was not carried out.
As familial adenomatous polyposis is known to involve a variety of extracolonic manifestations, this finding raises the suspicion of a possible variant of this syndrome including adrenal hemangioma. An extensive study based on a larger patient series with genetic exploration is necessary.
Key words: Hemangioma, cavernous, adrenal gland, familial adenomatous polyposis
Introduction
Adrenal hemangioma (AH) is a rare vascular tumor that is often discovered incidentally. In the literature, it has been reported in association with other lesions, often with the adrenal gland or extra-adrenal malignant tumors.1-4To the best of our knowledge, It has never been reported in association with familial adenomatous polyposis (FAP), which may nevertheless have a variety of extra-colonic manifestations.5 Thus, screening for adenomatous polyposis coli (APC) gene mutation, involved in the genesis of FAP, has never been performed in AFP-HA association. We report an original case of a huge incidental AH in a patient with FAP discovered during routine follow-up.
Case Report
A 60-year old patient with a 10-year history of FAP, was admitted to the surgery department for prophylactic total colectomy. Pre-operative abdominal computed tomography (CT) scan revealed 18x17 cm heterogeneous retro-peritoneal cystic mass with calcifications. This lesion showed partial enhancement after contrast injection.
Based on these findings, an hydatid cyst, a metastatic cystic lesion or a sarcoma were considered. Tumor markers (CA19-9 and α-fetoprotein) were within normal limits. Serological hydatic test was negative. The patient underwent surgical cystectomy and coloproctectomy with ileoanal anastomosis. Per-operatively, the mass was well circumscribed, adhering to the left adrenal gland, but did not seem to infiltrate the surrounding tissues. Therefore, frozen section examination was considered non-useful. The mass was removed along with the colon. Macroscopic examination showed a cystic mass of 17.5×17 ×9 cm, with a smooth surface (Figure 1A). Sections disclosed an hemorrhagic content. The wall was irregular with voluminous adherent blood clots forming black and sometimes calcified buds. The adrenal gland was embedded in the cyst wall (Figure 1B). Gross appearance of the colectomy revealed over than 100 sessile and pediculated polyps that prevailed in the first 27 cm as well as the distal portion of the colon (Figure 2A). The polyps measured between 3 and 15 mm in diameter. Histologically, the cystic wall was thick and fibro-inflammatory. It was consisted of anfractuous vascular cavities of different sizes (Figure 1C). They were lined with regular flat cells which were positive for vascular immuno-staining (CD34 and CD31) and negative for mesothelial markers (CK5/6 and calretinin), confirming their vascular differentiation (Figure 1D). Vascular cavities were separated by fibroblastic and myxoid septa. The adrenal tissue was embedded in the cyst wall. Resected samples of the colectomy showed that the polyps corresponded to tubular adenomas of different degrees of dysplasia without evidence of malignant transformation (Figure 2B).
Figure 1.
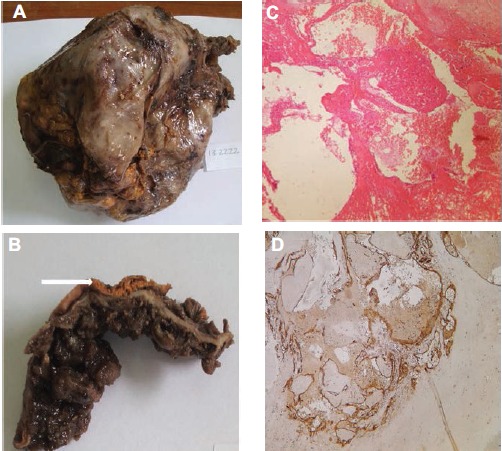
Macroscopic and histologic features of adrenal cavernous hemangioma: A) huge cyst with smooth surface; B) laminated adrenal tissue adhering to the external surface of the cyst wall (arrow). Note blood clots in the internal side; C) anfractuous vascular cavities separated by fibrous septa (HEx200); D) positive immunostaining of endothelial cells with CD34 (IHCx200).
Figure 2.
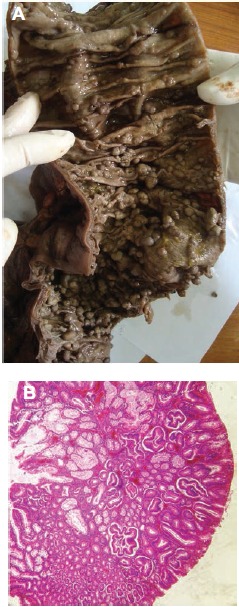
Macroscopic and histologic features of colonic adenomatous polyposis: A) numerous colonic polyps of various sizes; B) tubular adenoma with low-grade dysplasia (HEx200).
Screening for APC gene mutation was not carried out. The course was uneventful without signs of recurrence.
Discussion
Cavernous hemangiomas are rare benign tumors usually localized in the liver and the skin.5,6 Adrenal site is extremely rare occurring only in 0.01% of cases and accounts for 63 reported cases in the literature.6,7 The first report was published in 1955 by Johnson and Jeppesen.8
AH are often discovered as incidentalomas either by imaging studies or histologic examination.1,9 They are usually unilateral and are more frequent in women in the 5th or 7th decade.6,7,9 AH are usually asymptomatic but can be revealed by a painful palpable mass or by retroperitoneal hemorrhage.7
The diagnosis is often made by abdominal or pelvic CT scan, showing an hypodense, heterogenous mass with variable areas of hemorrhage or necrosis.6,7,9 Calcifications are noted in approximately two-thirds of cases but they are not specific since they may be observed in a variety of other adrenal lesions, including pheochromocytoma, hemorrhage, tuberculosis, adrenal cortical carcinoma, gastrointestinal metastasis and melanomas.7,9,10 Magnetic resonance imaging (MRI) is proven to be a valuable diagnostic tool for AH. Non-enhanced T1-weighted MRI shows heterogenous low-signal intensity and a high intensity signal in non-enhanced T2-weighted.7,10
The tumor size is variable. Surgically removed lesions were 10 cm in diameter on average, but may reach 30 cm.6 It is usually soft or fluctuant and when sectioned, it partially collapses due to the escape of blood and has a spongy appearance. Recent hemorrhages, organized thrombi, fibrosis and calcification may be seen.6,7
These lesions are usually well demarcated, encapsulated and located in the adrenal cortex. Morphologically, adrenal cavernous hemangioma has a comparable appearance as in the other organs. It is typically composed of blood-filled vascular channels of varied size lined by a single layer of flat endothelial cells supported by fibrous tissue. Thrombi in various stages of organization with areas of infarction may be present, and older lesions show dense fibrosis and calcifications. Small tumors may regress spontaneously by fibrosclerosis.7
It is uncommon to note islands of adipose tissue, which can mimic an angiomyolipoma.11,12 Cystic hemangioma, such as reported in our case, pose a problem of differential diagnosis with adrenal non-neoplastic cysts. These lesions include pseudocysts and true cysts whose lining is absent in the first case and of mesothelial type in the second. Adrenal hydatid cyst can mimic hemangioma radiologically but it has identifiable morphologic characteristics.
Adrenal lymphangioma is very rare and characterized by a large lymphatic channels in loose connective tissue stroma. The presence of peripheral lymphoid aggregates with often increased mast cells and the positive immunostaining with D2-40 of the lining cells can rectify the diagnosis.13
The pathogenesis of adrenal hemangiomas is unclear. In the skin, the brain or the liver, they are usually congenital and hereditary factors seems to play a role.9 Furthermore, cavernous hemangioma may integrate a congenital syndrome such as, Rendu Osler Weber disease, Von Hippel Lindau disease or Sturge-Weber syndrome. For the adrenal location, there is no evidence of an hereditary origin since all the published cases were sporadic and were not the subject of any genetic study.
AH may be associated with malignant extraadrenal lesions such as small-cell carcinoma of the lung, common bile duct cancer and gynecologic cancers.3,4 To our knowledge, its association with FAP, has never been reported. FAP is an autosomal dominant heredity disease which usual form is due to APC germinal gene mutation (5q21-q22).14
Adrenal tumors that are likely to be associated with FAP or its variants include corticoadrenal adenomas, pheochromocytomas and adrenal carcinomas.5,16-18 In these cases, genetic studies are limited and few APC gene mutations have been identified, mainly in multiples and bilateral adrenal adenomas.5,15,19 APC gene mutation in AH associated with FAP could integrate this tumor in syndromic variant of the familial polyposis.
Surgical resection is mandatory for adrenal hemangiomas whose size is greater than 6 cm, due to the risk of malignant transformation and hemorrhage. For tumors’ size ranging from 4 to 6 cm, surgical indication depends on patient’s age, physical condition, and history of other extra-adrenal tumor. Conservative attitude is recommended for tumors’ size less than 4 cm, based on radiologic follow-up. In fact, they may regress spontaneously.7
Conclusions
Adrenal cavernous hemangioma is a rare benign tumor, usually discovered incidentally on imaging. Its association with familial adenomatous polyposis has never been reported and raises the suspicion of a possible variant of FAP including this lesion. To confirm this hypothesis, an extensive study based on a large patient series with genetic exploration, is necessary.
References
- 1.Kinebuchi Y, Daimon H, Kawaguchi K. Adrenal cavernous hemangioma associated with myelolipomatous metaplasia. Int J Urol 2016;23:106-8. [DOI] [PubMed] [Google Scholar]
- 2.Arkadopoulos N, Kyriazi M, Yiallourou AI, et al. A rare coexistence of adrenal cavernous hemangioma with extramedullar hemopoietic tissue: a case report and brief review of the literature. World J Surg Oncol 2009;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alcázar J, Márquez A, Rosales M. An unusual cause of adrenal mass in a patient with operable non-small-cell pulmonary carcinoma. Arch Bronconeumol 1998;34:513-4. [DOI] [PubMed] [Google Scholar]
- 4.Chudácek Z, Kohoutek V. Simultaneousoccurrence of a cavernous adrenal gland hemangioma and a bile duct-liver carcinoma. Rofo 1980;132:460-2. [PubMed] [Google Scholar]
- 5.Groen EJ, Roos A, Muntinghe FL, et al. Extra-intestinal manifestations of familial adenomatous polyposis. Ann Surg Oncol 2008;15:2439-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De la villéon B, Goudard Y, Peroux E, et al. L’hémangiome caverneux: un incidentalome rare de la surrénale. Progr Urol 2011;21:961-4. [DOI] [PubMed] [Google Scholar]
- 7.Harzallah L, Zouari L, Ben Cherifa L, et al. Hémangiome surrénalien: à propos d’un cas. Annal Endocrinol 2006;67:624-7. [DOI] [PubMed] [Google Scholar]
- 8.Johnson CC, Jeppesen FB. Hemangioma of the adrenal. J Urol 1955;74:573-7. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Dang Y, He R, Chen G. Rare cavernous hemangioma of adrenal gland: case report. Sao Paulo Med J 2014;132:249-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeLellis R, Lloyd R, Heitz P, Eng C. Tumors World Health Organization, International Agency for Research on Cancer. WHO classification of tumors of Endocrine Organs. Lyon: IARC Press; 2004. [Google Scholar]
- 11.Termote B, Verswijvel G, Palmers Y. Fat containing adrenal cavernous haemangioma: CT and MRI findings. JBR-BTR 2007;90:516-8. [PubMed] [Google Scholar]
- 12.Llado Carbonell C, Arango Toro O, Vesa Llanes J, et al. Hémangiome surrénalien : revue de la littérature. Progr Urol 1996;6:292-6. [PubMed] [Google Scholar]
- 13.Wagner L, Robert M, Faix A, et al. Diagnostic et traitement des tumeurs kystiques de la surrénale. A propos de 2 observations. Progr Urol 1996;6:940-3. [PubMed] [Google Scholar]
- 14.Bosman FT. World Health Organization, International Agency for Research on Cancer. WHO classification of tumors of the digestive system. 4th ed. Lyon: IARC Press; 2010. [Google Scholar]
- 15.Manfredi M. Hereditary hamartomatous polyposis syndromes. Gastroenterol Hepatol (NY) 2010;6:185-9612. [PMC free article] [PubMed] [Google Scholar]
- 16.Kartheuser A, Walon C, West S, et al. Familial adenomatous polyposis associated with multiple adrenal adenomas in a patient with a rare 3’ APC mutation. J Med Genet 1999;36:65. [PMC free article] [PubMed] [Google Scholar]
- 17.Chelaïfa K, Bouzaïdi K, Chouaïb S, et al. Adrenal Adenoma in a patient with Gardner’s Syndrome. Acta Radiol 2003;44:15. [DOI] [PubMed] [Google Scholar]
- 18.Traill Z, Tuson J, Woodham C. Adrenal carcinoma in a patient with Gardner’s syndrome: imaging findings. AJR Am J Roentgenol 1995;165:1460-1. [DOI] [PubMed] [Google Scholar]
- 19.Gaujoux S, Pinson S, Gimenez-Roqueplo AP, et al. Inactivation of the APC gene is constant in adrenocortical tumors from patients with familial adenomatous polyposis but not frequent in sporadic adrenocortical cancers. Clin Cancer Res 2010;16:5133-41. [DOI] [PubMed] [Google Scholar]


