Abstract
Retinal fibrosis, characterized by dysregulation of extracellular matrix (ECM) protein deposition by retinal endothelial cells, pigment epithelial cells, and other resident cell-types, is a unifying feature of several common retinal diseases. Fibronectin is an early constituent of newly deposited ECM and serves as a template for assembly of other ECM proteins, including collagens. Under physiologic conditions, fibronectin is found in all layers of Bruch’s membrane. Proliferative vitreoretinopathy (PVR), a complication of retinal surgery, is characterized by ECM accumulation. Among the earliest histologic manifestations of diabetic retinopathy (DR) is capillary basement membrane thickening, which occurs due to perturbations in ECM homeostasis. Neovascularization, the hallmark of late stage DR as well as exudative age-related macular degeneration (AMD), involves ECM assembly as a scaffold for the aberrant new vessel architecture. Rodent models of retinal injury demonstrate a key role for fibronectin in complications characteristic of PVR, including retinal detachment. In mouse models of DR, reducing fibronectin gene expression has been shown to arrest the accumulation of ECM in the capillary basement membrane. Alterations in matrix metalloproteinase activity thought to be important in the pathogenesis of AMD impact the turnover of fibronectin matrix as well as collagens. Growth factors involved in PVR, AMD, and DR, such as PDGF and TGFβ, are known to stimulate fibronectin matrix assembly. A deeper understanding of how pathologic ECM deposition contributes to disease progression may help to identify novel targets for therapeutic intervention.
Keywords: Fibronectin, retina, fibrosis, neovascularization, extracellular matrix, diabetic retinopathy, age-related macular degeneration, proliferative vitreoretinopathy
Introduction
A unifying feature of an array of retinal disorders, particularly at late stages of disease states, is the dysregulated accumulation of extracellular matrix (ECM) protein characteristic of retinal fibrosis. This can occur as the result of an aberrant wound-healing response, as in the case of post-surgical complications of proliferative vitreoretinopathy (PVR) and epiretinal membrane (ERM) formation. In the most severe cases, these fibroproliferative membranes can lead to tractional retinal detachment. The role of fibrosis in other retinal diseases is less well-characterized, but such conditions are only increasing in prevalence, including diabetic retinopathy (DR) and age-related macular degeneration (AMD).
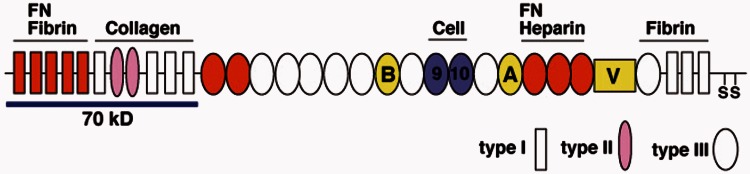
Fibronectin, an important constituent of the vertebrate ECM, is ubiquitously expressed in human tissues. It is a 230–270 kDa glycoprotein, secreted as a dimer, which ranges in size due to alternative splicing events (Figure 1).1 The N-terminal portion of the fibronectin molecule contains a self-assembly domain that allows for individual fibronectin dimers to come together in a process termed fibrillogenesis, resulting in the assembly of a three-dimensional (3D) matrix.2 In contrast to collagen self-assembly, fibronectin fibrillogenesis is a non-spontaneous cell-mediated process.3 Fibronectin is secreted in a compact conformation and must be engaged by cell-surface integrins, most notably α5β1 integrin, in order to reveal the cryptic self-association domains necessary to form a definitive 3D matrix.4 In vitro studies have demonstrated that other ECM proteins, including types I, III, and IV collagen, depend on a previously assembled fibronectin matrix for incorporation into the ECM.5–8 As such, fibronectin matrix assembly may play a key role in a range of fibrotic diseases, representing an early step in pathologic ECM protein accumulation. In summarizing the existing literature on fibronectin in retinal homeostasis and pathology, we hope to lay the groundwork for further elucidating the mechanism by which fibronectin may contribute to fibrotic disease progression in PVR, DR, AMD, and other diseases affecting the retina.
Figure 1.
Fibronectin. Fibronectin is a multi-domain glycoprotein consisting of 3 different modules (types I, II, and III) comprising several binding sites including an FN self-association domain, collagen-binding domain, cell-binding domain, and heparin-binding domain. Three alternatively spliced regions include the extra domain-A (EDA) and EDB regions, both type III repeats, and a variable (V) region. The protein is secreted as a dimer, linked by disulfide bonds (adapted from Mao and Schwarzbauer1). (A color version of this figure is available in the online journal.)
Fibronectin in the retina
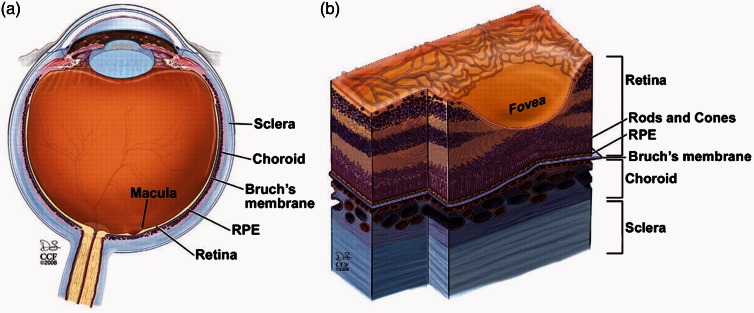
The importance of fibronectin in retinal homeostasis begins with embryonic development, during which fibronectin can be found in the choroid, retinal pigment epithelium (RPE), and transiently in the neuroblastic layer. The choroid and RPE serve essential functions in supporting and nourishing the retina itself and the neuroblastic layer contains retinal progenitor cells that differentiate into a host of cell types, including the rod and cone photoreceptors of the retina (Figure 2).9 Artificial disruption of fibronectin during development has catastrophic consequences for the structure of the retina overall.10 The inner limiting membrane stains strongly for fibronectin in the postnatal period. The same authors documented a role for the fibronectin receptor, α5β1 integrin, in neurite outgrowth from retinal ganglion cells grown on various ECM substrates in vitro as a model for retinal ganglion cell regeneration in the mature retina.11 While there is no appreciable fibronectin staining in the inner limiting membrane of healthy adult retinas, age-matched samples from diabetic patients show prominent fibronectin staining of the inner limiting membrane.12,13
Figure 2.
Anatomy of the retina. The thin sheet of neural tissue that comprises the retina lines the globe of the eye, overlying the retinal pigment epithelium (RPE), Bruch’s membrane, and the vessels of the choroid. The sclera is a connective tissue layer that encases all of these structures. The macula is the portion of the retina responsible for central vision, owing to the high concentration of cone cells within the fovea – a depression within the macula (adapted from Yuan et al.94). (A color version of this figure is available in the online journal.)
Bruch’s membrane
Bruch’s membrane, which underlies the basal surface of the RPE and together with it comprise the blood-retinal barrier, itself consists predominantly of ECM protein.14,15 Indeed, fibronectin has been identified in each of the five layers of Bruch’s membrane – the RPE basal lamina itself, the inner collagenous layer, the elastic layer, the outer collagenous layer, and finally the basement membrane of the choriocapillaries, which as the outermost layer of Bruch’s membrane underlies the basal surface of the choroidal endothelium.16 In the basement membrane of retinal vessels, fibronectin is a key structural component, as it is in vascular basement membranes in other tissues.17–19 The central three layers of Bruch’s membrane are physically linked by collagen fibers that can traverse the elastic layer, linking the inner and outer collagenous layers.20 Along with fibronectin, the collagenous layers share types I, III, and V collagen as constituents, in addition to proteoglycans, amongst which lipoproteins accumulate with age.21 The impact of Bruch’s membrane ECM components on the retina itself is perhaps most directly mediated by the RPE basal lamina, which is engaged by cell-surface integrins of the RPE cells themselves.22–24 Amongst these, the α5 integrin subunit has been visualized in sections of human retina, which together with the β1 subunit form the so-called fibronectin receptor.25 The importance of fibronectin as a structural component of the RPE and its basement membrane is underscored by in vitro data, which demonstrate that RPE cells spread more on fibronectin than laminin, type I collagen, or even the major basement membrane protein type IV collagen.26
Complications of vitreoretinal surgery
As many as 10% of surgeries for retinal detachment fail due to the development of proliferative vitreoretinopathy (PVR) post-operatively.27 It is the most common cause of recurrent retinal detachment after primary retinal detachment repair, accounting for 75% of such cases; however, the surgical management of PVR is challenging.28 A deeper understanding of the mechanism of disease will be important to help reduce the development of PVR, since at present surgical success in treating PVR is modest at best.29 Furthermore, due to the increased rate of complications related to PVR with successive surgeries, non-surgical treatments are needed.30 The disease process is characterized by a fibrocellular proliferation that occurs in the post-operative period. These proliferations form ERMs that can exert traction on the retina, compromising visual acuity, and potentially precipitating repeat retinal detachment.31 Primary prevention of PVR is an important goal, as at present, patients requiring additional surgery have poor visual potential.32
Early animal models of PVR were established, in part, based on the observations that fibronectin was a constituent of both epiretinal and subretinal membranes and that platelet-derived growth factor (PDGF) receptor and ligand staining were increased in PVR patient samples as compared to control samples of RPE.33–36 Intravitreal administration of fibronectin together with PDGF was sufficient to induce intraocular membrane formation and resultant retinal detachment.37 Multiple cell types have been identified within such induced ERM’s, as well as those isolated from clinical PVR samples, including RPE cells, glial cells, macrophages, fibroblasts, and myofibroblasts. Myofibroblasts from PVR are α-smooth muscle actin positive and have been shown to express extra domain (ED)-A fibronectin, a splice variant of fibronectin known to be enriched in pathologic fibrosis and important in promoting the myofibroblastic phenotype.38–40 RPE cells treated with transforming growth factor (TGF)-β2, a pro-fibrotic growth factor known to be elevated in the vitreous of eyes with PVR, are induced to express ED-A fibronectin and this effect is further amplified by co-treatment with connective tissue growth factor (CTGF).41 Importantly, ED-containing fibronectin is necessarily cellular in origin, as opposed to the plasma-derived splice variant of fibronectin, which implicates the aforementioned resident cell types of PVR in its production in situ. Thus, both plasma fibronectin, which appears as a result of vascular leakage, and cell-derived fibronectin can be found in PVR.42
Amongst the different cellular constituents of PVR, RPE cells have been noted to be the most consistently present in clinical samples and the most abundant.43 An important step in the progression from an initial defect in the native RPE cell layer to the development of PVR is the transition of quiescent RPE cells to detached, migratory cells, which divide readily and contribute to ECM accumulation.41 A relevant consequence of this shift is an altered integrin expression profile in PVR-derived RPE. The α5 integrin is present in PVR tissue, but the same authors observed no staining in the RPE of normal retina44,45 An intriguing potential explanation for this discrepancy in the integrin expression profile of healthy versus PVR-derived RPE cells may be related to changes in gene expression with disease. In vitro quiescent RPE cells, analogous to those in normal retinal tissue, express less than one-tenth the level of both α5 and β1 integrins than their actively proliferating counterparts.25 These data suggest that the RPE cells of PVR eyes may be better equipped to engage fibronectin protein and thus to initiate fibronectin matrix assembly leading to ECM accumulation.
Past efforts at enhancing outcomes of PVR surgery used anti-mitotic agents or molecules thought to bind culprit plasma-derived initiators of PVR, namely 5-fluorouracil and heparin, respectively. Despite initially promising results, these were later found to result in worse visual outcomes than surgery without adjuvant therapy.46,47 Efforts to prevent PVR formation using more targeted therapies are warranted. Elevated PDGF levels are associated with development of PVR following surgery for retinal detachment.48 A treatment strategy aimed at inhibiting a PDGF family ligand, which showed promise in vitro by inhibiting the migration of RPE cells, was observed to be ineffective when cells were plated on fibronectin.49 This suggests that novel therapies aimed at inhibiting fibronectin deposition could be synergistic with growth factor-directed therapies, like anti-PDGF biologics that have shown promise in a mouse model of PVR and in a phase 1 clinical trial for neovascular AMD.50,51 One proposed method of inhibiting fibronectin deposition in the context of PVR is the use of a novel single chain variable fragment (scFv) antibody designed to inhibit fibronectin self-association as well as the fibronectin cell-binding domain, which has been examined in vitro.52 Recent studies have validated the use of genomic data and analysis of biomarkers from samples taken during retinal detachment surgery, in combination with traditional clinical parameters, to better identify patients at risk for development of PVR.53,54 Such methods will be crucial in selecting retinal detachment patients who might benefit from the application of novel preventative strategies administered intraoperatively or in the immediate postoperative period.
Diabetic retinopathy
Among the earliest changes that occur in the microvasculature of diabetic patients is thickening of the endothelial basement membrane as a result of increased deposition of type IV collagen, laminin, and fibronectin.55 Animal models of DR, as well as samples from diabetic patients, show increased fibronectin staining in the retinal microvasculature and increased expression of fibronectin at the mRNA level.17,56 In addition to fibronectin, type IV and other collagens are detected at elevated levels in the retinal endothelial basement membrane, as well as in the inner limiting membrane at the vitreoretinal interface.57 Increased gene expression and tissue staining for these ECM proteins have been observed in response to intravitreal vascular endothelial growth factor (VEGF) administration in a mouse model.58 These changes likely begin in a subclinical phase of the disease, prior to what would be termed background DR, and data suggest that there is a mechanistic link between such changes in basement membrane morphology, as well as composition, and disease progression. Excessive ECM deposition secondary to hyperglycemia has been linked to increased vascular permeability, a hallmark of the earliest clinically detectable phase of DR.59 Intravitreal injection of diabetic rats with antisense oligonucleotides to fibronectin, collagen IV, and laminin decreases hyperglycemia-induced vascular leakage.60 The distribution of proteoglycans and glycosaminoglycans associated with these ECM proteins, including heparan sulfate – known to interact with fibronectin and impact its binding properties – is significantly altered in diabetic patients.61,62 Increased vascular permeability is also thought to be hastened by elevated matrix metalloproteinase (MMP) activity, as documented in mouse models of DR.63
In the late stages of disease, termed proliferative DR, fibrovascular proliferations (and to a lesser extent avascular proliferations) contribute to tractional retinal detachment and increased risk of rhegmatogenous retinal detachment as well.64 Myofibroblasts have been identified in ERM’s harvested from patients with proliferative DR and their contractile phenotype is thought to play a role in exerting traction on the retina.65 Fibronectin has been shown to play a dual role in angiogenesis, the hallmark of proliferative DR.66 First, as a component of vascular basement membranes, fibronectin is structurally important to new vessel architecture.67 In addition, the III12-14 binding domain of fibronectin has been shown to interact with VEGF, the key modulator of neovascularization and currently the major therapeutic target in proliferative DR and neovascular AMD.68
Intravitreal injection of fibronectin siRNA to diabetic rats prevents the basement membrane thickening usually associated with DR as well as other markers of vascular compromise.17,69 These data help to validate fibronectin as a relevant therapeutic target in DR. Increased fibronectin expression, however, is not the only consequence of chronic hyperglycemia that may impact fibronectin matrix deposition. Indeed, increased staining for α5 integrin has been documented in the retina of patients with proliferative DR.45 In vitro, retinal endothelial cells were induced to express higher levels of α5 integrin, alongside elevated fibronectin and type IV collagen, when grown under high glucose conditions and were more adherent than cells grown under normal glucose conditions.70 In an in vitro model of diabetic nephropathy, high glucose induced increased integrin activity.5 A novel therapy currently under investigation that could impact fibronectin deposition is ALG-1001 (“Luminate,” Allegro Ophthalmics LLC, NCT02348918), an integrin inhibitor designed to effect αvβ3, αvβ5, and α5β1 integrins.71 This raises the possibility that a therapeutic strategy aimed at preventing fibronectin matrix assembly, which would address ECM changes downstream of both increased fibronectin gene expression and altered integrin activity, could offer a greater likelihood of altering the clinical course of disease.
Age-related macular degeneration
AMD is the most prevalent macular dystrophy in the USA and the impact on public health is expected to worsen as the population ages.72 The disease impacts Bruch’s membrane, resulting in thickening of this structure and the development of drusen, deposits which form between the RPE basement membrane and the inner collagenous zone.73 Other deposits that are hypothesized to be involved in the pathogenesis of AMD include basal linear deposits that form within Bruch’s membrane and basal laminar deposits, which form between the RPE and its BM (the innermost layer of Bruch’s membrane).74 While other basement membrane components, including type IV collagen and laminins, are consistently found in basal deposits, conflicting data exist regarding the presence of fibronectin.75–78 CTGF, a known mediator of basal deposit formation, has been shown to induce fibronectin production by RPE cells.79 Since inhibition of fibronectin matrix assembly in vitro also prevents collagen IV accumulation, which suggests that collagen IV deposition relies on a pre-existing fibronectin matrix, it is likely that fibronectin is present in these deposits at least at some stage.5
As in other tissues, the delicate balance of matrix turnover in Bruch’s membrane is impacted by age. Tissue inhibitors of matrix metalloproteinases (TIMPs) help to modulate MMP activity and are increased in the aging retina – a phenomenon that is amplified in AMD. Samples from patients with AMD exhibit increased staining for TIMP-3 as compared to samples from healthy age-matched controls.80 In addition, MMP-2 and MMP-9 activity is decreased in the early stages of AMD, which contributes to buildup of type IV collagen.81,82 In later stages of disease, when choroidal neovascularization is predominantly responsible for vision loss, there is a shift in the profile of proteinase activity in Bruch’s membrane as retinal endothelial cells begin to migrate and proliferate.83 MMP activity is also increased in RPE cells, which helps to facilitate reorganization of the surrounding ECM making it more permissive to neovascularization, with fibronectin signaling contributing to this shift.84,85 In addition, integrin αvβ3 is known to be important in the formation of new vessels, and the success of therapies directed at inhibiting αvβ3 in animal models of choroidal neovascularization underscores its importance.86,87 Fibronectin is a ligand of integrin αvβ3 and fibronectin matrix is known to impact αvβ3 activity.88,89 As such, it is likely that these integrin-directed therapies are impacting fibronectin in the retina and a deeper understanding of these effects would help to validate fibronectin as a therapeutic target.
Discussion
Preliminary in vitro studies have been performed to assess the feasibility of targeting fibronectin fibrillogenesis in an effort to treat PVR. Use of an scFv antibody to fibronectin prevented matrix assembly by RPE cells and collagen gel contraction by fibrosarcoma cells.52 Although to our knowledge fibronectin protein has not been targeted directly in experimental models of DR or AMD, integrin-directed therapies reviewed herein likely impact cell–fibronectin interactions and thus the fibronectin matrix.71,86,87 Furthermore, inhibition of fibronectin production at the mRNA level has shown great promise in animal models of DR.17,69 Based on these data, efforts at gaining a deeper understanding of the importance of fibronectin in the pathogenesis of these retinal diseases are warranted. The importance of fibronectin in sequelae related to age-related posterior vitreous detachment, the persistence of vitreomacular adhesions, and idiopathic macular hole all represent other avenues of investigation that must be further explored.90
The vitreous humor has a collagenous component, which gradually deteriorates with age and plays a role in liquefaction of the vitreous gel. Advances in proteomic analysis are allowing for novel insight into biomarkers within the vitreous for diseases ranging from PVR to idiopathic macular holes, retinal detachment, and DR.91 Mass spectroscopy in particular has helped to identify upstream mediators of ECM production, such as TGFβ in the vitreous, as well as a heretofore unrecognized contribution by ECM molecules, including TIMP1 and SPARC.92 Such in-depth analysis of in vivo samples may help shed more light on the role of fibronectin and other ECM proteins across the spectrum of retinal disease. Three different fragments of the collagen IV non-collagenous (NC1) head domain, Arresten, Canstatin, and Tumstatin, act as potent inhibitors of angiogenesis. This phenomenon demonstrates the importance of interactions between ECM constituents in neovascular disease.93 As the NC1 domain may be responsible for the interaction between type IV collagen and fibronectin, this further warrants closer investigation of the potential for fibronectin to serve as a therapeutic target in proliferative DR and neovascular AMD.5
The diseases reviewed here, PVR, DR, and AMD, are far from exhaustive as a list of the retinal pathologies in which ECM is important. Furthermore, none of these phenomena should be considered in isolation, particularly as they converge in fibroproliferative disease states that can contribute to retinal detachment and ultimately require surgery. An ability to manipulate the ECM of the retina using targeted molecular therapies would spare many patients the risks of surgery and improve outcomes for those that still require intervention. The retina represents a uniquely accessible tissue for the introduction of such potential therapies locally, via intravitreal injection. This fact should add greater urgency to the search for a deeper understanding of ECM in retinal disease, so that we can move closer to the use of such novel therapies to help patients with these vision-threatening conditions.
Acknowledgement
The authors would like to thank the NCI (to JES) and NIDDK (to CGM) for funding.
Authors’ contribution
CGM, JLP, GB, and JES conceived of the article; CGM and JES drafted the article; JLP and GB provided substantive revisions
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol 2005; 24: 389–99. [DOI] [PubMed] [Google Scholar]
- 2.Schwarzbauer JE. Identification of the fibronectin sequences required for assembly of a fibrillar matrix. J Cell Biol 1991; 113: 1463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh P, Carraher C, Schwarzbauer JE. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol 2010; 26: 397–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao Y, Schwarzbauer JE. Stimulatory effects of a three-dimensional microenvironment on cell-mediated fibronectin fibrillogenesis. J Cell Sci 2005; 118: 4427–36. [DOI] [PubMed] [Google Scholar]
- 5.Miller CG, Pozzi A, Zent R, Schwarzbauer JE. Effects of high glucose on integrin activity and fibronectin matrix assembly by mesangial cells. Mol Biol Cell 2014; 25: 2342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadler KE, Hill A, Canty-Laird EG. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol 2008; 20: 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol Biol Cell 2002; 13: 3546–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velling T, Risteli J, Wennerberg K, Mosher DF, Johansson S. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins alpha 11beta 1 and alpha 2beta 1. J Biol Chem 2002; 277: 37377–81. [DOI] [PubMed] [Google Scholar]
- 9.Turksen K, Aubin JE, Sodek J, Kalnins VI. Localization of laminin, type IV collagen, fibronectin, and heparan sulfate proteoglycan in chick retinal pigment epithelium basement membrane during embryonic development. J Histochem Cytochem 1985; 33: 665–71. [DOI] [PubMed] [Google Scholar]
- 10.Taylor L, Arnér K, Engelsberg K, Ghosh F. Scaffolding the retina: the interstitial extracellular matrix during rat retinal development. Int J Dev Neurosci 2015; 42: 46–58. [DOI] [PubMed] [Google Scholar]
- 11.Vecino E, Heller JP, Veiga-Crespo P, Martin KR, Fawcett JW. Influence of extracellular matrix components on the expression of integrins and regeneration of adult retinal ganglion cells. PLoS ONE 2015; 10: e0125250–e0125250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.To M, Goz A, Camenzind L, Oertle P, Candiello J, Sullivan M, Henrich PB, Loparic M, Safi F, Eller A, Halfter W. Diabetes-induced morphological, biomechanical, and compositional changes in ocular basement membranes. Exp Eye Res 2013; 116: 298–307. [DOI] [PubMed] [Google Scholar]
- 13.Ljubimov AV, Burgeson RE, Butkowski RJ, Couchman JR, Zardi L, Ninomiya Y, Sado Y, Huang ZS, Nesburn AB, Kenney MC. Basement membrane abnormalities in human eyes with diabetic retinopathy. J Histochem Cytochem 1996; 44: 1469–79. [DOI] [PubMed] [Google Scholar]
- 14.Call TW, Hollyfield JG. Sulfated proteoglycans in Bruch's membrane of the human eye: localization and characterization using cupromeronic blue. Exp Eye Res 1990; 51: 451–62. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Godino R, Pierce EA, Garland DL. Extracellular matrix alterations and deposit formation in AMD. In: Rickman CB, LaVail MM, Anderson RE, Grimm C, Hollyfield J, Ash J. (eds). Retinal degenerative diseases, Cham: Springer International Publishing, 2016, pp. 53–8. [DOI] [PubMed] [Google Scholar]
- 16.Das A, Frank RN, Zhang NL, Turczyn TJ. Ultrastructural localization of extracellular matrix components in human retinal vessels and Bruch's membrane. Archive Ophthalmol 1990; 108: 421–9. [DOI] [PubMed] [Google Scholar]
- 17.Roy S, Sato T, Paryani G, Kao R. Downregulation of fibronectin overexpression reduces basement membrane thickening and vascular lesions in retinas of galactose-fed rats. Diabetes 2003; 52: 1229–34. [DOI] [PubMed] [Google Scholar]
- 18.Cohen MP, Saini R, Klepser H, Vasanthi LG. Fibronectin binding to glomerular basement membrane is altered in diabetes. Diabetes 1987; 36: 758–63. [DOI] [PubMed] [Google Scholar]
- 19.Stenman S, Vaheri A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J Exp Med 1978; 147: 1054–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korte GE, D'Aversa G. The elastic tissue of Bruch's membrane. Connections to choroidal elastic tissue and the ciliary epithelium of the rabbit and human eyes. Archive Ophthalmol 1989; 107: 1654–8. [DOI] [PubMed] [Google Scholar]
- 21.Huang JD, Presley JB, Chimento MF, Curcio CA. Age-related changes in human macular Bruch's membrane as seen by quick-freeze/deep-etch. Exp Eye Res 2007; 85: 202–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campochiaro PA, Jerdon JA. The extracellular matrix of human retinal pigment epithelial cells in vivo and its synthesis in vitro. Invest Ophthalmol Vis Sci 1986; 27: 1615–21. [PubMed] [Google Scholar]
- 23.Chen L, Miyamura N, Ninomiya Y, Handa JT. Distribution of the collagen IV isoforms in human Bruch’s membrane. Br J Ophthalmol 2003; 87: 212–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aisenbrey S, Zhang M, Bacher D, Yee J, Brunken WJ, Hunter DD. Retinal pigment epithelial cells synthesize laminins, including laminin 5, and adhere to them through alpha3- and alpha6-containing integrins. Invest Ophthalmol Vis Sci 2006; 47: 5537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Proulx S, Guérin SL, Salesse C. Effect of quiescence on integrin alpha5beta1 expression in human retinal pigment epithelium. Mol Vis 2003; 9: 473–81. [PubMed] [Google Scholar]
- 26.Wu W-C, Chang Y-C, Wu K-Y, Chen S-Y, Hsieh M-C, Wu M-H, Wu H-J, Wu W-S, Kao Y-H. Pharmacological implications from the adhesion-induced signaling profiles in cultured human retinal pigment epithelial cells. Kaohsiung J Med Sci 2014; 30: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pastor JC. A brief review and re-thinking of proliferative vitreoretinopathy. Eur Ophthal Rev 2011; 5: 139–40. [Google Scholar]
- 28.Khan MA, Brady CJ, Kaiser RS. Clinical management of proliferative vitreoretinopathy: an update. Retina 2015; 35: 165–75. [DOI] [PubMed] [Google Scholar]
- 29.Asaria RH, Kon CH, Bunce C, Charteris DG, Wong D, Luthert PJ, Khaw PT, Aylward GW. How to predict proliferative vitreoretinopathy: a prospective study. Ophthalmology 2001; 108: 1184–6. [DOI] [PubMed] [Google Scholar]
- 30.Lai FHP, Lo ECF, Chan VCK, Brelen M, Lo WL, Young AL. Combined pars plana vitrectomy-scleral buckle versus pars plana vitrectomy for proliferative vitreoretinopathy. Int Ophthalmol 2016; 36: 217–24. [DOI] [PubMed] [Google Scholar]
- 31.Sadaka A, Giuliari GP. Proliferative vitreoretinopathy: current and emerging treatments. Clin Ophthalmol 2012; 6: 1325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pennock S, Rheaume M-A, Mukai S, Kazlauskas A. A novel strategy to develop therapeutic approaches to prevent proliferative vitreoretinopathy. Am J Pathol 2011; 179: 2931–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiscott P, Waller HA, Grierson I, Butler MG, Scott DL, Gregor Z, Morino I. Fibronectin synthesis in subretinal membranes of proliferative vitreoretinopathy. Br J Ophthalmol 1992; 76: 486–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robbins SG, Mixon RN, Wilson DJ, Hart CE, Robertson JE, Westra I, Planck SR, Rosenbaum JT. Platelet-derived growth factor ligands and receptors immunolocalized in proliferative retinal diseases. Invest Ophthalmol Vis Sci 1994; 35: 3649–63. [PubMed] [Google Scholar]
- 35.Campochiaro PA, Jerdan JA, Glaser BM. Serum contains chemoattractants for human retinal pigment epithelial cells. Archive Ophthalmol 1984; 102: 1830–3. [DOI] [PubMed] [Google Scholar]
- 36.Ioachim E, Stefaniotou M, Gorezis S, Tsanou E, Psilas K, Agnantis NJ. Immunohistochemical study of extracellular matrix components in epiretinal membranes of vitreoproliferative retinopathy and proliferative diabetic retinopathy. Eur J Ophthalmol 2005; 15: 384–91. [DOI] [PubMed] [Google Scholar]
- 37.Yeo JH, Sadeghi J, Campochiaro PA, Green WR, Glaser BM. Intravitreous fibronectin and platelet-derived growth factor. New model for traction retinal detachment. Archive Ophthalmol 1986; 104: 417–21. [DOI] [PubMed] [Google Scholar]
- 38.Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol 1998; 142: 873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abu El-Asrar AM, Missotten L, Geboes K. Expression of myofibroblast activation molecules in proliferative vitreoretinopathy epiretinal membranes. Acta Ophthalmol 2011; 89: e115–21. [DOI] [PubMed] [Google Scholar]
- 40.Bochaton-Piallat ML, Kapetanios AD, Donati G, Redard M, Gabbiani G, Pournaras CJ. TGF-beta1, TGF-beta receptor II and ED-A fibronectin expression in myofibroblast of vitreoretinopathy. Invest Ophthalmol Vis Sci 2000; 41: 2336–42. [PubMed] [Google Scholar]
- 41.Khankan R, Oliver N, He S, Ryan SJ, Hinton DR. Regulation of fibronectin-EDA through CTGF domain-specific interactions with TGFβ2 and its receptor TGFβRII. Invest Ophthalmol Vis Sci 2011; 52: 5068–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campochiaro PA, Jerdan JA, Glaser BM, Cardin A, Michels RG. Vitreous aspirates from patients with proliferative vitreoretinopathy stimulate retinal pigment epithelial cell migration. Archive Ophthalmol 1985; 103: 1403–5. [DOI] [PubMed] [Google Scholar]
- 43.Cui JZ, Chiu A, Maberley D, Ma P, Samad A, Matsubara JA. Stage specificity of novel growth factor expression during development of proliferative vitreoretinopathy. Eye (Lond) 2007; 21: 200–8. [DOI] [PubMed] [Google Scholar]
- 44.Brem RB, Robbins SG, Wilson DJ, O'Rourke LM, Mixon RN, Robertson JE, Planck SR, Rosenbaum JT. Immunolocalization of integrins in the human retina. Invest Ophthalmol Vis Sci 1994; 35: 3466–74. [PubMed] [Google Scholar]
- 45.Robbins SG, Brem RB, Wilson DJ, O'Rourke LM, Robertson JE, Westra I, Planck SR, Rosenbaum JT. Immunolocalization of integrins in proliferative retinal membranes. Invest Ophthalmol Vis Sci 1994; 35: 3475–85. [PubMed] [Google Scholar]
- 46.Asaria RH, Kon CH, Bunce C, Charteris DG, Wong D, Khaw PT, Aylward GW. Adjuvant 5-fluorouracil and heparin prevents proliferative vitreoretinopathy: results from a randomized, double-blind, controlled clinical trial. Ophthalmology 2001; 108: 1179–83. [DOI] [PubMed] [Google Scholar]
- 47.Wickham L, Bunce C, Wong D, McGurn D, Charteris DG. Randomized controlled trial of combined 5-Fluorouracil and low-molecular-weight heparin in the management of unselected rhegmatogenous retinal detachments undergoing primary vitrectomy. Ophthalmology 2007; 114: 698–704. [DOI] [PubMed] [Google Scholar]
- 48.Moysidis SN, Thanos A, Vavvas DG. Mechanisms of inflammation in proliferative vitreoretinopathy: from bench to bedside. Mediators Inflamm 2012; 2012: 815937–815937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan C-M, Chang H-H, Wang V-C, Huang C-L, Hung C-F. Inhibitory effects of resveratrol on PDGF-BB-induced retinal pigment epithelial cell migration via PDGFRβ, PI3K/Akt and MAPK pathways. PLoS ONE 2013; 8: e56819–e56819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akiyama H, Kachi S, Silva RLE, Umeda N, Hackett SF, McCauley D, McCauley T, Zoltoski A, Epstein DM, Campochiaro PA. Intraocular injection of an aptamer that binds PDGF-B: a potential treatment for proliferative retinopathies. J Cell Physiol 2006; 207: 407–12. [DOI] [PubMed] [Google Scholar]
- 51.Jaffe GJ, MD, GJJ, MD DE, Eliott D, Wells JA,, MD JAW, Prenner JL,, MD JLP, MD AP, Papp A,, MD SP, Patel S. A phase 1 study of intravitreous E10030 in combination with ranibizumab in neovascular age-related macular degeneration. Ophthalmology 2015; 123: 78–85. [DOI] [PubMed] [Google Scholar]
- 52.Sharma M, Tiwari A, Sharma S, Bhoria P, Gupta V, Gupta A, Luthra-Guptasarma M. Fibrotic remodeling of the extracellular matrix through a novel (engineered, dual-function) antibody reactive to a cryptic epitope on the N-terminal 30 kDa fragment of fibronectin. PLoS ONE 2013; 8: e69343–e69343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rojas J, Fernandez I, Pastor JC, MacLaren RE, Ramkissoon Y, Harsum S, Charteris DG, Van Meurs JC, Amarakoon S, Garcia-Arumi J, Ruiz-Moreno JM, Rocha-Sousa A, Brion M, Carracedo A, Genetics on PVR Study Group (web file). Predicting proliferative vitreoretinopathy: temporal and external validation of models based on genetic and clinical variables. Br J Ophthalmol 2015; 99: 41–8. [DOI] [PubMed] [Google Scholar]
- 54.Ricker LJAG, Kessels AGH, de Jager W, Hendrikse F, Kijlstra A, la Heij EC. Prediction of proliferative vitreoretinopathy after retinal detachment surgery: potential of biomarker profiling. Am J Ophthalmol 2012; 154: 347–354.e2–347–354.e2. [DOI] [PubMed] [Google Scholar]
- 55.Klaassen I, van Geest RJ, Kuiper EJ, van Noorden CJF, Schlingemann RO. The role of CTGF in diabetic retinopathy. Exp Eye Res 2015; 133: 37–48. [DOI] [PubMed] [Google Scholar]
- 56.Roy S, Cagliero E, Lorenzi M. Fibronectin overexpression in retinal microvessels of patients with diabetes. Invest Ophthalmol Vis Sci 1996; 37: 258–66. [PubMed] [Google Scholar]
- 57.Ljubimov AV, Burgeson RE, Butkowski RJ, Couchman JR, Zardi L, Ninomiya Y, Sado Y, Huang ZS, Nesburn AB, Kenney MC. Basement membrane abnormalities in human eyes with diabetic retinopathy. J Histochem Cytochem 1996; 44: 1469–79. [DOI] [PubMed] [Google Scholar]
- 58.Kuiper EJ, Hughes JM, van Geest RJ, Vogels IMC, Goldschmeding R, van Noorden CJF, Schlingemann RO, Klaassen I. Effect of VEGF-A on expression of profibrotic growth factor and extracellular matrix genes in the retina. Invest Ophthalmol Vis Sci 2007; 48: 4267–76. [DOI] [PubMed] [Google Scholar]
- 59.Chronopoulos A, Trudeau K, Roy S, Huang H, Vinores SA, Roy S. High glucose-induced altered basement membrane composition and structure increases trans-endothelial permeability: implications for diabetic retinopathy. Curr Eye Res 2011; 36: 747–53. [DOI] [PubMed] [Google Scholar]
- 60.Oshitari T, Polewski P, Chadda M, Li A-F, Sato T, Roy S. Effect of combined antisense oligonucleotides against high-glucose- and diabetes-induced overexpression of extracellular matrix components and increased vascular permeability. Diabetes 2006; 55: 86–92. [PubMed] [Google Scholar]
- 61.Ugarova TP, Zamarron C, Veklich Y, Bowditch RD. Conformational transitions in the cell binding domain of fibronectin. Biochemistry 1995; 34: 4457–66. [DOI] [PubMed] [Google Scholar]
- 62.Hewitt AT, Nakazawa K, Newsome DA. Analysis of newly synthesized Bruch's membrane proteoglycans. Invest Ophthalmol Vis Sci 1989; 30: 478–86. [PubMed] [Google Scholar]
- 63.Das A, McGuire PG, Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology 2015; 122: 1375–94. [DOI] [PubMed] [Google Scholar]
- 64.Roy S, Amin S, Roy S. Retinal fibrosis in diabetic retinopathy. Exp Eye Res 2016; 142: 71–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walshe R, Esser P, Wiedemann P, Heimann K. Proliferative retinal diseases: myofibroblasts cause chronic vitreoretinal traction. Br J Ophthalmol 1992; 76: 550–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Casaroli Marano RP, Preissner KT, Vilaró S. Fibronectin, laminin, vitronectin and their receptors at newly-formed capillaries in proliferative diabetic retinopathy. Exp Eye Res 1995; 60: 5–17. [DOI] [PubMed] [Google Scholar]
- 67.Eming SA, Hubbell JA. Extracellular matrix in angiogenesis: dynamic structures with translational potential. Exp Dermatol 2011; 20: 605–13. [DOI] [PubMed] [Google Scholar]
- 68.Martino MM, Hubbell JA. The 12th-14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. FASEB J 2010; 24: 4711–21. [DOI] [PubMed] [Google Scholar]
- 69.Roy S, Nasser S, Yee M, Graves DT, Roy S. A long-term siRNA strategy regulates fibronectin overexpression and improves vascular lesions in retinas of diabetic rats. Mol Vis 2011; 17: 3166–74. [PMC free article] [PubMed] [Google Scholar]
- 70.Roth T, Podestá F, Stepp MA, Boeri D, Lorenzi M. Integrin overexpression induced by high glucose and by human diabetes: potential pathway to cell dysfunction in diabetic microangiopathy. Proc Natl Acad Sci USA 1993; 90: 9640–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Agarwal A, Afridi R, Hassan M, Sadiq MA, Sepah YJ, Do DV, Nguyen QD. Novel therapies in development for diabetic macular edema. Curr Diab Rep 2015; 15: 652–652. [DOI] [PubMed] [Google Scholar]
- 72.Friedman DS, O'Colmain BJ, Muñoz B, Tomany SC, McCarty C, de Jong PTVM, Nemesure B, Mitchell P, Kempen J, Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Archive Ophthalmol 2004; 122: 564–72. [DOI] [PubMed] [Google Scholar]
- 73.Kliffen M, van der Schaft TL, Mooy CM, de Jong PT. Morphologic changes in age-related maculopathy. Microsc Res Tech 1997; 36: 106–22. [DOI] [PubMed] [Google Scholar]
- 74.Curcio CA, Millican CL. Basal linear deposit and large drusen are specific for early age-related maculopathy. Archive Ophthalmol 1999; 117: 329–39. [DOI] [PubMed] [Google Scholar]
- 75.van der Schaft TL, Mooy CM, de Bruijn WC, Bosman FT, de Jong PT. Immunohistochemical light and electron microscopy of basal laminar deposit. Graefes Arch Clin Exp Ophthalmol 1994; 232: 40–6. [DOI] [PubMed] [Google Scholar]
- 76.Hageman GS, Mullins RF. Molecular composition of drusen as related to substructural phenotype. Mol Vis 1999; 5: 28–28. [PubMed] [Google Scholar]
- 77.Lommatzsch A, Hermans P, Müller KD, Bornfeld N, Bird AC, Pauleikhoff D. Are low inflammatory reactions involved in exudative age-related macular degeneration? Morphological and immunhistochemical analysis of AMD associated with basal deposits. Graefes Arch Clin Exp Ophthalmol 2008; 246: 803–10. [DOI] [PubMed] [Google Scholar]
- 78.Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Archive Ophthalmol 2004; 122: 598–614. [DOI] [PubMed] [Google Scholar]
- 79.Nagai N, Klimava A, Lee W-H, Izumi-Nagai K, Handa JT. CTGF is increased in basal deposits and regulates matrix production through the ERK (p42/p44mapk) MAPK and the p38 MAPK signaling pathways. Invest Ophthalmol Vis Sci 2009; 50: 1903–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kamei M, Hollyfield JG. TIMP-3 in Bruch’s membrane: changes during aging and in age-related macular degeneration. Invest Ophthalmol Vis Sci 1999; 40: 2367–75. [PubMed] [Google Scholar]
- 81.Nita M, Strzałka-Mrozik B, Grzybowski A, Mazurek U, Romaniuk W. Age-related macular degeneration and changes in the extracellular matrix. Med Sci Monit 2014; 20: 1003–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leu ST, Batni S, Radeke MJ, Johnson LV, Anderson DH, Clegg DO. Drusen are cold spots for proteolysis: expression of matrix metalloproteinases and their tissue inhibitor proteins in age-related macular degeneration. Exp Eye Res 2002; 74: 141–54. [DOI] [PubMed] [Google Scholar]
- 83.Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol 2003; 48: 257–93. [DOI] [PubMed] [Google Scholar]
- 84.Hoffmann S, He S, Ehren M, Ryan SJ, Wiedemann P, Hinton DR. MMP-2 and MMP-9 secretion by rpe is stimulated by angiogenic molecules found in choroidal neovascular membranes. Retina 2006; 26: 454–61. [DOI] [PubMed] [Google Scholar]
- 85.An E, Lu X, Flippin J, Devaney JM, Halligan B, Hoffman EP, Hoffman E, Strunnikova N, Csaky K, Hathout Y. Secreted proteome profiling in human RPE cell cultures derived from donors with age related macular degeneration and age matched healthy donors. J Proteome Res 2006; 5: 2599–610. [DOI] [PubMed] [Google Scholar]
- 86.Kamizuru H, Kimura H, Yasukawa T, Tabata Y, Honda Y, Ogura Y. Monoclonal antibody-mediated drug targeting to choroidal neovascularization in the rat. Invest Ophthalmol Vis Sci 2001; 42: 2664–72. [PubMed] [Google Scholar]
- 87.Hammes HP, Brownlee M, Jonczyk A, Sutter A, Preissner KT. Subcutaneous injection of a cyclic peptide antagonist of vitronectin receptor-type integrins inhibits retinal neovascularization. Nat Med 1996; 2: 529–33. [DOI] [PubMed] [Google Scholar]
- 88.Danen EHJ, Sonneveld P, Brakebusch C, Fässler R, Sonnenberg A. The fibronectin-binding integrins alpha5beta1 and alphavbeta3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J Cell Biol 2002; 159: 1071–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mao Y, Schwarzbauer JE. Accessibility to the fibronectin synergy site in a 3D matrix regulates engagement of alpha5beta1 versus alphavbeta3 integrin receptors. Cell Commun Adhes 2006; 13: 267–77. [DOI] [PubMed] [Google Scholar]
- 90.Steel DHW, Lotery AJ. Idiopathic vitreomacular traction and macular hole: a comprehensive review of pathophysiology, diagnosis, and treatment. Eye (Lond) 2013; 27: S1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rocha AS, Santos FM, Monteiro JP, Castro-de-Sousa JP, Queiroz JA, Tomaz CT, Passarinha LA. Trends in proteomic analysis of human vitreous humor samples. Electrophoresis 2014; 35: 2495–508. [DOI] [PubMed] [Google Scholar]
- 92.Murthy KR, Goel R, Subbannayya Y, Jacob HK, Murthy PR, Manda SS, Patil AH, Sharma R, Sahasrabuddhe NA, Parashar A, Nair BG, Krishna V, Prasad TK, Gowda H, Pandey A. Proteomic analysis of human vitreous humor. Clin Proteomics 2014; 11: 29–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mundel TM, Kalluri R. Type IV collagen-derived angiogenesis inhibitors. Microvasc Res 2007; 74: 85–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yuan X, Gu X, Crabb JS, Yue X, Shadrach K, Hollyfield JG, Crabb JW. Quantitative proteomics: comparison of the macular Bruch membrane/choroid complex from age-related macular degeneration and normal eyes. Mol Cell Proteomics 2010; 9: 1031–46. [DOI] [PMC free article] [PubMed] [Google Scholar]