Abstract
Objective: To investigate the effects of Bisphenol A (BPA) on prolactin (PRL) release, pituitary cell proliferation, prolactinoma formation in estrogen-sensitive Fischer 344 (F344) rats. Materials and methods: Four-week-old female F344 rats were orally administered with different concentrations of BPA or intraperitoneal injection of estradiol benzoate (estradiolbenzoate, E2) for 12 weeks. Bodyweight, blood RPL level and pituitary weights were observed and recorded. Real-time PCR, western blot and immunohistochemistry analysis were used to detect the mRNA and protein levels of the proliferation markers, including proliferating cell neclear antigen (PCNA), pituitary tumor-transforming gene (PTTG) and its relevant marker ERα. Plasma and urine BPA concentration in patients with prolactinoma and healthy participants were measured as well. Results: Body weights of the rats treated with BPA were significantly decreased compared with those in the control group. The plasma PRL level and the pituitary weights of the rats were higher than those in the control group after BPA treatment. Compared with the control group, the pituitary mRNA and protein expression levels of PCNA and PTTG were significantly increased after BPA treatment. Moreover, ERα expression level was enhanced by the treatment of BPA in comparison with that of the control group. Finally, the plasma BPA concentration in the prolactin tumor patients was significantly higher than that in the healthy participants. Conclusion: BPA can significantly promote pituitary cell proliferation and prolactin secretion in F344 rats, which may have impact on the proliferation and secretion of pituitary cell function through the ERα pathway.
Keywords: Prolactinomas, bisphenol A, cell proliferation, prolactin secretion, estradiol
Introduction
Prolactinoma or is the most common tumor (representing 40% of all pituitary adenoma) of the pituitary gland that produces a hormone called prolactin [1]. The most common manifestations of prolactinoma are infertility and sexual dysfunction [2,3]. Current studies suggested that estrogen (E2) were involved in progression of prolactinoma [4-7]. In F344 rats prolactinoma model, the estrogen receptor antagonist fulvestrant can effectively suppress the growth of prolactinoma [5].
Bisphenol A (BPA), a monomer of plastics, is widely used in the manufacture of polycarbonate plastics and epoxy resins, which leads to a close exposure to human via food and beverage storage containers, baby bottles, dental-sealants as well as other materials [8]. There were reports suggested that BPA could bind hormone receptors to act as a selective ER modulator, which involved in multiple endocrine-regulated pathways [9-11]. Moreover, BPA can accelerate estrus, which promotes the growth of prostate and alter prepubertal mammary gland development [12-14]. Data from mice also suggested that BPA could permanently change the female genital tract and affect morphology and function of the ovaries in [15]. Furthermore, BPA can significantly increase the incidence of cancers of endocrine target organs, including prostate, breast, testis and ovary and were reported to induce breast and endometrial cancer via estrogen receptor-α (ER-α), ER-β or other non-genomic pathways [16,17].
Previous reports suggested that high concentrations of BPA could induce cell growth and prolactin secretion via triggering membrane ER-α mediated Ca2+ fluxes and prolactin releasing in GH3/B6 pituitary tumor cells [18,19]. Moreover, report also demonstrated that BPA promotes proliferation of prolactin cells and lead to hyperprolactinemia [17]. However, the effects of long-term exposure to BPA is still unclear and little attention has been paid for elucidating the relationship between BPA and prolactinoma [16,20].
In this study, we examined BPA’s role in occurrence of prolactinoma. Different concentrations of BPA were used to treating pituitary cell to observing proliferation and the formation of prolactinoma in female F344 rats. The relationship between BPA concentrations in serum and prolactinoma compared between prolactinoma patients and healthy subjects as well.
Materials and methods
Ethic statement
The study was approved by Animal Care and Ethical Committee of Shandong University and accepted by patients involved in this trial. All participating subjects were formally informed for the purpose of using there sample of this study and a letter of consent was signed by every subject involved. For the animal experiments, all measures were taken to minimize the suffering of the animals.
Animals
Four weeks old F344 female rats (Vital River Laboratory Animal Technology Co. Ltd, Bingjing, China), with weights of 53.64 ± 3.68 g, were used in the study. Animal rooms were maintained on a 12:12 light-dark cycle starting at 07:00 am and kept at a temperature of 22°C-23°C. All animals were free access to food and tap water. BPA (>99% purity; Sigma-Aldrich, Inc., St. Louis, MO) was dissolved in dimethylsulfoxide. E2 were purchased from Hangzhou Animal Medicine Co., Ltd, China.
Clinical trials part
A total of 120 patients were enrolled in our study. Participants were divided into prolactinoma group and healthy controls. Tissue samples were collected during March, 2015 and December, 2015. Inclusion criteria: 1) prolactinoma patients and healthy volunteers; 2) with normal liver and kidney function; 3) without taking estrogen dopamine pathway antidepressants and other drugs; 4) no history of head trauma. Exclusion criteria: 1) long-term use of contraceptive drugs; 2) with liver and kidney function injury. The serum and midstream urine were collected for further examination. Fluorescence-high performance liquid chromatography mass spectrometry was used to detect plasma and urine BPA concentration.
Animal experiment
60 female F344 rats were randomly divided into six groups with 10 rats in each group. The groups listed as follows: control group (with only corn oil), experimental groups and positive control group. The experimental groups were daily fed with BPA 50 mg/kg/d group (named as BPA50 group), BPA 200 mg/kg/d group (named as BPA200 group) and BPA 400 mg/kg/d group (named as BPA400 group). Rats with E2 intraperitoneal injection were set as positive group (2 mg/kg/5 d, named as E2 group).
Immunohistochemical staining
Rats were anesthetized after 6 weeks of indicated treatment via isoflurane inhalation. The blood samples were collected by angular vein plexus for PRL test via ELISA at indicated time points. The body weight changes were monitored daily. HE staining and immumohistochemical staining were conducted to examine the expression of PRL, PCNA and ER-α.
Real-time PCR
The RNA isolation from pituitary cells were conducted by using TRizol (Invitrogen) according to manufacturer’s instruction. Synthesis of cDNA was conducted by using AMV reverse transcriptase (Promega, Madison, WI, USA) according to instruction of manufacturer. Real-time PCR (qPCR) detection for transcripts of targeting genes with SYBR Green Mix (Life technologies) were described previously [21,22]. Transcripts of β-actin were also amplified from the same sample to serve as an internal control. Relative gene expression was quantified by 2-∆∆CT method as previously described [23]. Primers for qPCR detection were listed as Table 1.
Table 1.
qPCR Primers and their sequence used in this study
| Primer name | Sequence (5’ to 3’) |
|---|---|
| PCNA-F1 | ATATGCCGGGACCTTAGCCA |
| PCNA-R1 | TCGCAGAAAACTTCACCCCG |
| PTTG-F1 | ATTGCAGGTTTCAACGCCAC |
| PTTG-R1 | AGCCTTCCTGCTGGCTTT |
| ERα-F1 | ATCCCCAAAGCAAACAGGACTT |
| ERα-R1 | TCAAAGAGCTAAGCCAGTCGC |
| ERβ-F1 | CTGGACAGGGATGAGGGGAA |
| ERβ-R1 | TCAGCTTCCGGCTACTTTCTG |
| β-actin-F1 | AGCCATGTACGTAGCCATCC |
| β-actin-R1 | ACCCTCATAGATGGGCACAG-3 |
Western blot analysis
Tissue samples were washed three times with PBS and protein was extracted by BCA kit according to the manufacturer’s instructions. Then the samples were lysed by the Laemmli Sample Buffer as previously described [22,24]. The protein of lysate was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for Western blot as previously described [24]. Briefly, after SDS-PAGE, the separated proteins were transferred onto PVDF membrane and probed with rabbit anti-ERɑ, PCNA, and mouse anti-PTTG (dilution 1:200) (Abcam Inc., Cambridge, MA, USA) monoclonal antibody. Specific reactions were detected using goat anti-rabbit IgG or goat anti-mouse IgG conjugated with horseradish peroxidase (Sigma, St. Louis, MO) and revealed by a chemiluminescence substrate (Bio-Rad Laboratories, Hercules, CA, USA). The membrane was also blotted with GAPDH antibody (Santa Cruz) to normalize the protein loading. The chemiluminescence signal was recorded by the ChemiDoc MP imaging system (Bio-Rad Laboratories). The luminescence signal was captured and analyzed by using the ImageLab Program (Version 6.1).
Statistical analysis
SPSS 10.0 statistical software was used to for statistical analysis, quantitative data were presented as mean ± SD. The Student t-test, one-way analysis of variance, Dunnett’s test, and χ2 test were conducted for analyzing data. Correlation analysis was applied to observe the correlation between bisphenol A and prolactinoma. P<0.05 was considered to be statistically significant.
Results
BPA exposure increased pituitary weight and plasma PRL level
To investigate if rats exposing to BPA will display any abnormalities in lactotropic hormone secretion, F344 rats from indicated group were sacrificed and the pituitary weight and plasma PRL levels were measured. As demonstrated in Figure 1A-C, both pituitary weights and plasma levels of PRL were significantly elevated in rats treated with BPA or E2 compared to normal controls. Notably, with intervention of BPA for 6 weeks, the serum PRL levels of BPA50, BAP200, BAP400 and E2 group are significantly higher than control group and the PRL level of E2 group demonstrated the highest level (Figure 1B). After drug intervention for 12 weeks, the serum PRL level of BPA groups or E2 group was further increases (Figure 1C).
Figure 1.
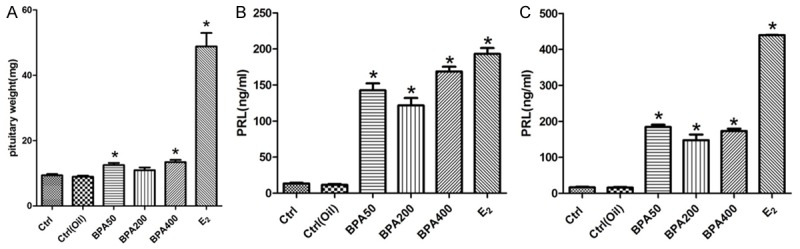
BPA exposure increased pituitary weight plasma PRL level in rat. A. The pituitary weight changes after rat treat with BPA 50 mg/kg/d, BPA 200 mg/kg/d, BPA 400 mg/kg/d and E2. B. The PRL level in the serum of the rats from indicated groups with different treatment 6 weeks. C. The PRL level in the serum of the rats with indicated treatment. Experiment for each sample was repeated for at least three times. Significant differences between control group experimental groups are shown by “*” to indicate significant difference (P<0.05).
BPA and E2 exposure promoted the formation of pituitary tumor
With indicated treatments for 12 weeks, no obvious abnormal rat pituitary tissues were found in the control group or solvent control group. However, in BPA50 group (with BPA 50 mg/kg/d), there were four rats (4/10) demonstrated tumor changes in pituitary with disappearance of normal morphology and increased adenohypophysis (Figure 2A). Moreover, disordered arrangement of cells, diversified tumor cell morphology such as nuclear abnormity and nuclear hyperchromatism were observed as well (Figure 2A). Red blood cell overflow phenomenon was visible. On the other hand, there are 3 rats and one rat with pituitary tumor were identified in BPA400 group and BPA200 group, respectively. The pituitary cell proliferation is visible grown from the pituitary gland (Figure 2A). Moreover, the pituitary PRL immunohistochemical manifested the pituitary tumor were prolactinoma. The PRL immunohistochemical score among BPA and E2 groups was obviously higher than that of control group or corn oil group. These results further indicated that treatment with BPA or E2 could induce prolactinoma formation (Figure 2B).
Figure 2.
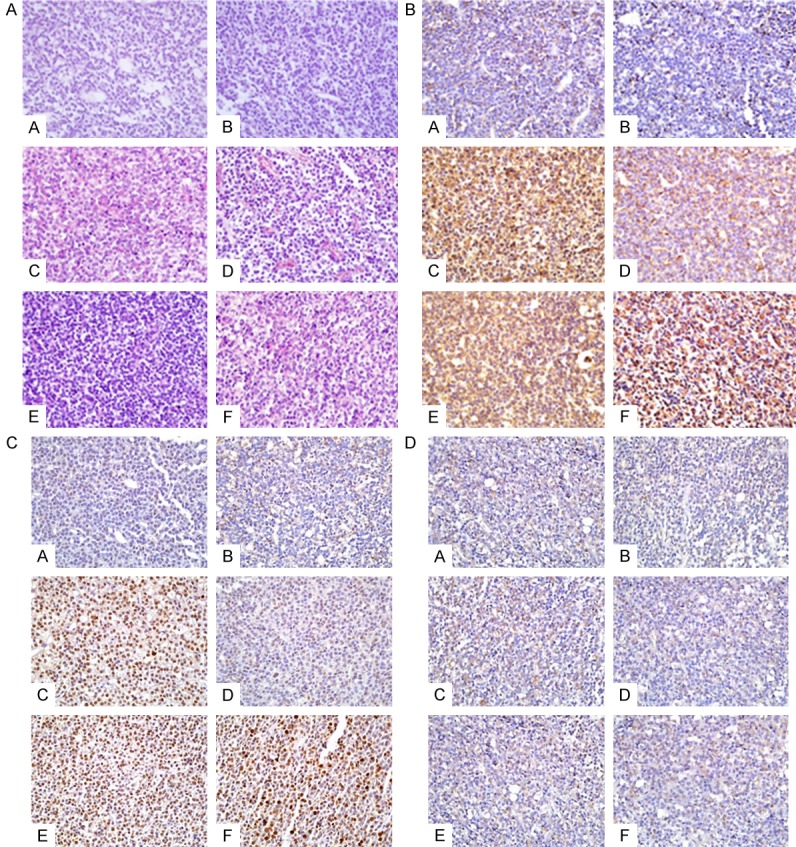
BPA and E2 exposure promoted the formation of pituitary tumor. A. The HE staining of rat pituitary gland Indicated that BPA and E2 could promoted the formation of pituitary tumor; B. The immunohistochemical staining of pituitary PRL level; C. The immunohistochemical staining for pituitary PCNA; D. The immunohistochemical staining for pituitary ERα. Group set: A (Control), B (Oil control), C (BPA50), D (BPA200), E (BPA400), F (E2).
The PCNA immunohistochemical score of rat pituitary treated with BPA and E2 group was obviously higher than those of control group and corn oil group, the difference was statistically significant (Figure 2C). The ERα expression in BPA50 group was obviously increased, which is higher than the control groups. However, no significant difference of ERα immunohistochemical score was found in BPA200, 400 and E2 group compared with the control group and the corn oil group (Figure 2D).
BPA treatment up-regulate ERα, ERβ PTTG and PCNA in rat pituitary
Moreover, ERα expression in BPA50 and E2 group was significantly increased as well. The expression of ERβ in BPA50 group and BPA200 group was obviously increased (Figure 3A). However, no significant difference was found in the BPA400 and E2 groups compared with control group and the corn oil group (Figure 3B), To further confirm our result, we also conducted a quantities analysis for the density of our WB data with repeating the experiments for at least 3 times (Figure 4). Based on our data, the PCNA protein expressions in BPA50, 400 and E2 group were obviously higher. However, the PCNA protein expression in BPA200 group was similar to control group and the corn oil group. On the other hand, the PTTG protein expression in BPA50, 400 and E2 group was also higher than control groups. However, the PTTG protein expression in BPA200 has no statistical significance difference with the control group and the corn oil group.
Figure 3.
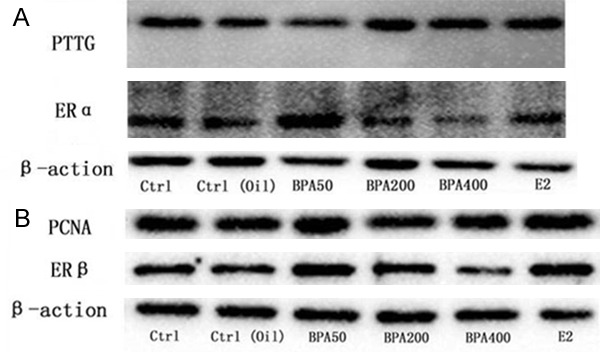
The western blot results of rat pituitary PTTG, PCNA, ERα, ERβ. A: Western blot analysis for PTTG and ERα; B: Western blot analysis for PCNA and ERβ.
Figure 4.
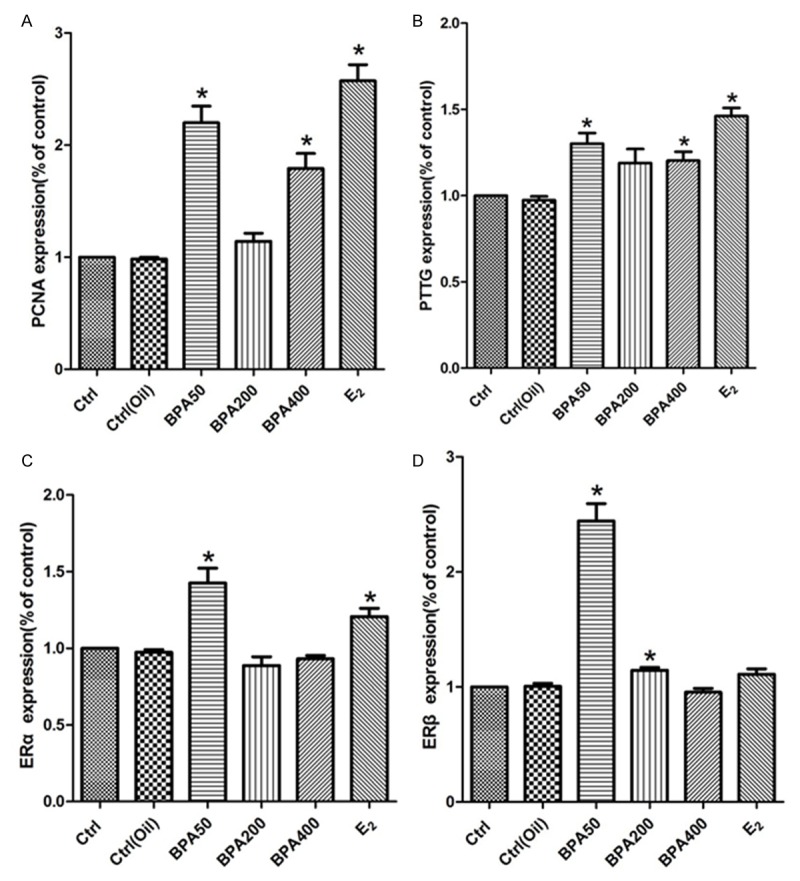
Quantification analysis of the WB blot data for different genes. A: Quantification analysis of rat pituitary PCNA. B. Quantification analysis of rat pituitary PTTG. C: Quantification analysis of rat pituitary ERα. D: Quantification analysis of rat pituitary ERβ.
The qPCR quantification of mRNA of indicated genes was also conducted for confirming our result. The mRNA expression levels of PCNA and PTTG in BPA 50, 200, 400 and E2 group were significantly higher than those in the control group and the corn oil group, the difference was statistically significant (Figure 5A and 5B). The ERα mRNA expression level in BPA50, 400 and E2 group was obviously higher than that of control group and the corn oil group, the difference was statistically significant. However, no significant difference of the ERα mRNA expression level was found in BPA200 group compared to that of control group and the corn oil group (Figure 5C). The ERβ mRNA in BPA50, 200 and E2 group was obviously higher than that of control group and corn oil group, the difference was statistically significant (Figure 5D).
Figure 5.
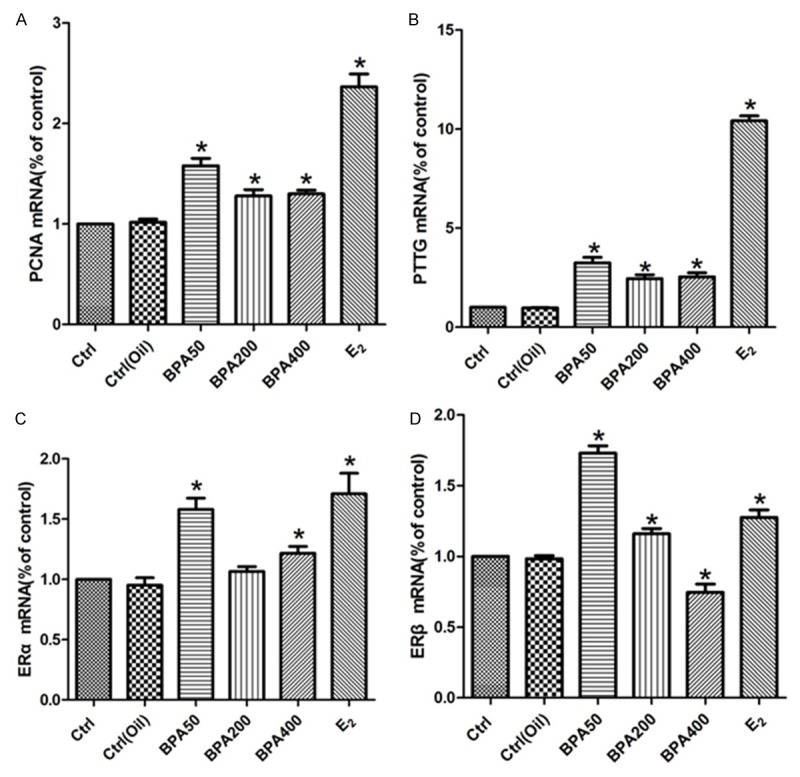
qPCR analysis for mRNA expression of indicated genes among different groups. A. The results of PCNA mRNA expression in pituitaries; B. The results of PTTG mRNA expression in pituitaries; C. The results of ERα mRNA expression in pituitaries; D. The results of ERβ mRNA expression in pituitaries. Experiment for each sample was repeated for at least three times. Significant differences between control group experimental groups are shown by “*” to indicate significant difference (P<0.05).
Serum BPA concentration was significant elevated in prolactinoma patients
To confirm our expectation of the relationship between BPA and occurrence of prolactinoma, we further examine the BPA concentration both in serum and urine from prolactinoma patients. As demonstrated in Figure 6A the BPA level (ng/mL) in the serum of the prolactinoma patients was significantly higher than normal control group, which implied a co-relationship between each other. However, no statistically significant difference was found of urine BPA concentration (Figure 6B).
Figure 6.
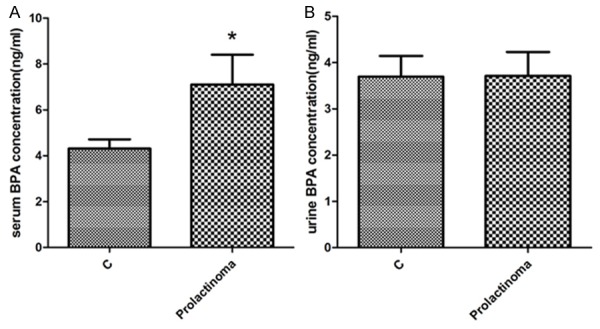
Serum BPA concentration was significant evaluated in prolactinoma patients. A. The BPA concentration in serum of the prolactinoma patients and healthy controls; B. The BPA concentration in urine from the prolactinoma patients and healthy control; Experiment for each sample was repeated for at least three times. Significant differences between control group experimental groups are shown by “*” to indicate significant difference (P<0.05).
Discussion
In the present study, we examined the effect of the pituitary cell proliferation and prolactin secretion for female F344 rats treated with different concentrations of BPA. Similarly to the previous researches [25,26], our results showed that BPA exposure increased plasma PRL level and lactotropic cell proliferation in the pituitary gland. Moreover, our data confirmed that the long-term consequences of this proliferation caused neoplasm formation. Moreover, the BPA concentration in the serum of the prolactinoma patients was significantly higher than control group.
Our data suggested that BPA exposure increases plasma PRL level, lactotropic cell proliferation and pituitary growth. It should be noted that, PRL levels were significantly increased in BPA50 and BPA400 group compared with that in BPA200 group, showing u-shaped pattern, which was different from the previous observation [17,27]. Such difference may be due to the different experimental designs or different species used in study, or the doses of BPA. Moreover, the unique non-monotonic dose responses and variant signaling patterns caused by E2 and BPA suggest that complex and multiple signaling pathways or binding partners could be involved [28].
The mechanisms of gene-BPA interactions have not yet fully clarified [29]. Previous reports suggested that BPA selectively binding to classic ER (ERα and ERβ) and acts as an ER modulator [30,31]. ERα and ERβ play a significant role in steroidogenesis, follicle growth, and endometrial cycle [32]. In in vivo study, BPA could up-regulate mRNA expression of ERα [33]. Our data showed the ERα expression in BPA50 group was evaluated which was consisted with previous report [33]. Interestingly, the effect of BPA on the expressions of ERα/ERβ was inconsistent with its impact on PRL secretion and pituitary cell proliferation. The may be due to the BPA acts on pituitary cells through a variety of pathways except ERα/ERβ-dependent genomic pathway, which may need further investigation.
BPA is widely used and ubiquitous in the environment. In the general population, BPA has been detected in various tissues and body flow, including serum, breast milk, urine umbilical cord blood and saliva [34-36]. Moreover, women who used oral contraceptives for menstrual irregularities have a 7-to 8-fold higher incidence of prolactinoma, which suggests exogenous estrogens may stimulate incipient prolactinoma to grow [17,37]. Therefore, we hypothesized that BPA could promote the formation of human prolactinoma. We found serum BPA concentration of the prolactinoma patients was significantly higher than that in the normal control groups.
In summary, we systematically investigated the relationship between the occurrence and progression of the prolactinoma with different doses of BPA. It is clear to conclude that BPA can significantly promote rat pituitary cell proliferation, prolactin secretion, and promote the formation of prolactinoma. Further investigation are needed to elucidate whether long-term exposure to BPA may have an increased incidence of prolactinoma, and the underlying mechanisms of BPA on the pituitary cells through various strategies.
Acknowledgements
This study is supported by grants from Natural Science Foundation of China (Grant No. 81272181) and Science and Technology development program of Shandong province (Grant No. 2012GGE27080).
Disclosure of conflict of interest
None.
References
- 1.Glezer A, Bronstein MD. Prolactinomas. Endocrinol Metab Clin North Am. 2015;44:71–78. doi: 10.1016/j.ecl.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Gillam MP, Molitch ME, Lombardi G, Colao A. Advances in the treatment of prolactinomas. Endocr Rev. 2006;27:485–534. doi: 10.1210/er.2005-9998. [DOI] [PubMed] [Google Scholar]
- 3.Schlechte JA. Clinical practice. Prolactinoma. N Engl J Med. 2003;349:2035–2041. doi: 10.1056/NEJMcp025334. [DOI] [PubMed] [Google Scholar]
- 4.Takekoshi S, Yasui Y, Inomoto C, Kitatani K, Nakamura N, Osamura RY. A Histopathological Study of Multi-hormone Producing Proliferative Lesions in Estrogen-induced Rat Pituitary Prolactinoma. Acta Histochem Cytochem. 2014;47:155–164. doi: 10.1267/ahc.14029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao L, Gao H, Gui S, Bai G, Lu R, Wang F, Zhang Y. Effects of the estrogen receptor antagonist fulvestrant on F344 rat prolactinoma models. J Neurooncol. 2014;116:523–531. doi: 10.1007/s11060-013-1351-8. [DOI] [PubMed] [Google Scholar]
- 6.Leng L, Zhang Y. Effects of an estrogen receptor antagonist on proliferation, prolactin secretion and growth factor expression in the MMQ pituitary prolactinoma cell line. J Clin Neurosci. 2011;18:1694–1698. doi: 10.1016/j.jocn.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Miyajima K, Takekoshi S, Itoh J, Kakimoto K, Miyakoshi T, Osamura RY. Inhibitory effects of anti-VEGF antibody on the growth and angiogenesis of estrogen-induced pituitary prolactinoma in Fischer 344 Rats: animal model of VEGF-targeted therapy for human endocrine tumors. Acta Histochem Cytochem. 2010;43:33–44. doi: 10.1267/ahc.09034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.North EJ, Halden RU. Plastics and environmental health: the road ahead. Rev Environ Health. 2013;28:1–8. doi: 10.1515/reveh-2012-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alonso-Magdalena P, Ropero AB, Soriano S, Garcia-Arevalo M, Ripoll C, Fuentes E, Quesada I, Nadal A. Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways. Mol Cell Endocrinol. 2012;355:201–207. doi: 10.1016/j.mce.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Burns KA, Arao Y, Luh CJ, Korach KS. Differential estrogenic actions of endocrine-disrupting chemicals bisphenol A, bisphenol AF, and zearalenone through estrogen receptor alpha and beta in vitro. Environ Health Perspect. 2012;120:1029–1035. doi: 10.1289/ehp.1104689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, Matsushima A, Okada H, Shimohigashi Y. Distinction of the binding modes for human nuclear receptor ERRgamma between bisphenol A and 4-hydroxytamoxifen. J Biochem. 2010;148:247–254. doi: 10.1093/jb/mvq056. [DOI] [PubMed] [Google Scholar]
- 12.Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999;401:763–764. doi: 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- 13.Timms BG, Howdeshell KL, Barton L, Bradley S, Richter CA, vom Saal FS. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc Natl Acad Sci U S A. 2005;102:7014–7019. doi: 10.1073/pnas.0502544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munoz-de-Toro M, Markey CM, Wadia PR, Luque EH, Rubin BS, Sonnenschein C, Soto AM. Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice. Endocrinology. 2005;146:4138–4147. doi: 10.1210/en.2005-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markey CM, Wadia PR, Rubin BS, Sonnenschein C, Soto AM. Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biol Reprod. 2005;72:1344–1351. doi: 10.1095/biolreprod.104.036301. [DOI] [PubMed] [Google Scholar]
- 16.Seachrist DD, Bonk KW, Ho SM, Prins GS, Soto AM, Keri RA. A review of the carcinogenic potential of bisphenol A. Reprod Toxicol. 2016;59:167–182. doi: 10.1016/j.reprotox.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soto AM, Sonnenschein C. Environmental causes of cancer: endocrine disruptors as carcinogens. Nat Rev Endocrinol. 2010;6:363–370. doi: 10.1038/nrendo.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wozniak AL, Bulayeva NN, Watson CS. Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-alpha-mediated Ca2+ fluxes and prolactin release in GH3/B6 pituitary tumor cells. Environ Health Perspect. 2005;113:431–439. doi: 10.1289/ehp.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chun TY, Gorski J. High concentrations of bisphenol A induce cell growth and prolactin secretion in an estrogen-responsive pituitary tumor cell line. Toxicol Appl Pharmacol. 2000;162:161–165. doi: 10.1006/taap.1999.8840. [DOI] [PubMed] [Google Scholar]
- 20.Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Cien Saude Colet. 2012;17:407–434. doi: 10.1590/s1413-81232012000200015. [DOI] [PubMed] [Google Scholar]
- 21.Patel D, Opriessnig T, Stein DA, Halbur PG, Meng XJ, Iversen PL, Zhang YJ. Peptide-conjugated morpholino oligomers inhibit porcine reproductive and respiratory syndrome virus replication. Antiviral Res. 2008;77:95–107. doi: 10.1016/j.antiviral.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel D, Nan Y, Shen M, Ritthipichai K, Zhu X, Zhang YJ. Porcine reproductive and respiratory syndrome virus inhibits type I interferon signaling by blocking STAT1/STAT2 nuclear translocation. J Virol. 2010;84:11045–11055. doi: 10.1128/JVI.00655-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Nan Y, Wang R, Shen M, Faaberg KS, Samal SK, Zhang YJ. Induction of type I interferons by a novel porcine reproductive and respiratory syndrome virus isolate. Virology. 2012;432:261–270. doi: 10.1016/j.virol.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Velasco-Marinero E, Herrero-Payo JJ, Carretero-Gonzalez J. Changes in pituitary and prolactin cells of Wistar rats after two dental fillings with bisphenolic resins. Arch Oral Biol. 2011;56:592–598. doi: 10.1016/j.archoralbio.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Brannick KE, Craig ZR, Himes AD, Peretz JR, Wang W, Flaws JA, Raetzman LT. Prenatal exposure to low doses of bisphenol A increases pituitary proliferation and gonadotroph number in female mice offspring at birth. Biol Reprod. 2012;87:82. doi: 10.1095/biolreprod.112.100636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goloubkova T, Ribeiro MF, Rodrigues LP, Cecconello AL, Spritzer PM. Effects of xenoestrogen bisphenol A on uterine and pituitary weight, serum prolactin levels and immunoreactive prolactin cells in ovariectomized Wistar rats. Arch Toxicol. 2000;74:92–98. doi: 10.1007/s002040050658. [DOI] [PubMed] [Google Scholar]
- 28.Jeng YJ, Kochukov MY, Watson CS. Membrane estrogen receptor-alpha-mediated nongenomic actions of phytoestrogens in GH3/B6/F10 pituitary tumor cells. J Mol Signal. 2009;4:2. doi: 10.1186/1750-2187-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Machtinger R, Orvieto R. Bisphenol A, oocyte maturation, implantation, and IVF outcome: review of animal and human data. Reprod Biomed Online. 2014;29:404–410. doi: 10.1016/j.rbmo.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Maruyama K, Nakamura M, Tomoshige S, Sugita K, Makishima M, Hashimoto Y, Ishikawa M. Structure-activity relationships of bisphenol A analogs at estrogen receptors (ERs): discovery of an ERalpha-selective antagonist. Bioorg Med Chem Lett. 2013;23:4031–4036. doi: 10.1016/j.bmcl.2013.05.067. [DOI] [PubMed] [Google Scholar]
- 31.Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147:S56–69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- 32.Caserta D, Ciardo F, Bordi G, Guerranti C, Fanello E, Perra G, Borghini F, La Rocca C, Tait S, Bergamasco B, Stecca L, Marci R, Lo Monte G, Soave I, Focardi S, Mantovani A, Moscarini M. Correlation of endocrine disrupting chemicals serum levels and white blood cells gene expression of nuclear receptors in a population of infertile women. Int J Endocrinol. 2013;2013:510703. doi: 10.1155/2013/510703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chao HH, Zhang XF, Chen B, Pan B, Zhang LJ, Li L, Sun XF, Shi QH, Shen W. Bisphenol A exposure modifies methylation of imprinted genes in mouse oocytes via the estrogen receptor signaling pathway. Histochem Cell Biol. 2012;137:249–259. doi: 10.1007/s00418-011-0894-z. [DOI] [PubMed] [Google Scholar]
- 34.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110:A703–707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fung EY, Ewoldsen NO, St Germain HA Jr, Marx DB, Miaw CL, Siew C, Chou HN, Gruninger SE, Meyer DM. Pharmacokinetics of bisphenol A released from a dental sealant. J Am Dent Assoc. 2000;131:51–58. doi: 10.14219/jada.archive.2000.0019. [DOI] [PubMed] [Google Scholar]
- 37.Shy KK, McTiernan AM, Daling JR, Weiss NS. Oral contraceptive use and the occurrence of pituitary prolactinoma. JAMA. 1983;249:2204–2207. [PubMed] [Google Scholar]


