Abstract
The pathogenesis of metastasis of colon cancer (Cca) is to be further investigated. The dysfunction of apoptotic mechanism plays a role in the cancer cell over growth. This study tests a hypothesis by which intestinal bacterium-derived cyp27a1 prevents apoptosis in colon cancer cells. In this study, the levels of cyp27a1 in human stool samples were assessed by enzyme-linked immunosorbent assay. The apoptosis of Cca cells was observed by flow cytometry. The expression of cyp27a1 was assessed by real time RT-PCR and Western blotting. We observed higher levels of cyp27a1 in the stool samples of Cca patients than that from healthy subjects. Cca colon epithelial biopsy contained high levels of cyp27a1 protein, but not the cyp27a1 mRNA. Cyp27a1 prevented Cca cell apoptosis induced by vitamin D3. In conclusion, intestinal bacterium-derived cyp27a1 facilitates Cca survival by inhibiting Cca cell apoptosis.
Keywords: Colon cancer, vitamin D, Cyp27a1, apoptosis
Introduction
Colon cancer (Cca) is the cancer in the colon. It is due to the abnormal growth of cells that have the ability to invade or spread to other parts of the body [1]. Signs and symptoms may include blood in the stool, a change in bowel movements, weight loss, and feeling tired all the time [2]. The pathogenesis of Cca is unclear [3]. In the cases with early diagnosis and early treatment, the therapeutic results are quite encouraging [4]. However, because of the anatomical feature, a large part of Cca patients are diagnosed at the advanced stage, among which a large portion of the patients with remote Cca metastasis; the therapeutic results of those patients is relatively poor currently [5,6].
It is suggested that dysfunction of the apoptotic mechanism contributes to the pathogenesis of cancer [7,8]. Apoptosis is a process of programmed cell death that occurs in multicellular organisms [9]. Apoptotic cells have characteristic cell morphology changes, including blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, chromosomal DNA fragmentation, and global mRNA decay [10,11]. In healthy human beings, the body cells die regularly, while cancer cells proliferate fast or out of control [12]. Such a phenomenon is implicated as the dysfunction of the apoptotic mechanism; the causative factors are unclear yet.
A number of factors can regulate apoptosis [13], such as the binding of nuclear receptors by glucocorticoids [14], heat [15], radiation [16], nutrient deprivation [17], viral infection [18], hypoxia [19]. In addition, the activated vitamin D3 (VD3) can also induce cancer cell apoptosis [20]. VD3 can be converted to its bioactive form by cytochrome p450 27B1 (cyp27b1) [21]; the activated form of VD3 can be inactivated by cyp27a1 [22]. It seems that deregulation of cyp27a1 may be associated with the reduction of cancer cell apoptosis. Yet, the regulation of cyp27a1 in cancer patients remains less understood. In this study, we observed high levels of cyp27a1 in the stool samples of Cca patients. Data from in vitro study showed that cyp27a1 inhibited Cca cell apoptosis induced by VD3.
Materials and methods
Ethic statement
This study was approved by the Human Ethic Committee at Harbin Medical University. All the experimental procedures were carried out in accordance with the approved guidelines. An informed written consent was obtained from each human subject.
Collection of human stool samples
From each human subject, stool samples (about 5 g/sample) were collected into a sterile plastic bag immediately after release. Immediately, a portion of the samples was processed for protein extraction. A portion of the samples was processed for bacterial culture. Human subjects consisted of 32 Cca patients and 20 healthy subjects. The Cca (19 males; 13 females; age: 35-68 years old) was diagnosed by clinical doctors and pathologists, and treated with the routine procedures in our hospital.
Extracting fecal DNA and protein
The fecal samples mixed with RNAprotect Bacteria Reagent (Qiagen) were homogenized to a uniform consistency, and RNA was routinely extracted from 0.3 g of the fresh fecal materials using the RNeasy Mini Kit (Qiagen), following the manufacturer’s instructions. Protein was extracted from 0.5 g of the fecal materials using a protein extracting kit (Merck Millipore), following the manufacturer’s instructions. The protein was quantified using the BCA method with a Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific), following the manufacturer’s instructions. Approximately 1.0 × 109 cells grown in BM2 were harvested at an OD600 = 0.5, mixed with RNAprotect Bacteria Reagent (Qiagen), and stored at -80°C. Isolation of total RNA and assessment of its quantity and quality were performed as previously described (26).
Assessment of cyp27a1
Proteins were extracted from the stool samples and quantitated with the BCA method. The contents of cyp27a1 were determined by enzyme-linked immunoassay (ELISA) with commercial reagent kits (Biocompare), following the manufacturer’s instructions.
Real time quantitative RT-PCR (qPCR)
To quantify the expression of cyp27a1 in intestinal bacteria, the extracted bacterial RNA was reversely converted to cDNA with a reverse transcription kit (QuantiTect Reverse Transcription Kit, Qiagen). The cDNA was amplified in a Bio-Rad CFX 384 Real-time system with the SYBR Green Master Mix (Invitrogen) and a pair of primer of cyp27a1 (tgccttctctgagcctgaaa and gcatctccagctctgcaatc), or β-actin (cgcaaagacctgtatgccaa and cacacagagtacttgcgctc). The results were calculated with the 2-ΔCt method, and presented as fold change against a control group.
Western blotting
To determine the levels of protein, the total protein was fractioned by SDS-PAGE and transferred onto a PVDF membrane. After blocking with 5% skim milk, the membrane was incubated with the primary antibodies and followed by incubating with the second antibodies (labeled with peroxidase). Washing with TBST (Tris-buffered saline Tween 20) was performed after each time of incubation. The membrane was developed with the enhanced chemiluminescence and photographed with an image processing system (UVI, Cambridge, UK).
Cell culture
T84 cells (a human Cca cell line) were cultured in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 0.1 mg/ml streptomycin and 2 mM L-glutamine. The cell viability was greater than 99% before using for further experiments as assessed by Trypan blue exclusion assay. The cell number was quantified with a flow cytometer.
Assessment of apoptotic cells
T84 cells were dissociated from the culture flask with trypsin-EDTA (0.25%; Thermo Fisher Scientific). The cells were stained with an Annexin V reagent kit and propidium iodide (5 µg/ml) (Sigma Aldrich), following the manufacturer’s instructions. The rate of apoptotic cells was analyzed by flow cytometry.
RNA interference (RNAi)
RNAi of cyp27a1 in T84 cells were performed with a cyp27a1 shRNA kit (Santa Cruz Biotec), following the manufacturer’s instructions. The RNAi results were assessed by Western blotting.
Statistics
Data are presented as mean ± SD. The difference between two groups was assessed by the Student t test, ANOVA if more than two groups. P<0.05 was set as a criterion of significance.
Results
High levels of cyp27a1 are detected in the stool of patients with Cca
We collected the stool samples from Cca patients and analyzed by ELISA. The results showed that cyp27a1 was detected in the stool samples, which was higher in Cca patients than in healthy subjects (Figure 1A). We assumed that the cyp27a1 was produced by intestinal bacteria. To test this, we isolated bacteria from the stool samples. The bacteria were analyzed by RT-qPCR and Western blotting. The results showed that the bacteria expressed cyp27a1 at both mRNA and protein levels, which was higher in Cca patients than in healthy subjects (Figure 1B, 1C).
Figure 1.

Cca patient stool bacteria express high levels of cyp27a1. A: The bars indicate the cyp27a1 protein levels (by ELISA) in stool samples of 32 Cca patients and 20 healthy subjects. B and C: The bars indicate the mRNA levels of cyp27a1 (B: By RT-qPCR) and the immune blots indicate the protein levels of cyp27a1 (C: By Western blotting) in bacteria isolated from the stool samples. Data are presented as mean ± SD. *, P<0.01, compared with healthy subjects. Samples from individual patients were analyzed separately. Antibody of cyp27a1 was purchased from Santa Cruz Biotec (Santa Cruz, CA).
Colon epithelium from Cca patients contains higher cyp17a1 than normal subjects
Considering the cyp27a1 produced by intestinal bacteria might be absorbed by the intestinal epithelial cells, we collected colon epithelial samples from Cca patients and non-cancer subjects. The samples were analyzed by RT-qPCR and Western blotting. As shown by Figure 2, significantly higher protein levels of cyp27a1 were detected in samples from Cca patients as compared with samples from non-cancer subjects, while the mRNA levels of cyp27a1 were not different between Cca patients and non-cancer subjects. The results suggest that the cyp27a1 detected in the epithelial samples is not produced by the epithelial cells, but absorbed from the bacterium-derived cyp27a1 of the intestinal tract.
Figure 2.

Colon epithelial cells absorb cyp27a1 from the intestinal tract. A: The immune blots indicate the cyp27a1 protein levels in colon epithelial samples (analyzed by Western blotting). The samples were collected from 20 non-cancer subjects and 20 Cca patients. B: The bars indicate the integrated density of the immune blots of panel A (summarized from all the readouts of the individual samples). C: The bars indicate the cyp27a1 mRNA levels of the samples (analyzed by RT-qPCR). Data of bars are presented as mean ± SD. *, P<0.01, compared with the non-cancer group.
Cyp27a1 indirectly speeds up Cca cell growth
To determine the significance of the phenomenon that the epithelial cells absorbing more cyp27a1 in Cca patients than in non-cancer subjects as shown by Figure 2, we tested the effects of cyp27a1 on Cca cell growth. T84 cells (a colon cancer cell line) were cultured in the presence of VD3 or/and cyp27a1. Addition of VD3 to the culture significantly suppressed the cell growth, which was abolished by the presence of cyp27a1 (Figure 3). The results indicate that VD3 can slow down the cancer cell growth, which can be antagonized by the presence of cyp27a1.
Figure 3.

Cyp27a1 interferes with the effect of VD3 on suppression of cancer cell growth. The bars indicate the number of T84 cancer cells at time points from 0 hr to 144 hr. Control: T84 cells were cultured in the presence of PMA (40 ng/ml). VD3: In the presence of VD3 (10 nM) and PMA (Phorbol 12-myristate 13-acetate; Sigma Aldrich). VD3/cyp27a1: In the presence of VD3, PMA and cyp27a1 (10 nM). Data of bars are presented as mean ± SD. *, P<0.01, compared with the control group. VD3 was purchased from Sigma Aldrich (St. Louis., MO). Cyp27a1 was provided by Sangon Biotech (Shanghai, China).
Cyp27a1 interferes with VD3-induced Cca cell apoptosis
The data of Figure 3 implicate that VD3 may induce Cca cell apoptosis. To test this, we analyzed the cells treated with the procedures of Figure 3. As shown by flow cytometry data, the presence of VD3 significantly induced Cca cell apoptosis, which was abolished by the presence of cyp27a1 (Figure 4A-F). Since cyp27b1 can induce cancer cell apoptosis via activating VD3 [23], cyp27a1 can inactivate the activated VD3 [24], we inferred that the role of cyp27a1 in experiments of Figure 4A-C was inhibition of the Cca cell apoptosis induced by cyp27b1. To test this, we added VD3 to cyp27b1-deficient Cca cell (Figure 4G) culture. Indeed, the VD3-induced Cca cell apoptosis (as shown by Figure 4B) was abolished (Figure 4D, 4E).
Figure 4.
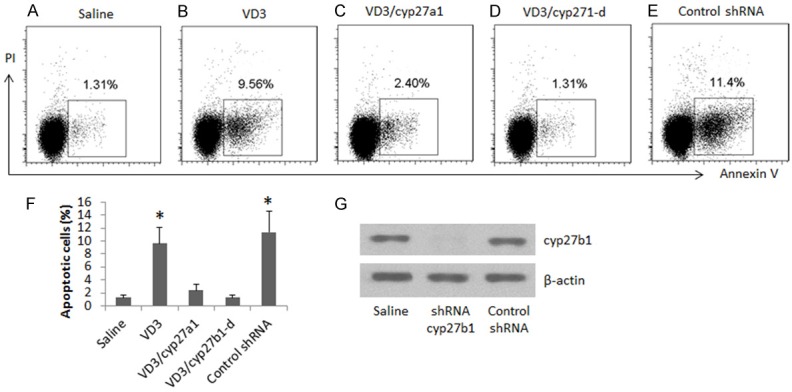
Cyp27a1 inhibits Cca cell apoptosis. (A-E) The gated dot plots indicate the frequency of apoptotic Cca cells. The Cca cells (T84 cells) were cultured for 3 days. The culture condition is denoted on the X xis of (F) (the summarized data of apoptotic cells in A-E). The cells were stained with Annexin V and propidium iodide, and analyzed by flow cytometry. VD3: 10 nM. Cyp27a1: 10 nM. Cyp27b1-d: cyp27b1-deficient Cca cells (generated by RNAi as shown by G).
Discussion
The therapeutic effect on advanced Cca is poor currently. The present study revealed a novel phenomenon that the stool samples of Cca patients contained high levels of cyp27a1, which could be absorbed by the colon epithelial cells. Cyp27a1 inhibited Cca cell apoptosis induced by VD3. The results implicate that cyp27a1 indirectly facilitate Cca growth.
The epithelial surfaces of the body are colonized by a large number of microorganisms, which represent the so-called normal microbiome. Cumulative studies suggest that the activities of the microbiome are associated with cancer genesis and progression [25]. Under physiological condition, the immune system develops different mechanisms to keep a balance with the microbiome [26]. If these mechanisms are impaired, it may cause tumor growth [27]. The present data provide previous unknown data that intestinal bacteria over produce cyp24a1 in Cca patients, which may be one of the reasons to contribute the genesis of Cca. The underlying mechanism still needs further investigation.
It is reported that the bioactive form of VD3 can inhibit cancer growth. Wierzbicka et al indicate that VD3 analogues have low calcemic activity, such as calcipotriol or 20(OH)D3, are very promising candidates for Cca therapy [28]. Ben-Eltriki et al indicate that calcitriol (the bioactive form of VD3) has cancer preventive function by inducing cancer cell apoptosis [29]. Our data are in line with those previous studies by showing that exposure of Cca cells to VD3 resulted in Cca cell apoptosis, in which cyp27b1 played a critical role since that was abolished by knockdown of cyp27b1.
The present data also revealed that cyp27a1 blocked the VD3-induced Cca apoptosis. Cyp27a1 is another enzyme of VD3; it inactivates the bioactive form of VD3, the calcitriol. Diesing et al also found that upon exposure to VD3, breast cancer cells secreted cyp27a1 to degrade cancitriol and suggested that this is a self-protective function of cancer cells [30]. Our data show that there is another source of cyp27a1 in the body; intestinal bacteria can produce cyp27a1, which can be absorbed by the epithelial cells. Actually, Cca cells are transformed from the intestinal epithelial cells. The large quantity of cyp27a1 in the intestinal epithelial cells may be one of the factors blocking the epithelial cell apoptosis.
In summary, the present data show that intestinal bacteria produce a large quantity of cyp27a1, which blocks the intestinal epithelial cell apoptosis.
Acknowledgements
This study was supported by grants of Natural Science Foundation of Heilongjiang Province (H201384); Harbin medical university scientific research innovation fund (2016LCZX26) and Heilongjiang provincial health and Family Planning Commission research subject (2016-128).
Disclosure of conflict of interest
None.
Authors’ contribution
YCJ, XZ, CSZ, DW and YZ performed the experiments, analyzed data and reviewed the manuscript. CL designed the project, supervised the experiments and wrote the paper.
References
- 1.Adler J, Robertson DJ. Interval Colorectal Cancer After Colonoscopy: exploring Explanations and Solutions. Am J Gastroenterol. 2015;110:1657–1664. doi: 10.1038/ajg.2015.365. [DOI] [PubMed] [Google Scholar]
- 2.Kalady MF, Heald B. Diagnostic approach to hereditary colorectal cancer syndromes. Clin Colon Rectal Surg. 2015;28:205–214. doi: 10.1055/s-0035-1564432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Sabatino A, Lenti MV, Giuffrida P, Vanoli A, Corazza GR. New insights into immune mechanisms underlying autoimmune diseases of the gastrointestinal tract. Autoimmun Rev. 2015;14:1161–1169. doi: 10.1016/j.autrev.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Steele SR, Chang GJ, Hendren S, Weiser M, Irani J, Buie WD, Rafferty JF Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Dis Colon Rectum. 2015;58:713–725. doi: 10.1097/DCR.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 5.Brzozowa M, Michalski M, Wyrobiec G, Piecuch A, Dittfeld A, Harabin-Słowińska M, Boroń D, Wojnicz R. The role of Snail1 transcription factor in colorectal cancer progression and metastasis. Contemp Oncol (Pozn) 2015;19:265–270. doi: 10.5114/wo.2014.42173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang S, Han X, Wei B, Fang J, Wei H. RSPO2 enriches LGR5(+) spheroid colon cancer stem cells and promotes its metastasis by epithelial-mesenchymal transition. Am J Transl Res. 2016;8:354–364. [PMC free article] [PubMed] [Google Scholar]
- 7.Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21:485–495. doi: 10.1093/carcin/21.3.485. [DOI] [PubMed] [Google Scholar]
- 8.Janknecht R. Multi-talented DEAD-box proteins and potential tumor promoters: p68 RNA helicase (DDX5) and its paralog, p72 RNA helicase (DDX17) Am J Transl Res. 2010;2:223–234. [PMC free article] [PubMed] [Google Scholar]
- 9.Roos WP, Thomas AD, Kaina B. DNA damage and the balance between survival and death in cancer biology. Nat Rev Cancer. 2016;16:20–33. doi: 10.1038/nrc.2015.2. [DOI] [PubMed] [Google Scholar]
- 10.Cotter TG. Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer. 2009;9:501–507. doi: 10.1038/nrc2663. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Jing J, Yu S, Song M, Tan H, Cui B, Huang L. Advanced glycation endproducts induce apoptosis of endothelial progenitor cells by activating receptor RAGE and NADPH oxidase/JNK signaling axis. Am J Transl Res. 2016;8:2169–2178. [PMC free article] [PubMed] [Google Scholar]
- 12.Buchheit CL, Weigel KJ, Schafer ZT. Cancer cell survival during detachment from the ECM: multiple barriers to tumour progression. Nat Rev Cancer. 2014;14:632–641. doi: 10.1038/nrc3789. [DOI] [PubMed] [Google Scholar]
- 13.Gong X, Wang H, Ye Y, Shu Y, Deng Y, He X, Lu G, Zhang S. miR-124 regulates cell apoptosis and autophagy in dopaminergic neurons and protects them by regulating AMPK/mTOR pathway in Parkinson’s disease. Am J Transl Res. 2016;8:2127–2137. [PMC free article] [PubMed] [Google Scholar]
- 14.Shen C, Gu W, Cai GQ, Peng JP, Chen XD. Autophagy protects meniscal cells from glucocorticoids-induced apoptosis via inositol trisphosphate receptor signaling. Apoptosis. 2015;20:1176–1186. doi: 10.1007/s10495-015-1146-9. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed K, Tabuchi Y, Kondo T. Hyperthermia: an effective strategy to induce apoptosis in cancer cells. Apoptosis. 2015;20:1411–1419. doi: 10.1007/s10495-015-1168-3. [DOI] [PubMed] [Google Scholar]
- 16.Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14:199–208. doi: 10.1038/nrc3672. [DOI] [PubMed] [Google Scholar]
- 17.Sharma K, Le N, Alotaibi M, Gewirtz DA. Cytotoxic autophagy in cancer therapy. Int J Mol Sci. 2014;15:10034–10051. doi: 10.3390/ijms150610034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo X, Fu YX. The tragic fate of group 3 innate lymphoid cells during HIV-1 infection. J Clin Invest. 2015;125:3430–3432. doi: 10.1172/JCI83823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marignol L, Rivera-Figueroa K, Lynch T, Hollywood D. Hypoxia, notch signalling, and prostate cancer. Nat Rev Urol. 2013;10:405–413. doi: 10.1038/nrurol.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Y, Johnson CS, Trump DL. Mechanistic insights of vitamin D anticancer effects. Vitam Horm. 2016;100:395–431. doi: 10.1016/bs.vh.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 22.Jones G, Prosser DE, Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res. 2014;55:13–31. doi: 10.1194/jlr.R031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banks M, Holick MF. Molecular Mechanism(s) involved in 25-Hydroxyvitamin D’s antiproliferative effects in CYP27B1-transfected LNCaP cells. Anticancer Res. 2015;35:3773–3779. [PubMed] [Google Scholar]
- 24.Szymczak I, Pawliczak R. The active metabolite of vitamin D3 as a potential immunomodulator. Scand J Immunol. 2016;83:83–91. doi: 10.1111/sji.12403. [DOI] [PubMed] [Google Scholar]
- 25.Russo E, Taddei A, Ringressi MN, Ricci F, Amedei A. The interplay between the microbiome and the adaptive immune response in cancer development. Therap Adv Gastroenterol. 2016;9:594–605. doi: 10.1177/1756283X16635082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goc J, Hepworth MR, Sonnenberg GF. Group 3 innate lymphoid cells: regulating host-commensal bacteria interactions in inflammation and cancer. Int Immunol. 2016;28:43–52. doi: 10.1093/intimm/dxv056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Huycke MM. Colorectal cancer: role of commensal bacteria and bystander effects. Gut Microbes. 2015;6:370–376. doi: 10.1080/19490976.2015.1103426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wierzbicka JM, Binek A, Ahrends T, Nowacka JD, Szydłowska A, Turczyk Ł, Wąsiewicz T, Wierzbicki PM, Sądej R, Tuckey RC, Slominski AT, Chybicki J, Adrych K, Kmieć Z, Żmijewski MA. Differential antitumor effects of vitamin D analogues on colorectal carcinoma in culture. Int J Oncol. 2015;47:1084–1096. doi: 10.3892/ijo.2015.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ben-Eltriki M, Deb S, Adomat H, Tomlinson Guns ES. Calcitriol and 20(S)-protopanaxadiol synergistically inhibit growth and induce apoptosis in human prostate cancer cells. J Steroid Biochem Mol Biol. 2016;158:207–219. doi: 10.1016/j.jsbmb.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Diesing D, Cordes T, Fischer D, Diedrich K, Friedrich M. Vitamin D--metabolism in the human breast cancer cell line MCF-7. Anticancer Res. 2006;26:2755–2759. [PubMed] [Google Scholar]


