Abstract
Objective: This study aimed to determine whether the human immunodeficiency virus (HIV) exists in giant idiopathic esophageal ulcers in the patients with acquired immune deficiency syndrome (AIDS). Methods: 16 AIDS patients with a primary complaint of epigastric discomfort were examined by gastroscopy. Multiple and giant esophageal ulcers were biopsied and analyzed with pathology staining and reverse transcription-polymerase chain reaction (RT-PCR) to determine the potential pathogenic microorganisms, including HIV, cytomegalovirus (CMV) and herpes simplex viruses (HSV). Results: HIV was detected in ulcer samples from 12 out of these 16 patients. Ulcers in 2 patients were infected with CMV and ulcers in another 2 patients were found HSV positive. No obvious cancerous pathological changes were found in these multiple giant esophageal ulcer specimens. Conclusion: HIV may be one of the major causative agents of multiple benign giant esophageal ulcers in AIDS patients.
Keywords: AIDS, esophageal ulcer, HIV, HSV, CMV, endoscopy
Introduction
Esophageal ulcer (EU) is a common comorbidity in AIDS patients [1,2]. EU can be diagnosed when a 5 mm or larger mucosa continuity is lost in its major dimension upon endoscopy [1]. The inflammatory lesions caused by different pathogens may lead to esophageal mucosa paragraphs, which can damage the submucosa layers and even the muscles. Current treatment guidelines for EU in AIDS patients differ among patients because their ulcerous lesions are caused by various etiologies, including viral, bacterial and fungal [2]. Thus, finding the exact causative agent of these ulcerous lesions is very important for treatment [3]. CMV, HSV and fungal organisms are generally considered the main causes of EU in AIDS patients. However, idiopathic ulcers were found to be associated with HIV infection [2,4]. In our previous study, we found that HIV viruses existed in the absence of CMV, HSV or other pathogens in multiple giant esophagus ulcers in AIDS patients [4]. These ulcerous were generally defined as non-cancerous lesions by pathological standards [4]. In Brunaldi’s study, 41 out of 399 AIDS patients were found to have EU. Among the patients with EU, 29 were found with CMV infection and 7 with HSV infection. In order to determine whether HIV viruses are the major contributor to the development of giant esophageal ulcers in AIDS patients, we examined 16 AIDS patients who were admitted to the hospital with epigastric discomfort (odynophagia, thoracalgia or dysphagia) as the chief complaint and had multiple giant esophageal ulcers by gastroscopy. We found that HIV viruses were closely related to these giant-sized, non-cancerous ulcers.
Materials and methods
Patients and laboratory tests
AIDS patients with gastroenterological symptoms as chief complaints (such as retrosternal pain after eating, epigastric burning pain, odynophagia and dysphagia) were chosen to have endoscopy examinations in Shanghai Public Health Clinical Center from July 2011 to June 2015 (Table 1). All patients were given consent forms and those who refused to take biopsies or had a history of antiviral treatment were excluded from the study. According to the above criteria, sixteen AIDS patients with gastroscopy-diagnosed EU were included in this study. All sixteen patients were tested for CD4 and CD8 T cells, serum HIV load, white blood cell (WBC) counts before and after endoscopy.
Table 1.
Clinical data of the enrolled patients
| Patient No. | Sex | Age | Reasons for endoscopic examination | Duration of HIV infection |
|---|---|---|---|---|
| 1 | F | 34 | Retrosternal pain after eating for 2 months | 1 month |
| 2 | F | 26 | Retrosternal pain after eating for 2 weeks | 2 months |
| 3 | F | 31 | Fever with epigastric burning pain for 1 week | 1 day |
| 4 | F | 35 | Intermittent fever for 2 months, with odynophagia and thoracalgia | 1 month |
| 5 | M | 25 | Intermittent fever for 2 months, with retrosternal pain after eating | 1 week |
| 6 | M | 40 | Dysphagia and epigastric discomfort for 1 week | 1 month |
| 7 | M | 56 | Intermittent fever for 2 months, with retrosternal pain after eating for 1 week | 1 month |
| 8 | M | 28 | Fever with odynophagia, and epigastric discomfort for 3 months | 3 months |
| 9 | M | 34 | Fever with odynophagia, and epigastric discomfort for 4 months | 4 months |
| 10 | M | 77 | Odynophagia, and epigastric discomfort for 1 week | 1 week |
| 11 | M | 41 | Epigastric burning pain for 1 week | 3 years |
| 12 | M | 42 | Thoracalgia and epigastric discomfort for 1 month, intensified symptoms for 1 week | 1 week |
| 13 | M | 49 | Thoracalgia and dyspagia for 2 weeks | 6 months |
| 14 | M | 55 | Thoracalgia while eating for 2 weeks | 1 month |
| 15 | M | 45 | Thoracalgia and abdominal pain after eating for 3 weeks | 1 year |
| 16 | M | 28 | Thoracalgia and epigastric discomfort for 3 months | 6 months |
Endoscopy
Endoscopy was performed and photographed with a Gastroscope-CV260 (Olympus, Japan). In order to maintain consistency, the endoscopies for all 16 patients were done by the same physician. EU was defined when there was at least a 5 mm disruption in mucosal continuity. Lesions of the EU were described and categorized by the following standards: extension (number and size), depth (deep or shallow), location of EU (proximal, middle, distal, middle/distal). Three pieces of tissue were biopsied from the margins of the ulcers. 0.9% NaCl saline was used to wash the bottom of the ulcers before biopsy. Some sampling points were bleeding. All samples were then washed thoroughly with 1X phosphate-buffered saline (PBS) to clean-up any blood contamination. One of the specimens was used for hematoxylin-eosin (HE) staining. Another specimen was used for HIV RNA detection by RT-PCR, and the last specimen was used for CMV DNA and HSV 1 DNA detection by PCR.
Histopathological test
The specimens from ulcerous tissues were fixed in 10% formalin and paraffin-embedded. The tissues were then cut into sections with a thickness of 3 µm and stained with HE staining for further pathological observation under a microscope.
RT-PCR for HIV
The mRNA was extracted from the biopsy tissues from AIDS patients according to the manufacturer’s recommendations of miRNeasy Mini Kit (QIAGEN, Hilden, German); All tissue samples were washed with PBS for at least 3 times before being used for extracting RNA. The quality of mRNA was evaluated by the OD260/OD280 ratio. Only those samples with the ratio range between 1.8 and 2.0 were used for RT-PCR or PCR test. cDNA was generated from 1 μg of total RNA using the RT2 microRNA First Strand Kit (Promega, American) according to manufacturer’s standard procedures: reverse transcription at 42°C for 30 min, then 30°C for 3 min and initial denaturation at 72°C for 10 min. The HIV-specific primers were designed as follows: The pair of primers for the first-round PCR reaction : 5’-TGG AAA TGT GGA AAG GAA C-3’ and 5’-CCT GTA TGC AGA CCC CAA TAT GTT-3’; The pair of primers for the secondary PCR reaction: 5’-ACT GAG AGA CAG GCT AAT TTT TTA GGG A-3’ and 5’-CTC CTA GTG GGA TRT GTA CTT CYG ARC TTA-3’. The RT-PCR product was a 1316-base pair fragment in HIV pol gene. The peripheral blood from a HIV positive patient was used as the positive control for each RT-PCR reaction, and an equal volume of PBS solution was used as a negative control for each reaction.
PCR for CMV and HSV
DNA was extracted from the biopsy tissues from AIDS patients according to the manufacturer’s recommendations of miDNAeasy Mini Kit (Qiagen, Hilden, German). The quality of DNA was evaluated by the OD260/OD280 ratio. Only those samples with the ratio range between 1.8 and 2.0 were used for PCR test. The net PCR was employed to detect CMV and HSV in EUs of the patients according to the method reported by Dr. Victória [5]. The CMV-specific primers were as follows: Outer primers: F: 5’-ACATGGAATCCAGGATCTGGTGCC 3’; R: 5’-CCCTATGATATGCCACGAAAACCG 3’, 30 cycles of denaturation at 95°C for 30 s, annealing at 58°C for 45 s and extension at 72°C for 30 s (PCR product size is 612 bp). Inner primers: F: 5’-CAACACGTAACGTCTTCTGAAGCC 3’; R: 5’-TAGACCACCATGATGCCCTCATCC 3’, 30 cycles of denaturation at 95°C for 30 s, annealing at 57°C for 45 s and extension at 72°C for 30 s (PCR product size is 224 bp). The HSV-1 specific primers were designed as follows: Outer primers: F: 5’-TGC TGG AGG ATC ACG AGT TTG 3’; R: 5’-CAT CGT CTT TGT TGG GAA CTT 3’, 30 cycles of denaturation at 95°C for 30 s, annealing at 61°C for 45 s and extension at 72°C for 30 s (PCR product size is 663 bp). Inner primers: F: 5’-TGCAGAGCAACCCCATGAAG 3’; R: 5’-ATGACCATGTCGGTGACCTTGG 3’, 30 cycles of denaturation at 95°C for 30 s, annealing at 60°C for 45 s and extension at 72°C for 30 s (PCR product size is 241 bp). DNAs extracted from the peripheral blood of CMV or HSV positive patients were used as the positive controls for each PCR reaction. Equal volume of PBS solution was used as negative controls.
Results
Clinical laboratory test of AIDS patients
HIV in serum from 16 patients was higher than the normal value. CD4+ lymphocyte counts of all these patients were lower than the normal value, and 9 out of 16 patients’ CD8+ lymphocyte counts were lower than the normal value. ESR in all 16 patients was higher than normal. 8 patients were HBsAb positive. 5 patients were anti-CMV IgG positive, but anti-CMV IgM negative and anti-EBV IgM negative. T-SPOT.TB AgA(ESAT-6) and T-SPOT.TB AgB(EFR-10) were both negative among these patients (Table 2).
Table 2.
Clinic laboratory test results of the 16 AIDS patients
| No. | HIV RNA (copies/ml) | CD4 (cells/μl) | CD8 (cells/μl) | ESR (mm/h) | HBsAg (IU/ml) | HBsAb (mIU/ml) | Anti-HCV (S/CO) | Anti-TP (S/CO) | Anti-CMV Ig M | Anti-CMV Ig G | Anti-EBV Ig M | T-SPOT.TB AgA (ESAT-6) | T-SPOT.TB AgB (EFR-10) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 351000 | 1 | 357 | 92 | 0.00 | 23.63 | 0.10 | 0.08 | (-) | (-) | (-) | <0 | <0 |
| 2 | 17200 | 7 | 97 | 60 | 0.02 | 56.00 | 0.03 | 0.02 | (-) | (+) | (-) | <0 | <0 |
| 3 | 2600 | 12 | 73 | 58 | 0.04 | 322.22 | 14.60 | 34.86 | (-) | (-) | (-) | <0 | <0 |
| 4 | 285000 | 16 | 80 | 70 | 0.04 | 19.80 | 0.03 | 0.01 | (-) | (-) | (-) | <0 | <0 |
| 5 | 138000 | 9 | 110 | 52 | 0.02 | 44.84 | 0.05 | 0.05 | (-) | (-) | (-) | <0 | <0 |
| 6 | 322000 | 10 | 72 | 23 | 0.03 | 4.12 | 0.05 | 0.05 | (-) | (-) | (-) | <0 | <0 |
| 7 | 98100 | 12 | 69 | 25 | 0.03 | 2.00 | 0.10 | 29.26 | (-) | (+) | (-) | <0 | <0 |
| 8 | 182000 | 26 | 426 | 54 | 0.01 | 148.65 | 0.07 | 10.63 | (-) | (+) | (-) | <0 | <0 |
| 9 | 210000 | 23 | 384 | 55 | 0.04 | 15.90 | 0.03 | 0.35 | (-) | (-) | (-) | <0 | <0 |
| 10 | 293000 | 172 | 783 | 30 | 1638 | 0.00 | 0.01 | 0.06 | (-) | (-) | (-) | <0 | <0 |
| 11 | 6170 | 80 | 732 | 28 | 700.00 | 0.00 | 0.10 | 0.20 | (-) | (-) | (-) | <0 | <0 |
| 12 | 330000 | 47 | 383 | 54 | 0.03 | 7.59 | 0.11 | 32.97 | (-) | (-) | (-) | <0 | <0 |
| 13 | 16500 | 30 | 74 | 41 | 0.02 | 15.57 | 0.09 | 0.14 | (-) | (+) | (-) | <0 | <0 |
| 14 | 646000 | 12 | 69 | 25 | 0.04 | 0.8 | 0.04 | 15.57 | (-) | (+) | (-) | <0 | <0 |
| 15 | 540 | 23 | 160 | 18 | 0.04 | 1000 | 0.13 | 0.09 | (-) | (-) | (-) | <0 | <0 |
| 16 | 3410 | 23 | 616 | 21 | 0.01 | 0.00 | 0.10 | 0.05 | (-) | (-) | (-) | <0 | <0 |
Normal range of lab test (references): HIV RNA: <40; CD4: 410~1590; CD8: 190~1140; ESR 0.00~15.00; HBsAg: <0.05; HBsAb: <10; Anti-HCV: <1; Anti-TP: <1; Anti-CMV Ig M negative (-); Anti-CMV Ig G negative (-); Anti-EBV Ig M: negative (-); T-SPOT.TB AgA (ESAT-6): <6 (no reaction); T-SPOT.TB AgB (EFR-10): <6 (no reaction).
Gastroscopic findings of the EU patients with AIDS
In these 16 patients, giant-sized ulcers with similar shapes could be found in the esophageal mucosa from the oropharynx to stomach cardia. Most of the ulcers were found in the middle of the esophagus. Some ulcers were located directly facing each other and others were located deep within the esophageal mucosa. The sizes of these deep ulcers varied significantly from 1 to 10 cm long and 1 to 3 cm wide. Some of these deep ulcers even extended from the middle of the esophagus to the stomach cardia. No obvious mucosal hyperemia and edema were observed around the margins of the ulcers. The bottom of ulcers was usually very clean without apparent attachment or hemorrhage. Some ulcers were flat and others were uneven with white moss on the surface (Figure 1).
Figure 1.
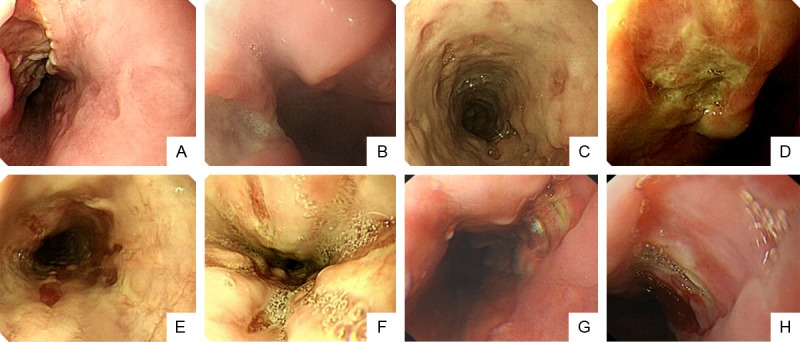
Appearances of esophagus ulcers in HIV infected patients. Endoscopic photos were taken from all 16 patients with EU, and their representative endoscopic images were shown and categorized into the following A to H types. A. Esophagus huge gap-like ulcers, sharp edge, dry-river presentation, the depth of the ulcer reached the muscular layer, which is long and wide with hyperemia and edema but clean bottom, huge superficial ulcers are also found on the opposite side. B. Esophagus huge connecting ulcer covered with white moss, peripheral esophageal mucosal congestion and edema. C. Esophagus with multiple coin-like depressed ulcers surrounding the entire esophagus, red muscle is visible at the bottom of the large mucous membrane and the bottom has no obvious moss. D. Burn like esophageal huge deep ulcer with light yellow moss. E. Multiple bar or oval-like ulcers, partially fused to a larger slice of ulcer, deeper to the submucosal, muscle layer is visible. F. Gap-like ulcers which goes along the direction of the esophagus with thin moss. G. A huge ulcer in the distal esophagus with clean bottom and even edges. H. Esophagus with huge ulcers, nearly 1/4 of the entire esophagus is occupied, part of the ulcer is covered with moss and clean.
One of the patients who had colonic ulcers and proliferative lesions also suffered from ileocecal hemorrhage to the sigmoid flexure simultaneously. The endoscopy images of non-cancerous ulcers could easily be differentiated from those of the cancerous ulcers.
Pathological and molecular biology test results of the biopsies
The sixteen patients with chronic non-atrophic gastritis determine by pathological biopsy were tested for the helicobacter pylori (H. pylori) infection. All patients were tested with the 13C Breath Test machine (HY-IREXA, Hua You Ming Kang Technology, Guangzhou, China) at fasting state and were also tested with the Urea [13C] Breath Test Kit (H20061169, Product Number B14200158288, Beijing Boran Pharmaceutical Co Ltd, China). They blew up the first collection bag, took one 13C-Urea capsule, and waited for half an hour to blow up the second collection bag. The two bags of breath samples were put into the 13C Breath Test machine for H. pylori testing. The test results of H. pylori were negative for all of these patients.
Pathological examination found focal and coagulative necrosis with inflammatory cell infiltration in the esophageal mucosal epithelial tissues. Muscle fibers were separated by inflammatory cells. Erosion formed on the surface and epithelial papilla showed hyperplasia without obvious atypia (Figure 2).
Figure 2.
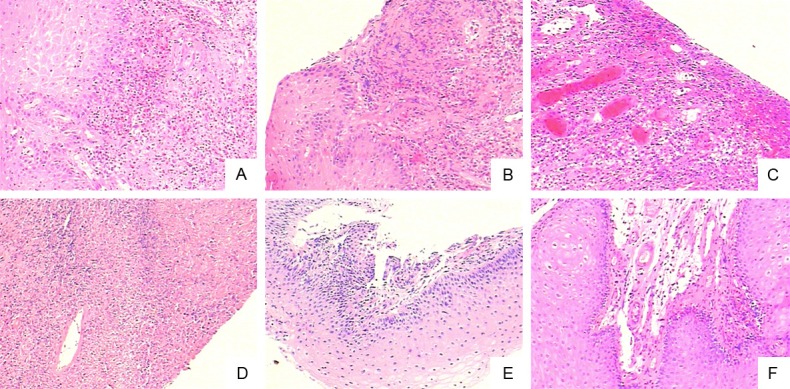
Characteristic changes in pathology in esophageal mucosal epithelial tissues from AID patients with giant EUs (HE 10×10). A. Infiltration of eosinophils under the squamous epithelium in esophageal mucosal epithelial tissue. B. Infiltration of inflammatory cells under mucosal with partial epithelial shedding. C. Epithelial infiltration of inflammatory cells with formation of the ulcers. D. Large area necrosis and inflammatory cells infiltration in esophageal mucosa. E. Squamous is infiltrated with inflammatory cells. F. Infiltration of submucosal inflammatory cells and obvious edema.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR): In esophageal mucosal biopsy specimens from these 16 patients, 12 of them were tested positive for HIV RNA positive with the expression of HIV-pol gene (Figure 3, from Lane 1 to 12). Two other patients were tested with CMV positive but HIV negative (Figure 3, Lanes 13 and 14). Another two were HSV positive but HIV negative (Lanes 15 and 16).
Figure 3.
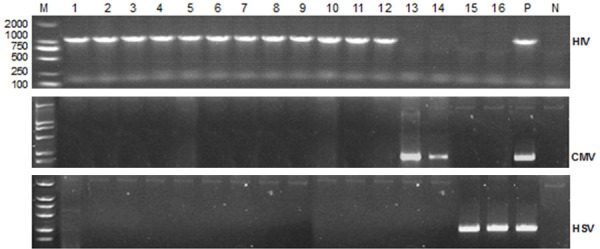
RT-PCR of HIV pol gene, HSV and CMV specific genes for esophageal lesion biopsy specimens. HIV pol gene fragment size is 1316 bp, CMV and HSV gene fragments are 224 bp and 241 bp, respectively. M is DNA marker DL 2000, lane 1-16 are samples from the esophageal ulcer biopsy tissues in AIDS patients, P is the positive control of HIV, HSV and CMV, N is negative control.
Discussion
Esophageal ulcer is a common complication in patients with human immunodeficiency virus infection at different disease stages. HIV attacks CD4+ T cells and causes damage to the gut barrier function. As a result, this increases the incidence of tumor and opportunistic infection [6]. There are two main pathogens which may cause digestive tract ulcers in AIDS patients, CMV [7] and HSV [8]. Wilcox has analyzed the pathogens in 100 AIDS patients who had esophageal ulcers. They found that 45% of the ulcers were caused by CMV infection, 40% were idiopathic, and only 5% by HSV [9]. However, Borges’ study showed that the pathogens in esophageal ulcer are HIV (53.3%), CMV (20%), HPV (13.3%), and Haemophilus Ducreyi (6.7%) [10]. Obviously, HIV may also be a direct cause of esophageal ulcer in AIDS patients. Idiopathic esophageal ulcer was known as the chronic HIV-associated esophageal ulcerations. It was usually diagnosed in patients with CD4 cell counts less than 100/µL [11].
It was reported that the biological performance of several types of esophageal ulcers were quite similar to tumors [12]. The multiple giant ulcers in the esophagus and colon in AIDS patients could be easily misdiagnosed as tumors and were removed surgically. Currently the most definitive way to judge whether the ulcers are benign or malignant is with pathology [13]. We reported here a general method to recognize and differentiate these special ulcers in order to avoid unnecessary removal of the affected organs. The treatment for benign ulcers is quite different from that for malignant ulcers. Misdiagnosis will have serious consequences on patients’ follow-up treatment and prognosis. Therefore, great attention should be paid in distinguishing benign and malignant esophageal ulcers when patients are undergoing endoscopy. Compared with benign esophageal ulcers, ulcerative esophageal carcinomas present with irregular shapes and multiple sizes and are mainly found in the middle part of the esophagus. There are irregular bulges on the edge and inflammatory exudate at the bottom of these ulcerative esophageal carcinomas. The two endoscopic characteristics of the non-tumor multiple giant esophageal ulcers in AIDS patients are: 1) The ulcers are huge and deep, with or without whitish moss on a flat bottom; 2) The mucosa between the ulcers is normal.
The common bowel mucosal lesions in AIDS patients are colitis and colonic ulcers [14]. Neither Epstein-barr virus (EBV) nor CMV was found in the esophagus and colon tissues in our patients reported here. Although the virology diagnosis mainly depends on pathological examinations of the biopsy from these lesions, other researchers found that PCR was also effective to search for the pathogenic viruses [11,15]. Since HIV has been found to be related to the formation of esophageal ulcers, clinicians should be on a high alert for these types of ulcers in HIV patients.
HIV is a kind of retrovirus whose viral genome consists of two identical positive-stranded RNA. The HIV viruses mainly infect immune cells expressing molecular CD4, such as CD4+ T cells, dendritic cells (DC) and macrophages. As CD8+ cells are not affected by HIV, the percentages and the numbers of CD8+ cells in 43.75% patients were normal (7/16). According to Britta Siegmund’s study, in one AIDS patient with EU, the CD8+ cells count was normal in peripheral blood but the author found more CD8+ cells infiltrating the esophageal mucosa. The CD8+ cells most likely caused further damage to the esophageal mucosa and also inhibited the HIV virus [1].
The intestinal mucosal immune system includes three parts: Peyer patches, mucosal lamina propria, and lymphocytes in the epidermis. They are all the main targets of HIV infection. As a result, ulcers tend to form in the lymphoid aggregation sites. Siegmund found that in the early invasion phage of HIV viruses, the infiltration of activated T cells plays a key role in the formation of the giant ulcers [15]. Due to the reduced number of CD4+ cells by HIV infection, lymph tissues such as Peyer patches underwent significant atrophy. The germinal center was almost completely invisible and only a few DCs were present in the germinal center [16]. Thus, as HIV progresses, the digestive tract becomes thinner and thinner, parts of vessels become more exposed, and the gut barrier function becomes more compromised. Ulcer, hemorrhage and other opportunistic infections may develop as a result of these pathological changes. It is also known that chronic inflammatory infiltration with necrosis can be found in both the esophageal ulcers and colonic ulcers. The AIDS patients’ esophageal ulcers accompanied inflammatory infiltration and apoptotic cells [17]. Apoptosis plays an influential role in the occurrence of the idiopathic esophageal ulcers in AIDS patients and this did not happen in HIV negative patients [17].
The epidemiologic studies suggested that more than half of the world’s population were infected with Helicobacter pylori (H. pylori). However, the results of the H. Pylori in all 16 patients in this study are negative. Possible explanations include: 1) acid secretion in AIDS patients may be compromised, leading to increased pH in the stomach, which inhibits H. pylori proliferation; 2) the antibiotics that AIDS patients used for treating HIV concomitantly inhibited H. pylori proliferation; 3) a relationship exists between H. pylori infection and the number of the lymphocytes present in the mucosa. H. pylori infection in AIDS patients are lower in those patients whose CD4 counts are less than 100 [16].
Ulcer incidence in intestines of AIDS patients is high. The mucosal lesions in intestine are usually more than that in the esophagus. However, we found more esophageal ulcers in this study. This is probably because that AIDS patients usually pay less attention to lower gastrointestinal syndromes than syndromes such as dysphagia, retrosternal burning feeling. Patients usually do not undergo colonoscopy unless the broken vessels in ulcers which leads to hematochezia. Once the ulcer forms, it is hard to self-heal. Giant ulcers appear to be even more difficult to heal.
Opportunistic infections are one of the major factors of mortality in AIDS patients. The common opportunistic pathogenic micro-organisms are candida, mycobacterium tuberculosis, pneumocystis carinii, cryptococcus, histoplasma capsulatum, toxoplasma gondii, cryptosporidia, mycobacterium avium, among others [18]. Candida albicans is a common opportunistic infection in the digestive tract that can cause ulcers in the mouth, esophagus, colon and other areas. Acid-fast microorganisms such as cryptosporidia and atypical mycobacteria can cause gastrointestinal symptom such as diarrhea. We observed that one patient had abdominal pain and hematochezia. This patient also had a history of syphilis for 6 years. Serious inflammatory lesions, positive acid-fast stain, colonic ulcer bleeding and opportunistic infections all existed in this patient.
The highly sensitive nature of RT-PCR in detecting HIV RNA necessitates that we should exclude any contaminated HIV positive patient blood in order to reduce false positives. We flushed the sampling areas very carefully and tried to avoid causing any bleeding at sampling. All specimens were washed thoroughly to get rid of any blood contamination before they were used for RT-PCR reactions.
Conclusion
In conclusion, we found via endoscopy that apoptosis and inflammatory infiltration are the pathological signs of non-cancerous esophageal ulcers. There were also colonic multiple giant ulcers in AIDS patients. Differential diagnosis between benign and cancerous ulcers is very important because giant ulcers could look similar to the ulcerative carcinomas under an endoscope. A number of biopsies should be obtained in order to ascertain the definitive pathogens, such as HIV, CMV or HSV. Our results indicate that 75% (12/16) of AIDS patients have ulcers infected with HIV. Therefore, HIV may be the causative agent for these multiple giant non-cancerous esophageal ulcers. Our data seem to differ from other reports. This may be due to the heterogeneity of AIDS patients in different ethnicities and environments. Another factor that may lead to such differences is the antiviral treatment of HIV in AIDS patients [10]. In addition, the risk of opportunistic infections increases in AIDS patients due to their compromised immunity. This should be particularly noticed in AIDS patients with multiple and giant esophagus ulcers.
Acknowledgements
The study was supported by the Chinese 12th Five-Year Plan for a special study fund on hepatitis B and AIDS (2013ZX10004216-001-001) and the Biological Medicine Guide Fund of Shanghai Science and Technology Committee (124119a7801).
Disclosure of conflict of interest
None.
References
- 1.Wilcox CM, Rodgers W, Lazenby A. Prospective comparison of brush cytology, viral culture, and histology for the diagnosis of ulcerative esophagitis in AIDS. Clin Gastroenterol Hepatol. 2004;2:564–567. doi: 10.1016/s1542-3565(04)00239-3. [DOI] [PubMed] [Google Scholar]
- 2.Dragean CA, Bogdan I, Azzouzzi K, Goncette L. Giant idiopathic ulcer of esophagus in the context of acquired immunodeficiency syndrome (AIDS) JBR-BTR. 2013;96:72–74. doi: 10.5334/jbr-btr.212. [DOI] [PubMed] [Google Scholar]
- 3.Brunaldi MO, Rezende RE, Garcia SB, Machado AA, Modena JL, Zucoloto S. Esophageal ulcer in Brazilian patients with HIV: prevalence and comparative analysis among diagnostic methods. AIDS Patient Care STDS. 2010;24:311–316. doi: 10.1089/apc.2009.0299. [DOI] [PubMed] [Google Scholar]
- 4.Wang LW, Ma YL, Lv BL, Xu YH, Zhou MZ, Qiu CL, Huang SP, Shen YZ, Zhang RF, Cheng JL. One case with multiple huge esophageal ulcers caused by HIV infection and literature review. Chinese Journal of Digestive Endoscopy. 2012:166–167. [Google Scholar]
- 5.Victoria JM, Guimaraes AL, da Silva LM, Kalapothakis E, Gomez RS. Polymerase chain reaction for identification of herpes simplex virus (HSV-1), cytomegalovirus (CMV) and human herpes virus-type 6 (HHV-6) in oral swabs. Microbiol Res. 2005;160:61–65. doi: 10.1016/j.micres.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Chow DC, Be SH, Eickhoff L, Soloway GN, Saul Z. Primary esophageal lymphoma in AIDS presenting as a nonhealing esophageal ulcer. Am J Gastroenterol. 1996;91:602–603. [PubMed] [Google Scholar]
- 7.Sheth A, Boktor M, Diamond K, Lavu K, Sangster G. Complete esophageal obliteration secondary to cytomegalovirus in AIDS patient. Dis Esophagus. 2010;23:E32–E34. doi: 10.1111/j.1442-2050.2010.01095.x. [DOI] [PubMed] [Google Scholar]
- 8.Goodgame RW. Viral infections of the gastrointestinal tract. Curr Gastroenterol Rep. 1999;1:292–300. doi: 10.1007/s11894-999-0112-5. [DOI] [PubMed] [Google Scholar]
- 9.Wilcox CM, Schwartz DA, Clark WS. Esophageal ulceration in human immunodeficiency virus infection. Causes, response to therapy, and long-term outcome. Ann Intern Med. 1995;123:143–149. doi: 10.7326/0003-4819-123-2-199507150-00010. [DOI] [PubMed] [Google Scholar]
- 10.Borges MC, Colares JK, Lima DM, Fonseca BA. Advantages and pitfalls of the polymerase chain reaction in the diagnosis of esophageal ulcers in AIDS patients. Dig Dis Sci. 2009;54:1933–1939. doi: 10.1007/s10620-008-0584-4. [DOI] [PubMed] [Google Scholar]
- 11.Wallace MR, Brann OS. Gastrointestinal manifestations of HIV infection. Curr Gastroenterol Rep. 2000;2:283–293. doi: 10.1007/s11894-000-0020-1. [DOI] [PubMed] [Google Scholar]
- 12.Odemis B, Ataseven H, Basar O, Ertugrul I, Yuksel O, Turhan N. Ulcer in the basis of Zenker’s diverticulum mimicking esophageal malignancy. J Natl Med Assoc. 2006;98:1177–1180. [PMC free article] [PubMed] [Google Scholar]
- 13.Wilcox CM, Straub RF, Schwartz DA. Prospective evaluation of biopsy number for the diagnosis of viral esophagitis in patients with HIV infection and esophageal ulcer. Gastrointest Endosc. 1996;44:587–593. doi: 10.1016/s0016-5107(96)70014-7. [DOI] [PubMed] [Google Scholar]
- 14.Averbach M, Cutait R, Correa P, Duarte MI, Leite K, Borges JL. [Colorectal diseases in AIDS patients and endoscopic findings] . Arq Gastroenterol. 1998;35:104–109. [PubMed] [Google Scholar]
- 15.Siegmund B, Moos V, Loddenkemper C, Wahnschaffe U, Engelmann E, Zeitz M, Schneider T. Esophageal giant ulcer in primary human immunodeficiency virus infection is associated with an infiltration of activated T cells. Scand J Gastroenterol. 2007;42:890–895. doi: 10.1080/00365520601127299. [DOI] [PubMed] [Google Scholar]
- 16.Lichterfeld M, Lorenz C, Nischalke HD, Scheurlen C, Sauerbruch T, Rockstroh JK. Decreased prevalence of Helicobacter pylori infection in HIV patients with AIDS defining diseases. Z Gastroenterol. 2002;40:11–14. doi: 10.1055/s-2002-19637. [DOI] [PubMed] [Google Scholar]
- 17.Houghton JM, Korah RM, Kim KH, Small MB. A role for apoptosis in the pathogenesis of AIDS-related idiopathic esophageal ulcers. J Infect Dis. 1997;175:1216–1219. doi: 10.1086/593671. [DOI] [PubMed] [Google Scholar]
- 18.Sengupta D, Lal S, Shrinivas Opportunistic infection in AIDS. J Indian Med Assoc. 1994;92:24–26. [PubMed] [Google Scholar]


