Abstract
Asthma is a chronic airway disease common around the world. The burden of this disease could be reduced with new and effective treatments. Here, the efficacy of a polysaccharide extract from the Boletus edulis (BEP) mushroom, which has demonstrated anti-inflammatory properties, was tested in a mouse model of asthma. Five groups of BaLB/C mice were developed; one group served as a control and did not have asthma induction. The other four groups of mice were sensitized by ovalbumin challenge. FinePointe™ RC animal airway resistance and pulmonary compliance was used to assess airway function in asthma models. Three of the 4 model groups received treatments: one received pravastatin, one received dexamethasone, and one received BEP. Histopathology of lung tissues was performed using H&E and AB-PAS staining. Levels of cytokines IL-4 and IFN-g were detected using ELISA, qRT-PCR, and Western blotting. Cyclophilin A was measured by Western blot, and flow cytometry was used to determine the proportion of CD4+CD25+FOXP3+ Treg cells. BEP treatment resulted in improvements in lung pathology, IL-4 level (P<0.05), and IFN-γ level (P<0.05) similar to traditional dexamethasone treatment. Further, the proportion of anti-inflammatory CD4+CD25+FOXP3+ Treg cells significantly increased (P<0.05) compared to untreated asthma models, and expression of cyclophilin A significantly decreased (P<0.05). Thus, Boletus edulis polysaccharide reduces pro-inflammatory responses and increases anti-inflammatory responses in mouse models of asthma, suggesting this may be a novel treatment method.
Keywords: Boletus edulis polysaccharide (BEP), asthma, mouse models, anti-inflammatory responses, CD4+CD25+FOXP3+ Treg cells
Introduction
Asthma is one of the most common chronic respiratory diseases worldwide [1]. Its characteristic symptoms include wheezing, difficulty breathing, repetitive paroxysmal cough, and airway hyper-responsiveness [2]. Airway inflammation, which is both the central pathogenic feature and the principal clinical manifestation, induces airflow obstruction and bronchial hyper-responsiveness [3]. This process has a complex pathogenesis involving both genetic and environmental factors. One of the mechanisms underlying airway inflammation is an imbalance in T helper immune cells [4-10]. In asthma, T helper type 2 (Th2) cells are functionally upregulated, while Th1 cells are inhibited, which enables Th2 cytokines to promote inflammation. Interleukin-4 (IL-4), secreted by Th2 cells, induces airway inflammation by activating eosinophils as well as promoting IgE secretion [11]. The level of IL-4 is increased in bronchoalveolar lavage fluid of patients with asthma [12]. Further, when Th1 cells are suppressed they cannot secrete IFN-γ to inhibit IgE secretion; IgE is upregulated in allergy and asthma, a process that promotes inflammation [13].
However, a Th1/Th2 imbalance is not the sole mechanism underlying asthma pathogenesis; other immunological pathways regulate airway inflammation. In particular, regulatory T cells (Treg), especially those producing the proteins CD4, CD25, and FOXP3 (CD4+CD25+FOXP3+), contribute to this process [14-17]. Treg cells are essential for inducing and maintaining immunological tolerance to foreign and self-antigens (including allergens). CD4+CD25+FOXP3+ Treg cells are a subset of CD4+T lymphocytes, which can inhibit immune response and induce immune tolerance by secreting inhibitory cytokines (IL-10, TGF-β, and IL-35) or mediating cell-cell contact [15,16]. Given the increasing incidence of asthma around the world [1], finding new ways to ameliorate the immune response is critical to reducing the burden of this disease.
The edible fungus Boletus edulis produces a polysaccharide (BEP, molecular weight 113 kDa) that exhibits a variety of biological activities, including anti-tumor, immune stimulation, and anti-oxidation [18-22]. Given its demonstrated anti-inflammatory activities, we hypothesized that it may be useful in altering the immune response in asthma. Here, the effects of BEP were assessed in a mouse model of asthma by investigating the IL-4 and IFN-γ content and immune cell counts (CD4+CD25+FOXP3+ Treg cells) after BEP administration and in comparison with traditional asthma therapies. The findings will provide new ideas for the treatment of asthma.
Methods
Experimental animals
Seventy-five female BaLB/c mice ranging in weight from 8-20 g were purchased from Shanghai Lab Animal Research Center (Shanghai, China). The mice were divided into 5 groups: 15 mice in the control group, 15 in the untreated asthma group, 15 in the pravastatin group, 15 in the Boletus edulis polysaccharide group, and 15 in the dexamethasone group.
Mouse model of asthma
To establish a mouse model of asthma, mice were sensitized using the ovalbumin (OVA) method presented by Szefler et al. [23]. Briefly, after being divided into 5 groups and adapted to the environment for 7 days, each mouse in the 4 non-control groups was injected with 25 μg OVA (albumin, from chicken egg white, SIGMA-ALDRICH, batch number: 080M7012V) and 1 mg aluminium hydroxide gel (Imject Alum, Thermo, batch number: NE169583) (dissolved in PBS, 200 μL) on days 0 and 7. Mice in the control group were mock-sensitized by intraperitoneal injection of an equivalent volume of PBS, with the same injection site as the asthma groups. Next, mice in the 4 non-control groups were stimulated to induce asthma beginning on day 14 by being placed in a closed container and inhaling 6% OVA solution by ultrasonic atomization (402A ultrasonic atomizer, Yuwell Medical Equipment, Jiangsu, China) for 30 minutes once daily for 7 consecutive days. Mice in the control group inhaled PBS, at the same frequency and for the same duration as the asthma group. On day 21, 5 mice were taken from each group to be assessed for airway response. On day 22, the remaining mice were euthanized to measure inflammatory markers.
Treatment modalities
Mice in each group were administered a mock treatment or chemical treatment as follows: (1) Control group received atomized PBS only; (2) Untreated asthma group, atomized PBS; (3) Pravastatin group, pravastatin atomization inhalation at a dose of 3.33 mg/kg (pravastatin sodium tablets, Daiichi Sankyo Pharmaceutical, Shanghai, batch number: 110150), once daily beginning on day 7 of model construction; (4) Boletus edulis polysaccharide group: intragastric administration of BEP (Edible Fungus Institute of Lishui City, Zhejiang, China) at a dose of 300 mg/kg, once per day, beginning on day 7 of model construction; (5) Dexamethasone group, intraperitoneal injection of dexamethasone at 2 mg/kg (dexamethasone sodium phosphate, Cisen Pharmaceutical, batch number: 1211146411) on half an hour before each stimulation, until the end of the experiment.
Determination of indexes and methods
Airway hyper-responsiveness was assessed on 5 mice per group using the FinePointe™ RC animal airway resistance and pulmonary compliance system (BUXCO, USA). Within 24 hours after the final stimulation procedure, each mouse was injected intraperitoneally with 60 mg/kg pentobarbital sodium. Then, a tracheal incision was introduced for intubation connection of the animal airway resistance and pulmonary compliance detection system. Respiratory frequency was set to 160 times/min. Mice were administered an atomized inhalation of 10 μL acetylcholine (Shanghai Joe Feather Biological Science and Technology Co., Ltd, Shanghai, China), at ascending concentrations of 0 mg/mL, 1 mg/mL, 2 mg/mL, 4 mg/mL, 8 mg/mL, and 16 mg/mL. The maximum airway resistance within 3 minutes after atomizing inhalation was measured to indicate maximum resistance of the mouse at the corresponding concentration of acetylcholine. At the end of the experiment, the mice were euthanized by an overdose of anesthetics.
The remaining 10 mice per group were subjected to other measures. Bronchoalveolar lavage fluid (BALF) was collected from mice that were first anesthetized then killed by intraperitoneal bleeding. The thorax was accessed to ligate the furcation root of the right lung, and the mouse received tracheal intubation. Next, 0.5 mL cold PBS were introduced and suction was performed 2-3 times, with a recovery rate of about 80%, to obtain BALF. BALF samples were mixed and centrifuged at 1500 rpm for 10 minutes, then the supernatant was stored at -80°C for detection of cytokines; the precipitate from the above centrifugation step and freeze at -80°C for PCR and immunoblotting. Finally, the lung lobe was collected, fixed with 4% formalin, and embedded in paraffin for sectioning. Sections were stained with hematoxylin and eosin or Alcian blue/periodic acid-schiff, and samples were examined under light microscope.
ELISA
Enzyme-linked immunosorbent assays (ELISA) were used to detect cytokines in BALF with IL-4 (Lot: L1304010390), IL-17 (Lot: L130426057), and IFN-gamma (Lot: L130426058) kits (USCN, Wuhan, China). 96-well- Plates with were prepared with blank wells and standards. Standard wells received 50 μL standard substances for IL-4, IL-17 or IFN-gamma at different concentrations. Sample wells received 50 μL of BALF. Standard wells and sample wells received 100 μL horseradish peroxidase (HRP)-labeled antibody. Plates were sealed and incubated at 37°C for 60 min. Wells were washed and patted dry 5 times. Next, 50 μL each of substrate A and B were added, and plates were incubated at 37°C for 15 min. All wells then received 50 μL stop buffer, and OD value was measured on a microplate reader (SPECTRA max Plus 384, Molecular Devices) at 450 nm within 15 min.
Protein extraction and western blotting
The BALF was centrifuged (600g×) at 4°C for 5 minutes to collect cells and extract total protein. BCA total protein quantification assay kit (Beyotime Biotechnology, P0012) was used to measure the protein concentration, and 30 μg total protein were separated by 10% SDS-PAGE. Samples were transferred to PVDF membrane (Millipore Corporation, IPVH00010), sealed with TBST containing 5% skim milk powder, and incubated for 1 hour at room temperature. Membrane was washed with PBST, then primary antibodies for proteins of interest or internal reference [anti-IFN-g (Santa Cruz Biotech, SC52557); anti-IL-4 (Abcam, AB11524); anti-b-actin (Santa Cruz Biotech, SC47778)] were added. Membrane was incubated with shaking at room temperature for 1 hour, washed with PBST, and incubated with secondary antibody [goat anti-rat IgG-HRP (Bioworld, SC2006); goat anti-mouse IgG-HRP (Bioworld, BS12478)] at room temperature with shaking for 1 hour. Staining was developed with an ECL Plus chemiluminescence kit (Millipore, Bedford, MA, USA). ChemiDoc XRS+ System (Bio-RAD) was used to visualize immunoblots, with gray value analysis used to determine relative expression of proteins.
Quantitative RT-PCR
The BALF was centrifuged to obtain cell precipitates for total RNA extraction using high-purity total RNA rapid extraction kits from Shanghai Generay Biotech Co. Ltd (GK3012). After detection of purity and solubility of RNA solution, Revert Aid First Strand cDNA synthesis kit (Fermentas Corporation, K1622) was used to reverse transcribe in a 20 μL reaction system comprising 4 μL 5× Reaction Buffer, 1 μL of 20 U/μL Ribolock TM RNase Inhibitor; 2 μL 10 mM dNTP mix, and 1 μL RevertAid TM M-MmLv Reverse Transcriptase. Reaction mix was heated at 25°C for 5 minutes, then 42°C for 60 minutes, and at 70°C for 5 minutes, then terminated. cDNA was diluted and stored at -70°C.
qRT-PCR amplification was performed using the following primer sequences. Mouse Actin forward, GAGACCTTCAACACCCCAGC and mouse Actin reverse, ATGTCACGCACGATTTCCC were based on the target Genbank ID 11461 to produce PCR fragments of 263 bp. The mouse ILIl-4 forward, TCATCCTGCTCTTCTTTCTCG, and mouse ILIl-4 reverse, TGGCGTCC CTTCTCCTGT, were based on targeting Genbank ID 16189 to produce PCR fragments of 114 bp. Mouse IFN-γ forward, TGTCCTCAACTGCTCTCCAC, and mouse IFN-γ reverse, GAGGCATCAACTGA CAGGTCT, were based on Genbank ID 15977 to produce PCR fragments of 179 bp. IQ SYBR Green Supermix (12.5 μL, Bio-Rad, 170-8882AP) was used in a total 25 μL reaction including 1 μL each of 10 µM primers and 10.5 μL cDNA. Reactions were run at 50°C for 3 minutes, 95°C for 3 minutes, and 40 cycles of 95°C for 10 seconds and 59°C for 20 seconds. The 2-∆∆Ct method to determine relative expression levels of mRNA.
Detection of CD4+CD25+FOXP3+ Treg cells
BALF was assessed under a microscope; approximately 106 cells were collected by centrifugation at 1500 rpm for 5 minutes. Each sample was probed with 0.125 μg anti-mouse CD4 (Abcam, Shanghai, China) and 0.06 μg anti-mouse CD25 antibody (Abcam, Shanghai, China) and incubated at 4°C away from light for 30 minutes. Samples were washed with flow cytometry staining buffer twice and 1 mL resuspended cells was added to Fixation/Permeabilization working solution and incubated at 4°C overnight. Samples were washed with 1× permeabilization buffer twice, centrifuged, and added with flow cytometry staining buffer to a total of 100 μL reaction volume. 0.5 μg Fc blocking agent (CD16/32, Abcam) were added, and samples were incubated at 4°C away from light for 15 minutes. Next, 0.5 μg anti-mouse/rat FOXP3 antibody (Abcam) were added, and samples were incubated at 4°C away from light for 30 minutes. Samples were washed twice with 1× permeabilization buffer, re-suspended with 500 μL 1× permeabilization buffer, and detected by flow cytometer.
Statistical methods
All data were analyzed using SPSS17.0 software. The results are expressed as mean ± standard deviation (x̅±s). The inter-group comparisons of means were performed using univariate analysis of variance and pairwise comparison between groups, with P<0.05 as significantly different.
Results
Mouse model of asthma: airway resistance
The mouse model of asthma was induced using standard atomized exposure to OVA [23]. We measured airway resistance to confirm induction of asthma. Mice exposed to OVA inhalation exhibited significant airway resistance at acetylcholine concentrations of 1, 2, 4, 8, and 16 mg/mL (P<0.05; Table 1). To determine whether drug treatment resulted in improved airway resistance, we compared airway resistance after acetylcholine challenge between untreated mice with asthma and various treatment groups (Table 1). Compared with the untreated asthma model group, the model mice treated with pravastatin had significantly less airway resistance at an acetylcholine concentration of 16 mg/mL (P<0.05). Similarly, model mice treated with Boletus edulis polysaccharide (BEP) had significantly less airway resistance at acetylcholine concentrations of 1 and 16 mg/mL (P<0.05). The airway resistance of model mice treated with dexamethasone was significantly less at acetylcholine concentrations of 2, 4, 8, and 16 mg/mL (P<0.05).
Table 1.
Airway resistance in mice with asthma following treatment (x̅±s, cmH2O·sec/mL)
| Treatment Group | 1 mg/mL | 2 mg/mL | 4 mg/mL | 8 mg/mL | 16 mg/mL |
|---|---|---|---|---|---|
| Control | 0.07±0.04 | 0.20±0.07 | 0.26±0.06 | 0.36±0.10 | 0.51±0.16 |
| Untreated asthma | 0.64±0.27* | 0.82±0.29* | 1.19±0.21* | 1.47±0.27* | 1.63±0.05* |
| Pravastatin | 0.13±0.04# | 0.29±0.09# | 0.66±0.25# | 0.62±0.10# | 0.62±0.28# |
| Boletus edulis polysaccharide | 0.11±0.22 | 0.23±0.28 | 0.91±0.18 | 0.90±0.18# | 1.31±0.18# |
| Dexamethasone | 0.15±0.07# | 0.40±0.24 | 0.78±0.53 | 0.84±0.05# | 1.41±0.04# |
P<0.01, compared with normal group;
P<0.05, compared with model group.
Lung tissue pathology
To determine whether treatment with asthma drugs (pravastatin and dexamethasone) or BEP resulted in improvements in lung pathology following acetylcholine challenge, we compared H&E-stained tissue samples between untreated asthma model mice and treated asthma model mice. Control mice had lung tissues, including pulmonary alveoli, bronchi of the lung, and pulmonary interstitium, with clear structure and absence of congestion in the alveolar wall, inflammatory exudate in the alveolar and bronchial lumen, obvious lesion of epithelial cells in the lung bronchi, increase of mucous cells, or significant peribronchial inflammatory cell infiltration (Figure 1A). In untreated mice with asthma, significant lesions were observed in the lung tissues, as were inflammatory cell infiltration around the bronchus and in the alveolar septum, eosinophils and lymphocytes around the bronchus, thickening of the alveolar wall, and loss of the alveolar structure (Figure 1B). Further, one mouse exhibited severe bleeding in the lung. In mice treated with pravastatin, there was a reduced lesion degree compared to untreated mice; the degree of peribronchial inflammatory cell infiltration and proportion of eosinophils were also lower. Similarly, the alveolar wall was mildly thickening, and did not exhibit bleeding, congestion, or other symptoms (Figure 1C). Mice treated with BEP had a reduced lesion degree compared to untreated controls (Figure 1D). Three mice had a lesion degree similar to that of the dexamethasone group (Figure 1E); but the remaining 5 mice had more lesions compared with the dexamethasone group. The inflammatory cell infiltration mainly occurred around the bronchi and blood vessels, with lymphocytes as the main cell types; there were fewer eosinophils, no obvious bleeding, and no congestion in the alveolar lumen. Mice treated with dexamethasone exhibited a reduced degree of lesions compared with the untreated group, and were similar to mice treated with pravastatin (Figure 1E). The alveolar wall was thickened, but did not exhibit bleeding, congestion, or other symptoms.
Figure 1.

Histology of lung tissues in asthma mouse models. A: Control; B: Untreated asthma model; C: Asthma model treated with pravastatin; D: Asthma model treated with Boletus edulis polysaccharide; E: Asthma model treated with dexamethasone (×400).
Lung tissues were also visualized with AB-PAS staining to detect acidic mucosubstances (blue) and neutral mucosubstances (red), or both (purple, most common). No obvious mucosubstance was detected in the bronchus in the control group (Figure 2A), while purple mucosubstance was seen in bronchia of lung tissues in the untreated asthma model group (Figure 2B). In contrast, the mucosubstances detected in lung tissues in mice treated with pravastatin (Figure 2C), BEP (Figure 2D), and dexamethasone (Figure 2E) were reduced to different degrees compared with the untreated asthma model group. Mice treated with dexamethasone or pravastatin exhibited less mucosubstance staining than the BEP-treated mice.
Figure 2.

Mouse lung tissues stained with PAS. A: Control; B: Untreated asthma model; C: Asthma model treated with pravastatin; D: Asthma model treated with Boletus edulis polysaccharide; E: Asthma model treated with dexamethasone (×400).
Levels of IL-4 and IFN-γ in BALF and lung tissues
Inflammatory markers IL-4 and IFN-γ were assessed in both BALF and lung tissues. The levels of IL-4 in BALF (Table 2) and in lung tissues (Figure 3) were lower in the untreated asthma group compared with control group, but tended to increase in each treatment group compared to the untreated group (P<0.05). In contrast, the IFN-γ levels in BALF (Table 2) and in lung tissues (Figure 3) were increased in the untreated asthma model group compared with the control group, but tended to decrease in each treatment group compared to the untreated group (P<0.05).
Table 2.
IL-4 and IFN-γ levels in BALF and lung tissues (pg/mL)
| Treatment Group | BALF | Lung tissues | ||
|---|---|---|---|---|
|
| ||||
| IL-4 | IFN-γ | IL-4 | IFN-γ | |
| Control | 48.19±5.33 | 273.89±44.98 | 429.53±34.49 | 915.97±144.05 |
| Untreated asthma | 35.20±5.55* | 350.59±72.80* | 296.63±45.51* | 1322.18±226.75* |
| Pravastatin | 39.18±4.19*,# | 303.99±51.34# | 358.43±68.78*,# | 1243.66±101.58*,# |
| Boletus edulis polysaccharide | 40.88±6.48*,# | 292.97±49.12# | 349.00±41.49*,# | 1123.72±365.82# |
| Dexamethasone | 44.21±8.55# | 288.61±25.81# | 342.58±47.06*,# | 1100.22±139.77# |
| F | 6.355 | 3.275 | 9.604 | 5.086 |
| P | 0.001 | 0.019 | 0.001 | 0.002 |
P<0.01, compared with normal group;
P<0.05, compared with model group.
Figure 3.

Protein expression of IL-4 and IFN-γ in lung tissues. Relative protein expression of cytokines in the lung of mouse models of asthma after indicated treatment as detected by Western blotting and normalized against b-actin. Notes: *P<0.01, compared to control group; #P<0.05, compared to untreated asthma model group.
The protein levels were validated at the gene level by quantitative RT-PCR. In accordance with the observed protein levels, mRNA expression of IL-4 in lung tissues in the untreated model was lower than in the control group (Figure 4), but tended to increase in each treatment group compared to the untreated mice (P<0.05). mRNA expression of IFN-γ in lung tissues in the untreated group was higher compared to the control group, but tended to decrease in each treatment group compared to the untreated mice (P<0.05).
Figure 4.

mRNA expression of IL-4 and IFN-γ in lung tissues. Relative mRNA expression of cytokines in the lung of mouse models of asthma after indicated treatment as detected by qRT-PCR. Notes: #P<0.05, compared to untreated asthma model group.
Protein expression of cyclophilin a in lung tissues
Cyclophilin A is another protein that reflects the level of inflammation in asthma; thus, cyclophilin A was assessed in the mouse models in this study. Western blot test results showed that the protein expression of cyclophilin A in lung tissues was higher in the untreated asthma model group compared with the control group, but tended to decrease in each treatment group compared to the untreated mice (P<0.05), as shown in Figure 5.
Figure 5.
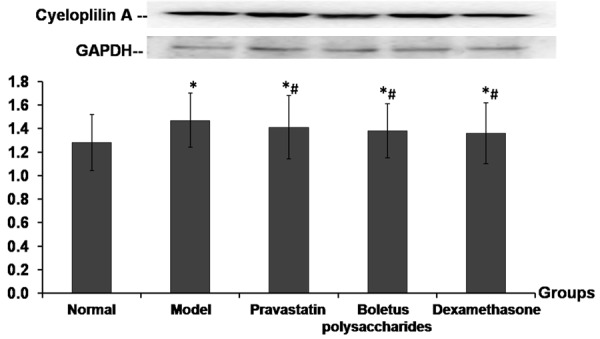
Protein expression of cycloplilin A in lung tissues. Relative protein expression of cyclophilin A in the lung of mouse models of asthma after indicated treatment as detected by Western blotting and normalized against GAPDH. Notes: *P<0.01, compared to control group; #P<0.05, compared to untreated asthma model group.
Proportion of CD4+CD25+FOXP3+ Treg cells in BALF
BALF cell contents were sorted by flow cytometry to determine the proportion of CD4+CD25+FOXP3+ Treg cells. The CD4+T cells (Treg/T) and cells in lymphocytes population (Treg/Cell) were lower in the untreated asthma model group compared to the control group, but tended to increase in each treatment group compared to the untreated model group (P<0.05), as shown in Figure 6.
Figure 6.
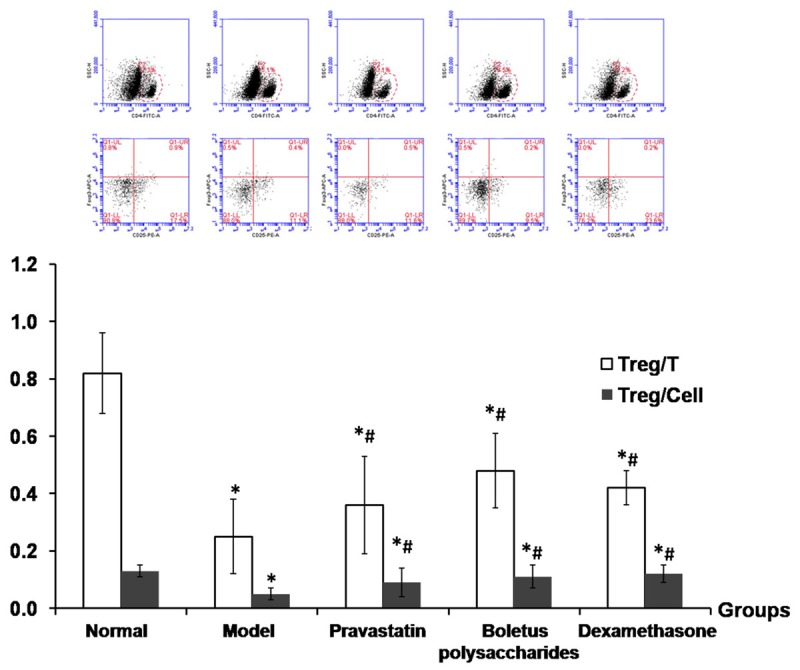
Proportion of CD4+CD25+FOXP3+ Treg cells in BALF. The proportions of CD4+CD25+FOXP3+ Treg cells were measured in bronchoalveolar lavage fluid from the lung of mouse models of asthma after indicated treatment. Notes: *P<0.01, compared to control group; #P<0.05, compared to untreated asthma model group.
Discussion
Asthma has become a serious public health concern, and thus research effort is often devoted to identifying new and effective methods for the treatment of asthma. In this study, we tested the potential for Boletus edulis polysaccharide to reduce asthma severity in a mouse model. The findings reveal that BEP may be a viable treatment alternative to current asthma therapies.
OVA stimulation is a useful method to induce asthma in mice [23]. Here, OVA stimulation resulted in symptoms associated with asthma, especially increased airway resistance and lung pathology, demonstrating successful modeling. The features of asthma observed in this model were ameliorated, at least in part, by treatment with pravastatin, dexamethasone, or BEP. In particular, BEP treatment resulted in the greatest improvement in airway resistance. BEP was also able to induce reductions in inflammatory cell infiltration that were comparable with those of dexamethasone treatment. Further, mucosubstance deposition in lung tissues was reduced in BEP-treated mice compared with untreated mice.
Inflammation is central to asthma pathology, as the main cause of airflow obstruction and airway hyper-responsiveness [24]. Imbalances of Th1/Th2 cells can promote inflammatory processes. Activated Th1 and Th2 cells secrete the cytokines IFN-γ and IL-4, respectively. IFN-γ promotes resistance to infection, antitumor activity, and immune regulation by activating macrophages, improving expression of MHC class I and class II molecules, and promoting antigen presentation [25]. IFN-γ is also an IL-4 antagonistic factor that can inhibit the IgE-mediated immune response that promotes bronchial asthma [26]. Further, IFN-γ can promote Th1 cell differentiation, inhibit the reaction of Th2 cells, inhibit eosinophil gathering in the airway mucosa and IgE production in the blood, and inhibits human bronchial epithelial cell proliferation [27,28].
IL-4 is the only cytokine for B cells producing IgE [29,30]. In the alveolar lavage fluid of patients with moderate and severe asthma inflammation, the generation of IL-4 and IgE are directly related [31]. In addition, secretion of IL-4 in asthma patients with acute episodes is significantly higher than in remission patients and normal controls [32,33]. Similarly, IL-4 concentration increases in serum of children with asthma, while IFN-γ concentration decreases [32,33]. Accordingly, the BALF and lung tissues of mice treated with BEP exhibited higher IL-4 and lower IFN-γ (protein and mRNA) compared with the untreated asthma model group. However, these two indicators were both slightly more normalized in the dexamethasone treatment group.
Importantly, many clinical studies have found that imbalances cannot completely explain asthma pathogenesis. The importance of the regulatory T cells, which express specific cell surface markers CD4 and CD25 [34,35], has been recognized. Indeed, Treg cells can induce and maintain immune tolerance for foreign antigens and autoantigens (including allergen) through a variety of mechanisms, which are essential for controlling inflammation in asthma. In particular, CD4+CD25+FOXP3+ Treg cells play an important role in the pathogenesis of asthma [36]. Prior studies have reported that CD4+CD25+FOXP3+ Treg cells are significantly fewer in the peripheral blood and bronchoalveolar lavage fluid of children with asthma compared with healthy controls [37]. In the peripheral blood of patients with moderate and severe asthma, CD4+CD25+FOXP3+ Treg numbers are significantly lowered compared with the mild asthma and healthy control groups [38-42]. In addition, CD4+CD25+FOXP3+ Treg cells can reduce airway mucus secretion, inhibiting airway smooth muscle proliferation and collagen synthesis [38-42]. Together these findings highlight the importance of CD4+CD25+FOXP3+ Treg cells in controlling asthma airway inflammation. In our study, the proportion of CD4+CD25+FOXP3+ Treg cells in BALF with CD4+T cells (Treg/T) and cells in lymphocytes population (Treg/Cell) was significantly increased in mice treated with BEP compared with the untreated mice.
Cyclophilin protein (CyPA) belongs to the immunophilin protein family and is an intracellular receptor for cyclosporin A. It is also an extracellular chemokine stimulating factor, which has significant chemotactic enhancement effect for T cells and eosinophils [43]. The serum CyPA level in acute asthma patients is significantly higher than that in stationary patients and healthy controls [44]. In this study, the protein expression of CyPA of BEP-treated mice was lower than in untreated asthma model mice (P<0.05) in lung tissues.
In summary, OVA induction of asthma in mice was verified by clinical symptoms, lung pathology, and immune markers. Treatment of induced asthma with Boletus edulis polysaccharide resulted in improved pathology, airway resistance, and immune markers similar to dexamethasone treatment.
Acknowledgements
This study was supported by Zhejiang Provincial Natural Science Foundation of China (No.LY12H10003), Zhejiang Provincial Medical and health Foundation of China (No. 2013KYB303), Educational Committee of Zhejiang Province of China (Y201432632), and Lishui Municipal Science and Technology Project (2015RC08).
Disclosure of conflict of interest
None.
References
- 1.Kuschner WG. The asthma epidemic. N Engl J Med. 2007;356:1073. doi: 10.1056/NEJMc063596. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8:183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 3.Rosewich M, Zissler UM, Kheiri T, Voss S, Eickmeier O, Schulze J, Herrmann E, Dücker RP, Schubert R, Zielen S. Airway inflammation in children and adolescents with bronchiolitis obliterans. Cytokine. 2015;73:156–162. doi: 10.1016/j.cyto.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Brown VG, Ennis M. T cell cytokine production in childhood asthma. Curr Resp Med Rev. 2005;1:1–6. [Google Scholar]
- 5.Li LQ, Huo LL, Zhang XG, Yu JE. Progress in research on relationship between bronchial asthma and Th1/Th2 imbalance. Zhong Xi Yi Jie He Xue Bao. 2005;3:403–407. doi: 10.3736/jcim20050520. [DOI] [PubMed] [Google Scholar]
- 6.Barbas AS, Downing TE, Balsara KR, Tan HE, Rubinstein GJ, Holzknecht ZE, Collins BH, Parker W, Davis RD, Lin SS. Chronic aspiration shifts the immune response from Th1 to Th2 in a murine model of asthma. Eur J Clin Invest. 2008;38:596–602. doi: 10.1111/j.1365-2362.2008.01976.x. [DOI] [PubMed] [Google Scholar]
- 7.Ji NF, Xie YC, Zhang MS, Zhao X, Cheng H, Wang H, Yin KS, Huang M. Ligustrazine corrects Th1/Th2 and Treg/Th17 imbalance in a mouse asthma model. Int Immunopharmacol. 2014;21:76–81. doi: 10.1016/j.intimp.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Zhu M, Liang Z, Wang T, Chen R, Wang G, Ji Y. Th1/Th2/Th17 cells imbalance in patients with asthma with and without psychological symptoms. Allergy Asthma Proc. 2016;37:148–156. doi: 10.2500/aap.2016.37.3928. [DOI] [PubMed] [Google Scholar]
- 9.Wu Q, Tang Y, Hu X, Wang Q, Lei W, Zhou L, Huang J. Regulation of Th1/Th2 balance through OX40/OX40L signalling by glycyrrhizic acid in a murine model of asthma. Respirology. 2016;21:102–111. doi: 10.1111/resp.12655. [DOI] [PubMed] [Google Scholar]
- 10.Li W, Liu YJ, Zhao XL, Shang SQ, Wu L, Ye Q, Xu H. Th1/Th2 Cytokine profile and its diagnostic value in Mycoplasma pneumoniae pneumonia. Iran J Pediatr. 2016;26:e3807. doi: 10.5812/ijp.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gour N, Wills-Karp M. IL-4 and IL-13 signaling in allergic airway disease. Cytokine. 2015;75:68–78. doi: 10.1016/j.cyto.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, Martin RJ, Alam R. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol. 2014;134:1175–1186. doi: 10.1016/j.jaci.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Platts-Mills T. The role of immunoglobulin E in allergy and asthma. Am J Resp Crit Care Med. 2001;164:S1–S5. doi: 10.1164/ajrccm.164.supplement_1.2103024. [DOI] [PubMed] [Google Scholar]
- 14.Hu G, Liu Z, Zheng C. Antigen-non-specific regulation centered on CD25+Foxp 3+ Treg cells. Cell Mol Immunol. 2010;7:414–418. doi: 10.1038/cmi.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang SO, Kim HJ, Kim YJ, Kang MJ, Kwon JW, Seo JH, Kim HY, Kim BJ, Yu J, Hong SJ. Asthma prevention by Lactobacillus Rhamnosusrhamnosus in a mouse model is associated with CD4(+)CD25(+)Foxp3(+) T Cells. Allergy Asthma Immunol Res. 2012;4:150–156. doi: 10.4168/aair.2012.4.3.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan ZZ, Li L, Guo Y, He J. Roles of CD4+CD25+Foxp3+ regulatory T cells and IL-33 in the pathogenesis of asthma in children. Zhongguo Dang Dai Er Ke Za Zhi. 2014;16:1211–1214. [PubMed] [Google Scholar]
- 17.Yun X, Shang Y, Li M. Effect of Lactobacillus salivarius on Th1/Th2 cytokines and the number of spleen CD4+CD25+Foxp3+ Treg in asthma Balb/c mouse. Int J Clin Exp Pathol. 2015;8:7661–7674. [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang A, Xiao N, He P, Sun P. Chemical analysis and antioxidant activity in vitro of polysaccharides extracted from Boletus edulis. Int J Biol Macromol. 2011;49:1092–1095. doi: 10.1016/j.ijbiomac.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Luo A, Luo A, Huang J, Fan Y. Purification, characterization and antioxidant activities in vitro and in vivo of the polysaccharides from Boletus edulis bull. Molecules. 2012;17:8079–8090. doi: 10.3390/molecules17078079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding X, Hou YL, Hou WR. Structure elucidation and antioxidant activity of a novel polysaccharide isolated from Boletus speciosus Forst. Int J Biol Macromol. 2012;50:613–618. doi: 10.1016/j.ijbiomac.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Hou Y, Ding X, Hou W, Song B, Wang T, Wang F, Li J, Zeng Y, Zhong J, Xu T, Zhu H. Pharmacological evaluation for anticancer and immune activities of a novel polysaccharide isolated from Boletus speciosus Frost. Mol Med Rep. 2014;9:1337–1344. doi: 10.3892/mmr.2014.1976. [DOI] [PubMed] [Google Scholar]
- 22.Wang D, Sun SQ, Wu WZ, Yang SL, Tan JM. Characterization of a water-soluble polysaccharide from Boletus edulis and its antitumor and immunomodulatory activities on renal cancer in mice. Carbohydr Polym. 2014;105:127–134. doi: 10.1016/j.carbpol.2013.12.085. [DOI] [PubMed] [Google Scholar]
- 23.Jeon J, Kim Y, Kim H, Kang JS, Lee WJ. Anti-inflammatory effect of alloferon on ovalbumin-induced asthma. Immune Netw. 2015;15:304–312. doi: 10.4110/in.2015.15.6.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanania NA. Targeting airway inflammation in asthma: current and future therapies. Chest. 2008;133:989–998. doi: 10.1378/chest.07-0829. [DOI] [PubMed] [Google Scholar]
- 25.Muraille E, Leo O. Revisiting the Th1/Th2 paradigm. Scand J Immunol. 1998;47:1–9. doi: 10.1111/j.1365-3083.1998-47-1.00383.x. [DOI] [PubMed] [Google Scholar]
- 26.Zissler UM, Chaker AM, Effner R, Ulrich M, Guerth F, Piontek G, Dietz K, Regn M, Knapp B, Theis FJ, Heine H, Suttner K, Schmidt-Weber CB. Interleukin-4 and interferon-γ orchestrate an epithelial polarization in the airways. Mucosal Immunol. 2016;9:917–26. doi: 10.1038/mi.2015.110. [DOI] [PubMed] [Google Scholar]
- 27.Vicario M, Blanchard C, Stringer KF, Collins MH, Mingler MK, Ahrens A, Putnam PE, Abonia JP, Santos J, Rothenberg ME. Local B cells and IgE production in the oesophageal mucosa in eosinophilic oesophagitis. Gut. 2010;59:12–20. doi: 10.1136/gut.2009.178020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takami K, Takuwa N, Okazaki H, Kobayashi M, Ohtoshi T, Kawasaki S, Dohi M, Yamamoto K, Nakamura T, Tanaka M, Nakahara K, Takuwa Y, Takizawa H. Interferon-gamma inhibits hepatocyte growth factor-stimulated cell proliferation of human bronchialepithelial cells: upregulation of p27 (kip1) cyclin-dependent kinase inhibitor. Am J Respir Cell Mol Biol. 2002;26:231–238. doi: 10.1165/ajrcmb.26.2.4643. [DOI] [PubMed] [Google Scholar]
- 29.Maggi E, Del Prete GF, Tiri A, Macchia D, Parronchi P, Ricci M, Romagnani S. T cell clones providing helper function for IgE synthesis release soluble factor(s) that induce IgE production in human B cells: possible role for interleukin 4 (IL-4) Clin Exp Immunol. 1988;73:57–62. [PMC free article] [PubMed] [Google Scholar]
- 30.Kuhl K, Hanania NA. Targeting IgE in asthma. Curr Opin Pulm Med. 2012;18:1–5. doi: 10.1097/MCP.0b013e32834deebb. [DOI] [PubMed] [Google Scholar]
- 31.Gorska K, Krenke R, Domagala-Kulawik J, Korczynski P, Nejman-Gryz P, Kosciuch J, Hildebrand K, Chazan R. Comparison of cellular and biochemical markers of airway inflammation in patients with mild-to-moderate asthma and chronic obstructive pulmonary disease: an induced sputum and bronchoalveolar lavage fluid study. J Physiol Pharmacol. 2008;59:271–283. [PubMed] [Google Scholar]
- 32.Lee SY, Kim SJ, Kwon SS, Kim YK, Kim KH, Moon HS, Song JS, Park SH. Distribution and cytokine production of CD4 and CD8 T-lymphocyte subsets in patients with acute asthma attacks. Ann Allergy Asthma Immunol. 2001;86:659–664. doi: 10.1016/S1081-1206(10)62295-8. [DOI] [PubMed] [Google Scholar]
- 33.Charoenying Y, Kamchaisatian W, Atamasirikul K, Direkwattanachai C, Manuyakorn W, Benjaponpitak S. Cytokine response during exacerbation compared with stable phase in asthmatic children. Asian Pac J Allergy Immunol. 2010;28:35–40. [PubMed] [Google Scholar]
- 34.Langier S, Sade K, Kivity S. Regulatory T cells in allergic asthma. Isr Med Assoc J. 2012;14:180–183. [PubMed] [Google Scholar]
- 35.Stelmaszczyk-Emmel A. Regulatory T cells in children with allergy and asthma: it is time to act. Respir Physiol Neurobiol. 2015;209:59–63. doi: 10.1016/j.resp.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 36.Sjaheim TB, Bjørtuft Ø, Drabløs PA, Kongerud J, Halstensen TS. Increased bronchial density of CD25+Foxp3+ regulatory T cells in occupational asthma: relationship to current smoking. Scand J Immunol. 2013;77:398–404. doi: 10.1111/sji.12035. [DOI] [PubMed] [Google Scholar]
- 37.Chauhan A, Singh M, Agarwal A, Paul N. Correlation of TSLP, IL-33, and CD4+CD25+FOXP3+ Tregulatory (Treg) in pediatric asthma. J Asthma. 2015;52:868–872. doi: 10.3109/02770903.2015.1026441. [DOI] [PubMed] [Google Scholar]
- 38.Bakr SI, Mahran MZ, Soliman DA. Role of regulatory CD4+CD25+Foxp3+ T cells in bronchial asthma in Egyptian children. Egypt J Immunol. 2013;20:29–38. [PubMed] [Google Scholar]
- 39.Lee JH, Yu HH, Wang LC, Yang YH, Lin YT, Chiang BL. The levels of CD4+CD25+ regulatory T cells in paediatric patients with allergic rhinitis and bronchial asthma. Clin Exp Immunol. 2007;148:53–63. doi: 10.1111/j.1365-2249.2007.03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chenn BL, Dienger KM, Sproles AA, Shah JS, Köhl J, Belkaid Y, Wills-Karp M. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J Exp Med. 2005;202:1549–1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdulamir AS, Kadhim HS, Hafidh RR, Ali MA, Faik I, Abubakar F, Abbas KA. Severity of asthma: the role of CD25+, CD30+, NF-kappaB, and apoptotic markers. J Investig Allergol Clin Immunol. 2009;19:218–224. [PubMed] [Google Scholar]
- 42.Kearley J, Robinson DS, Lloyd CM. CD4+CD25+ regulatory T cells reverse established allergic airway inflammation and prevent airway remodeling. J Allergy Clin Immunol. 2008;122:617–624. doi: 10.1016/j.jaci.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colgan J, Asmal M, Neagu M, Yu B, Schneidkraut J, Lee Y, Sokolskaja E, Andreotti A, Luban J. Cyclophilin A regulates TCR signal strength in CD4+T cells via a proline-directed conformational switch in Itk. Immunity. 2004;21:189–201. doi: 10.1016/j.immuni.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Chen F, Gui S. The detection of serum Cyclophilin A in patient with Asthma and its clinical significance. Journal of Clinical Pulmonary Medicine. 2011;16:527–528. [Google Scholar]


