Abstract
Background
Autosomal dominant congenital mirror movements (CMM) is a neurodevelopmental disorder characterized by early onset involuntary movements of one side of the body that mirror intentional movements on the contralateral side; these persist throughout life in the absence of other neurological symptoms. The main culprit genes responsible for this condition are RAD51 and DCC. This condition has only been reported in a few families, and the molecular mechanisms linking RAD51 mutations and mirror movements (MM) are poorly understood.
Methods
We collected demographic, clinical, and genetic data of a new family with CMM due to a truncating mutation of RAD51. We reviewed the literature to identify all reported patients with CMM due to RAD51 mutations.
Results
We identified a heterozygous nonsense mutation c.760C>T (p.Arg254*) in eight subjects: four with obvious and disabling MM, and four with a mild phenotype. Including our new family, we identified 32 patients from 6 families with CMM linked to RAD51 variants.
Discussion
Our findings further support the involvement of RAD51 in CMM pathogenesis. Possible molecular mechanisms involved in CMM pathogenesis are discussed.
Keywords: Mirror movement, RAD51, motor control, corticospinal tract, corpus callosum, neurodevelopment
Introduction
Mirror movements (MM) are characterized by involuntary movements of one side of the body that mirror intentional movements on the contralateral side.1 They can be a manifestation of various congenital or acquired neurological disorders.2,3 Autosomal dominant congenital mirror movements (CMM) are characterized by early onset MM that persist throughout life in the absence of other neurological symptoms.1 Since CMM predominantly affects the hands, difficulty carrying out activities of daily living is limited to tasks that require independent movements of the two hands, but patients can also experience chronic upper limb pain.4,5 The pathophysiology is related to abnormal development of the motor network and likely involves corticospinal tract abnormalities leading to bilateral downstream transmission of the motor command and altered interhemispheric communication resulting in bilateral activation of primary motor areas.6–10
The main culprit genes responsible for this condition are RAD51 and DCC.11–14 Only a few families and sporadic cases have been linked to RAD51 mutations. Here, we report a new family with autosomal dominant CMM with a RAD51 mutation and review the literature for previously reported patients. We also discuss the possible pathogenic molecular mechanism in CMM-RAD51 patients.
Methods
Subjects
A family with CMM was identified during an ongoing study of genetic movement disorders in Norway. Affected subjects, obligatory carriers, and possibly affected family members (taking the pedigree into account) were invited to participate in the study. We collected demographic, clinical, and genetic data. Clinical evaluation and patient phenotyping including movement scoring was performed by a single physician (J.K.) who was blinded to the genotypes. Movements were rated according to the Woods and Teuber MM scale,15 where 0 means absence of MM; 1, barely discernible but repetitive movement; 2, either slight but unsustained repetitive movement or stronger but briefer repetitive movement; 3, strong and sustained repetitive movement; and 4, movement equal to that observed in the intended hand. Written informed consent was obtained from all included family members in accordance with the ethical agreement 2012/1451 of the Regional Ethical Committee in Norway.
Mutation analysis and genotyping
DNA was extracted from peripheral blood lymphocytes using standard techniques (QIAamp DNA Blood Mini Kit, Qiagen). The RAD51 and DCC genes exons and their flanking intronic sequences were amplified and sequenced by Sanger sequencing. Genotyping was done independently by a single investigator (OT) that was unaware of the phenotype.
Review of the literature
We conducted a review of published studies to identify all reported patients with CMM due to RAD51 mutations. We searched the PubMed database using the terms “RAD51 and mirror movements” from 1980 to September 2016. The reference lists of papers thus retrieved were also scanned for relevant articles.
Results
Study of the New RAD51-MM family
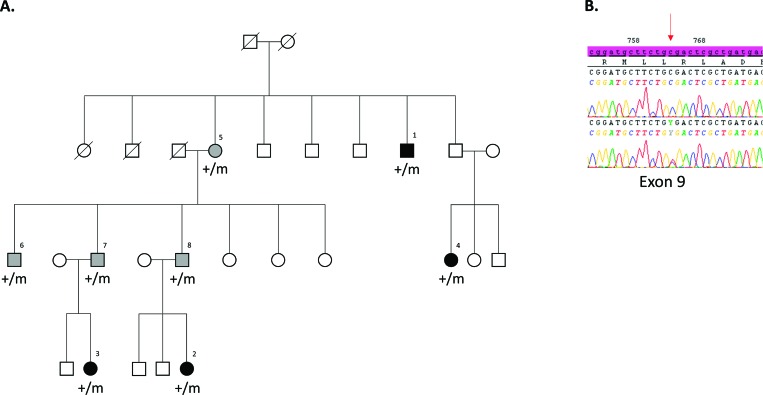
The characteristics of the patients are shown in Table 1. By direct sequencing of the RAD51 coding sequence (NM_002875.4), we identified a heterozygous nonsense mutation c.760C>T (p.Arg254*) in the eight subjects (Figure 1). Among them, four had obvious MM in their hands that disturbed their activities of daily living (subjects #1, #2, #3, #4). The remaining four mutation carriers had no complaints despite having mild MM when attempting to perform strictly unilateral movements (subjects #5, #6, #7; #8) (Video 1). No other non-neurological phenotype cosegregated with the RAD51 variants.
Table 1. Patient Characteristics.
| Patient-Sex/Age | MM Severity1/Location | Asymmetry | Associated Disorders | Functional Disability |
|---|---|---|---|---|
| 1-M/59 | 3/UL | No | No | Difficulties in fine bimanual activities |
| 2-F/5 | 3/UL | No | No | Difficulties in fine bimanual activities |
| 3-F/3 | 3/UL; 1/LL | No | No | Difficulties in fine bimanual activities |
| 4-F/22 | 3/UL; 1/LL | No | No | Difficulties in fine bimanual activities |
| 5-F/70 | 1/UL | R>L | Chronic fatigue | None |
| 6-M/45 | 1/UL | R>L | No | None |
| 7-M/44 | 1/UL | L>R | No | Chronic UL pain |
| 8-M/43 | 1/UL | R>L | No | None |
Abbreviations: F, Female; L, Left; LL, Lower Limbs; M, Male; MM, Mirror Movements; R, Right; UL, Upper Limbs.
1According to the Woods and Teuber MM severity scale: (0: No MM; 1: Barely discernible but repetitive MM; 2: Either slight but sustained MM, or stronger, but briefer MM; 3: Strong and sustained repetitive MM; 4: MM equal to that observed in the intended hand).
Figure 1. Identification of the R254* Mutation in the Norwegian Family. (A) Segregation of the mutation. Black symbols represent individuals with strong congenital mirror movements, and gray symbols represent individuals with mild congenital mirror movements. Circles indicate females; squares, males; symbols with a diagonal line, deceased; m: mutated allele, +: wild-type allele. (B) Sequence profile of the mutation in the heterozygous state in a congenital mirror movement patient compared with an unaffected subject. The red arrow points to sequence changes; electrophoregrams were obtained with SeqScape software (v2.6, Applied Biosystems).
Video 1. Illustrative video of severe and mild mirror movements. Two patients (subjects #2 and #3) with severe mirror movements (severity score = 3), and then a patient (subject #7) with mild mirror movements (severity score = 1). The red arrow indicates the side of the voluntary movement.
Review of the literature
We retrieved 11 papers that were analyzed in detail. Including our new family, we identified 32 CMM patients from 6 families with variants of RAD51 as summarized in Table 2.12,14,16 Thirty patients had a clearly pathogenic mutation, and two had missense variants that are possibly pathogenic.
Table 2. Summary of Reported CMM Patients with RAD51 Mutations.
| Families | Reference | Patient Origin | Number of Patients (M/F) | Asymptomatic Carriers (M/F) | Variants | In Silico Predictions (SIFT/Polyphen-2) | CADD score Raw Score Phred | ExAC frequency | Final prediction | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 | France | 9 (7/2) | 7 (3/4) | c.760C>T/p.Arg254* | - | 13.368440 | 42 | 0 | Pathogenic |
| 2 | 12 | Germany | 2 (1/1) | 0 | c.855dup/p.Pro286Thrfs*37 | - | 8.263609 | 35 | 0 | Pathogenic |
| 3 | 16 | USA | 11 (6/5) | 1 (0/1) | c.749G>A/p.Arg250Gln | Deleterious/possibly damaging | 7.716175 | 35 | 0 | Pathogenic |
| 4 | 14 | Italy | 1 (0/1) | 2 (1/1) | c.140A>G/p.His47Arg | Deleterious/benign | 2.124248 | 17.01 | 8,238.10-6 | Possibly pathogenic |
| 5 | 14 | France | 1 (0/1) | 1 (0/1) | c.406A>T/p.Ile136Phe | Deleterious/benign | 3.953950 | 23.6 | 0 | Possibly pathogenic |
| 61 | Norway | 8 (4/4) | 1 (1/0) | c.760C>T/p.Arg254* | - | 13.368440 | 42 | 0 | Pathogenic | |
Abbreviations: CADD, Combined Annotation Dependent Depletion; CMM, congenital mirror movements; ExAC, Exome Aggregation Consortium; F, Female; M, Male.
1Present report
Discussion
We report a new CMM family with a RAD51 truncating mutation, providing further support for the involvement of RAD51 in CMM pathogenesis. A review of all published cases of RAD51-linked CMM raises the possibility of a molecular pathogenesis other than mere haploinsufficiency.
In this paper, we report the first CMM family from Norway comprising eight affected members: four with strong MM, and four with a mild phenotype. All affected patients have a heterozygous nonsense mutation of RAD51, previously demonstrated to cause CMM in a large, unrelated French family.12 The exact age of onset is difficult to ascertain in CMM patients, including in the family described here. In our clinical experience, the age of onset corresponds to the period of manual skill development in toddlers, regardless of the culprit gene (DCC or RAD51). Males with a DCC mutation may be more prone to develop MM,11,13,14 whereas the sex ratio seems to be well balanced (18 males/14 females) in patients with CMM due to RAD51 mutations. In the Norwegian family described here, there was considerable intrafamilial variability in MM severity, and the available data were too scarce to properly discuss the penetrance. Typically, penetrance is incomplete, and phenotypic severity varies widely within families with CMM (including RAD51-CMM families),11–14 from asymptomatic carriers to subjects with strong, sustained MM. The incomplete penetrance and phenotypic variability of CMM due to RAD51 mutations may be related neither to age nor sex, but it could be linked to greater expression of RAD51 from the remaining normal allele or to other genetic, epigenetic, or environmental modifiers. In mildly affected subjects, it can be difficult to distinguish among mild genetically determined “pathologic” MM, physiologic MM, and secondary MM. Indeed, subtle mirroring can be seen in otherwise healthy subjects, particularly in young children or elderly subjects.17 Mild MM can similarly be observed in patients with various central nervous system (CNS) disorders, including poststroke hemiplegia,18 amyotrophic lateral sclerosis,19 multiple sclerosis,20 Parkinson’s disease,21–23 essential tremor,24,25 Huntington’s disease,26 and corticobasal degeneration.27 In this setting, the MM are acquired and therefore presumably absent before CNS disorder onset.
Since the RAD51 protein is well known for its important role in DNA repair through homologous recombination,28 its role in a developmental CNS disorder such as CMM was totally unexpected. The first RAD51 mutations discovered in CMM patients were nonsense or frameshift mutations.12 In particular, we described a French family carrying the same mutation as the Norwegian family (reported here), and the phenotypes are very similar.12 CMM patients in the French family exhibited a downregulation of RAD51 mRNA, corresponding to the degradation of the mutated mRNA by nonsense-mediated decay. Western blot analyses failed to detect any truncated protein in lymphoblastic cells of affected family members. Taking genotypic and phenotypic similarities into account, haploinsufficiency is likely the pathogenic mechanism accounting for CMM in both families. In keeping with this, Exome Aggregation Consortium (ExAC) data show that RAD51 is highly intolerant to loss of function (probability of loss of function intolerance = 0.99). Therefore, the prevailing hypothesis has been one of a haploinsufficiency leading to CMM in RAD51 patients. The recent finding that a missense pathogenic RAD51 mutation was responsible for CMM in a large family16 raises the possibility of pathophysiologic mechanisms other than mere haploinsufficiency. Interestingly, a dominant-negative effect of RAD51 mutations was recently proposed for patients affected with Fanconi’s anemia, another disorder linked to RAD51 mutations.29,30 To date, there is no report of a patient with both CMM and Fanconi’s anemia. Similarly, none of the RAD51 pathogenic variants have been described in both disorders. This suggests that CMM and Fanconi’s anemia are allelic disorders due to mutations with mechanistically distinct functional effects on RAD51 activity. Further functional studies are needed to investigate the genotype/phenotype correlation in patients with RAD51 mutations in depth and clarify the impact of nongenetic factors on the phenotype and penetrance.
Acknowledgments
The authors thank the family members who agreed to participate in this study.
Footnotes
Funding: We thank Merz-pharmaceuticals and the Vestre Viken Hospital Trust (JK) for financial support of this study.
Financial Disclosures: O.T., J.K., T.G., S.M., C.D., I.D., and C.D. have no disclosures. Aurélie Méneret received travel funding from Zambon, and Marta Ruiz received travel funding from The Dystonia Medical Research Foundation. Emmanuel Roze received research support from CNRS, INSERM (COSSEC), AP-HP (DRC-PHRC), Merz-Pharma, Orkyn, Aguettant, IP santé, Ultragenix, and UCB pharma; served on scientific advisory boards for Orkyn, Ultragenix, Retrophin, and Merz-pharma; received speech honoraria from Orkyn, Aguettant, Merz-Pharma, and Ultragenix; and received travel funding from Teva, Sanofi-Genzyme, the Dystonia Coalition, The Dystonia Medical Research Foundation, and the Movement Disorders Society.
Conflict of Interest: The authors report no conflict of interest related to this paper.
Ethics statement: Written informed consent was obtained from all included family members in accordance with the ethical agreement 2012/1451 of the Regional Ethical Committee in Norway. This study was performed in accordance with the ethical standards detailed in the Declaration of Helsinki. The authors’ institutional ethics committee has approved this study and all patients have provided written informed consent. All patients that appear on video have provided written informed consent; authorization for the videotaping and for publication of the videotape was provided.
References
- 1.Bonnet C, Roubertie A, Doummar D, Bahi-Buisson N, Cochen de Cock V, Roze E. Developmental and benign movement disorders in childhood. Mov Disord. 2010;25:1317–1334. doi: 10.1002/mds.22944. doi: 10.1002/mds.22944. [DOI] [PubMed] [Google Scholar]
- 2.Cox BC, Cincotta M, Espay AJ. Mirror movements in movement disorders: a review. Tremor Other Hyperkinet Mov (N Y) 2012:2. doi: 10.7916/D8VQ31DZ. doi: 10.7916/D8VQ31DZ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farmer SF. Mirror movements in neurology. J Neurol Neurosurg Psychiatry. 2005;76:1330. doi: 10.1136/jnnp.2005.069625. doi: 10.1136/jnnp.2005.069625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meneret A, Trouillard O, Brochard V, Roze E. Congenital mirror movements caused by a mutation in the DCC gene. Dev Med Child Neurol. 2015;57:776. doi: 10.1111/dmcn.12810. doi: 10.1111/dmcn.12810. [DOI] [PubMed] [Google Scholar]
- 5.Meneret A, Welniarz Q, Trouillard O, Roze E. Congenital mirror movements: from piano player to opera singer. Neurology. 2015;84:860. doi: 10.1212/WNL.0000000000001290. doi: 10.1212/WNL.0000000000001290. [DOI] [PubMed] [Google Scholar]
- 6.Cincotta M, Ziemann U. Neurophysiology of unimanual motor control and mirror movements. Clin Neurophysiol. 2008;119:744–762. doi: 10.1016/j.clinph.2007.11.047. doi: 10.1016/j.clinph.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 7.Gallea C, Popa T, Billot S, Meneret A, Depienne C, Roze E. Congenital mirror movements: a clue to understanding bimanual motor control. J Neurol. 2011;258:1911–1919. doi: 10.1007/s00415-011-6107-9. doi: 10.1007/s00415-011-6107-9. [DOI] [PubMed] [Google Scholar]
- 8.Gallea C, Popa T, Hubsch C, et al. RAD51 deficiency disrupts the corticospinal lateralization of motor control. Brain. 2013;136:3333–3346. doi: 10.1093/brain/awt258. doi: 10.1093/brain/awt258. [DOI] [PubMed] [Google Scholar]
- 9.Peng J, Charron F. Lateralization of motor control in the human nervous system: genetics of mirror movements. Curr Opin Neurobiol. 2013;23:109–118. doi: 10.1016/j.conb.2012.08.007. doi: 10.1016/j.conb.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Welniarz Q, Dusart I, Gallea C, Roze E. One hand clapping: lateralization of motor control. Front Neuroanat. 2015;9:75. doi: 10.3389/fnana.2015.00075. doi: 10.3389/fnana.2015.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srour M, Riviere JB, Pham JM, et al. Mutations in DCC cause congenital mirror movements. Science. 2010;328:592. doi: 10.1126/science.1186463. doi: 10.1126/science.1186463. [DOI] [PubMed] [Google Scholar]
- 12.Depienne C, Bouteiller D, Meneret A, et al. RAD51 haploinsufficiency causes congenital mirror movements in humans. Am J Hum Genet. 2012;90:301–307. doi: 10.1016/j.ajhg.2011.12.002. doi: 10.1016/j.ajhg.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Depienne C, Cincotta M, Billot S, et al. A novel DCC mutation and genetic heterogeneity in congenital mirror movements. Neurology. 2011;76:260–264. doi: 10.1212/WNL.0b013e318207b1e0. doi: 10.1212/WNL.0b013e318207b1e0. [DOI] [PubMed] [Google Scholar]
- 14.Meneret A, Depienne C, Riant F, et al. Congenital mirror movements: mutational analysis of RAD51 and DCC in 26 cases. Neurology. 2014;82:1999–2002. doi: 10.1212/WNL.0000000000000477. doi: 10.1212/WNL.0000000000000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woods BT, Teuber HL. Mirror movements after childhood hemiparesis. Neurology. 1978;28:1152–1157. doi: 10.1212/wnl.28.11.1152. doi: 10.1212/WNL.28.11.1152. [DOI] [PubMed] [Google Scholar]
- 16.Franz EA, Chiaroni-Clarke R, Woodrow S, et al. Congenital mirror movements: phenotypes associated with DCC and RAD51 mutations. J Neurol Sci. 2015;351:140–145. doi: 10.1016/j.jns.2015.03.006. doi: 10.1016/j.jns.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Koerte I, Eftimov L, Laubender RP, et al. Mirror movements in healthy humans across the lifespan: effects of development and ageing. Dev Med Child Neurol. 2010;52:1106–1112. doi: 10.1111/j.1469-8749.2010.03766.x. doi: 10.1111/j.1469-8749.2010.03766.x. [DOI] [PubMed] [Google Scholar]
- 18.Nelles G, Cramer SC, Schaechter JD, Kaplan JD, Finklestein SP. Quantitative assessment of mirror movements after stroke. Stroke. 1998;29:1182–1187. doi: 10.1161/01.str.29.6.1182. doi: 10.1161/01.STR.29.6.1182. [DOI] [PubMed] [Google Scholar]
- 19.Wittstock M, Meister S, Walter U, Benecke R, Wolters A. Mirror movements in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2011;12:393–397. doi: 10.3109/17482968.2011.577223. doi: 10.3109/17482968.2011.577223. [DOI] [PubMed] [Google Scholar]
- 20.Cabib C, Llufriu S, Martinez-Heras E, Saiz A, Valls-Sole J. Enhanced mirror activity in ‘crossed' reaction time tasks in multiple sclerosis. Clin Neurophysiol. 2016;127:2001–2009. doi: 10.1016/j.clinph.2016.01.017. doi: 10.1016/j.clinph.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Espay AJ, Li JY, Johnston L, Chen R, Lang AE. Mirror movements in parkinsonism: evaluation of a new clinical sign. J Neurol Neurosurg Psychiatry. 2005;76:1355–1358. doi: 10.1136/jnnp.2005.062950. doi: 10.1136/jnnp.2005.062950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Espay AJ, Morgante F, Gunraj C, Chen R, Lang AE. Mirror movements in Parkinson's disease: effect of dopaminergic drugs. J Neurol Neurosurg Psychiatry. 2006;77:1194–1195. doi: 10.1136/jnnp.2005.086892. doi: 10.1136/jnnp.2005.086892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ottaviani D, Tiple D, Suppa A, et al. Mirror movements in patients with Parkinson's disease. Mov Disord. 2008;23:253–258. doi: 10.1002/mds.21825. doi: 10.4103/0972-2327.150606. [DOI] [PubMed] [Google Scholar]
- 24.Louis ED, Rios E, Henchcliffe C. Mirror movements in patients with essential tremor. Mov Disord. 2009;24:2211–2217. doi: 10.1002/mds.22749. doi: 10.1002/mds.22749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louis ED, Gillman A. Mirror movements in essential tremor: prevalence and relationship to mini-mental status test scores. Tremor Other Hyperkinet Mov (N Y) 2012:2. doi: 10.7916/D8348J4S. doi: 10.7916/D8348J4S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Georgiou-Karistianis N, Hoy KE, Bradshaw JL, et al. Motor overflow in Huntington's disease. J Neurol Neurosurg Psychiatry. 2004;75:904–906. doi: 10.1136/jnnp.2003.016733. doi: 10.1136/jnnp.2003.016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher CM. Alien hand phenomena: a review with the addition of six personal cases. Can J Neurol Sci. 2000;27:192–203. doi: 10.1017/s0317167100000834. doi: 10.1017/S0317167100000834. [DOI] [PubMed] [Google Scholar]
- 28.Godin SK, Sullivan MR, Bernstein KA. Novel insights into RAD51 activity and regulation during homologous recombination and DNA replication. Biochem Cell Biol. 2016:1–12. doi: 10.1139/bcb-2016-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ameziane N, May P, Haitjema A, et al. A novel Fanconi anaemia subtype associated with a dominant-negative mutation in RAD51. Nat Commun. 2015;6:8829. doi: 10.1038/ncomms9829. doi: 10.1038/ncomms9829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang AT, Kim T, Wagner JE, et al. A Dominant Mutation in Human RAD51 Reveals Its Function in DNA Interstrand Crosslink Repair Independent of Homologous Recombination. Mol Cell. 2015;59:478–490. doi: 10.1016/j.molcel.2015.07.009. doi: 10.1016/j.molcel.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]