Abstract
Background
Vincristine is an integral treatment component of many childhood tumors with potentially dose-limiting sensory and/or motor neuropathy. Results from a pilot study on the incidence of vincristine-induced peripheral neuropathy (VIPN) as well as the efficacy and safety of glutamine in reducing signs and symptoms of VIPN in children with cancer are presented.
Methods
Fifty-six patients between the ages of 5–21 with newly diagnosed leukemia, lymphoma, extracranial solid tumor or medulloblastoma and expected to receive a minimum cumulative dose of 6 mg/m2 of vincristine over a 30-week period were eligible. Patients’ neurological functioning was monitored every 3 weeks using clinical history, exam, and assessment of motor functioning. Upon identification of neuropathy, patients were randomized to either glutamine (6 g/m2 per dose twice daily, maximum 10 g/dose) or placebo for a 3-week period followed by 3-week wash out period (Time 3).
Results
Forty-nine patients were fully evaluable and 100 % developed neuropathy per study definitions. No significant differences in demographics or side effects were noted between the randomized groups. The distribution of sensory neuropathy scores between the two groups was statistically significant after the intervention (p = 0.022). Children receiving glutamine also rated their quality of life (QoL) as 8.42 points higher on the PedsQL total score than those receiving placebo (p = 0.031).
Conclusions
Glutamine supplementation is well tolerated and associated with improvements in sensory function and self-reported overall quality of life. Future studies are warranted to confirm the efficacy of glutamine for the treatment of vincristine-related sensory neuropathy in pediatric cancer patients.
Keywords: Glutamine, Integrative medicine, Supportive care, Neuropathy, Quality of life
Background
Vincristine is a vinca alkaloid derived from the periwinkle plant, which causes disruption of microtubule function through binding to tubulin resulting in mitotic arrest in replicating cells and cell death [1, 2]. Vincristine is an integral component of treatment for many childhood hematologic and solid malignancies. The dose-limiting toxicity of vincristine therapy is neurotoxicity, typically mixed sensory, and motor neuropathy but also including dysautonomia with characteristic jaw pain, numbness, paresthesias, fine motor clumsiness, cranial neuropathies, neuropathic pain, obstipation, loss of deep tendon reflexes, and foot-hand drop. These symptoms often progress, significantly impacting quality of life (QoL) and leading to profound motor weakness [3]. Current management is limited to dose reductions or delays in treatment.
That vincristine-induced peripheral neuropathy (VIPN) is an established toxicity of anticancer treatment is widely accepted; however, there is limited data documenting its incidence and severity in pediatric oncology due to considerable variability in the assessment of neuropathy, with several emerging measures, but little consensus to date [4–7]. In large comprehensive clinical trials in acute lymphoblastic leukemia (ALL) utilizing the National Cancer Institute Common Toxicity Criteria (NCI-CTC), severe VIPN of grades 2–4 has been documented with rates of 22–29 % [8]. With more sensitive exams, some small studies estimate incidence of symptoms and signs of VIPN approaching 100 % [6]. Among individuals with cancer 12 years and older, a recent meta-analysis of 13,683 patients documents that between 19 and 39 % suffer from neuropathic pain [9]. Importantly, despite general understanding of nearly uniform reversibility, more recent studies also suggest that the prevalence of VIPN extends into survivorship. Two small pilot studies among survivors of ALL found that approximately 30 % had abnormal nerve conduction studies up to 3 years post-therapy completion [10, 11], and a longer-term study of survivors of extracranial solid tumors treated between 1962 and 2002 demonstrated sensory (20 %) and motor (17.5 %) impairment by the modified total neuropathy score with a median follow up from diagnosis of 25 years [12].
In many of the existing studies, the assessment of VIPN has been limited to clinical assessment, most often utilizing the NCI-CTC. While this has been a widely used and systematic approach for assessment and documentation, it is inherently limited in its ability to capture all aspects of neuropathy of pediatric cancer patients and survivors [6]. Improved documentation of the incidence of neuropathy using assessments that are sensitive to change over time is needed so that clinical trials of innovative interventions may be designed and tested.
Proposed mechanisms underlying chemotherapy-induced peripheral neuropathy are multiple and incompletely elucidated, thereby limiting the discovery of effective prevention and treatment strategies. Hypotheses include primarily direct axonal damage but also mitochondrial dysregulation and changes in the gene expression of various pain mediators encompassing neurotransmitters and ion channels, as well as growth factors and cytokines [1, 13]. Vincristine administered weekly has antiangiogenic properties, and experimental models suggest that chemotherapy-induced neuropathy may actually be due to damage to the peripheral nerve microvasculature or vasa vasorum [14, 15].
Preliminary laboratory and human data has suggested that glutamine may have a promising role in the prophylaxis and treatment of chemotherapy-induced neuropathy thereby enabling patients to receive optimal doses and frequency of anticancer agents [16–20]. While glutamine’s specific role as a neuroprotectant is unknown, one proposed mechanism of action postulated has been related to circulating nerve growth factor (NGF) levels. Pilot studies have demonstrated a correlation between the severity of chemotherapy-induced neuropathy and declining levels of NGF in patients undergoing chemotherapy with various neurotoxic agents [21]. Additionally, glutamine has been shown to upregulate NGF mRNA in an animal model [22]. Another attractive hypothesis suggests that glutamine enhances microtube formation and/or stability [17, 23]. The low toxicity profile of glutamine per Micromedex and its suggested lack of interference with cytotoxic drugs make it an attractive supportive care agent in anticancer protocols. The primary aim of this study was to describe the incidence of VIPN in pediatric patients with cancer utilizing both standard-of-care and simple motor skill testing borrowed from neuropsychological assessment batteries. The secondary aims included (a) assessing the safety of glutamine in the pediatric oncology population, (b) investigating the efficacy of glutamine in preventing the progression and/or promoting resolution of VIPN, (c) exploring the effect of glutamine on measures of quality of life in children undergoing anticancer therapy, and (d) preliminarily examining the association of neuropathy with serum biomarkers (nerve growth factor and vascular endothelial growth factor).
Method
We conducted a longitudinal observation study, which included a double-blind, randomized, placebo-controlled intervention to investigate the efficacy of glutamine in the treatment of VIPN in children with cancer. All patients between the age of 5 and 21 who were diagnosed with leukemia or solid tumors and expected to receive a cumulative dose of 6 mg/m2 of vincristine over a 30-week period (or >6 mg/m2 if individual vincristine doses were capped at 2 mg) according to their primary cancer treatment protocol were eligible. Patients with CNS tumors other than medulloblastoma, focal neurologic findings, CNS metastasis, recurrent disease, already exposed to >8 mg/m2 of vincristine, or with ≥grade 2 neurologic toxicity by the National Cancer Institute-Common Toxicity Criteria, version 3 (NCI-CTCv3) [24], were excluded.
Subjects were consented according to Columbia University Institutional Review Board-approved guidelines, and all human investigations and interventions were in accordance with an assurance filed and approved by the U.S. Department of Health and Human Services. Following study enrollment and prior to the next initiation of an intensive vincristine-containing course, baseline assessment of the subject’s neurological status (Time 0) was performed with clinical exam and history, as well as a focused battery of neuropsychological tests intended to screen for motor functioning. Motor and sensory neuropathies were graded using the NCI-CTCv3 that included the assessment of limb functioning, including deep tendon reflexes and gait observation. (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf). The neuropsychological tests selected were the Purdue Pegboard (PPB) (fine motor), grip strength (GS) (motor strength), and the Symbol Digit (SD) Test (psychomotor) [25–27]. These assessments were performed by a psychologist and required about 10 min of the participant’s time. Subjects’ neurological functioning was monitored every 3 weeks (± 7 days) for the onset of neuropathy.
Peripheral neurologic toxicity was defined as either an increase in one clinical toxicity grade by the NCI-CTCv3 on physical exam or one-half standard deviation (SD) reduction from the subject’s baseline score on any two of the three borrowed neuropsychological instruments that measure motor functioning, representing a clinically meaningful decline. Interval assessments also included a measure of quality of life (QoL) using the PedsQL 4.0 parent-report and self-report versions to monitor their physical, emotional, social, and school QoL [28, 29]. The data from the neuropsychological instruments were scored utilizing age-based norms that yielded standardized z-scores, with a mean of 0 and an SD of 1, while the QoL measure yields scores ranging from 0 to 100, and higher scores represent better functioning across all measures administered.
Following identification of study defined peripheral neuropathy, the subjects were randomized at day 0 (Time 1) to receive either glutamine or placebo (L-glycine) at a dose of 6 g/m2 twice daily (up to a maximum of 10 g/dose) for 21 days (Time 1). Glutamine and the placebo were similar in texture, aroma, and taste (Thorne Research Inc., Dover, Idaho) and were dispensed by the research pharmacy in a powder formulation to be mixed in juice for oral administration. Only the research pharmacy and a single study coordinator were unblinded. Subject families were contacted once weekly during the supplementation period to answer questions designed to monitor compliance and adverse effects, while patient and/or parent completed a daily compliance diary. When possible, pre- and post-intervention study medication jar weights were used to corroborate self-reported compliance. Subjects were asked to return for a complete study assessment visit following the initial 21-day supplementation period (Time 2) and after an additional 21-day “wash out” period on day 42 (Time 3) at which point the study concluded. Blood specimens were collected at baseline study entry (Time 0), and then at each study visit following identification of VIPN (times 1–3) for analysis of serum glutamine, nerve growth factor (NGF), and vascular endothelial growth factor (VEGF). The specimen collection and methodology has been previously reported [18].
Statistical analysis
Descriptive statistics for both the glutamine and the placebo groups were used to summarize demographic information and diagnosis. The two groups were compared on these variables by using the independent two-sample t test for continuous variables and the chi-square test for independence for categorical variables (or Fisher’s exact test for sparse data).
The main analysis compared the glutamine and placebo group on motor and sensory neuropathy scores from the NCI CTC v3, neuropsychological assessment scores (i.e., Purdue Pegboard Test, Symbol Digit Modalities Test, grip strength test), and QoL assessment scores (i.e., PedsQL) at the three time points.
To compare the motor and sensory neuropathy scores between the glutamine and the placebo group at each time point, the Wilcoxon-Mann-Whitney test was performed. Ordinal logistic regression analysis was used to assess the association between the change in motor or sensory neuropathy scores and the use of glutamine at the three time points. One-way analysis of variance (ANOVA) was used to compare the groups on mean change in QoL test scores between time points. A two-sample t test was used to compare mean VEGF and NGF levels by group at each time point. The analyses were conducted with SPSS version 20.0 software (SPSS IBM, Armonk, NY). A p value of .05 or less was considered to be statistically significant.
Results
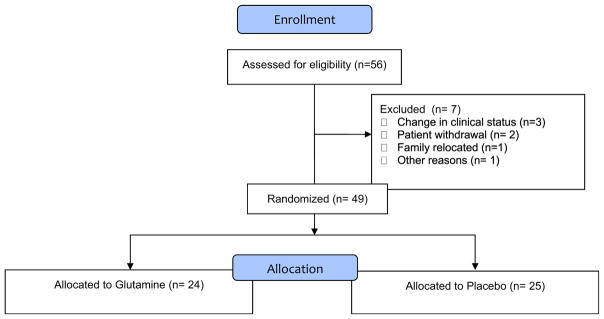
Fifty-six patients were enrolled, of which 49 were evaluable. The CONSORT diagram is presented in Fig. 1. Reasons for removal from study after randomization were change in clinical status (N = 3), family withdrawal (N = 2), family relocation (N = 1), and other (N = 1). Table 1 presents the demographics of the study participants. No significant difference between the placebo and control group were observed for any of the demographic variables.
Fig. 1.
CONSORT
Table 1.
Demographics by glutamine and placebo group
| Characteristics | Allb (n = 56) | Glutamine (n = 24) | Placebo (n = 25) | p |
|---|---|---|---|---|
| Gender | NS | |||
| Female | 29 | 14 | 12 | |
| Male | 27 | 10 | 13 | |
| Age (years)a | NS | |||
| Median | 11.0 | 11.0 | 10.0 | |
| Range | 4.0–19.0 | 5.0–19.0 | 4.0–17.0 | |
| Race | NS | |||
| White | 15 | 6 | 8 | |
| Black | 7 | 2 | 4 | |
| Hispanic | 30 | 15 | 12 | |
| Other | 4 | 1 | 1 | |
| Diagnosis | NS | |||
| ALL | 33 | 13 | 16 | |
| Non-Hodgkin Lymphoma | 2 | 0 | 1 | |
| Lymphoma | 1 | 1 | 0 | |
| Ewing Sarcoma | 3 | 2 | 1 | |
| Wilms’ Tumor | 5 | 1 | 3 | |
| Rhadomyosarcoma | 6 | 4 | 2 | |
| Other | 6 | 3 | 2 |
NS not statistically significant at 0.05 level
Age at date of consent
Including seven patients who were removed from study prior to randomization or completion of the 3 time points
Of the 49 evaluable patients treated with >6 mg/m2 vincristine within a 30-week time frame, 78 % developed peripheral neuropathy as defined by CTCv3 and 100 % by the study criteria which included the assessment of motor functioning. The mean cumulative vincristine dose received at randomization was not significantly different between the glutamine and placebo groups (11.1 mg ± 4.6 vs. 9.5 mg ± 3.8, respectively). Interestingly, 26 (53 %) of the neuropathy classifications were by clinical history and examination alone using the NCI-CTCv3, 11 (22 %) by battery testing alone, and 12 (25 %) by both examinations. There were no significant correlations between the score on the NCI-CTCv3 and any testing instrument at the time of identification of peripheral neuropathy (time 1), suggesting that these measure different aspects of sensory and motor functioning. While no single neuropsychological testing measure emerged as most sensitive to detecting change over time, using both hands simultaneously to complete the Purdue Pegboard test demonstrated a low correlation with the sensory neuropathy scores (r = −0.30) at time 2. Additionally, no demographic variable appeared to influence the development of neuropathy.
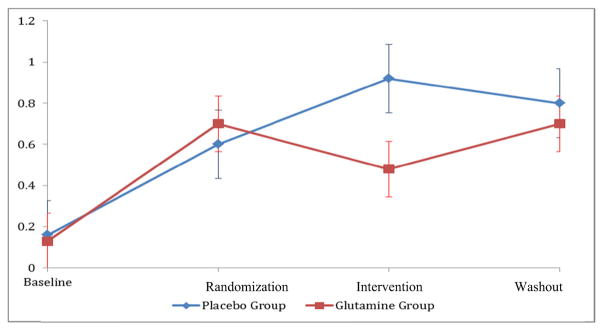
As per clinical exam using the NCI-CTCv3, a statistically higher number of children progressed on the sensory neuropathy scale in the placebo compared to the glutamine group (p = 0.02) from time 1 to time 2 (Tables 2 and 3) based upon an increase in their NCI-CTCv3 score. Applying ordinal logistic regression, we continued to observe a significant change in sensory neuropathy scores, measured by the NCI-CTCv3 that was significantly associated with the use of glutamine between time 1 and time 2. The glutamine group had 3.48 times higher odds than did the placebo group to see a drop in the sensory neuropathy score going from time 1 to time 2 (p = 0.03) (Tables 4 and 5). Following the 21-day wash-out period (time 3), no significant difference between the two groups was observed as the groups converged more closely together. When we evaluated neuropathy by NCI-CTCv3 results, we observed a difference in sensory neuropathy scores, but not on motor neuropathy scores, in the glutamine group on clinical assessment as compared with the placebo group (Fig. 2, Tables 2, 3, 4, and 5). Serial battery testing data demonstrated no significant differences between the two groups at any time point post-randomization as defined by a 0.5 SD decline on any two of the assessment measures.
Table 2.
Sensory neuropathy scores by group by time
| Groups | Sensory neuropathy score | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 0 | 1 | 2 | 3 | 4 | 5 | p valuea | |
| Time 1 (day 0) | |||||||
| Glutamine | 8 | 15 | 1 | 0 | 0 | 0 | 0.52 |
| Placebo | 10 | 15 | 0 | 0 | 0 | 0 | |
| Time 2 (day 21) | |||||||
| Glutamine | 13 | 10 | 1 | 0 | 0 | 0 | 0.02 |
| Placebo | 6 | 15 | 4 | 0 | 0 | 0 | |
| Time 3 (day 42) | |||||||
| Glutamine | 12 | 9 | 2 | 1 | 0 | 0 | 0.50 |
| Placebo | 10 | 11 | 3 | 1 | 0 | 0 | |
Estimated using the Wilcoxon-Mann-Whitney test
Table 3.
Motor neuropathy scores by group by time
| Groups | Motor neuropathy score | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 0 | 1 | 2 | 3 | 4 | 5 | p valuea | |
| Time 1 (day 0) | |||||||
| Glutamine | 19 | 4 | 1 | 0 | 0 | 0 | 0.198 |
| Placebo | 16 | 5 | 4 | 0 | 0 | 0 | |
| Time 2 (day 21) | |||||||
| Glutamine | 17 | 5 | 2 | 0 | 0 | 0 | 0.559 |
| Placebo | 20 | 2 | 3 | 0 | 0 | 0 | |
| Time 3 (day 42) | |||||||
| Glutamine | 17 | 5 | 1 | 1 | 0 | 0 | 0.477 |
| Placebo | 16 | 3 | 6 | 0 | 0 | 0 | |
Estimated using the Wilcoxon-Mann-Whitney test
Table 4.
Change in sensory neuropathy scores by group
| Groups | Decrease | No change | Increase | Sig.a |
|---|---|---|---|---|
| Time 1 (day 0) vs time 2 (day 21) | ||||
| Glutamine | 9 | 11 | 4 | p = .03 |
| Placebo | 3 | 13 | 9 | |
| Total | 12 | 24 | 13 | |
| Time 2 (day 21) vs time 3 (day 42) | ||||
| Glutamine | 6 | 9 | 9 | NS |
| Placebo | 6 | 16 | 3 | |
| Total | 12 | 25 | 12 | |
| Time 1 (day 0) vs time 3 (day 42) | ||||
| Glutamine | 6 | 13 | 5 | NS |
| Placebo | 4 | 14 | 7 | |
| Total | 10 | 27 | 12 | |
Estimated using ordinal logistic regression
Table 5.
Change in motor neuropathy scores by group
| Groups | Decrease | No change | Increase | Sig.a |
|---|---|---|---|---|
| Time 1 (day 0) vs time 2 (day 21) | ||||
| Glutamine | 3 | 16 | 5 | NS |
| Placebo | 6 | 16 | 3 | |
| Total | 9 | 32 | 8 | |
| Time 2 (day 21) vs time 3 (day 42) | ||||
| Glutamine | 4 | 16 | 4 | NS |
| Placebo | 1 | 18 | 6 | |
| Total | 5 | 34 | 10 | |
| Time 1 (day 0) vs time 3 (day 42) | ||||
| Glutamine | 4 | 14 | 0 | NS |
| Placebo | 6 | 11 | 8 | |
| Total | 10 | 25 | 8 | |
Estimated using ordinal logistic regression
Fig. 2.
Comparison of sensory neuropathy exam scores
Being in the glutamine group was also associated with a significant difference in self-reported, but not parent-reported, overall quality of life over the previous 1 month following supplementation. A significant group effect on the PedsQL 4.0 total QoL change score between time 2 (day 21) and time 3 (day 42). Specifically, children in the glutamine group scored 8.42 points higher than did the placebo group on PedsQL total QoL summary score at time 3 (looking back over the previous 1 month), when compared with time 2 [F(1, 40) = 5.03, p = 0.03].
Compliance and side effects
Compliance with the administration of glutamine and placebo was similar between the two groups with 82.5 % (range 10–100) and 76.2 % (range 11–100) of the prescribed doses administered, respectively (NS). Glutamine was well tolerated with only mild patient-reported side effects in 2 % of the patients while on the supplementation with resolution of most symptoms upon discontinuation (Table 6). No significant difference in adverse effects was observed with glutamine compared to placebo.
Table 6.
Side effects by glutamine and placebo group
| Side effects | Week 1 Day 0–day 7 |
Week 2 Day 8–day 14 |
Week 3 Day 15–day 21 |
p value | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Glutamine (n = 23) | Placebo (n | = 25) Glutamine (n = 22) | Placebo (n | = 25) Glutamine (n | = 23) Placebo (n = 24) | ||
| None | 16 | 20 | 20 | 22 | 19 | 23 | NS |
| Diarrhea | 0 | 0 | 0 | 0 | 0 | 0 | NS |
| Nausea | 2 | 1 | 1 | 0 | 1 | 0 | NS |
| Vomiting | 0 | 0 | 1 | 0 | 0 | 0 | NS |
| Constipation | 0 | 0 | 0 | 0 | 0 | 0 | NS |
| Other | 1 | 3 | 0 | 2 | 1 | 0 | NS |
| Decreased appetite | 0 | 0 | 0 | 0 | 0 | 0 | NS |
| Headaches | 0 | 1 | 0 | 1 | 0 | 0 | NS |
| Loose bowels | 1 | 0 | 0 | 0 | 0 | 1 | NS |
| Gas | 1 | 0 | 0 | 0 | 0 | 0 | NS |
| Stomach ache | 2 | 0 | 1 | 0 | 0 | 0 | NS |
| Soft stools | 0 | 0 | 0 | 0 | 0 | 0 | NS |
| Bad taste | 1 | 0 | 0 | 0 | 2 | 0 | NS |
NS not significant at 0.05 level
Biological markers of neuropathy
Analysis of serum glutamine, NGF, and VEGF at each of the designated time points did not reveal significant associations between candidate serum biomarkers and the development of VIPN. This suggests that the effect of glutamine on sensory neuropathy was not mediated through NGF or VEGF. Analysis of these serum biomarkers by diagnosis did not alter the findings (data not shown).
Conclusion
To the authors’ knowledge, we present the results of the first clinical study that describes the incidence of VIPN in children and adolescents with cancer utilizing both clinical and non-invasive objective motor assessment. Using only CTCv3 criteria, 78 % of the patients developed evidence of neuropathy; using both measures, the incidence of detectable neuropathy was 100 %. Our findings are consistent with documentation of early sensory electrophysiological abnormalities in 82 % of children in the midst of receiving four to eight doses of vincristine dose for ALL [30], and the finding that 100 % of children ALL and solid tumors had a score exceeding zero on a pediatric modified total neuropathy score (Ped-mTNS) [5, 6], or in the total neuropathy score (TNSr) for ALL survivors, on average 7.4 years post-treatment [10]. Our study adds to the body of literature highlighting the under-recognized prevalence of VIPN and underscores the need for effective interventions that either prevent or mitigate the effects of vincristine on the peripheral nervous system.
While the primary endpoint was measured using the NCI CTC v3, as an exploratory aim, we also evaluated the utility of selected neuropsychological assessments to screen for motor functioning abnormalities; however, the CTC served as the primary benchmark for describing incidence of neuropathy. The finding that 22 % of our sample met criteria by battery testing alone suggests that the recognition of developing peripheral neuropathy symptoms among a proportion of patients may not be fully documented with the reliance upon history and physical exam, graded by a singular form of evaluation such as the NCI-CTC or Balis Scales [31–33]. It is important to point out the variability among assessment measures and questionnaires available at the time of study initiation, warranting the need for further research to identify measures sufficiently sensitive and specific to identify peripheral neuropathy among this population. It is therefore possible that the brief 10-min motor screening assessments provided a more structured and objective indication of subclinical evidence of vincristine-induced peripheral neuropathy, which could provide additional information to the clinician for proactive intervention. These assessments offer an alternative to other combined subjective and objective measures of nerve function such as the total neuropathy score-pediatric vincristine (TNS-PV) [32] and the Ped-mTNS [5], recently developed and validated for use in children aged 5 and older as sensitive indicators of functionality. It is important to consider using these more clinimetric scales in future studies to extend our preliminary results. It is also notable that in a recent multi-centered comparative reliability and validation study of the TNS-PV in patients with acute lymphocytic leukemia, the TNS-PV and the NCI-CTC sensory scale used in our study were found to be the most responsive to changes in peripheral neuropathy over time [32]. Consequently, based on our results, it is highly recommended that patients anticipated to be exposed to >6 mg/m2 VCR be proactively and serially screened for symptoms of peripheral neuropathy utilizing neurological and self-report measures and neuropsychological screening tools when possible to ensure an accurate clinical assessment.
In addition, we found that glutamine may provide a protective effect for sensory, but not motor vincristine-induced neuropathic signs and symptoms, evidenced by change in both clinical and screening assessments. This result stands in contrast to a recent multi-center trial performed in 250 children with cancer which did not find a beneficial effect of glutamic acid for the prevention of vincristine-induced neuropathy [34]. There are several possible reasons for these disparate results. First, this was a single-institution study with a small sample that was assessed and monitored for compliance by a consistent study team. Additionally, our study was not restricted to patients undergoing treatment for leukemia. Consequently, in the Children’s Oncology Group trial, corticosteroid-induced myopathy symptoms may have obscured a benefit of glutamic acid for vincristine-related neuropathy.
However, it is also plausible that the difference between the results of the two studies relates to either the form of the amino acid or dosing utilized. The dose used in the current study was determined by prior adult studies that also described a significant beneficial effect of glutamine on paclitaxel-induced peripheral neuropathy [18]. Finally, the longitudinal effect of supplementation with glutamine on patient-reported QoL should not be overlooked, as these findings are discrete from clinician-derived data and may have greater relevance when managing the acute- and late-effects of treatment for childhood cancer. The lack of observed benefit of glutamine for motor neuropathy may be related to the insensitivity of physical exam in detecting improvements in lower-extremity motor function, especially as lower limb function generally shows more impairment related to VCR use than do upper limb function.
Unfortunately, changes in the candidate biomarkers NGF and VEGF did not correlate with the development or degree of neuropathy, or the apparent benefit of glutamine administration. Recently, genome-wide association studies have identified a single nucleotide genetic polymorphism (T) in the promoter region of the CEP72 gene that is significantly associated with the development of NCI-CTC grade 2 or higher VIPN. CEP72 encodes a centrosomal protein critical for microtubule organization. The homozygous risk allele (TT versus CC/TC) correlates with lower expression of CEP72 and increased sensitivity to vincristine toxicity in patients and cytotoxicity in relevant preclinical in vitro models of normal neuronal tissues and leukemia [8]. While these results illuminate the direct axonal damaging effects of vincristine and offer the important potential to personalize dosing in order to mitigate severity of neuropathy, further studies will be required to determine whether such an approach actually compromises antitumor efficacy. Along a similar line of reasoning, Egbelakin et al. previously performed a pharmacogenomic analysis of the cytochrome P450 CYP3A5 genotype in children with ALL [35]. Active CYP3A5 enzyme expressers experienced less VIPN than did non-expressers, who consequently required more dose reductions or omissions but also demonstrated faster vincristine metabolism and clearance, potentially resulting in lower overall active drug exposure. While such genetic predisposition to toxicity may lead to better understanding of the mechanisms underlying VIPN and help identify patients at risk, supportive care strategies to ameliorate neuropathic symptoms that appear intrinsic to the cytotoxic action of the agent will still be required.
Despite the limitations, the results from this pilot study remain encouraging given the excellent compliance with administration of the glutamine and placebo, as well as the uniform assessments that included both physician exam and non-invasive screening in addition to self- and parent-report, at systematic time points. These preliminary results require collaborative studies to confirm the efficacy and safety of glutamine with a larger sample of pediatric cancer patients that utilize discrete measures of sensory and motor functioning to more clearly delineate the specific symptoms and risk factors associated with acute VIPN and incorporate long-term disease-related survival patient-reported outcomes. Additionally, future work should consider the inclusion of mechanistic studies to further our understanding of why glutamine may impart a beneficial effect on sensory neuropathy. The potential benefits of this and future interventions for chemotherapy-induced peripheral neuropathy are of paramount importance to pediatric cancer as survivors seek to resume their daily activities at the level commensurate with their pre-diagnosis abilities and overall level of functioning.
Supplementary Material
Acknowledgments
The authors would like to thank the families, nurses, and research staff who participated in this trial. We would like to recognize Gabrielle’s Angel Foundation for Cancer Research and the Tamarind Foundation for supporting this research.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Gidding CEM, Kellie SJ, Kamps WA, de Graaf SSN. Vincristine revisited. Crit Rev Oncol Hematol. 1999;29:267–287. doi: 10.1016/s1040-8428(98)00023-7. [DOI] [PubMed] [Google Scholar]
- 2.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 3.Legha SS. Vincristine neurotoxicity. Pathophysiology and management. Medical toxicology. 1986;1:421–427. doi: 10.1007/BF03259853. [DOI] [PubMed] [Google Scholar]
- 4.Smith EML, Cohen JA, Pett MA, Beck SL. The reliability and validity of a modified Total neuropathy score-reduced and neuropathic pain severity items when used to measure chemotherapy-induced peripheral neuropathy in patients receiving Taxanes and Platinums. Cancer Nurs. 2010;33:173–183. doi: 10.1097/NCC.0b013e3181c989a3. [DOI] [PubMed] [Google Scholar]
- 5.Gilchrist LS, Tanner L. The pediatric-modified total neuropathy score: a reliable and valid measure of chemotherapy-induced peripheral neuropathy in children with non-CNS cancers. Support Care Cancer: Off J Multinatl Assoc Support Care Cancer. 2013;21:847–856. doi: 10.1007/s00520-012-1591-8. [DOI] [PubMed] [Google Scholar]
- 6.Gilchrist LS, Marais L, Tanner L. Comparison of two chemotherapy-induced peripheral neuropathy measurement approaches in children. Support Care Cancer : Off J Multinatl Assoc Support Care Cancer. 2014;22:359–366. doi: 10.1007/s00520-013-1981-6. [DOI] [PubMed] [Google Scholar]
- 7.Vasquez S, Guidon M, McHugh E, Lennon O, Grogan L, Breathnach OS. Chemotherapy induced peripheral neuropathy: the modified total neuropathy score in clinical practice. Ir J Med Sci. 2014;183:53–58. doi: 10.1007/s11845-013-0971-5. [DOI] [PubMed] [Google Scholar]
- 8.Diouf B, Crews KR, Lew G, et al. Association of an inherited genetic variant with vincristine-related peripheral neuropathy in children with acute lymphoblastic leukemia. JAMA. 2015;313:815–823. doi: 10.1001/jama.2015.0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett MI, Rayment C, Hjermstad M, Aass N, Caraceni A, Kaasa S. Prevalence and aetiology of neuropathic pain in cancer patients: a systematic review. Pain. 2012;153:359–365. doi: 10.1016/j.pain.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 10.Ramchandren S, Leonard M, Mody RJ, et al. Peripheral neuropathy in survivors of childhood acute lymphoblastic leukemia. J Peripher Nerv Syst: JPNS. 2009;14:184–189. doi: 10.1111/j.1529-8027.2009.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain P, Gulati S, Seth R, Bakhshi S, Toteja GS, Pandey RM. Vincristine-induced neuropathy in childhood ALL (acute lymphoblastic leukemia) survivors: prevalence and electrophysiological characteristics. J Child Neurol. 2014;29:932–937. doi: 10.1177/0883073813491829. [DOI] [PubMed] [Google Scholar]
- 12.Ness KK, Jones KE, Smith WA, et al. Chemotherapy-related neuropathic symptoms and functional impairment in adult survivors of extracranial solid tumors of childhood: results from the St. Jude lifetime cohort study. Arch Phys Med Rehab. 2013;94:1451–1457. doi: 10.1016/j.apmr.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fehrenbacher JC. Chemotherapy-induced peripheral neuropathy. In: Theodore JP, Gregory D, editors. Progress in molecular biology and translational science. New York: Academic Press; 2015. pp. 471–508. [DOI] [PubMed] [Google Scholar]
- 14.Kirchmair R, Tietz AB, Panagiotou E, et al. Therapeutic angiogenesis inhibits or rescues chemotherapy-induced peripheral neuropathy: taxol- and thalidomide-induced injury of vasa nervorum is ameliorated by VEGF. Molecular therapy. J Am Soc Gene Ther. 2007;15:69–75. doi: 10.1038/sj.mt.6300019. [DOI] [PubMed] [Google Scholar]
- 15.Kirchmair R, Walter DH, Ii M, et al. Antiangiogenesis mediates cisplatin-induced peripheral neuropathy: attenuation or reversal by local vascular endothelial growth factor gene therapy without augmenting tumor growth. Circulation. 2005;111:2662–2670. doi: 10.1161/CIRCULATIONAHA.104.470849. [DOI] [PubMed] [Google Scholar]
- 16.Jackson DV, Jr, Rosenbaum DL, Carlisle LJ, Long TR, Wells HB, Spurr CL. Glutamic acid modification of vincristine toxicity. Cancer Biochem Biophys. 1984;7:245–252. [PubMed] [Google Scholar]
- 17.Boyle FM, Wheeler HR, Shenfield GM. Glutamate ameliorates experimental vincristine neuropathy. J Pharmacol Exp Ther. 1996;279:410–415. [PubMed] [Google Scholar]
- 18.Vahdat L, Papadopoulos K, Lange D, et al. Reduction of paclitaxel-induced peripheral neuropathy with glutamine. Clin Cancer Res: Off J Am Assoc Cancer Res. 2001;7:1192–1197. [PubMed] [Google Scholar]
- 19.Stubblefield MD, Vahdat LT, Balmaceda CM, Troxel AB, Hesdorffer CS, Gooch CL. Glutamine as a neuroprotective agent in high-dose paclitaxel-induced peripheral neuropathy: a clinical and electrophysiologic study. Clin Oncol. 2005;17:271–276. doi: 10.1016/j.clon.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Wang WS, Lin JK, Lin TC, et al. Oral glutamine is effective for preventing oxaliplatin-induced neuropathy in colorectal cancer patients. Oncologist. 2007;12:312–319. doi: 10.1634/theoncologist.12-3-312. [DOI] [PubMed] [Google Scholar]
- 21.De Santis S, Pace A, Bove L, et al. Patients treated with antitumor drugs displaying neurological deficits are characterized by a low circulating level of nerve growth factor. Clin Cancer Res: Off J Am Assoc Cancer Res. 2000;6:90–95. [PubMed] [Google Scholar]
- 22.Gwag BJ, Sessler FM, Robine V, Springer JE. Endogenous glutamate levels regulate nerve growth factor mRNA expression in the rat dentate gyrus. Mol Cells. 1997;7:425–430. [PubMed] [Google Scholar]
- 23.Boyle FM, Wheeler HR, Shenfield GM. Amelioration of experimental cisplatin and paclitaxel neuropathy with glutamate. J Neuro-Oncol. 1999;41:107–116. doi: 10.1023/a:1006124917643. [DOI] [PubMed] [Google Scholar]
- 24.DCTD N, NIH, DHHS, editor. Cancer therapy evaluation program common terminology criteria for adverse events, version 3.0. 2003 Mar 31; ed. ( http://ctep.cancer.gov) Publish Date: August 9, 2006.
- 25.Smith A. Symbol digit modalities test. Western Psychological Corporation; Los Angeles: 1991. [Google Scholar]
- 26.Reddon JR, Gill DM, Gauk SE, Maerz MD. Purdue Pegboard: test-retest estimates. Percept Mot Skills. 1988;66:503–506. doi: 10.2466/pms.1988.66.2.503. [DOI] [PubMed] [Google Scholar]
- 27.Reitan R, Davison L. Clinical neuropsychology: current status and applications. Washington, D.C: V.H. Winston; 1974. [Google Scholar]
- 28.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the pediatric quality of life inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr: Off J Ambul Pediatr Assoc. 2003;3:329–341. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 30.Reinders-Messelink HA, Van Weerden TW, Fock JM, et al. Mild axonal neuropathy of children during treatment for acute lymphoblastic leukaemia. Eur J Paediatr Neurol: EJPN: Off J Eur Paediatr Neurol Soc. 2000;4:225–233. doi: 10.1053/ejpn.1999.0310. [DOI] [PubMed] [Google Scholar]
- 31.Cornblath DR, Chaudhry V, Carter K, et al. Total neuropathy score: validation and reliability study. Neurology. 1999;53:1660–1664. doi: 10.1212/wnl.53.8.1660. [DOI] [PubMed] [Google Scholar]
- 32.Lavoie Smith EM, Li L, Hutchinson RJ, et al. Measuring vincristine-induced peripheral neuropathy in children with acute lymphoblastic leukemia. Cancer Nurs. 2013;36:E49–E60. doi: 10.1097/NCC.0b013e318299ad23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lavoie Smith EM, Li L, Chiang C, et al. Patterns and severity of vincristine-induced peripheral neuropathy in children with acute lymphoblastic leukemia. J Peripher Nerv Syst. 2015;20(1):37–46. doi: 10.1111/jns.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egbelakin A, Ferguson MJ, MacGill EA, et al. Increased risk of vincristine neurotoxicity associated with low CYP3A5 expression genotype in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2011;56(3):361–367. doi: 10.1002/pbc.22845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradfield SM, Sandler E, Geller T, Tamura RN, Krischer JP. Glutamic acid not beneficial for the prevention of vincristine neurotoxicity in children with cancer. Pediatr Blood Cancer. 2015;62:1004–1010. doi: 10.1002/pbc.25384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.