Abstract
Objective:
Compare reconstruction outcomes for various lateral skull base closure techniques.
Study Design:
Retrospective medical records review.
Setting:
University-based tertiary referral center.
Patients:
Patients who underwent resections of tumors involving the lateral skull base requiring reconstruction beyond primary closure.
Intervention(s):
Reconstructive techniques, from rotational flaps to free tissue transfer.
Main Outcome Measure(s):
Outcome data including wound complications, cerebrospinal fluid (CSF) leakage, and need for surgical revision were tabulated.
Results:
Eighty-six patients underwent lateral skull base tumor resection and reconstruction. Procedures were primarily lateral temporal bone resections but also included subtotal temporal bone, total temporal bone, and infratemporal fossa resections. Cutaneous malignancy was the most common resection indication (83%) and the temporalis rotational flap was the most commonly employed reconstructive option (30%). When free tissue transfer techniques were used, the radial forearm, anterolateral thigh, and latissimus dorsi were the most frequent donor sites. Patients with T2 disease were more likely to undergo temporalis flaps, whereas patients with T4 disease were more likely to undergo free flap reconstruction. Major complications were uncommon (~8%), the most frequent being stroke (~3%). The postoperative wound complication rate was approximately 45%. The majority involved minor dehiscences and were managed conservatively. Patients with T4 disease were more likely to have wound complications (p < 0.05). Radial forearm free flaps were less likely to have wound complications when compared with other reconstruction techniques (p < 0.05).
Conclusions:
Many factors go into planning lateral skull base reconstruction. Free flaps were more often used for T4 disease. Radial forearm free flaps tended to have lower wound complication rates when compared with other techniques.
Keywords: Lateral skull base, Lateral skull base resection, Reconstruction outcomes, Skull base reconstruction, Temporal bone
Resection of tumors of the lateral skull base often results in defects that are not amenable to primary closure. There are numerous techniques for defect reconstruction including local flaps, pedicled flaps, and free tissue transfer (1–3). Reconstructions must be able to fill the soft-tissue/bony defect, replace cutaneous tissue, provide watertight closure in cases of dural violation, and be composed of well-vascularized tissue (1,4). Management algorithms have been proposed looking at factors such as defect size, extent of disease, cerebrospinal fluid (CSF) leakage, and tissue irradiation; however, there are no universally accepted guidelines for lateral skull base reconstruction (5,6).
Reconstructing the lateral skull base entails specific challenges compared with other regions of the head and neck. When using regional pedicled flaps, the distance from the donor site to skull base defect can reach the upper limits of reliable flap length. For example, the upper limit of the pectoralis major myocutaneous pedicled flap is generally considered to be the external auditory canal; however, many lateral skull base defects extend significantly above this level, limiting the usefulness of this flap (1,3,7). Another frequent issue with lateral skull base resections is the potential for CSF leakage (4). Though it is not typically necessary to reconstruct bony skull base defects, reconstructions often must provide a watertight seal that can withstand the pressure of CSF and resist the effect of gravity.
As head and neck reconstructive surgery has evolved, there has been a trend towards the more frequent use of pedicled flaps or free tissue transfers (1,4,5,8,9). One of the major advantages of these reconstructive options is their improved resistance to wound complications compared with local or regional flaps in patients treated with previous definitive or postoperative adjuvant radiotherapy. These techniques also provide the capability to fill and span large and deep defects.
Proper selection of an effective reconstructive technique that minimizes the risk of complications and maximizes the chance of reconstructive success is an important aspect of surgical planning. Recent reports looking at complications following reconstructions of lateral skull base defects demonstrate rates ranging from 15 to 50% (1,5,6,8–11). Though there has been a trend towards lower complication rates with the increased use of free tissue transfers, a difference between specific types of reconstruction has not been shown. This study aims to compare complications among different types of flap techniques and to identify factors that influence optimal reconstruction of lateral skull base defects.
METHODS
A retrospective medical records review was performed for the period of July 2003 to April 2015 with the approval of the University of Iowa Hospitals and Clinics Biomedical Institutional Review Board.
Subjects were included in the study if they underwent lateral skull base resection (CPT codes 69,535 and 69,970), required reconstruction of the defect beyond primary closure, and had medical records available for review. Exclusion criteria included sleeve resection as the method of extirpation and/or the surgical defect was closed primarily. Data were collected and tabulated in a database using Microsoft Excel (Redmond, CA) and included basic demographic information, type of resection, oncologic staging of the index lesion(s) as documented in the medical record using the appropriate system for the site of lesion and histology (12–14), defect size, type of reconstruction, and complications including major (e.g., stroke, myocardial infarction, and death) and minor (e.g., hematoma, surgical site infection).
We did not analyze hearing rehabilitation as a function of reconstructive technique. All patients eligible for and electing to receive an osseointegrated implant were successful users and none of the reconstructive techniques prohibited use of an osseointegrated implant.
Descriptive statistics were calculated and trends were assessed using the Fisher’s exact and χ2 tests, where appropriate. Multivariable analysis was performed using the analysis of variance technique. Results were considered significant if the probability was less than 0.05. Overall survival curves were constructed using the Kaplan–Meier estimator method. All calculations were performed using functions in Microsoft Excel.
RESULTS
Eighty-six subjects who underwent 92 flap reconstructions were identified that met inclusion criteria and did not meet the exclusion criteria. Demographic data are presented in Table 1 and included 69 men and 17 women. Common comorbidities included diabetes (21%), cardiovascular disease (17%), and peripheral vascular disease (11%).
TABLE 1.
Demographics
| n | % | |
|---|---|---|
| Patients | 86 | |
| Male | 69 | 80.2 |
| Female | 17 | 19.8 |
| Age (yr) | ||
| Mean (SDa) | 67.1 (13.7) | |
| Range | 20.6–89.4 | |
| Tobacco use | ||
| History of use | 54 | 62.8 |
| Negative history | 32 | 37.2 |
| Pack-years | ||
| Mean (SDa) | 31.3 (22.3) | |
| Range | 2–100 | |
| Continued use after surgery | 13 | |
| Comorbidities | ||
| Diabetes mellitus | 18 | 20.9 |
| Cardiovascular disease | 15 | 17.4 |
| Peripheral vascular disease | 9 | 10.5 |
| Immunosuppressedb | 7 | 8.1 |
Standard deviation.
Includes subjects on chronic immunosuppressive medication (e.g., chronic prednisone usage).
Table 2 lists the oncologic and reconstructive details. The majority of subjects had a cutaneous malignancy of the periauricular skin or cartilaginous external auditory canal that involved the temporal bone requiring resection. Fifty-five subjects (64%) had previously undergone surgical treatment of their tumor, most commonly wide local excision or subtotal resection. A smaller number of subjects (29, 34%) had a history of radiation to the head and neck region, most commonly definitive radiation therapy.
TABLE 2.
Resection and reconstruction details
| n | % | n | % | ||
|---|---|---|---|---|---|
| Flap reconstructionsa | 92 | Local/regional flaps | 44 | 47.8% | |
| Subjects | 86 | Temporalis rotational flap | 28 | 30.4% | |
| Index lesion | Three with submental flaps | ||||
| Squamous cell carcinoma | 44 | 51.2% | One with sternocleidomastoid flap | ||
| Basal cell carcinoma | 25 | 29.1% | Submental flap | 7 | 7.6% |
| Meningioma | 4 | 4.7% | Cervicofacial advancement flap | 2 | 2.2% |
| Adenoid cystic | 3 | 3.5% | Sternocleidomastoid flap | 2 | 2.2% |
| Adenocarcinoma | 2 | 2.3% | Latissimus dorsi ped flap | 2 | 2.2% |
| Epithelial-myoepithelial carcinoma | 1 | 1.2% | Pectoralis Major pedicle flap | 2 | 2.2% |
| Epitheliod malignant peripheral nerve sheath tumor | 1 | 1.2% | Posterior scalp flap | 1 | 1.1% |
| Melanoma | 1 | 1.2% | |||
| Merkel cell | 1 | 1.2% | Free tissue transfer | 48 | 52.2% |
| Mucoepidermoid carcinoma | 1 | 1.2% | Radial forearm free flap | 16 | 17.4% |
| Oncocytic salivary duct neoplasm | 1 | 1.2% | Two with temporalis flaps | ||
| Paraganglioma | 1 | 1.2% | Anterolateral thigh | 14 | 15.2% |
| Undifferentiated carcinoma | 1 | 1.2% | Latissimus dorsi | 13 | 14.1% |
| Parascapularfasciocutaneous free flap | 2 | 2.2% | |||
| Laterality | Rectus abdominis free flap | 2 | 2.2% | ||
| Left | 49 | 57.0% | Lateral arm | 1 | 1.1% |
| Right | 37 | 43.0% | |||
| Previous interventions | |||||
| Resection typeb | History of radiation | 29 | 33.7% | ||
| Lateral temporal bone | 53 | 60.2% | Previous resection | 55 | 64.0% |
| Subtotal temporal bone | 20 | 22.7% | |||
| Total temporal bone | 1 | 1.1% | Postoperative adjuvant interventions | ||
| Miscellaneous | 14 | 15.9% | Radiation | 40 | 46.5% |
| Radiation + chemotherapy | 4 | 4.7% | |||
| Chemotherapy | 3 | 3.5% |
Extra reconstructions include two for recurrent disease, two for flap necrosis, and two for dehiscence.
Includes two additional resections for recurrent disease.
Fifty-one subjects had formal cancer staging available (Table 3). Locally advanced disease was common with T4 lesions being the most prevalent (35, 69%) as was advanced stage of disease (38, 75%).
TABLE 3.
Resection, reconstruction, and complication data by tumor stage
| Total | T2 | T3 | T4 | |
|---|---|---|---|---|
| Number (%) | 51 (100) | 10 (19.6) | 6(11.8) | 35 (68.6) |
| Resection (%) | ||||
| Lateral temporal bone | 29 | 10 (100) | 3 (50) | 16 (45.6) |
| Subtotal temporal bone | 17 | 0 (0) | 2 (33.3) | 15 (42.9) |
| Radical mastoidectomy | 5 | 0 (0) | 1 (16.7) | 4 (11.4) |
| Reconstruction (%) | ||||
| Local/regional | 23 (45.1) | 7 (70) | 4 (66.7) | 12 (34.3) |
| Temporalis | 18 (35.3) | 7 (70) | 2 (33.3) | 9 (25.7) |
| Submental | 2 (3.9) | 0 (0) | 2 (33.3) | 0 (0) |
| Other local | 3 (5.9) | 0 (0) | 0 (0) | 3 (8.6) |
| Free tissue transfer | 28 (54.9) | 3 (30) | 2 (33.3) | 23 (65.7) |
| Radial forearm | 8 (15.7) | 0 (0) | 1 (16.7) | 7 (20) |
| Anterolateral thigh | 8 (15.7) | 1 (10) | 1 (16.7) | 6 (17.1) |
| Latissimus dorsi | 9 (17.6) | 1 (10) | 0 (0) | 8 (22.9) |
| Other free tissue transfer | 3 (5.9) | 1 (10) | 0 (0) | 2 (5.7) |
| Complications (%) | ||||
| Major | 3 (5.9) | 0 (0) | 0 (0) | 3 (8.6) |
| Wound | 24 (47.1) | 2 (20) | 1 (16.7) | 21 (60) |
| Cerebrospinal fluid leak | 9 (17.6) | 0 (0) | 1 (16.7) | 8 (22.9) |
RESECTION/RECONSTRUCTION
The most common procedure performed was a lateral temporal bone (LTB) resection in 53 subjects (58%) followed by subtotal temporal bone resection (STTB) in 20 subjects (22%). The regional lymphatics were treated surgically when indicated based on evidence of regional extension and/or histology of the index lesion. Lymph node dissections were performed in 63 subjects (68.5%). Of those who underwent lymph node dissections, 28 of 63 (44.4%) were found to have nodal disease. Adjuvant radiotherapy was delivered to the primary site in 43 (50%) of subjects and this included the high-risk lymphatics. The flaps used for reconstruction of the lateral skull base defects are listed in Table 2. The temporalis rotational flap was the most commonly used pedicled flap (28 subjects, 30%). In addition to the temporalis flaps, three subjects had concurrent submental flaps with one subject undergoing a concurrent sternocleidomastoid (SCM) flap. Seven subjects (7.6%) underwent submental rotational flaps as their primary reconstruction technique. Other regional flaps included pedicled cervicofacial, latissimus dorsi, pectoralis major, SCM, and posterior scalp flaps. Free tissue transfer was used in 48 (52%) of operations (Table 2). The most commonly harvested donor site was the radial forearm (RFFF) (16, 17%) followed by the anterolateral thigh (ALT) (14, 15%) and latissimus dorsi (13, 14%) sites.
Defects resulting from the treatment of T2 lesions were primarily reconstructed using temporalis rotational flaps (seven of 10 subjects). Reconstruction techniques for T3 lesions included two temporalis flaps, two submental flaps, one radial forearm free flap, and one ALT free flap. The most frequent reconstruction option for T4 lesions was the temporalis rotational flap used in nine subjects though free tissue transfers were more common in aggregate: eight latissimus dorsi, seven radial forearm, and six ALT flaps. Less frequent reconstruction techniques for T4 disease included latissimus dorsi pedicled, pectoralis major pedicled, and posterior scalp flaps as well as rectus abdominis and parascapular fasciocutaneous free flaps. Though there was a trend toward using free tissue transfer with more advanced local disease, this was not statistically significant (Table 4).
TABLE 4.
Reconstruction data by stage, complications, and defect size
| T2 | T3 | T4 | p a | |
|---|---|---|---|---|
| Reconstruction type | ||||
| Local/Regional flap | 7 | 4 | 12 | 0.0712 |
| Free flap | 3 | 2 | 23 | |
| Complications | ||||
| Major | 0 | 0 | 3 | <0.01 |
| Minor | 2 | 1 | 21 | |
| CSF leak | 0 | 1 | 8 | |
| No complications | 8 | 4 | 3 | |
| Defect size | ||||
| 10–50 cm2 | 1 | 2 | 5 | 0.8764 |
| 51–100 cm2 | 1 | 1 | 7 | |
| >100 cm2 | 1 | 1 | 9 | |
| No complications | Minor | Major | ||
|
| ||||
| Reconstruction type | ||||
| Local/Regional flap | 21 | 20 | 4 | 0.9550 |
| Free flap | 21 | 21 | 5 | |
| Common free flapsb | 19 | 19 | 5 | 0.9764 |
| Common locoregionalc | 17 | 15 | 3 | |
| Uncommon | 7 | 7 | 1 | |
| Commond | 36 | 34 | 8 | 0.8802 |
| Uncommon | 6 | 7 | 1 | |
| Radial forearm | 15 | 1 | 0 | <0.01 |
| Anterolateral thigh | 4 | 11 | 1 | |
| Latissimus dorsi | 4 | 7 | 4 | |
| 10–50 cm2 | 51–100 cm2 | >100 cm2 | ||
|
| ||||
| Complications | ||||
| Major | 2 | 1 | 1 | 0.3830 |
| Wound | 6 | 7 | 11 | |
| CSF leak | 1 | 2 | 5 | |
| No complications | 13 | 7 | 7 | |
| Wound | 6 | 7 | 11 | 0.1918 |
| No complications | 13 | 7 | 7 | |
| Reconstruction type | ||||
| Local/Regional flap | 11 | 3 | 2 | <0.01 |
| Free flap | 8 | 11 | 16 | |
χ2 test.
Radial forearm, anterolateral thigh, and latissimus dorsi free tissue donor site.
Temporalis and submental island flaps donor sites.
Common free flaps and common locoregional flaps.
CSF indicates cerebrospinal fluid.
Reconstructive methods by preoperative and postoperative radiation status as well as concurrent lymph node dissection were examined (Supplemental Digital Content 1, http://links.lww.com/MAO/A473). There was no statistically significant difference in the flaps used based on previous treatment with radiation. Patients who underwent adjuvant radiation therapy were more likely to have undergone a free tissue transfer than a locoregional reconstruction. Patients who underwent lymph node dissection at the time of resection were also more likely to undergo free tissue transfer.
Figure 1 presents the reconstructed defect size for the most commonly employed flaps Smaller defects were reconstructed using submental island flaps, temporalis rotational flaps, and radial forearm free flaps while ALT and latissimus dorsi free tissue transfers were used for larger defects. Larger defects were more likely to be reconstructed using free tissue transfer compared with locoregional flaps (Table 4). There was no significant correlation between defect size and stage of disease.
FIG. 1.
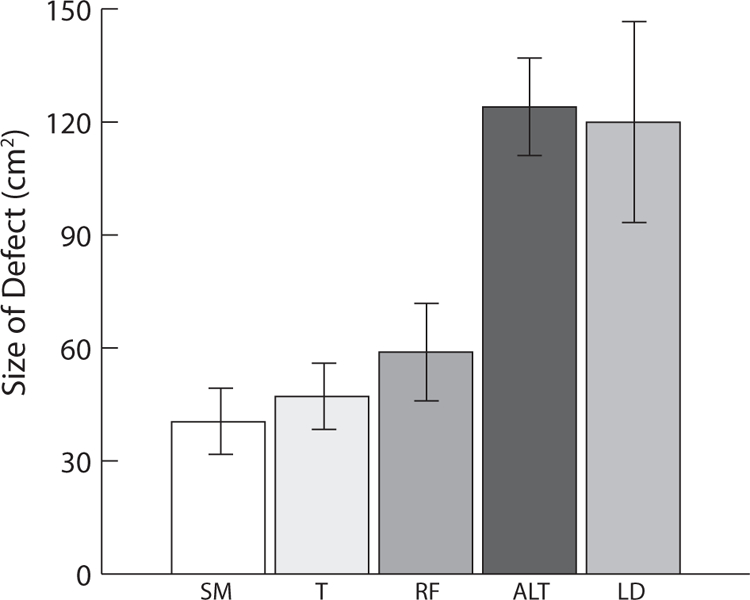
Reconstruction type by defect size. ALT indicates anterolateral thigh; LD, latissimus dorsi; RF, radial forearm; SM, submental; T, temporalis.
Facial nerve involvement was present in 41 subjects (48%). Of these, 63% had frank facial nerve involvement and 37% were found to have perineural invasion in final pathologic analysis. Thirty-eight subjects underwent sacrifice of the facial nerve. Of subjects with sacrificed facial nerves, 27 (71%) underwent procedures for facial reanimation including nerve grafting (11 sural, four great auricular, four lateral antebrachial cutaneous, and one vastus lateralis nerve graft), fascial slings (six subjects), and one primary neurorraphy.
COMPLICATIONS
Table 5 presents complication data. There were nine major complications identified in seven subjects (7.6%). The most common was stroke, which occurred in three subjects and resulted in one perioperative death. Two subjects developed meningitis and two additional subjects suffered non-ST elevation myocardial infarctions. One subject developed heparin-induced thrombocytopenia (HIT) resulting in the development of a pulmonary embolus and ischemic bowel.
TABLE 5.
Complication data by reconstruction type
| Total | Temporalis | Submental | Radial Forearm | Anterolateral Thigh | Latissimus Dorsi | Other Locala | Other Free Tissue Transferb | |
|---|---|---|---|---|---|---|---|---|
| Number performed (%) | 92 (100) | 28 (30.4) | 7 (7.6) | 16 (17.4) | 14 (15.2) | 13 (14.1) | 10 (10.9) | 4 (4.3) |
| Major complications (%) | 9 (9.8) | 3 (10.7) | 0 (0) | 0 (0) | 1 (7.1) | 4 (30.8) | 1 (10) | 0 (0) |
| Stroke | 3 | 1 | 1 | 1 | ||||
| NSTEMI | 2 | 1 | 1 | |||||
| Meningitis | 2 | 2 | ||||||
| HIT with PE, ischemic bowel | 1 | 1 | ||||||
| Death | 1 | 1 | ||||||
| Wound complications (%) | 41 (44.6) | 12 (42.9) | 3 (42.9) | 1 (6.3) | 11 (78.6) | 7 (53.8) | 5 (50) | 2 (50) |
| Dehiscence | 17 | 6 | 2 | 1 | 2 | 3 | 2 | 1 |
| Infection | 10 | 3 | 1 | 3 | 1 | 2 | ||
| Hematoma | 3 | 2 | 1 | |||||
| Seroma | 3 | 2 | 1 | |||||
| Venous congestion | 3 | 1 | 2 | |||||
| Arterial thrombus | 2 | 1 | 1 | |||||
| Hemorrhage | 2 | 1 | 1 | |||||
| Stitch abscess | 1 | 1 |
Other local flap donor sites included sternocleidomastoid, latissimus dorsi pedicled, pectoralis major pedicled, posterior scalp, and cervicofacial advancement flaps.
Other free tissue transfer donor sites included rectus abdominis, parascapular fasciocutaneous tissue, and lateral arm.
HIT indicates heparin-induced thrombocytopenia; NSTEMI, non-ST segment elevation myocardial infarction; PE, pulmonary embolism.
Ten subjects (10.9%) required at least one additional surgery in the perioperative period following surgical resection and reconstruction. These included explorations for complications such as arterial thrombus, vascular congestion, control of CSF leakage, and wound dehiscence. Three subjects (3.3%) underwent multiple surgeries during their initial hospitalization. This included two subjects who underwent exploration for venous congestion who later had flap necrosis and underwent removal of their initial flap and insertion of a pectoralis major pedicled flap. One subject had to be taken back to control wound hemorrhage and later during the hospitalization underwent multiple procedures including ileocecectomy and thrombectomy for complications secondary to development of HIT.
Postoperative wound complications occurred in 41 of 92 surgeries (44.6%); the most common wound complication was dehiscence, which occurred after 17 surgeries (18%). Most dehiscences were minor and managed with local wound care including packing, negative-pressure wound therapy, and/or simple debridement. Four wound dehiscences did not respond to conservative management and underwent further revision. The next most prevalent wound complication was a surgical site infection, which occurred after 10 surgeries (11%). These were primarily treated with antibiotics and local wound care.
Vascular complications with flaps occurred after 12 surgeries (13%). Free tissue transfer of the anterolateral thigh or latissimus dorsi donor sites accounted for nine of these complications. The vascular complications included three flaps with venous congestion, two with arterial thrombus, and two with hemorrhage, all of which required urgent exploration of the flap. Isolated hematomas developed in three reconstructions including two requiring surgical drainage and one that was treated conservatively. Seromas developed after three reconstructions requiring drainage at the bedside.
Dural defects were present during 16 surgeries (17%), and were managed with a variety of techniques including the use of collagen matrix, muscle plug, fat grafting, and use of temporalis muscle or fascia to occlude the defect. Dural closure was successful in 10 subjects, and six went on to have CSF leakage postoperatively. Three CSF leaks were successfully managed with CSF diversion. Two CSF leaks were initially managed with CSF diversion, but leakage persisted and ultimately required revision surgery. One CSF leak was initially managed with a rotational nape of neck fasciocutaneous flap.
Wound complication rates were compared with the major primary reconstructive techniques (Table 4). There was no significant difference in complication rates with free tissue transfer compared with locoregional reconstruction. There was a significant difference in complication rates between different types of free tissue transfer, with fewer complications seen using the radial forearm. When comparing common free flaps (RFFF, ALT, latissimus dorsi), common locoregional flaps (temporalis, submental), and uncommon flaps, there were no significant differences in wound complication rates. There was also no significant difference in major or minor complications when comparing for defect size. There was no increased risk of wound complications with concurrent lymph node dissection, or preoperative, or adjuvant radiation therapy (see Table, Supplemental Digital Content 1, http://links.lww.com/MAO/A473).
Wound complication as a function of disease extent is presented in Table 3. Wound complications were seen in two subjects with T2 disease (20%), one subject with T3 disease (17%), and 21 subjects with T4 disease (60%). Complications were statistically more common in those with advanced disease (Table 4). There were eight subjects with CSF leak following T4 disease, one following resection of T3 disease, and none following those with T2 disease.
Revision surgery due to a reconstructive complication was necessary in three subjects (3.5%). This included two free flaps that developed necrosis and were repaired with pedicled flaps as well as one temporalis rotational flap which developed bone exposure following radiation therapy and subsequently was repaired with a radial forearm free tissue transfer.
Subjects with a history of smoking or peripheral vascular disease had a higher wound complication rate (see Table, Supplemental Digital Content 2, http://links.lww.com/MAO/A474). There was a trend toward a higher complication rate in those with a history of diabetes. There was no significant difference for those with a history of CVD. Subjects who had an extensive smoking history (>50 pack-years) had a wound complication rate higher than those with a milder (2–10 or 15–45 pack-years) or no smoking history. Six out of seven of the subjects who had major complications had a history of tobacco use whereas only one with no previous tobacco use suffered from a major complication (cerebrovascular accident).
RECURRENCE/SURVIVAL
Residual disease was defined as either incomplete removal of tumor or evidence of tumor that presented less than 6 months after the reconstructive surgery. Recurrent disease was defined as disease that presented 6 months or greater after surgery. Residual disease was present in 12 of the 86 subjects (14%). Recurrent disease developed in 13 additional subjects (15%). Recurrences were mainly local while five subjects progressed to distant disease manifested as pulmonary metastases.
Mean follow-up period was 25.9 months. Follow-up ranged from 7 days (perioperative death) to 11.5 years. Median follow-up was 11.5 months. Overall survival for all subjects who underwent resection and reconstruction had a 2-year survival of 71.4% and a 5-year survival of 56.7% (see Figure, Supplemental Digital Content 3A, http://links.lww.com/MAO/A475). Subjects with Stage 4 disease showed a 2-year survival rate of 70.0% and a 5-year survival of 49.4% (see Figure, Supplemental Digital Content 3B, http://links.lww.com/MAO/A476).
DISCUSSION
Defects of the lateral skull base resulting from resection of tumor range in size, volume, shape, and requirements for reconstruction. Our results regarding these reconstructions over the last 12 years demonstrate the successful employment of a wide range of options for repairing these defects.
Slightly more than half of the reconstructions used free tissue transfer. This is consistent with the contemporary reconstructive literature, which demonstrates a trend towards more frequent use of free tissue transfer for repair of the lateral skull base (1,4,5,8,9).
One factor in the trend away from use of local rotational flaps has been the increased risk of wound complications when the regional tissue has been previously radiated or if there are plans for postoperative radiation. This was seen in one of our patients with a temporalis rotational flap, which developed bone exposure following radiation therapy and necessitated the use of free tissue transfer for reconstruction.
The major complication rate was low (9.8%) despite the number of extensive skull base surgeries. Two patients had multiple major complications, so the percentage of individual patients undergoing surgery developing complications was actually less (7.6%). The postoperative wound complication rate in this study (44.6%) is consistent with previously published studies (15–50%) (1,5,6,8–11). One explanation as to the why the current study’s wound complication rate was near the high end of the published complication rate range could be the inclusion herein of a large number of minor and easily managed dehiscences not accounted for in other studies. The vascular complication (13%) and CSF leak (14%) rates are similar to other published reports (1,5,6,8–11). Interestingly, only one out of five subjects with a lumbar drain placed intraoperatively for CSF leak developed a leak postoperatively, and three out of six subjects who did not have a lumbar drain placed during the initial surgery for a known leak had a leak postoperatively. This suggests a possible beneficial effect of inserting a lumbar drain intraoperatively if there is a CSF leak or dural defect during the initial resection. In total, seven out of the 10 subjects who had lumbar drains placed were successful in controlling the CSF leakage.
To our knowledge, there have been no large studies showing a difference in complication rates between specific reconstruction techniques. Our results indicate that resections reconstructed with RFFF tissue transfer may have a decreased rate of wound complications, though do not necessarily indicate that it should be the preferred technique for all defects. The increased complication rates in reconstructions using ALT or latissimus dorsi free flaps could be secondary to these flaps being used for more extensive, complicated defects, though our data failed to show an increased complication rate with greater defect surface areas. However, it is possible that defect surface area does not account for depth and complexity of the defect to be reconstructed. ALT and latissimus dorsi free flaps may have been used in more complicated wounds more prone to complications. The increased bulk of these flaps results in more gravitational pull on the wounds, which could increase the rate of dehiscence. The radial forearm donor site tissue is less bulky, which may decrease the amount of weight and thus tension on the closure. When working with more bulky flaps it is important to keep in mind the concept of wound tension and gravitational pull on the flap. Meticulous tissue approximation and using deep suspension sutures to support the weight of the flap are important factors to decrease the risk of dehiscence. RFFFs were only used in one subject with a dural defect, which could also contribute to the lower complication rate.
The RFFF is also a very vascular flap, which may have some benefits over reconstructions with temporalis rotational flaps. The option of utilizing a dual venous drainage system, including both the cephalic vein as well as the venae commitans may account for the lower incidence of vascular complications compared with free flaps such as the ALT and latissimus dorsi, which are limited to only one venous drainage system.
Submental island flaps have been increasingly used at our institution for lateral skull base reconstruction. A recently published study indicated that the submental island flap may be a favorable option for reconstruction of the lateral skull base in terms of complications, cosmesis, and length of hospital stay in select patients (15). The results from the seven patients in the present study are in agreement with this report and reinforce the utility and efficacy of this flap option for reconstruction of lateral skull base defects. This flap can be used along with other flaps, such as a temporalis rotational flap, to reconstruct complicated defects. There has been an evolving trend towards the use of supraclavicular flaps for reconstruction of the lateral skull base (16,17). Though no patient included in the present study had this flap performed, our institution has recently begun to use this form of reconstruction.
It is not surprising that our results show differences in both management and complications between T2 and T4 disease. Those with T4 disease were more likely to undergo more extensive resection, require reconstruction with free tissue transfer, and have wound complications postoperatively. This is likely due to an interaction between the increased resection complexities and increased required flap volumes and surface areas to properly close these defects.
Our results failed to show a significant effect on complication rates between free tissue transfer and locoregional reconstruction, common and uncommon flap techniques, and defect size. Together, this indicates that the complexity of the resections and reconstructions of the lateral skull base does not allow for one ideal technique for a given defect. It is important for the reconstructive surgeon to evaluate all options and use their clinical judgment to select a method that they feel would work best for the unique characteristics of each individual defect.
The survival data are comparable with data from previous studies (5,10). The similarity in the survival rates between all patients and those with formally staged Stage IV disease likely is related to the majority of patients included in the study would likely also have Stage IV disease if they had undergone formal staging. Limitations of the study include loss to follow-up and the retrospective nature of the study. Being a tertiary referral center with a large geographic catchment area, follow-up was an issue for many patients in the study, as many returned for initial follow-up, but once stable chose to follow-up closer to home. The retrospective nature of the study also limited the data to information contained in the medical record.
CONCLUSIONS
Many factors go into planning lateral skull base reconstruction. Free tissue transfer was more often used for T4 disease. ALT and latissimus dorsi free flaps were used for larger defects. The wound complication rate was higher for those with advanced disease. Radial forearm free flaps tended to have lower wound complication rates when compared with other techniques.
Continued research regarding the management of lateral skull base defects will be beneficial in evaluating the efficacy and complications of the different techniques. There continues to be advances and changes in technique of reconstruction including the increased use of the supraclavicular and submental island flaps. Prospective studies evaluating these changes as well as the size of defects, including volume, would be beneficial for creating a definitive management algorithm.
Supplementary Material
Acknowledgments
Funding Source: None.
Footnotes
No financial interests or relationships exist for any of the authors pertinent to the subject of this work.
No conflicts of interests exist for any of the authors pertinent to the subject of this work.
REFERENCES
- 1.Imola MJ, Sciarretta V, Schramm VL. Skull base reconstruction. Curr Opin Otolaryngol Head Neck Surg 2003;11:282–90. [DOI] [PubMed] [Google Scholar]
- 2.Marzo SJ, Benscoter B, Leonetti JP. Contemporary options for lateral skull base reconstruction following tumor extirpation. Curr Opin Otolaryngol Head Neck Surg 2011;19:330–4. [DOI] [PubMed] [Google Scholar]
- 3.Schusterman MA, Kroll SS. Reconstruction strategy for temporal bone and lateral facial defects. Ann Plast Surg 1991;26:233–42. [DOI] [PubMed] [Google Scholar]
- 4.Thurnher D, Novak CB, Neligan PC, et al. Reconstruction of lateral skull base defects after tumor ablation. Skull Base 2007; 17:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moncrieff MD, Hamilton SA, Lamberty GH, et al. Reconstructive options after temporal bone resection for squamous cell carcinoma. J Plast Reconstr Aesthet Surg 2007;60:607–14. [DOI] [PubMed] [Google Scholar]
- 6.Patel NS, Modest MC, Brobst TD, et al. Surgical management of lateral skull base defects. Laryngoscope 2016;126:1911–7. [DOI] [PubMed] [Google Scholar]
- 7.Resto VA, McKenna MJ, Deschler DG. Pectoralis major flap in composite lateral skull base defect reconstruction. Arch Otolaryngol Head Neck Surg 2007;133:490–4. [DOI] [PubMed] [Google Scholar]
- 8.Gal TJ, Kerschner JE, Futran ND, et al. Reconstruction after temporal bone resection. Laryngoscope 1998;108:476–81. [DOI] [PubMed] [Google Scholar]
- 9.Teknos TN, Smith JC, Day TA, et al. Microvascular free tissue transfer in reconstructing skull base defects: lessons learned. Laryngoscope 2002;112:1871–6. [DOI] [PubMed] [Google Scholar]
- 10.Dean NR, White HN, Carter DS, et al. Outcomes following temporal bone resection. Laryngoscope 2010;120:1516–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantravadi AV, Marzo SJ, Leonetti JP, et al. Lateral temporal bone and parotid malignancy with facial nerve involvement. Otolaryngol Head Neck Surg 2011;144:395–401. [DOI] [PubMed] [Google Scholar]
- 12.Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual, 7th ed. New York, NY: Springer; 2010. [Google Scholar]
- 13.Hirsch BE. Staging system revision. Arch Otolaryngol Head Neck Surg 2002;128:93–4. [PubMed] [Google Scholar]
- 14.Moody SA, Hirsch BE, Myers EN. Squamous cell carcinoma of the external auditory canal: an evaluation of a staging system. Am J Otol 2000;21:582–8. [PubMed] [Google Scholar]
- 15.Miller C, Hanley JC, Gernon TJ, et al. The submental island flap for reconstruction of temporal bone defects. Otol Neurotol 2015;36: 879–85. [DOI] [PubMed] [Google Scholar]
- 16.Emerick KS, Herr MW, Lin DT, et al. Supraclavicular artery island flap for reconstruction of complex parotidectomy, lateral skull base, and total auriculectomy defects. JAMA Otolaryngol Head Neck Surg 2014;140:861–6. [DOI] [PubMed] [Google Scholar]
- 17.Hunt JP, Buchmann LO. The supraclavicular artery flap for lateral skull and scalp defects: effective and efficient alternative to free tissue transfer. J Neurol Surg Rep 2014;75:e5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


