Abstract
Objective
To compare the pulmonary function, measured at birth and at hospital discharge, of infants whose mothers had been randomized to a single rescue course of antenatal steroids versus placebo.
Study design
Follow-up at hospital discharge of a randomized, double-blinded trial. In the original trial, pregnant women > 14 days after their initial course of AS and < 34 weeks’ gestation were randomized to rescue AS or placebo. 44 mothers (56 infants) received rescue AS and 41 mothers (57 infants) received placebo. Passive respiratory compliance (Crs) and resistance (Rrs), and functional residual capacity (FRC), were measured at birth and discharge to evaluate changes in pulmonary mechanics over time. Statistical analyses were based on intention-to-treat.
Results
We previously reported that infants in the rescue AS compared with the placebo group demonstrated an increase in Crs measured within 72 hours of birth (1.21 vs 1.01 mL/cm H2O/kg for placebo; p<0.05). Here we show that the Crs benefit in the treated group was sustained until discharge. Infants in the placebo group demonstrated improvement in Crs such that by discharge, there was no difference in Crs between the rescue AS and placebo group (1.18 vs 1.22 mL/cm H2O/kg).
Conclusions
Rescue AS significantly increases Crs measured within 72 hours of birth and this increase is sustained until hospital discharge. Preterm infants in the placebo group demonstrate a decreased initial Crs compared with the rescue AS group, but achieve a comparable Crs with the rescue AS group by discharge.
Keywords: passive respiratory compliance (Crs), functional residual capacity (FRC), passive respiratory resistance (Rrs), pulmonary function tests (PFTs), lung trajectory, rescue antenatal steroids, betamethasone, premature infants
Premature infants exhibit respiratory morbidities as a result of architectural and functional immaturities of the lung. Meta-analyses have definitively shown that a single course of antenatal steroids (AS) administered prior to preterm delivery at ≤34 weeks’ gestation improves a host of neonatal outcomes.1 These benefits are evident both in pulmonary function, with improved functional residual capacity (FRC) and passive respiratory compliance (Crs),2 and in clinical outcomes, with reduced rates of neonatal death, respiratory distress syndrome (RDS), intraventricular hemorrhage (IVH), necrotizing enterocolitis (NEC), duration of mechanical ventilation, and duration of oxygen therapy.1 Because of these clear benefits, treatment of women at risk for preterm deliveries at ≤34 weeks’ gestation with a single course of AS remains the standard of care.3
Complicating matters, however, is the fact that the beneficial effects of AS are also time-limited. The improvements in Crs and FRC are clearest in infants born 1–7 days after the first maternal AS dose, but wane thereafter.4 Ideally, all preterm infants would be delivered within this short window of maximal benefit, but accurately predicting the timing of preterm deliveries is difficult. As a result, some women treated with an initial course of AS remain undelivered beyond this window of maximal benefit, but remain at risk for preterm delivery. The safety and efficacy of repeated courses of AS remains an area of active investigation.
We recently reported that a single rescue course of AS significantly increases the initial Crs and decreases oxygen need in treated preterm infants versus controls.5 In a similarly- designed concurrent blinded study of 437 randomized patients, Garite et al showed improvement in a composite neonatal outcome (including RDS, O2 requirement at 30 days, grade III or IV IVH, culture-proven sepsis, periventricular leukomalacia, NEC, or perinatal death) without short-term risk.6 These studies provide evidence that rescue AS can significantly improve initial pulmonary mechanics and short-term neonatal outcomes. Concern remains about the potential negative effects on pulmonary function and lung growth after rescue AS and its effect on the trajectory of infant pulmonary function. A growing body of evidence demonstrates that lung function tracks along trajectories that are established in early childhood, and that these early trajectories are predictive of lung performance in early adulthood,7,8 but little is known about the early lung trajectories of infants born prematurely.9 The objective of this study was to compare the pulmonary function at birth and discharge of preterm infants randomized to a rescue course of AS versus placebo. Because AS have been shown to have the physiologic effect of inducing surfactant production in the lung prior to a preterm delivery,4 we hypothesized that the rescue AS infants would have a stable level of Crs through discharge, and the Crs of the placebo treated infants would increase over the hospitalization so there would be no difference in Crs between the groups at discharge.
Methods
This study is an extension of the previously published prospective, randomized, placebo-controlled study of the impact of rescue AS on pulmonary mechanics measured after delivery (ClinicalTrials.gov: NCT00669383).5 In brief, the study was conducted at the Neonatal Intensive Care Unit at Oregon Health & Science University (Portland, Oregon) and at Sacred Heart Hospital (Pensacola, Florida). Randomization was stratified by gestational age at rescue AS dosing (≤28 versus > 28 weeks’ gestation) and by multiple gestation (twins versus singletons). The study was approved by the Institutional Review Boards at each institution and informed consent obtained for each enrolled patient. Pregnant women were recruited if they met the following inclusion criteria: gestation between 26 to <34 weeks at the time of enrollment, ongoing risk of threatened preterm delivery as determined by their care providers, ≥14 days after receiving an initial course of AS, and provision of informed consent. Exclusion criteria included: multiple gestation greater than twins, chorioamnionitis, insulin-dependent diabetes mellitus, major chromosomal or fetal abnormality, chronic steroid use during pregnancy, or if the initial course of AS was administered at <24 weeks’ gestation.
Women enrolled in the study were randomized to the rescue AS arm (given two 12mg intramuscular injections of betamethasone [Celestone Soluspan; Shering-Plough Corporation, Kenilworth, NJ]) or the placebo arm (given two 25mg IM injections of cortisone acetate, an inactive steroid indistinguishable in appearance from betamethasone) at 24-hour intervals. Within 72 hours of birth, pulmonary function tests were performed to measure Crs, FRC, and Rrs (passive respiratory resistance). These measurements were repeated just prior to discharge, usually at 34–36 weeks of postmenstrual age.
All pulmonary function measurements were obtained using a computerized infant pulmonary function cart (Sensor-Medics 2600; Sensor-Medics Inc., Yorba Linda, CA) via mask or endotracheal tube. Tests were done with the infant supine and quietly asleep and were performed within 72 hours of birth (and prior to surfactant delivery, if required) and again near discharge. Crs and Rrs were measured using the single-breath occlusion technique to induce the Hering-Breuer reflex as previously described.2,4,10,11 Acceptable measurements required a stable end expiratory baseline, plateau pressure >100ms and varying <0.125 cm H2O, appropriate flow-volume loops by visual inspection, and at least 10 breaths with measured coefficient of variation <20% as per standard international guidelines.12–14
FRC was measured using the nitrogen washout technique.2,15,16 After creating a calibration curve for each patient, the FiO2 was increased from baseline to 100% at end expiration. The curve was then used to correlate the nitrogen washout to the infant’s FRC. The FRC accounts for dead space and is corrected for temperature, pressure, humidity, and the infant’s weight. Acceptable measurements were obtained on quietly sleeping, supine infants, with testing initiated at end expiration, had no evidence of leak on tracing of the washout, and demonstrated consistency with coefficient of variation of <10% on at least 3 measurements.
Anthropometric measurements (weight, head circumference and length) taken at birth and at hospital discharge were compared between groups, and corresponding Z-scores for these measurements were calculated.17 Clinical variables including surfactant administration, diagnosis of RDS (defined as clinical signs of respiratory distress with radiographic appearance and needing supplemental oxygen with FiO2 >0.21), respiratory distress with FiO2 requirement ≥0.30 and ≥0.40 at 24 hours of age, days on mechanical ventilation, and days on supplemental oxygen were also measured.
Statistical analyses
Analysis was performed on an intention-to-treat basis. For continuous variables, means were compared using the independent-samples Student t test. For categorical variables, the groups were compared using the χ2 test and Fisher exact test, as appropriate. To determine p-values and confidence intervals for the primary outcomes, we employed linear mixed modeling to account for the non-independence of covariates among twins and also to account for additional confounders.18 For this analysis, we used the same model, which adjusts for covariates including gestational age at birth, twin gestation, maternal smoking, rupture of membranes, and gestational diabetes, applied in a prior study of data from this dataset.5 We used SPSS for Windows, Version 21 (SPSS Inc, Chicago, IL) and SAS 9.1.3 (SAS Institute Inc, Cary, NC) for all analyses.
Results
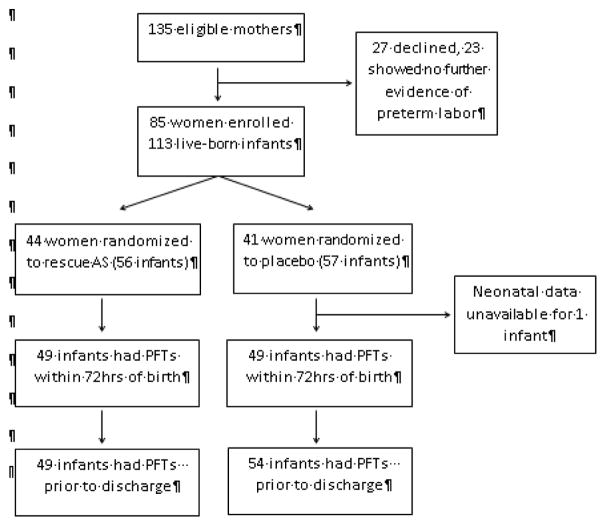
As previously reported, patients were recruited from June 2001 through May 2007. Of the 135 women approached to participate in this study, 27 declined participation, and 23 showed no further evidence of preterm labor. The remaining 85 women were randomized to the rescue AS arm (44 women) or the placebo arm (41 women). A total of 113 infants were born to these women and were included in the study, 56 from the rescue AS arm and 57 from the placebo arm (Figure 1; available at www.jpeds.com). The average gestational age at birth for the entire cohort was 32.1 weeks (30.9 weeks for infants ≤34 completed weeks’ gestation), and the average age at discharge was 36.3 weeks. The average time between the initial course of AS and treatment with rescue AS or placebo was 22.7 days, and the average time between treatment with rescue AS or placebo and delivery was 14.6 days (Table). In the rescue arm, 86.4% (38/44) of women received a full two-dose course of rescue AS.
Figure 1.
CONSORT flow diagram of study participants. Number of initial and discharge pulmonary function tests differs slightly within each group due to some measurements not meeting standard international guidelines. PFTs not meeting standard international guidelines for acceptable measurements were not included in the analysis.
Table.
Baseline demographic data characteristics of study participants. Footnote: Values are mean ± SD unless otherwise indicated.
| Baseline maternal characteristics | Rescue AS (n=44) | Placebo (n=41) | p |
|---|---|---|---|
| Age, years | 26.9 ± 7.5 | 28.6 ± 6.4 | ns |
| Gravida, n | 3.0 ± 2.1 | 3.0 ± 1.8 | ns |
| Parida, n | 1.2 ± 1.4 | 1.4 ± 1.3 | ns |
| Non-white race, n (%) | 14 (32) | 14 (34) | ns |
| ROM >24hrs, n (%) | 9 (20) | 6 (15) | ns |
| Vaginal delivery, n (%) | 20 (45) | 17 (41) | ns |
| Smoking per history, n (%) | 11 (25) | 2 (5) | < 0.05 |
| Chorioamnionitis, n (%) | 0 (0) | 2 (5) | ns |
| Twin Gestation, n (%) | 12 (27) | 16 (39) | ns |
| Time between 1st AS and study drug, days | 22.8 ± 9.3 | 22.9 ± 10.7 | ns |
| Time between study drug and delivery, days | 16.3 ± 18.6 | 14.6 ± 15.8 | ns |
| Baseline infant characteristics | Rescue AS (n=56) | Placebo (n=56) | |
|---|---|---|---|
| GA at birth, weeks | 31.9 ± 3.3 | 32.3 ± 2.9 | ns |
| Birth weight, g | 1806 ± 778 | 1830 ± 657 | ns |
| Age at first PFTs, hours | 29.5 ± 22.8 | 28.0 ± 21.5 | ns |
| Intubated at first PFTs, n (%) | 15 (27) | 18 (32) | ns |
| GA at last PFTs, weeks | 36.4 ± 2.0 | 36.2 ± 2.5 | ns |
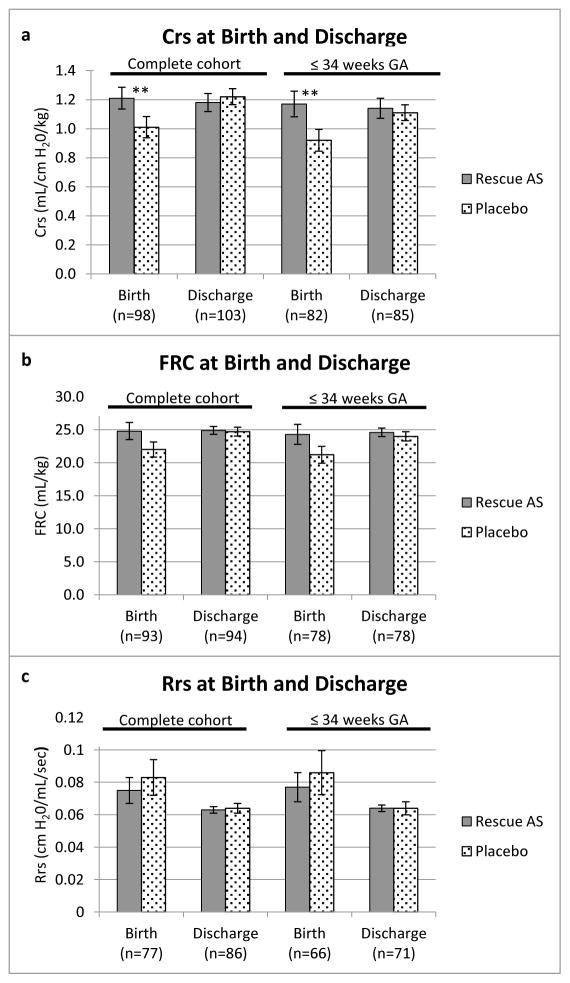
We previously reported that Crs at birth was significantly higher in the rescue AS group compared with placebo (1.21 vs 1.01 mL/cm H2O/kg; p value= 0.043).5 Here, we show that Crs at discharge was similar between the two groups (1.18 vs 1.22 mL/cm H2O/kg; p value is not significant). FRC was similar between the two groups at birth (24.8 vs 22.0 mL/kg) and at discharge (24.9 vs 24.7 mL/kg). There was no significant difference in Rrs between the two groups at birth (0.075 vs 0.083 cm H2O/mL/sec) or at discharge (0.063 vs 0.064 cm H2O/mL/sec) (Figure 2).
Figure 2.
Comparison of A, Crs, B, FRC, C, and Rrs at birth and discharge for infants in the entire cohort (left side of panels) and for the subset born ≤34 completed weeks’ gestation (right side of panels). ** p value <0.05. Crs is given in mL/cm H20/kg, FRC in mL/kg, and Rrs in cm H20/mL/sec. Error bars represent standard error.
For infants born ≤34 completed weeks’ gestation, Crs at birth was significantly higher in the rescue AS group compared with placebo (1.17 vs 0.90 mL/cm H2O/kg; p value= 0.0395). Crs at discharge was similar between the two groups (1.14 vs 1.11 mL/cm H2O/kg; p value is not significant). FRC was similar between the two groups at birth (24.3 vs 21.1 mL/kg) and at discharge (24.6 vs 24.0 mL/kg). Similarly, Rrs was similar between the two groups at birth (0.077 vs 0.086 cm H2O/mL/sec) and at discharge (0.064 vs 0.064 cm H2O/mL/sec) (Figure 2).
Discussion
This study demonstrates an improvement in Crs in the rescue AS group that is sustained from delivery until discharge in infants randomized to a single rescue course of AS versus placebo. At hospital discharge (34–36 weeks of corrected gestational age), there is no significant difference in Crs between the rescue AS and placebo groups.
Previously, we have shown that rescue AS, compared with placebo, improved Crs at birth by 20% in the entire cohort and by 30% in the infants born at ≤ 34 weeks of gestation. Infants treated with rescue AS also had decreased oxygen need. We have also shown that this benefit in respiratory compliance is most evident in infants ≤ 34 weeks’ gestation, and that for these more premature infants, it is associated with important clinical benefits including a lower risk of RDS.5 In this study, we built upon these results to show that the increased Crs after rescue AS was not only immediate (1.21 vs 1.01 mL/cm H2O/kg at birth; p<0.05) but also sustained until discharge. For infants ≤ 34 completed weeks’ gestation in the rescue AS arm, this early benefit is even larger (1.17 vs 0.90 mL/cm H2O/kg) and is similarly sustained until discharge. The mean initial Crs in the placebo group was 1.01 mL/cmH2O/kg and increased to 1.22 mL/cmH2O/kg at discharge which is about a 20% increase over the hospitalization, which is also likely clinically significant. In this randomized trial, there were no statistical differences in FRC or Rrs between the groups at delivery or discharge. These data show that rescue AS provide an immediate increase in Crs at birth to a level not achieved in the placebo group until later in their hospital course, and that this benefit in pulmonary function comes with a reduction in need for supplemental oxygen and a lower risk of RDS.
This study is of particular importance as it relates to concerns regarding the safety of repeat courses of AS. Previous studies in monkey and lamb models have associated the use of repeat courses of antenatal steroids with arrest of the normal program of alveolar septation,19–21 raising concerns that the early benefits might be offset by long-term deleterious consequences to pulmonary function. As we previously reported,5 Crs measured within 72 hours of birth is improved by a single rescue course of AS. We now report that by discharge there is no significant difference in Crs, FRC, or Rrs between the randomized groups. Thus, the early benefits of the rescue AS course do not appear to be offset by deficits in pulmonary function at the time of discharge. Furthermore, as we have previously shown, this single- course rescue AS regimen is not associated with differences in standardized Z scores for birth weight, birth HC, or birth length, though the initial study was not powered to show differences in these important anthropometric outcomes.5 Taken together, these findings suggest that rescue AS confer an immediate increase in respiratory compliance that is physiologic, but not supraphysiologic in degree, and lend support to the safety of a single repeat course of AS for preterm deliveries. We speculate that the initial increase in Crs in the rescue AS group may be due to reinduction of the enzyme system responsible for surfactant production.4 Although there was a trend for a higher FRC in the AS treated infants, it did not meet statistical significance and we speculate that the FRC measurements reflect structural changes which are persistent after a single course of AS. These findings also suggest that a limited course of rescue AS, similar to the single, two-dose regimen employed in this study, might allow optimal initial benefit to pulmonary function with minimal alteration to growth, although a direct comparison of different rescue AS regimens would be necessary to test this hypothesis fully.
A growing body of evidence suggests that pulmonary function tests (PFTs) in early childhood are predictive of future lung function. Stern et al showed that among healthy term newborns tested shortly after birth, those with the lowest quartile for Vmax FRC (maximal flow at FRC) were also in the lowest quartile for measurements of forced expiratory flows at multiple time points to 22 years of age.7 Belgrave et al showed a similar effect for measurements of SRaw (specific airway resistance) at 3 years of age to predict SRaw at age 11.8 These prospective cohort studies suggest that trajectories established early in childhood predict future lung function. Building upon these observational studies, our interventional study demonstrates that rescue AS induces a clear and sustained increase in Crs in the study group, and the placebo group, only remotely treated with AS, had a lower initial Crs that increased over the hospital course. Although the data in our study is focused only on two time points, birth and hospital discharge, it suggests that rescue AS can alter the trajectory of Crs in the premature lung in the early neonatal period. In light of the evidence that early lung trajectories are predictive of later lung function, we speculate that these early changes in Crs seen after rescue AS might change the trajectory of the premature infant’s pulmonary function, which could lead to improved long-term pulmonary outcomes.
This study’s strengths include its randomized, placebo-controlled design, its use of a limited two-dose course of AS, and its use of objective pulmonary function data performed by a single respiratory therapist to augment the data provided by similar studies with clinical outcomes. As there is increasing data that individuals continue to track along PFT percentiles established very early in life, it is important to quantify sequential PFTs to track therapeutic responses, particularly in the critical periods of rapid lung growth in infancy.7 However, we recognize several aspects of our study population that may limit the applicability of this study. By design, all the infants in this study had previously received an initial course of AS, and the study does not include a group untreated with AS. For this reason, the study’s conclusions on pulmonary function are most relevant to infants treated antenatally with AS. Similarly, the majority of the infants in this study were delivered at ≤34 weeks’ gestation with an average gestational age at birth of 32.1 weeks. Although our pulmonary function results are congruent with the clinical findings in other studies of rescue AS, the average age in our dataset is somewhat older, and should be interpreted accordingly. Notably, the impact of rescue AS on passive respiratory compliance was largest in the subset of infants born ≤34 weeks’ gestation with an average gestational age of 30.9 weeks’ gestation. Finally, despite randomization, there were more maternal smokers in the rescue AS arm than the placebo arm. Though maternal smoking is known to affect neonatal respiratory compliance,22 we adjusted for this and other variables in our statistical model.
This study provides a longitudinal perspective through initial neonatal discharge of the pulmonary function of infants treated with rescue AS compared with placebo from the time of birth until discharge. Rescue AS confer significant, measurable benefits in pulmonary function that are immediate, physiologic, and sustained throughout the initial hospitalization. Importantly, no differences in pulmonary function at discharge are seen for infants treated with rescue AS, compared with placebo, providing evidence in support of the safe use of a single two- dose regimen of rescue AS, though the sample size is small. Serial measurements of pulmonary function though early childhood will be needed to assess the effects of this early alteration in respiratory compliance trajectory on long-term pulmonary function and respiratory outcomes.
Acknowledgments
Supported by National Center for Advancing Translational Sciences/National Institutes of Health (NIH; UL1TR000128), NIH/National Heart Lung Blood Institute (K23 HL080231 and R01 HL105447), Office of Dietary Supplement, and American Lung Association (to C.M.).
Abbreviations
- AS
Antenatal steroids
- Crs
passive respiratory compliance
- FRC
functional residual capacity
- Rrs
passive respiratory resistance
- RDS
respiratory distress syndrome
- IVH
intraventricular hemorrhage
- NEC
necrotizing enterocolitis
- PFTs
pulmonary function tests
Footnotes
The authors declare no conflicts of interest.
Trial registration ClinicalTrials.gov: NCT00669383
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3:CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 2.McEvoy C, Bowling S, Williamson K, Stewart M, Durand M. Functional residual capacity and passive compliance measurements after antenatal steroid therapy in preterm infants. Pediatr Pulmonol. 2001;6:425–30. doi: 10.1002/ppul.1070. [DOI] [PubMed] [Google Scholar]
- 3.National Institutes of Health Consensus Development Panel. Antenatal corticosteroids revisited: repeat courses - National Institutes of Health Consensus Development Conference Statement; Obstet Gynecol; August 17–18, 2000; 200. pp. 144–50. [DOI] [PubMed] [Google Scholar]
- 4.McEvoy C, Schilling D, Spitale P, Peters D, O’Malley J, Durand M. Decreased respiratory compliance in infants less than or equal to 32 weeks’ gestation, delivered more than 7 days after antenatal steroid therapy. Pediatrics. 2008;121:e1032–8. doi: 10.1542/peds.2007-2608. [DOI] [PubMed] [Google Scholar]
- 5.McEvoy C, Schilling D, Peters D, Tillotson C, Spitale P, Wallen L, et al. Respiratory compliance in preterm infants after a single rescue course of antenatal steroids: a randomized controlled trial. Am J Obstet Gynecol. 2010;202:544, e1–9. doi: 10.1016/j.ajog.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garite TJ, Kurtzman J, Maurel K, Clark R Obstetrix Collaborative Research Network. Impact of a “rescue course” of antenatal corticosteroids: a multicenter randomized placebo-controlled trial. Am J Obstet Gynecol. 2009;200:248, e1–9. doi: 10.1016/j.ajog.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370:758–64. doi: 10.1016/S0140-6736(07)61379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belgrave DCM, Buchan I, Bishop C, Lowe L, Simpson A, Custovic A. Trajectories of lung function during childhood. Am J Respir Crit Care Med. 2014;189:1101–9. doi: 10.1164/rccm.201309-1700OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stocks J, Hislop A, Sonnappa S. Early lung development: lifelong effect on respiratory health and disease. Lancet Respir Med. 2013;1:728–42. doi: 10.1016/S2213-2600(13)70118-8. [DOI] [PubMed] [Google Scholar]
- 10.Håland G, Carlsen KCL, Sandvik L, Devulapalli CS, Munthe-Kaas MC, Pettersen M, et al. Reduced lung function at birth and the risk of asthma at 10 years of age. N Engl J Med. 2006;355:1682–9. doi: 10.1056/NEJMoa052885. [DOI] [PubMed] [Google Scholar]
- 11.LeSouef PN, England SJ, Bryan AC. Passive respiratory mechanics in newborns and children. Am Rev Respir Dis. 1984;129:552–6. [PubMed] [Google Scholar]
- 12.Bates JH, Schmalisch G, Filbrun D, Stocks J. Tidal breath analysis for infant pulmonary function testing. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. European Respiratory Society/American Thoracic Society. Eur Respir J. 2000;16:1180–92. doi: 10.1034/j.1399-3003.2000.16f26.x. [DOI] [PubMed] [Google Scholar]
- 13.Morris MG, Gustafsson P, Tepper R, Gappa M, Stocks J ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. The bias flow nitrogen washout technique for measuring the functional residual capacity in infants. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. Eur Respir J. 2001;17:529–36. doi: 10.1183/09031936.01.17305290. [DOI] [PubMed] [Google Scholar]
- 14.Gappa M, Colin AA, Goetz I, Stocks J ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. European Respiratory Society/American Thoracic Society. Passive respiratory mechanics: the occlusion techniques. Eur Respir J. 2001;17:141–8. doi: 10.1183/09031936.01.17101410. [DOI] [PubMed] [Google Scholar]
- 15.Yuksel B, Greenough A, Chan V, Russell RR. Comparison of helium dilution and nitrogen washout measurements of functional residual capacity in premature infants. Pediatr Pulmonol. 1993;16:197–200. doi: 10.1002/ppul.1950160310. [DOI] [PubMed] [Google Scholar]
- 16.Cotton RB, Olsson T, Law AB, et al. The physiologic effects of surfactant treatment on gas exchange in newborn premature infants with hyaline membrane disease. Pediatr Res. 1993;34:495–501. doi: 10.1203/00006450-199310000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Fenton TR, Sauve RS. Using the LMS method to calculate z-scores for the Fenton preterm infant growth chart. Eur J Clin Nutr. 2007;61:1380–5. doi: 10.1038/sj.ejcn.1602667. [DOI] [PubMed] [Google Scholar]
- 18.Brown H, Prescott R. Applied Mixed Models in Medicine. Chichester, UK: John Wiley & Sons; 1999. [Google Scholar]
- 19.Willet KE, Jobe AH, Ikegami M, Newnham J, Brennan S, Sly PD. Antenatal endotoxin and glucocorticoid effects on lung morphometry in preterm lambs. Pediatr Res. 2000;48:782–8. doi: 10.1203/00006450-200012000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Willet KE, Jobe AH, Ikegami M, Kovar J, Sly PD. Lung morphometry after repetitive antenatal glucocorticoid treatment in preterm sheep. Am J Respir Crit Care Med. 2001;163:1437–43. doi: 10.1164/ajrccm.163.6.2003098. [DOI] [PubMed] [Google Scholar]
- 21.Johnson JW, Mitzner W, London WT, Palmer AE, Scott R, Kearney K. Glucocorticoids and the rhesus fetal lung. Am J Obstet Gynecol. 1978;130:905–16. doi: 10.1016/0002-9378(78)90267-3. [DOI] [PubMed] [Google Scholar]
- 22.McEvoy CT, Schilling D, Clay N, Jackson K, Go MD, Spitale P, et al. Vitamin C supplementation for pregnant smoking women and pulmonary function in their newborn infants: a randomized clinical trial. JAMA. 2014;311:2074–82. doi: 10.1001/jama.2014.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]