Abstract
The dopamine (DA) D2 receptor (D2R) is a G protein-coupled receptor (GPCR) that is a common target for antipsychotic drugs (APDs). D2Rs signal through canonical G protein pathways but also through non-canonical beta-arrestin2 (βarr2) dependent pathways, a property referred to as functional selectivity or biased signaling. Antagonism of D2R signaling in the striatum is thought to be the primary mode of action of APDs. However, most APDs are not clinically effective at reversing cortical-related symptoms such as cognitive or motivational deficits in schizophrenia. The mechanistic underpinnings of these cortical deficits are largely unknown. Here we review the current mechanistic bases for GPCR functional selectivity/biased signaling and how these new concepts might be leveraged for therapeutic gain to correct deficits in cortico-striatal DA neurotransmission in schizophrenia.
Introduction
Dopamine (DA) is a catecholamine neurotransmitter that is involved in many physiological processes in the CNS and dysregulation of its function has been implicated in many CNS disorders such as schizophrenia. DA mediates its effects by binding to G protein-coupled receptors (GPCRs) belonging to the D1 or D2 class of receptors that activate intracellular signaling cascades. Like most GPCRs the classification of DA receptors was originally based on their coupling to either the stimulatory Gαs or the inhibitory Gαi G proteins (1). The D1 class of Gαs-coupled receptors consists of D1R and D5R whereas the D2 class of Gαi-coupled receptors includes D2R, D3R and D4R (2). G protein mediated GPCR signaling is rapidly desensitized by the initial phosphorylation of receptor by GPCR kinases (GRKs) followed by interaction with β-arrestins (βarr) (3, 4), which leads to inhibition of G protein signaling and subsequent internalization, dephosphorylation and recycling of competent receptors to the plasma membrane (5). In most cellular systems DA receptors interact with GRKs (GRK2, 3, 5 and 6) and βarrs (βarr1 and βarr2) (6–8) and are desensitized and internalized through this cooperative mechanism. However, in recent years a new mode of G protein-independent GPCR signaling has emerged that is mediated via β-arrestins through their ability to scaffold various signaling molecules such as kinases and phosphatases (9, 10). For D1Rs and D2Rs it has been shown that βarr2 but not βarr1 mediates this independent signaling pathway by scaffolding signaling molecules such as extracellular signal regulated kinase (ERK), protein kinase B (PKB or AKT) and protein phosphatase 2A (PP2A) (11, 12), which regulate certain DA-dependent physiological processes (13, 14). D2Rs are the common targets for all antipsychotic drugs (APDs) and selective D2R-βarr2 signaling can be leveraged to discover novel therapeutic avenues in schizophrenia, which will be the focus of this review.
Antipsychotic drugs and dopamine D2 receptor pharmacology
DA receptor-mediated signaling has been implicated in many CNS processes such as cognition, motor control and reward (15–17) and dysfunctional DA receptor signaling has been implicated in many CNS disorders including schizophrenia (18–21). The DA hypothesis of schizophrenia was conceptualized from the original works of Carlsson and Lindqvist, and Van Rossum, (22–26). Subsequently, seminal observations by Seeman’s and Snyder’s groups that neuroleptics or APDs bound to DA receptors (27–29) and that psychostimulants which increase brain DA exacerbated psychotic symptoms (30–33) crystallized the idea of a hyperdopaminergic state of DA in schizophrenia. Following the classification of DA receptors as D1 and D2, it was revealed that all APDs bound to D2Rs but not D1Rs and that they blocked D2Rs to inhibit hyperdopaminergia (1, 34, 35). It was later discovered in the 1980s that clozapine, then a newer antipsychotic, had lower affinity for D2Rs but higher affinities for the serotonin 5-HT2A receptor (36). Based on the relative binding affinities for D2 versus 5HT2A receptors, clinical APDs were either termed “typical” or “first generation” APDs (haloperidol and chlorpromazine) or “atypical” or “second generation” APDs (clozapine, risperidone and olanzapine). For both types of APDs however, binding to the D2R is a common property and it was shown that these APDs mediated their actions predominantly by acting as antagonists or inverse agonists at D2Rs (37, 38). Although both types of APDs are clinically effective there are significant differences in their therapeutic and side-effect profiles. Schizophrenia is characterized by positive (hallucinations, delusions), negative (alogia, anhedonia, avolition) and cognitive symptoms. The typical APDs are effective at targeting the positive symptoms of schizophrenia but have several motor-related side-effects called extrapyramidal symptoms (EPS). Although EPS induced by typical APDs is a result of excessive D2R binding in striatal regions, it is thought that the therapeutic effectiveness also requires striatal D2R binding (20). Atypical APDs have overcome some of the problems with typical APDs in the clinic and are relatively better at targeting the symptoms of schizophrenia without inducing EPS. However the atypical APDs have their own distinct side-effect profile, such as weight gain, agranulocytosis and hypotension. Unfortunately, none of the APDs efficiently target the cognitive dysfunction observed in schizophrenia, which precede the positive and negative symptoms.
Several pieces of evidence argued against hyperdopaminergia as the major biochemical manifestation in schizophrenia (39) and evidence from Pycock et al and Weinberger et al (40, 41) led to a revision of the DA hypothesis to include cortical hypodopaminergia in addition to sub-cortical hyperdopaminergia. Based on this updated DA hypothesis, it was understood that all APDs which are essentially D2R blockers would reverse only sub-cortical hyperdopaminergia but not cortical hypodopaminergia. To target these opposite phenomena simultaneously in a pharmacological manner one would have to devise an APD that is an antagonist and an agonist at the same time. To achieve this, partial agonism was the novel approach used since pharmacologically partial agonists are also antagonists as they block binding of the endogenous ligand. Aripiprazole, synthesized by Otsuka pharmaceuticals is a partial agonist at D2Rs but retains most of the properties of other atypical APDs such as 5HT2AR binding. Aripiprazole was termed a “third generation” APD and has been shown to be effective in the clinic with a lower risk of EPS and metabolic side-effects. However, even aripiprazole with its partial agonist activity has failed to reverse the cortical deficits in schizophrenia. Therefore, even after 60 years of development in APD therapeutics these drugs are only partially effective and lack complete efficacy to all symptoms of schizophrenia. New concepts in GPCR signaling provide a compelling strategy for overcoming these unmet needs in the pharmaceutical intervention of schizophrenia.
The discovery that D2Rs can signal not only through G protein pathways but also through the ability of β-arrestin2 (βarr2) to scaffold distinct signaling complexes has revealed novel avenues for pharmacological targeting of D2Rs and APD therapy. The initial observations that GPCRs, like the β2-adrenergic and the mu-opiate receptors, had the ability to signal both in vitro and in vivo through β-arrestins paved the way to explore this concept for other GPCRs (9, 42, 43). Our group provided the first evidence that DA-dependent behaviors could also be mediated via a βarr2-dependent manner at the D2R (11) through the ability of βarr2 to scaffold protein phosphatase 2A (PP2A), AKT and glycogen synthase kinase 3β (GSK3β) in the mouse striatum. However, the absence of βarr2 (βarr2 knockout) resulted in disruption of this signaling complex and a reduction in DA-dependent locomotion (11, 44), which essentially mimics the actions of APDs. Interestingly, in in vitro assays all clinically effective APDs block D2R-βarr2 recruitment (45), which is consistent with our previous findings in βarr2KO mice. It has also been shown that G protein-mediated signaling via DARPP32 but not βarr2 signaling via GSK3β exacerbates the manifestation of haloperidol-induced catalepsy (14, 46). Interestingly, for the handful of GPCR signaling systems in which G protein- versus β-arrestin- pathways have been critically examined, invariably these two signaling modes mediate distinct cellular and behavioral paradigms (47–49). Therefore, these data suggested that selectively targeting the D2R-βarr2 pathway and leveraging the concept of functional selectivity might be beneficial in APD development and might present with added benefits as will be discussed below.
Molecular and mechanistic bases for functional selectivity at D2R
Functional selectivity
The concept of functional selectivity or biased signaling for GPCRs originally arose more than 20 years ago from cellular observations of distinct pharmacological profiles of ligands or the ability of certain GPCRs to engage multiple G proteins (50–52). This concept has now been broadened to account for the realization that GPCRs have the ability to mediate signaling not only through activation of G proteins but also through the ability of β-arrestins to scaffold distinct signaling events (48). We now recognize that the ability of a ligand to act as agonist, partial agonist or antagonist not only depends on a) its chemical structure (53) but also b) on the conformation of receptor itself (54) and 3) perhaps more importantly on the complement of intracellular signal transducer proteins such as G proteins, GRKs and β-arrestins expressed in a particular cell type (Fig 1) (42, 55, 56). Considering that the majority of GPCRs are activated in vivo by a single endogenous ligand and naturally occuring GPCR mutants that are functionally selective for either of the pathways are rare, it is likely that most in vivo manifestations of GPCR functional selectivity rely on the cellular complement of signal transducers. Below we briefly review how each of these three aspects of functional selectivity have been recently addressed for the D2R and how they might impact therapeutic approaches for treatment of schizophrenia.
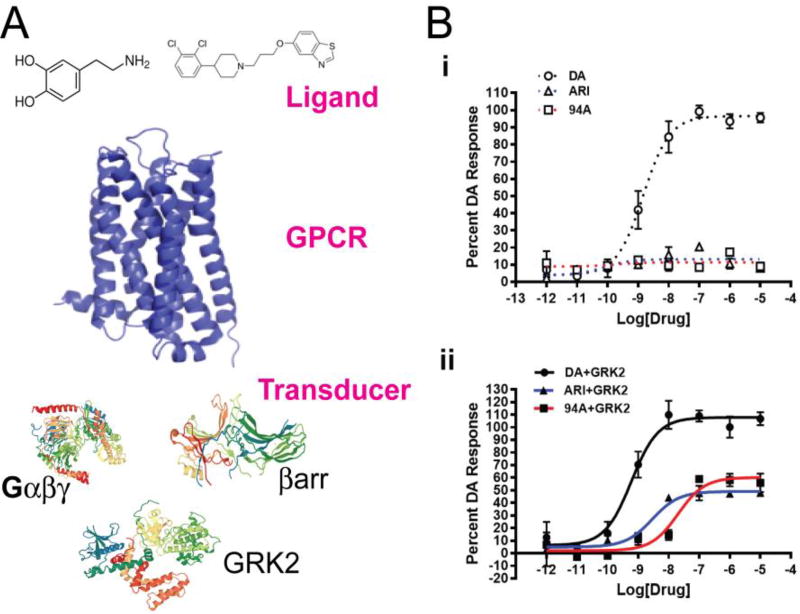
Figure 1. Functional Selectivity at dopamine D2Rs.
A) Functional selectivity at D2Rs can be regulated by the chemical structure of the ligand (shown dopamine and UNC9994) or conformations of the receptor or the complement of intracellular transducer proteins such as G proteins (Gαβγ), GPCR kinases (GRK) or β-arrestins (βarr). B) Bioluminiscence resonance energy transfer (BRET) assay for detection of βarr2 recruitment to D2Rs by dopamine (DA), aripiprazole (ARI) or UNC9994 (94A) in HEK293T cells with i) endogenous or ii) over-expression of GRK2 shows that over-expression of GRK2 renders ARI and 94A as partial agonists.
Ligand-induced functional selectivity at D2Rs
Dopamine (DA) is the endogenous ligand for D2R, which is equally effective at activating both Gαi-mediated cAMP inhibition and βarrestin2 recruitment and can regulate DA-dependent physiological processes. To decipher the actions of DA on each D2R pathway, functionally selective ligands might prove extremely useful since they could preferentially engage one pathway or the other. One of the first examples of functionally selective ligands for D2Rs was the synthetic D1/D2R catechol agonist dihydrexidine, which displays agonist activity at the Gαi-mediated inhibition of adenylyl cyclase versus antagonism at the Gβγ-mediated activation of K+ channels (50, 51). However, there were no examples of functionally selective ligands for G protein versus βarr2 pathways.
Therefore, based on the updated DA hypothesis and the goal of generating βarr2 functionally selective D2R partial agonists, our group in collaboration with the Roth and Jin laboratories at UNC Chapel Hill and the Mount Sinai School of Medicine developed the first βarr2 biased partial agonists using aripiprazole as a scaffold template (57, 58). These partial agonists, namely UNC9975 and UNC9994, showed minimal activity at the D2R-mediated Gαi pathway but were efficacious partial agonists/antagonists at the β-arrestin2 pathway using various cellular assays. UNC9994 but not UNC9975 is as efficacious as aripiprazole at the βarr2 pathway but does not have ideal PK properties (58). These compounds are also efficacious in animal models that predict APD activity since they inhibited hyperlocomotion and PPI disruption by amphetamine and phencyclidine (PCP) in mice. Additionally, UNC9994 lost its activity in global βarr2KO mice suggesting that its APD-like activity is mediated via βarr2. Interestingly, these compounds induce less catalepsy-like behavior than classical neuroleptics in animal models. Although these tool compounds were not markedly different than aripiprazole in any of the behavioral paradigms, the importance of one of these βarr2 biased ligands, UNC9994 was only appreciated subsequent to the examination of their functional profile in a brain region- and neuron-specific fashion providing a clear example of intracellular transducer protein-dependent biased signaling. This is discussed in detail in the DA hypothesis and functional selectivity section.
Engineered functionally selective Dopamine D2R receptors
In mammals D2Rs are expressed either on DA synthesizing neurons where they function as auto-receptors or in the striatum and cortical and limbic regions, where they function as postsynaptic receptors. D2Rs are expressed much more prominently in the striatum than in cortical and limbic regions. D2Rs can regulate a plethora of physiological processes such as locomotion, reward, habit-learning and working memory (8). Historically the function of D2Rs has been established through the use of pharmacological agents and more recently using genetic deletion approaches (59–61). To complement the functionally selective ligand approach an alternative strategy is to generate mutant receptors that activate only one pathway or the other (62, 63). Our group used a novel approach called “Evolutionary Trace” (ET) (64) to identify biased D2R mutants with varying range of bias for the G protein or the βarr2 pathway (65). Interestingly, residues near the second intracellular loop (IL) and in transmembrane 3 of GPCRs have been found from crystallographic studies to interact with the C terminal of the α subunit of G proteins and also postulated from modeling studies to interact with β-arrestins (66). Combinatorial mutations in the second intracellular loop A135R and M140D generated a βarr-biased D2R ([βarr]D2R), whereas L125N and Y133L generated a G protein biased D2R ([Gprot]D2R). These two D2R mutants displayed >90% bias for their respective pathway using standard cellular assays (65, 67). These mutants when virally overexpressed in D2R positive medium spiny neurons (MSNs) in the striatum of mice potentiated amphetamine-induced hyperlocomotion ([Gprot]D2R < [βarr]D2R = [WT]D2R). These in vivo data might suggest that the D2R-βarr2 pathway plays a more prominent role than the D2R-Gprot pathway in the response to amphetamine, which essentially reproduces what has been found with deletion of βarr2 in these same neurons (Urs et al., under review). Current efforts to reconstitute these D2R mutants in mice lacking D2Rs in various neuronal populations from different brain regions are gradually unraveling the contributions of each pathway to a variety of DA-dependent behaviors (Rose et al., in preparation).
In vivo determinants of functional selectivity
The classical G protein-dependent D2R signaling requires the ability of the receptor to promote the exchange of the guanine nucleotides on heterotrimeric G proteins. D2Rs preferentially activate Gi/o subtypes of G proteins but considering the multiplicity of the Gαi/o subunit family (Gαi1, Gαi2, Gαi3, and Gαo) and the large number of combinations of Gβγ subunits that can complex with the Gαi/o subunits, it is likely that these factors play an important role in the various select actions of D2Rs in the brain (68–71). Interestingly Gαo is the most highly expressed Gαi subtype in the brain (72) and is responsible for many of the G protein mediated signal transduction downstream of D2R (73). Additionally, D2Rs can signal through Gβγ subunits to activate K+ channels and regulate membrane excitability and dendritic arborization (74–76). In the striatum where D2Rs are highly expressed, several other proteins might influence G protein signaling. For example, Gαolf, a subtype of the Gαs stimulatory G protein family mainly found in the olfactory epithelium as well as the Regulator of G protein Signaling 9 subtype2 (RGS9–2), which accelerates inactivation of Gα subunits, are highly enriched in the striatum (77, 78). It is clear that D2R is able to engage both Gαi and Gαo subunits and this may yield diverse functional outcomes, however, targeting D2R activation of one G protein subtype (i.e. creating a biased ligand for Gαo) has not been realized, partly because of the difficulty in separating Gαo and Gαi signaling as the proteins are closely related.
In contrast to the considerable texture of Gα and Gβγ’s ability to mediate D2R signaling, separating G protein-mediated versus GRK/β-arrestin-dependent signaling mechanisms may be relatively simpler to achieve in vivo. Both modes of signaling show interdependence since, the Gβγ subunit participates in recruiting GPCR kinases like GRK2 and 3 to the activated receptor following receptor activation (79). However, the fact that GRK-mediated phosphorylation of the receptor enhances β-arrestin interaction with receptor and β-arrestin can scaffold signal complexes, clearly differentiates it from canonical G protein signaling and makes GRK/β-arrestin-dependent signaling sensitive to the cellular expression of GRKs and β-arrestins.
The expression patterns of GRKs and β-arrestins in the brain are unique. GRK2 and 3 and GRK6 to a certain extent, have significantly lower expression in the striatum compared to other brain regions in rat (80), mice and humans, with GRK2 showing the largest regional differences between striatum and prefrontal cortex (Urs et al., under review). Interestingly, genetic deletion of GRK6 or GRK2 has been shown to render mice supersensitive to dopaminergic agonists or reduce sensitivity to cocaine (81, 82). Expression of multiple GRK subtypes in the same cell is significant since different patterns of phosphorylation (most likely on D2R’s long third intracellular loop) may yield different functional outcomes, as has been shown for other GPCRs (83). Like GRK2, βarr2 but not βarr1 expression is lower in the striatum compared to other brain regions like the prefrontal cortex in mice (84) and humans (Urs and Caron, unpublished observations).The possible implications of varying expression patterns of GRK2 and βarr2 is highlighted by our in vitro data showing that D2R ligands such as aripiprazole behave as partial agonists only in the presence of βarr2 and GRK2 over-expression (57).
It is apparent that GPCR functional selectivity can be studied using synthetic ligands or artificially generated mutant receptors but these do not contribute to in vivo endogenous determinants of functional selectivity. Therefore, varied expression patterns of transducer proteins such as G proteins, GRKs and β arrestins can not only regulate functional selectivity of ligands in cellular systems but also regulate functional selectivity in vivo, which is often referred to as “system bias”. In the next section we will highlight the importance of in vivo functional selectivity or system bias at D2Rs and its potential relevance to schizophrenia therapeutics.
DA hypothesis of schizophrenia and functional selectivity
The DA hypothesis of schizophrenia posits cortical hypodopaminergia that manifests in sub-cortical hyperdopaminergia and these opposite phenomena are inter-dependent. Brain imaging studies in schizophrenia patients have shown that amphetamine-induced DA release is enhanced in the striatum (31, 85) (hyperdopaminergia), whereas a recent study has demonstrated, for the first time, cortical hypodopaminergia in schizophrenia patients (86). Given the lack of efficacy of first and second generation APDs towards cortical-related symptoms of schizophrenia, third generation APDs or partial agonists such as aripiprazole were developed with the hope that they could correct both these opposite DA related phenomena. However, in the end these partial agonists have been only marginally different than other antipsychotics in their ability to reverse cortical-related symptoms. Regrettably, the development of third generation APDs preceded our current understanding of functional selectivity at GPCRs.
As mentioned above, in an attempt to leverage our new appreciation for the concept of G protein- versus β-arrestin-dependent functional selectivity at GPCRs, we embarked several years ago in a large multi-center effort to develop partial agonist tool compounds at the D2R-βarr2 pathway based on the scaffold of the unbiased APD aripiprazole and two tool compounds UNC9975 and UNC9994 were derived. Pharmacologically these compounds do not elicit any Gαi agonist activity at D2Rs but have partial agonist activity at βarr2 recruitment. Aripiprazole on the other hand is a partial agonist at both D2R-mediated Gαi and βarr2 pathways. The in vitro efficacy of UNC9994 is similar to aripiprazole but UNC9975 is a very weak partial D2R-βarr2 agonist. However, the partial agonist activity at D2R-βarr2 recruitment for aripiprazole and UNC9994 is observed only when over-expressing GRK2 (Fig 1Bii) but not GRK6 (data not shown) in a bioluminiscence resonance energy transfer (BRET)-based reporter assay in HEK293T cells. In the same assay with endogenous levels of GRK2, both aripiprazole and UNC9994 showed no agonist activity (Fig. 1Bi) but are efficacious antagonists. We have recently made the interesting observation that levels of βarr2 and GRK2 vary between different brain regions expressing D2Rs (Urs et al., under review). Specifically, in the striatum βarr2 and GRK2 (but not βarr1 and GRK6) levels are significantly lower to those in the prefrontal cortex (PFC) in mice and humans. Interestingly, HEK293T cells have low levels of GRK2 and βarr2 similar to that of the striatum. Therefore, we hypothesized that aripiprazole and UNC9994 could act as partial agonists in the PFC but antagonists in the striatum.
To test this hypothesis we used mice where we could delete βarr2 or inactivate GRK2 in a region specific manner i.e in the PFC or striatum or both. To mimic the conditions postulated in the DA hypothesis of schizophrenia we used two common pharmacological drugs - amphetamine, a psychostimulant that reverses DA flow through the DA transporter (DAT) and mimics striatal hyperdopaminergia, and phencyclidine (PCP), a NMDA receptor antagonist that mimics cortical dysfunction. In the amphetamine model we observed that only UNC9994 but not aripiprazole lost its APD-like activity when βarr2 was deleted in striatal D2R-expressing neurons suggesting that striatal D2R-βarr2 antagonism is sufficient for APD-like activity. However, for PCP, UNC9994 lost its activity only when βarr2 was deleted in both cortico-striatal D2R neurons while aripiprazole was still active. This suggests a cortico-striatal role for βarr2 in APD-like activity. Furthermore, local PFC injection of UNC9994 but not aripiprazole inhibits PCP-induced locomotion in a GRK2-depdendent manner, suggesting that D2R-βarr2 biased partial agonism in the PFC is sufficient to reverse the behavioral effects of PCP.
The next pertinent question is what might be the PFC neuronal subtype mediating these effects. Post-mortem brain analyses of schizophrenia patients have shown a reduction in the mRNA expression of the gamma aminobutyric acid (GABA) synthesizing enzyme glutamic acid decarboxylase 67 (GAD67) (87–89) and the calcium-binding protein parvalbumin (PV) (90, 91), which are two markers of GABAergic fast-spiking interneurons (FSI). These findings have led to the hypothesis that disruption of FSI function in the PFC in schizophrenia can lead to altered excitation-inhibition balance (92, 93) (Fig. 2, middle panel) that can be evidenced as disrupted cortical gamma rhythms resulting in cognitive impairment (94, 95). In the PFC D2Rs are expressed on fast-spiking interneurons (FSI), a subset of GABA inhibitory interneurons that modulate firing of glutamatergic pyramidal neurons (Fig. 2, upper panel). The D2R agonist quinpirole increases FSI excitability in adult rodents in a similar fashion as D1R agonists (96), suggesting that this excitatory effect is mediated by a G protein-independent pathway, since D1Rs activate the stimulatory Gαs and D2Rs activate the inhibitory Gαi. The βarr2-biased D2R ligand UNC9994 and to some extent quinpirole but not aripiprazole enhances firing of PV+ FSIs of the PFC in a D2R-, GRK2- and βarr2-dependent manner (UNC9994>Quinpirole>Aripiprazole) suggesting that D2R-βarr2 drives FSI excitability. This enhanced excitability of PV+ FSIs by UNC9994 can be attributed to the relative balance between Gαi/o and βarr2 partial agonist activities. UNC9994 has no Gαi/o agonist (neuronal dampening) activity (57), whereas quinpirole and aripiprazole are Gαi/o agonist and partial agonist, respectively. These data are perhaps the first piece of evidence that suggest why aripiprazole might not have been successful in the clinic in reversing cortical-related symptoms in schizophrenia. Aripiprazole is unbiased and its D2R-βarr2 partial agonism (increased FSI firing) is counteracted by its reciprocal Gαi/o partial agonism (inhibition of FSI firing). Consistent with our behavioral observations, UNC9994 did not mimic the inhibitory effect of the D2R agonist quinpirole on striatal D2R+ MSNs, rather showing antagonist-like properties in striatal MSNs. Therefore, although behaviorally UNC9994 has the same effect as classical antipsychotics (i.e inhibition of psychomotor responses), it has opposite effects on neuronal firing in the PFC versus striatum. Consistent with the DA hypothesis and the above observations with UNC9994, genetic deletion of D2R also has opposite effects in PFC versus striatum on the PCP response (Urs et al., under review)
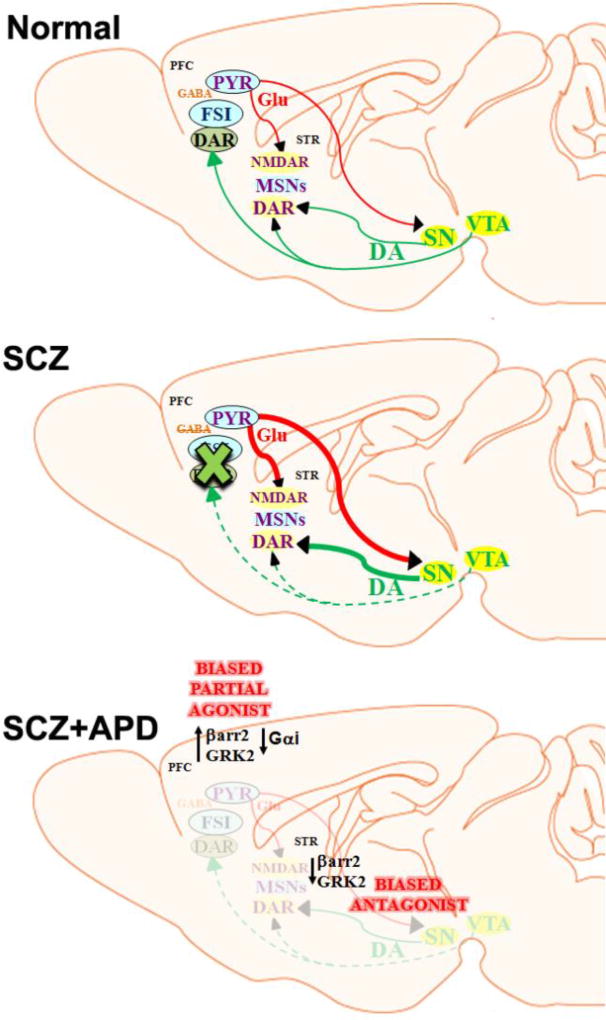
Figure 2. Simplified circuit schematic of the dopamine (DA) hypothesis of schizophrenia (SCZ) and implications of biased D2R signaling.
As depicted in sagittal sections of a mouse brain, under normal conditions (upper panel) DA in the prefrontal cortex (PFC) through DA receptors (DARs) regulates firing of fast-spiking GABAergic parvalbumin+ interneurons (FSIs) that regulate rhythmic firing of glutamate (Glu) releasing pyramidal neurons (PYR). The PYR neurons presumably regulate neuronal activity of either striatal (STR) medium spiny neurons (MSNs), which express NMDA receptors (NMDARs) and DARs, or midbrain substantia nigra pars compacta (SN) neurons that also release DA onto striatal MSNs. In conditions that mimic schizophrenia (SCZ, middle panel), a loss of PFC DA (hypodopaminergia) or inhibition of neuronal activity of FSIs leads to over-excitability of PYRs. The enhanced activity of PYRs could presumably lead to enhanced striatal MSN activity directly or through increased DA release (hyperdopaminergia) in the striatum by SN neurons. A β-arrestin2 (βarr2) biased partial agonist like UNC9994 that acts as an agonist in the PFC but as an antagonist in the striatum could represent an ideal APD-like compound that could simultaneously reverse both hypo- and hyperdopaminergia in schizophrenia (SCZ+APD, lower panel). The proposed mechanism of such a compound would be to enhance βarr2/GRK2 but reduce Gαi activity in the PFC and inhibit βarr2/GRK2 activity in the striatum. Normal activity (
 ), enhanced activity (
), enhanced activity (
 ) and reduced activity (
) and reduced activity (
 ).
).
Our data show that the same pharmacological agent UNC9994 can act as a D2R-βarr2 agonist in PFC FSIs but a D2R-βarr2 antagonist in striatal D2 MSNs, highlighting the feasibility of a pharmacological approach in which system bias-dependent functional selectivity and partial agonism can restore activity in oppositely affected systems. Unlike current antipsychotics, a dual action antipsychotic-like compound such as the UNC9994 tool compound would be ideal since it could simultaneously counteract cortical hypodopaminergia (D2R-βarr2 agonism) and striatal hyperdopaminergia (D2R-βarr2 antagonism) in schizophrenia (Fig.2, bottom panel). In the future, it will be interesting to evaluate whether UNC9994 will be effective at reversing cortical-related symptoms such as cognitive or motivational deficits in an appropriate animal model.
In conclusion, we demonstrate how new concepts of G protein- and βarrestin-biased functional selectivity at a GPCR can be utilized to develop tool compounds that afford distinct cell and region specific pharmacological profiles to potentially improve APD therapeutic approaches for schizophrenia.
References
- 1.Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979;277:93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- 2.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiological reviews. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 3.Benovic JL, Strasser RH, Caron MG, Lefkowitz RJ. Beta-adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science (New York, NY. 1990;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson SS, Downey WE, 3rd, Colapietro AM, Barak LS, Menard L, Caron MG. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science (New York, NY. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- 6.Kim KM, Valenzano KJ, Robinson SR, Yao WD, Barak LS, Caron MG. Differential regulation of the dopamine D2 and D3 receptors by G protein-coupled receptor kinases and beta-arrestins. The Journal of biological chemistry. 2001;276:37409–37414. doi: 10.1074/jbc.M106728200. [DOI] [PubMed] [Google Scholar]
- 7.Tiberi M, Nash SR, Bertrand L, Lefkowitz RJ, Caron MG. Differential regulation of dopamine D1A receptor responsiveness by various G protein-coupled receptor kinases. The Journal of biological chemistry. 1996;271:3771–3778. doi: 10.1074/jbc.271.7.3771. [DOI] [PubMed] [Google Scholar]
- 8.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 9.Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science (New York, NY. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 10.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science (New York, NY. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 11.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Urs NM, Daigle TL, Caron MG. A Dopamine D1 Receptor-Dependent beta-Arrestin Signaling Complex Potentially Regulates Morphine-Induced Psychomotor Activation but not Reward in Mice. Neuropsychopharmacology. 2011;36:551–558. doi: 10.1038/npp.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, et al. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132:125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 14.Urs NM, Snyder JC, Jacobsen JP, Peterson SM, Caron MG. Deletion of GSK3beta in D2R-expressing neurons reveals distinct roles for beta-arrestin signaling in antipsychotic and lithium action. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:20732–20737. doi: 10.1073/pnas.1215489109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wise RA. Dopamine, learning and motivation. Nature Reviews Neuroscience. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 16.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Hornykiewicz O. Dopamine (3-hydroxytyramine) in the central nervous system and its relation to the Parkinson syndrome in man. Dtsch Med Wochenschr. 1962;87:1807–1810. doi: 10.1055/s-0028-1114024. [DOI] [PubMed] [Google Scholar]
- 18.Goldman-Rakic PS. The relevance of the dopamine-D(1) receptor in the cognitive symptoms of schizophrenia. Neuropsychopharmacology. 1999;21:S170–S180. [Google Scholar]
- 19.Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abi-Dargham A, Laruelle M. Mechanisms of action of second generation antipsychotic drugs in schizophrenia: insights from brain imaging studies. Eur Psychiatry. 2005;20:15–27. doi: 10.1016/j.eurpsy.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlsson A, Lindqvist M, Magnusson T. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature. 1957;180:1200. doi: 10.1038/1801200a0. [DOI] [PubMed] [Google Scholar]
- 23.Carlsson A, Lindqvist M, Magnusson T, Waldeck B. On the presence of 3-hydroxytyramine in brain. Science (New York, NY. 1958;127:471. doi: 10.1126/science.127.3296.471. [DOI] [PubMed] [Google Scholar]
- 24.Carlsson A, Rasmussen EB, Krist Jansen P. The urinary excretion of adrenaline and noradrenaline by schizophrenic patients during reserpine treatment. J Neurochem. 1959;4:318–320. doi: 10.1111/j.1471-4159.1959.tb13211.x. [DOI] [PubMed] [Google Scholar]
- 25.Carlsson A, Lindqvist M. Effect of Chlorpromazine or Haloperidol on Formation of 3methoxytyramine and Normetanephrine in Mouse Brain. Acta Pharmacol Toxicol (Copenh) 1963;20:140–144. doi: 10.1111/j.1600-0773.1963.tb01730.x. [DOI] [PubMed] [Google Scholar]
- 26.van Rossum JM. The significance of dopamine-receptor blockade for the mechanism of action of neuroleptic drugs. Arch Int Pharmacodyn Ther. 1966;160:492–494. [PubMed] [Google Scholar]
- 27.Matthysse S. Antipsychotic drug actions: a clue to the neuropathology of schizophrenia? Fed Proc. 1973;32:200–205. [PubMed] [Google Scholar]
- 28.Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261:717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- 29.Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science (New York, NY. 1976;192:481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- 30.van Kammen DP, Docherty JP, Bunney WE., Jr Prediction of early relapse after pimozide discontinuation by response to d-amphetamine during pimozide treatment. Biol Psychiatry. 1982;17:233–242. [PubMed] [Google Scholar]
- 31.Angrist B, Peselow E, Rubinstein M, Wolkin A, Rotrosen J. Amphetamine response and relapse risk after depot neuroleptic discontinuation. Psychopharmacology (Berl) 1985;85:277–283. doi: 10.1007/BF00428187. [DOI] [PubMed] [Google Scholar]
- 32.Lieberman JA, Kane JM, Gadaleta D, Brenner R, Lesser MS, Kinon B. Methylphenidate challenge as a predictor of relapse in schizophrenia. Am J Psychiatry. 1984;141:633–638. doi: 10.1176/ajp.141.5.633. [DOI] [PubMed] [Google Scholar]
- 33.Davidson M, Keefe RS, Mohs RC, Siever LJ, Losonczy MF, Horvath TB, et al. L-dopa challenge and relapse in schizophrenia. Am J Psychiatry. 1987;144:934–938. doi: 10.1176/ajp.144.7.934. [DOI] [PubMed] [Google Scholar]
- 34.Spano PF, Govoni S, Trabucchi M. Studies on the pharmacological properties of dopamine receptors in various areas of the central nervous system. Adv Biochem Psychopharmacol. 1978;19:155–165. [PubMed] [Google Scholar]
- 35.Madras BK. History of the discovery of the antipsychotic dopamine D2 receptor: a basis for the dopamine hypothesis of schizophrenia. J Hist Neurosci. 2013;22:62–78. doi: 10.1080/0964704X.2012.678199. [DOI] [PubMed] [Google Scholar]
- 36.Meltzer HY, Matsubara S, Lee JC. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J Pharmacol Exp Ther. 1989;251:238–246. [PubMed] [Google Scholar]
- 37.Zhang W, Bymaster FP. The in vivo effects of olanzapine and other antipsychotic agents on receptor occupancy and antagonism of dopamine D1, D2, D3, 5HT2A and muscarinic receptors. Psychopharmacology (Berl) 1999;141:267–278. doi: 10.1007/s002130050834. [DOI] [PubMed] [Google Scholar]
- 38.Weiner DM, Burstein ES, Nash N, Croston GE, Currier EA, Vanover KE, et al. 5-hydroxytryptamine2A receptor inverse agonists as antipsychotics. J Pharmacol Exp Ther. 2001;299:268–276. [PubMed] [Google Scholar]
- 39.Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 40.Pycock CJ, Kerwin RW, Carter CJ. Effect of lesion of cortical dopamine terminals on subcortical dopamine receptors in rats. Nature. 1980;286:74–76. doi: 10.1038/286074a0. [DOI] [PubMed] [Google Scholar]
- 41.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 42.Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science (New York, NY. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 43.Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- 44.Beaulieu JM, Tirotta E, Sotnikova TD, Masri B, Salahpour A, Gainetdinov RR, et al. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci. 2007;27:881–885. doi: 10.1523/JNEUROSCI.5074-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masri B, Salahpour A, Didriksen M, Ghisi V, Beaulieu JM, Gainetdinov RR, et al. Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:13656–13661. doi: 10.1073/pnas.0803522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bateup HS, Santini E, Shen W, Birnbaum S, Valjent E, Surmeier DJ, et al. Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14845–14850. doi: 10.1073/pnas.1009874107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 48.Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends in pharmacological sciences. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 49.Walters RW, Shukla AK, Kovacs JJ, Violin JD, DeWire SM, Lam CM, et al. beta-Arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. The Journal of clinical investigation. 2009;119:1312–1321. doi: 10.1172/JCI36806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kilts JD, Connery HS, Arrington EG, Lewis MM, Lawler CP, Oxford GS, et al. Functional selectivity of dopamine receptor agonists. II. Actions of dihydrexidine in D2L receptor-transfected MN9D cells and pituitary lactotrophs. J Pharmacol Exp Ther. 2002;301:1179–1189. doi: 10.1124/jpet.301.3.1179. [DOI] [PubMed] [Google Scholar]
- 51.Mottola DM, Kilts JD, Lewis MM, Connery HS, Walker QD, Jones SR, et al. Functional selectivity of dopamine receptor agonists. I. Selective activation of postsynaptic dopamine D2 receptors linked to adenylate cyclase. J Pharmacol Exp Ther. 2002;301:1166–1178. doi: 10.1124/jpet.301.3.1166. [DOI] [PubMed] [Google Scholar]
- 52.Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science (New York, NY. 1991;252:802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- 53.Rajagopal S, Bassoni DL, Campbell JJ, Gerard NP, Gerard C, Wehrman TS. Biased agonism as a mechanism for differential signaling by chemokine receptors. The Journal of biological chemistry. 2013;288:35039–35048. doi: 10.1074/jbc.M113.479113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kenakin T, Christopoulos A. Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nature reviews. 2013;12:205–216. doi: 10.1038/nrd3954. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, et al. Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7157–7162. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raehal KM, Walker JK, Bohn LM. Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther. 2005;314:1195–1201. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- 57.Allen JA, Yost JM, Setola V, Chen X, Sassano MF, Chen M, et al. Discovery of beta-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:18488–18493. doi: 10.1073/pnas.1104807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen X, Sassano MF, Zheng L, Setola V, Chen M, Bai X, et al. Structure-functional selectivity relationship studies of beta-arrestin-biased dopamine D(2) receptor agonists. J Med Chem. 2012;55:7141–7153. doi: 10.1021/jm300603y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, et al. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature. 1995;377:424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- 60.Brami-Cherrier K, Anzalone A, Ramos M, Forne I, Macciardi F, Imhof A, et al. Ablation of D2 autoreceptors causes epigenetic reprogramming of cortical neurons. Mol Psychiatry. 2014;19:1153. doi: 10.1038/mp.2014.144. [DOI] [PubMed] [Google Scholar]
- 61.Bello EP, Mateo Y, Gelman DM, Noain D, Shin JH, Low MJ, et al. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat Neurosci. 2011;14:1033–1038. doi: 10.1038/nn.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lan H, Teeter MM, Gurevich VV, Neve KA. An intracellular loop 2 amino acid residue determines differential binding of arrestin to the dopamine D2 and D3 receptors. Mol Pharmacol. 2009;75:19–26. doi: 10.1124/mol.108.050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lan H, Liu Y, Bell MI, Gurevich VV, Neve KA. A dopamine D2 receptor mutant capable of G protein-mediated signaling but deficient in arrestin binding. Mol Pharmacol. 2009;75:113–123. doi: 10.1124/mol.108.050534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lichtarge O, Bourne HR, Cohen FE. An evolutionary trace method defines binding surfaces common to protein families. J Mol Biol. 1996;257:342–358. doi: 10.1006/jmbi.1996.0167. [DOI] [PubMed] [Google Scholar]
- 65.Peterson SM, Pack TF, Wilkins AD, Urs NM, Urban DJ, Bass CE, et al. Elucidation of G-protein and beta-arrestin functional selectivity at the dopamine D2 receptor. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:7097–7102. doi: 10.1073/pnas.1502742112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shukla AK, Manglik A, Kruse AC, Xiao K, Reis RI, Tseng WC, et al. Structure of active beta-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide. Nature. 2013;497:137–141. doi: 10.1038/nature12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peterson SM, Pack TF, Caron MG. Receptor, Ligand and Transducer Contributions to Dopamine D2 Receptor Functional Selectivity. PLoS One. 2015;10:e0141637. doi: 10.1371/journal.pone.0141637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lambright DG, Noel JP, Hamm HE, Sigler PB. Structural determinants for activation of the alpha-subunit of a heterotrimeric G protein. Nature. 1994;369:621–628. doi: 10.1038/369621a0. [DOI] [PubMed] [Google Scholar]
- 69.Nobles M, Benians A, Tinker A. Heterotrimeric G proteins precouple with G protein-coupled receptors in living cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18706–18711. doi: 10.1073/pnas.0504778102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wall MA, Coleman DE, Lee E, Iniguez-Lluhi JA, Posner BA, Gilman AG, et al. The structure of the G protein heterotrimer Gi alpha 1 beta 1 gamma 2. Cell. 1995;83:1047–1058. doi: 10.1016/0092-8674(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 71.Wong YH, Conklin BR, Bourne HR. Gz-mediated hormonal inhibition of cyclic AMP accumulation. Science (New York, NY. 1992;255:339–342. doi: 10.1126/science.1347957. [DOI] [PubMed] [Google Scholar]
- 72.Sternweis PC, Robishaw JD. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. The Journal of biological chemistry. 1984;259:13806–13813. [PubMed] [Google Scholar]
- 73.Jiang M, Spicher K, Boulay G, Wang Y, Birnbaumer L. Most central nervous system D2 dopamine receptors are coupled to their effectors by Go. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3577–3582. doi: 10.1073/pnas.051632598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cazorla M, Shegda M, Ramesh B, Harrison NL, Kellendonk C. Striatal D2 receptors regulate dendritic morphology of medium spiny neurons via Kir2 channels. J Neurosci. 2012;32:2398–2409. doi: 10.1523/JNEUROSCI.6056-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Uchida S, Akaike N, Nabekura J. Dopamine activates inward rectifier K+ channel in acutely dissociated rat substantia nigra neurones. Neuropharmacology. 2000;39:191–201. doi: 10.1016/s0028-3908(99)00111-2. [DOI] [PubMed] [Google Scholar]
- 76.Vallar L, Meldolesi J. Mechanisms of signal transduction at the dopamine D2 receptor. Trends in pharmacological sciences. 1989;10:74–77. doi: 10.1016/0165-6147(89)90082-5. [DOI] [PubMed] [Google Scholar]
- 77.Rahman Z, Gold SJ, Potenza MN, Cowan CW, Ni YG, He W, et al. Cloning and characterization of RGS9-2: a striatal-enriched alternatively spliced product of the RGS9 gene. J Neurosci. 1999;19:2016–2026. doi: 10.1523/JNEUROSCI.19-06-02016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Herve D. Identification of a specific assembly of the g protein golf as a critical and regulated module of dopamine and adenosine-activated cAMP pathways in the striatum. Front Neuroanat. 2011;5:48. doi: 10.3389/fnana.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pitcher JA, Inglese J, Higgins JB, Arriza JL, Casey PJ, Kim C, et al. Role of beta gamma subunits of G proteins in targeting the beta-adrenergic receptor kinase to membrane-bound receptors. Science (New York, NY. 1992;257:1264–1267. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- 80.Erdtmann-Vourliotis M, Mayer P, Ammon S, Riechert U, Hollt V. Distribution of G-protein-coupled receptor kinase (GRK) isoforms 2, 3, 5 and 6 mRNA in the rat brain. Brain Res Mol Brain Res. 2001;95:129–137. doi: 10.1016/s0006-8993(01)03046-3. [DOI] [PubMed] [Google Scholar]
- 81.Gainetdinov RR, Bohn LM, Sotnikova TD, Cyr M, Laakso A, Macrae AD, et al. Dopaminergic supersensitivity in G protein-coupled receptor kinase 6-deficient mice. Neuron. 2003;38:291–303. doi: 10.1016/s0896-6273(03)00192-2. [DOI] [PubMed] [Google Scholar]
- 82.Daigle TL, Ferris MJ, Gainetdinov RR, Sotnikova TD, Urs NM, Jones SR, et al. Selective Deletion of GRK2 Alters Psychostimulant-Induced Behaviors and Dopamine Neurotransmission. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nobles KN, Xiao K, Ahn S, Shukla AK, Lam CM, Rajagopal S, et al. Distinct phosphorylation sites on the beta(2)-adrenergic receptor establish a barcode that encodes differential functions of beta-arrestin. Sci Signal. 2011;4:ra51. doi: 10.1126/scisignal.2001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Attramadal H, Arriza JL, Aoki C, Dawson TM, Codina J, Kwatra MM, et al. Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. The Journal of biological chemistry. 1992;267:17882–17890. [PubMed] [Google Scholar]
- 85.Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol. 1999;13:358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- 86.Slifstein M, van de Giessen E, Van Snellenberg J, Thompson JL, Narendran R, Gil R, et al. Deficits in Prefrontal Cortical and Extrastriatal Dopamine Release in Schizophrenia: A Positron Emission Tomographic Functional Magnetic Resonance Imaging Study. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2014.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 88.Thompson M, Weickert CS, Wyatt E, Webster MJ. Decreased glutamic acid decarboxylase(67) mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J Psychiatr Res. 2009;43:970–977. doi: 10.1016/j.jpsychires.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 89.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 90.Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- 91.Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, et al. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 2012;62:1574–1583. doi: 10.1016/j.neuropharm.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends in neurosciences. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cho KK, Hoch R, Lee AT, Patel T, Rubenstein JL, Sohal VS. Gamma rhythms link prefrontal interneuron dysfunction with cognitive inflexibility in Dl×5/6(+/−) mice. Neuron. 2015;85:1332–1343. doi: 10.1016/j.neuron.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tseng KY, O'Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2007;17:1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]