RNA-Seq-based membrane proteomics provides insights into the biology of the oleaginous microalga Ettlia oleoabundans, allowing the identification of the novel proteins PSBS, MPH1, and RP2-CLC.
Abstract
Ettlia oleoabundans is a nonsequenced oleaginous green microalga. Despite the significant biotechnological interest in producing value-added compounds from the acyl lipids of this microalga, a basic understanding of the physiology and biochemistry of oleaginous microalgae is lacking, especially under nitrogen deprivation conditions known to trigger lipid accumulation. Using an RNA sequencing-based proteomics approach together with manual annotation, we are able to provide, to our knowledge, the first membrane proteome of an oleaginous microalga. This approach allowed the identification of novel proteins in E. oleoabundans, including two photoprotection-related proteins, Photosystem II Subunit S and Maintenance of Photosystem II under High Light1, which were considered exclusive to higher photosynthetic organisms, as well as Retinitis Pigmentosa Type 2-Clathrin Light Chain, a membrane protein with a novel domain architecture. Free-flow zonal electrophoresis of microalgal membranes coupled to liquid chromatography-tandem mass spectrometry proved to be a useful technique for determining the intracellular location of proteins of interest. Carbon-flow compartmentalization in E. oleoabundans was modeled using this information. Molecular phylogenetic analyses of protein markers and 18S ribosomal DNA support the reclassification of E. oleoabundans within the trebouxiophycean microalgae, rather than with the Chlorophyceae class, in which it is currently classified, indicating that it may not be closely related to the model green alga Chlamydomonas reinhardtii. A detailed survey of biological processes taking place in the membranes of nitrogen-deprived E. oleoabundans, including lipid metabolism, provides insights into the basic biology of this nonmodel organism.
Ettlia oleoabundans (taxonomic synonym of Neochloris oleoabundans) is a unicellular edaphic green microalga that belongs to the Chlorophyta phylum and is currently placed within the Chlorophyceae class (Chantanachat and Bold, 1962; Deason et al., 1991). It is a nonsequenced microalga classified as oleaginous due to its high lipid content (up to 56% [w/w] of its dry weight; Gouveia et al., 2009). Several abiotic stress conditions, such as high temperature (Yang et al., 2013), high salinity (Arredondo-Vega et al., 1995), and nitrogen deficiency (Tornabene et al., 1983; Li et al., 2008; Pruvost et al., 2009; Garibay-Hernández et al., 2013), trigger neutral lipid accumulation in this microalga. E. oleoabundans is a highly versatile organism, as it can grow in freshwater, wastewater (Levine et al., 2011; Wang and Lan, 2011; Yang et al., 2011; Olguín et al., 2015), and in culture media with salt concentrations up to seawater levels (Arredondo-Vega et al., 1995; Baldisserotto et al., 2012; Popovich et al., 2012). Moreover, it is able to grow under phototrophic, mixotrophic (Giovanardi et al., 2013; Baldisserotto et al., 2016), and heterotrophic (Wu et al., 2011; Morales-Sánchez et al., 2013) conditions. Owing to its high lipid content and growth versatility, E. oleoabundans is an organism of biotechnological interest. However, a basic understanding of its physiology is currently lacking, as most reports have focused on improving the lipid yield and productivity of E. oleoabundans under nitrogen deficiency conditions through different culture strategies and on evaluating how other environmental factors additionally control lipid production. At present, only a few reports have assessed the biology and biochemistry behind nitrogen deficiency and lipid accumulation in E. oleoabundans (Rismani-Yazdi et al., 2012; Benvenuti et al., 2015; Baldisserotto et al., 2016; Matich et al., 2016).
Rapidly developing postgenomics, systems biology approaches such as transcriptomics, proteomics, and metabolomics have become essential for understanding the physiology of different organisms, including microalgae (Jinkerson et al., 2011; Ndimba et al., 2013). Algal proteomics has been performed primarily with the model green alga Chlamydomonas reinhardtii for the analysis of subcellular compartments (Schmidt et al., 2006; Atteia et al., 2009; Terashima et al., 2010) and the characterization of the proteome under stress conditions (Chen et al., 2010; Baba et al., 2011; Castruita et al., 2011; Mühlhaus et al., 2011), including nitrogen starvation (Schmollinger et al., 2014; Valledor et al., 2014; Wase et al., 2014). Although C. reinhardtii currently provides the best model for microalgal lipid research (Liu and Benning, 2013), it is not an oleaginous species and may not represent the physiology of other species of biotechnological interest, as microalgae comprise an extremely diverse group of photosynthetic microorganisms (Hu et al., 2008). In recent years, a limited number of proteomics studies have been performed on nonmodel oleaginous strains such as Chlorella protothecoides (Gao et al., 2014), Chlorella sorokiniana (Ma et al., 2013), Chlorella vulgaris (Guarnieri et al., 2011, 2013), Isochrysis galbana (Song et al., 2013), and Nannochloropsis oceanica (Dong et al., 2013). However, proteomic analysis of nonmodel microalgae is still challenging, as the lack of a sequenced genome in most of them compromises the quality and quantity of the generated data (Ndimba et al., 2013; Wang et al., 2014).
Most of the proteomic studies performed on nonmodel microalgae are limited to the analysis of total protein extracts; therefore, the most abundant soluble proteins will be overrepresented. These approaches overlook the role played by microalgal membrane proteins, which have remained understudied despite the fact that the majority of lipid metabolism proteins have been proposed to be membrane associated (Natter et al., 2005; Joyard et al., 2010; Wang and Benning, 2012) and that nitrogen starvation is known to exert ultrastructural changes in microalgal cells (Moellering and Benning, 2010), including membrane lipid remodeling, turnover, and degradation (Li et al., 2012, 2014; Yoon et al., 2012).
In this study, microsomal membranes from E. oleoabundans cells submitted to nitrogen deprivation were analyzed via an RNA sequencing (RNA-Seq)-based proteomics approach (Wang et al., 2014) using the E. oleoabundans transcriptome generated by Rismani-Yazdi et al. (2012). To overcome the typical limitations of membrane proteomics due to the heterogenous, hydrophobic, and low-abundance nature of membrane proteins (Tan et al., 2008), a gel-free shotgun proteomics strategy was employed. In parallel, free-flow zonal electrophoresis (FFZE), a liquid-based matrix-free separation technology (Barkla et al., 2007; Wildgruber et al., 2014), was coupled to shotgun proteomics and uniquely employed to assess the intracellular location of novel identified proteins as well as to provide a detailed survey of biological processes related to energy and carbon flux in nitrogen-deprived E. oleoabundans, giving insights into the basic biology of this organism. Molecular phylogenetic analysis of proteins identified in this work and of 18S ribosomal DNA (rDNA) raised concerns regarding the taxonomic status of E. oleoabundans, as they support a close alliance between E. oleoabundans and species of the Trebouxiophyceae class that is contrary to its current classification.
RESULTS AND DISCUSSION
E. oleoabundans Membrane Proteome
To study the membrane proteome of the nonsequenced oleaginous microalga E. oleoabundans, microsomes from 4-d nitrogen-deprived cultures were isolated and subsequently analyzed using gel-free liquid chromatography-tandem mass spectrometry (LC-MS/MS).
As a first approach, product ion data were searched against the Viridiplantae protein database (TaxID 33090, unknown version; 677,107 entries) using the Mascot search program (Matrix Science). A total of 45 proteins (1,057 spectra) were identified with two or more unique peptides, from which only 30 were detected in at least two of four biological replicates. The small number of identified proteins can be attributed to the typical limitations of studying membrane proteins (Tan et al., 2008) but mostly to the lack of sequence data for nonsequenced organisms (Ndimba et al., 2013; Wang et al., 2014), such as E. oleoabundans. To overcome these limitations, an RNA-Seq-based proteomics strategy was established, using as a guide the E. oleoabundans de novo sequenced transcriptome (Rismani-Yazdi et al., 2012). The E. oleoabundans transcriptome comprises 56,550 nonredundant transcripts and was obtained from cells cultured under both nitrogen-replete and nitrogen-deprived conditions (Rismani-Yazdi et al., 2012). In order to generate an E. oleoabundans protein database, an in silico six-frame translation of the transcriptome was performed, yielding 54,652 nonredundant putative protein sequences. The E. oleoabundans protein database (unknown version; 53,921 entries) was merged with the Viridiplantae database and subsequently used for peptide and protein identification. Using this approach, 551 proteins (18,902 spectra) were identified, from which only 404 complied with the stringency described above. This was a 13.5-fold increase in identifications over using only the Viridiplantae database. This result shows that use of the E. oleoabundans translated transcriptome significantly improved protein identification and highlights the advantages of integrating de novo transcriptomic and proteomic analyses to study nonmodel microalgae (Guarnieri et al., 2011).
In order to describe the composition of the E. oleoabundans membrane proteome, the sequences from the 404 identified proteins were analyzed with transmembrane helix (HMMTOP version 2.0 and TMHMM version 2.0) and beta-barrel membrane protein (MCMBB and TMBETADISC-RBF) prediction programs (Krogh et al., 2001; Tusnády and Simon, 2001; Bagos et al., 2004; Ou et al., 2008). Proteins predicted to possess a transmembrane region by any of the four prediction programs were considered as integral membrane proteins. This analysis demonstrated that 57% of the E. oleoabundans membrane proteome is composed of integral membrane proteins; the remaining 43% can be classified as peripheral membrane proteins that do not transverse the membrane but may be associated with the membrane surface to varying extents (Tan et al., 2008).
Functional Annotation of the E. oleoabundans Membrane Proteome
To address the biological significance of the identified membrane proteins, we initially performed a functional annotation based on sequence similarity using the Blast2GO suite (Götz et al., 2008). From the total of 404 proteins, 391 returned a significant BLASTP match (E value cutoff ≤ 0.001) against the National Center for Biotechnology Information (NCBI) nonredundant protein sequences database. The majority of the proteins (92%) had best-hit homologs in species from the trebouxiophycean class, whereas only 3% possessed best-hit homologs with members of the chlorophycean class (Supplemental Fig. S1). This result resembles those obtained from the analysis of the E. oleoabundans transcriptome (Rismani-Yazdi et al., 2012), suggesting a closer proximity of E. oleoabundans to the trebouxiophycean Chlorella spp.
Although automatic annotations have been shown to be more reliable than generally believed (Škunca et al., 2012) and there is a demonstrated good performance of Blast2GO (Götz et al., 2008), functional misannotation in computational analysis remains a significant concern (Schnoes et al., 2009). To improve protein annotation, each of the 404 identified proteins were manually curated as described in “Materials and Methods.”
Following manual curation, the cellular location of 85% of the identified proteins was predicted (Supplemental Fig. S2A). The majority of the membrane proteins (41%) were located in the chloroplast, not surprising as this organelle occupies most of the microalga’s cell volume (Giovanardi et al., 2013). Despite this, most of the cellular compartments were represented in the membrane proteome, including the lipid droplets (LDs), which are structures induced by nitrogen deprivation (Davis et al., 2012; Popovich et al., 2012; Giovanardi et al., 2013). Cytoplasmic proteins comprised only 9% of the membrane proteome (Supplemental Fig. S2A). The majority of identified proteins (82%) were categorized into known biological processes (Supplemental Fig. S2B), which were grouped into three broad categories: protein and nitrogen metabolism (28%), energy production and homeostasis (23%), and carbon metabolism (15%). All the identified proteins are described in Supplemental Tables S1 to S15 and classified according to the biological process to which they are related. The corresponding protein homologs in the model organisms C. reinhardtii and Arabidopsis (Arabidopsis thaliana) also are indicated, as well as the predictions and the experimental evidence for the cellular locations of these proteins.
Novel Occurrence of the Photosynthesis-Related Proteins, Photosystem II Subunit S and Maintenance of Photosystem II under High Light1, in Green Microalgae
Photosystem II Subunit S (PSBS; or 22-kD protein) and Maintenance of Photosystem II under High Light1 (MPH1) are proteins related to well-characterized photoprotective responses that have been considered exclusive to higher photosynthetic organisms.
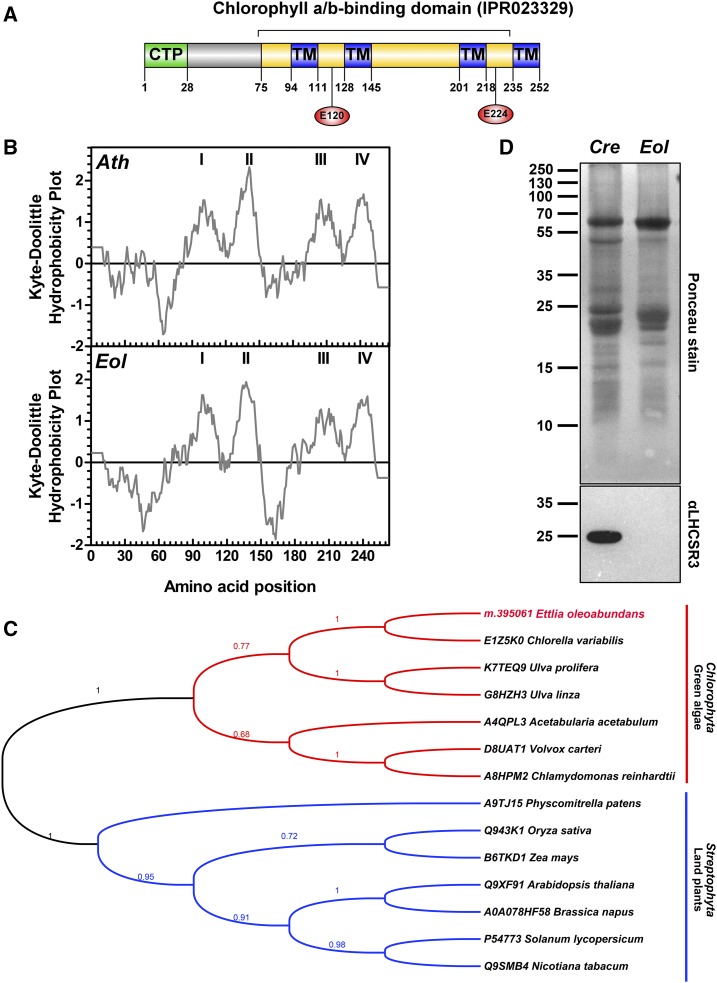
In this work, the E. oleoabundans PSBS protein (EoPSBS; m.395061; Supplemental Table S1) was identified in all four biological replicates with up to seven unique peptides and a maximum coverage of 29% of the predicted protein sequence. EoPSBS is composed of 252 amino acids with an estimated molecular mass of 28 kD considering the predicted chloroplast transit peptide (CTP; Fig. 1A) and of 25 kD following transit peptide cleavage. Similar to Arabidopsis PSBS (AtPSBS), EoPSBS comprises a chlorophyll a/b-binding protein domain (Fig. 1A), four membrane-spanning α-helices (Kyte-Doolittle [Fig. 1B] and HMMTOP version 2.0 [Fig. 1A]; Supplemental Fig S3A), and a predicted N-terminal CTP (28 amino acids; PredAlgo; Fig. 1A; Supplemental Fig. S3A). The two symmetrically arranged lumen-exposed Glu residues that are necessary for the PSBS pH-sensing mechanism and, thus, its function in land plants (Glu-122 and Glu-226 in AtPSBS; Li et al., 2002, 2004) are both conserved in EoPSBS (Glu-120 and Glu-224; Fig. 1A; Supplemental Fig. S3A). EoPSBS homologs from higher plants and green algae species were retrieved from the UniProtKB database (Fig. 1C; Supplemental Fig. S3A), although PSBS transcripts (Miller et al., 2010; Gerotto and Morosinotto, 2013) but not the corresponding protein (Bonente et al., 2008) had been identified in green microalgae until very recently, when PSBS was detected in C. reinhardtii upon high light acclimation (Correa-Galvis et al., 2016; Tibiletti et al., 2016). EoPSBS shares 75% identity with the predicted protein from Chlorella variabilis (class Trebouxiophyceae) and 46% with the corresponding proteins from chlorophycean species; in contrast, only 30% identity is shared with PSBS from land plants (Supplemental Fig. S3B). Phylogenetic analysis showed that PSBS is conserved along the green lineage (Viridiplantae); however, PSBS from green algae and land plants clustered into two distinct clades (Fig. 1C). This result suggests that PSBS was present in the common ancestor of extant green algae and land plants but that it evolved separately in these two phylogenetic groups, as has been suggested in previous evolutionary analyses of genomic and transcriptomic PSBS sequences (Koziol et al., 2007; Bonente et al., 2008; Gerotto and Morosinotto, 2013).
Figure 1.
Analysis of qE effector proteins (PSBS and LHCSR) in nitrogen-depleted E. oleoabundans. A, Protein architecture of EoPSBS. The identified protein domain signatures (InterPro; yellow), the predicted CTP (PredAlgo; green), and the transmembrane domains (TM; HMMTOP version 2.0; blue) are indicated. The conserved residues involved in the PSBS pH-sensing mechanism are shown (red circles). B, Hydrophobicity comparison of PSBS from E. oleoabundans (Eol) and Arabidopsis (Ath). Probable transmembrane domains (values greater than 0) are shown in the Kyte-Doolittle hydrophobicity plots (window size, 19). C, Phylogenetic analysis of PSBS homologs. Aligned sequences (Supplemental Fig. S3) were submitted for maximum likelihood (ML) analysis. The topology of the ML tree with the highest log likelihood (−3,122.6386) is shown. Bootstrap maximum likelihood (MLb) values are shown next to the branches. UniProtKB accession numbers for PSBS homologs are provided. D, Immunological detection of LHCSR in microsomes from E. oleoabundans and C. reinhardtii (Cre). The 12.5% (w/v) SDS-PAGE acrylamide gel was loaded with 20 μg of protein per lane.
Vascular plants rely on PSBS for the pH-regulated activation of the energy-dependent feedback deexcitation component (qE) of nonphotochemical quenching (NPQ) for photoprotection (Li et al., 2000; Niyogi and Truong, 2013). In contrast, eukaryotic algae, except for red algae, cryptophytes (Dittami et al., 2010), and peridinin-containing dinoflagellates (Boldt et al., 2012), commonly depend on LHC-Like Protein Stress Related (LHCSR; LI818 or LHCSX) for qE induction (Peers et al., 2009; Bailleul et al., 2010). Despite pH regulation of qE being restricted to LHCSR in C. reinhardtii, recent evidence has shown that its full NPQ capacity is also dependent on PSBS, whose substoichiometric accumulation is a prerequisite for further activation of the LHCSR-dependent qE mechanism (Correa-Galvis et al., 2016). These two mechanisms apparently overlapped at some point during evolution, as both proteins, LHCSR and PSBS, also have been identified in organisms that represent transitional states between green algae and vascular plants, where, contrary to what has been found in C. reinhardtii, these proteins function independently and additively in qE regulation (Alboresi et al., 2010; Gerotto et al., 2012; Mou et al., 2013; Zhang et al., 2013). In view of this, we searched for the presence of LHCSR in E. oleoabundans to determine if both qE effector proteins were present. An LHCSR homolog was not identified by searching the transcriptome (Rismani-Yazdi et al., 2012) and the corresponding in silico-translated proteome of E. oleoabundans. The absence of LHCSR was further confirmed by western-blot analysis of microsomes from nitrogen-deprived E. oleoabundans (Fig. 1D). The absence of an LHCSR homolog in E. oleoabundans questions the conservation of qE mechanisms within green microalgae.
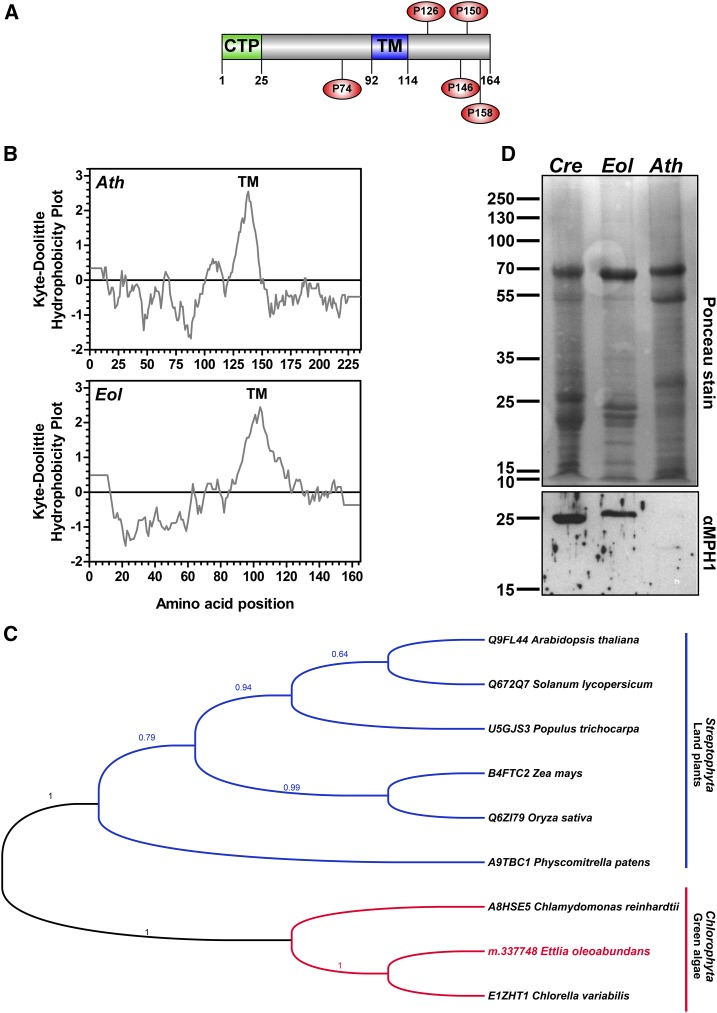
MPH1 is a Pro-rich intrinsic thylakoid protein that participates in the protection and stabilization of PSII against photooxidative damage in Arabidopsis under high-light stress (Liu and Last, 2015a, 2015b). Identification of an MPH1 homolog in the membranes of nitrogen-deprived E. oleoabundans (EoMPH1; m.337748; Supplemental Table S1) also was unexpected, since MPH1 has been reported as a protein specific to land plants (Liu and Last, 2015a, 2015b). In this study, EoMPH1 was identified in three out of four biological replicates, with up to three unique peptides and a maximum coverage of 19% of the predicted protein sequence. EoMPH1 is composed of 164 amino acids with an estimated molecular mass of 16.2 kD considering the CTP (Fig. 2A) and of 13.6 kD following its cleavage. It shares 15% to 20% sequence identity with MPH1 sequences from higher plants (Supplemental Fig. S4B), including structural features predicted for AtMPH1 (Liu and Last, 2015b): a single transmembrane domain (Kyte-Doolittle [Fig. 2B] and TMHMM version 2.0 [Fig. 2A]; Supplemental Fig. S4A), an N-terminal CTP (25 amino acids; PredAlgo; Fig. 2A; Supplemental Fig. S4A), and a high Pro content (6% of the protein), from which some interspersed Pro residues are conserved (Fig. 2A; Supplemental Fig. S4A). Based on the presence of MPH1 in E. oleoabundans, we searched for unreported homologs from other green microalgae species. The results identified predicted sequences from C. variabilis (class Trebouxiophyceae) and C. reinhardtii (class Chlorophyceae), which shared 68% and 28% identity, respectively, with EoMPH1 (Supplemental Fig. S4). Phylogenetic analysis showed that MPH1 sequences from green algae and land plants clustered into two distinct clades (Fig. 2C). To confirm the presence of MPH1 in membranes of green microalgae, microsomes from E. oleoabundans and C. reinhardtii were probed using a polyclonal antibody raised against AtMPH1 (Liu and Last, 2015b). A single band of approximately 25 kD was identified on the blot in both cases (Fig. 2D). Considering that both homologs have a predicted molecular mass close to 13 kD, this result suggests that microalgal MPH1 may form dimers. Contrary to this finding, a single band corresponding to the AtMPH1 predicted molecular mass (20 kD) was identified (Fig. 2D) in Arabidopsis membranes, whose low intensity may be attributed to its low abundance.
Figure 2.
MPH1 sequence analysis and identification in green microalgae membranes. A, Protein architecture of EoMPH1. The predicted CTP (PredAlgo; green) and transmembrane domain (TM; TMHMM version 2.0; blue) are indicated. Conserved Pro residues are shown (red circles). B, Hydrophobicity comparison of MPH1 from E. oleoabundans (Eol) and Arabidopsis (Ath). Probable transmembrane domains (values greater than 1.6) are shown in the Kyte-Doolittle hydrophobicity plots (window size, 19). C, Phylogenetic analysis of MPH1 homologs. Aligned sequences (Supplemental Fig. S4) were submitted for ML analysis. The topology of the ML tree with the highest log likelihood (−2,177.6951) is shown. MLb values are shown next to the branches. UniProtKB accession numbers for MPH1 homologs are provided. D, Immunological detection of MPH1 in microsomes from Arabidopsis (positive control; red box), E. oleoabundans, and C. reinhardtii (Cre). The 12.5% (w/v) SDS-PAGE acrylamide gel was loaded with 20 μg of protein per lane.
Retinitis Pigmentosa Type 2-Clathrin Light Chain, a Novel Domain Architecture Protein Identified in E. oleoabundans Membranes
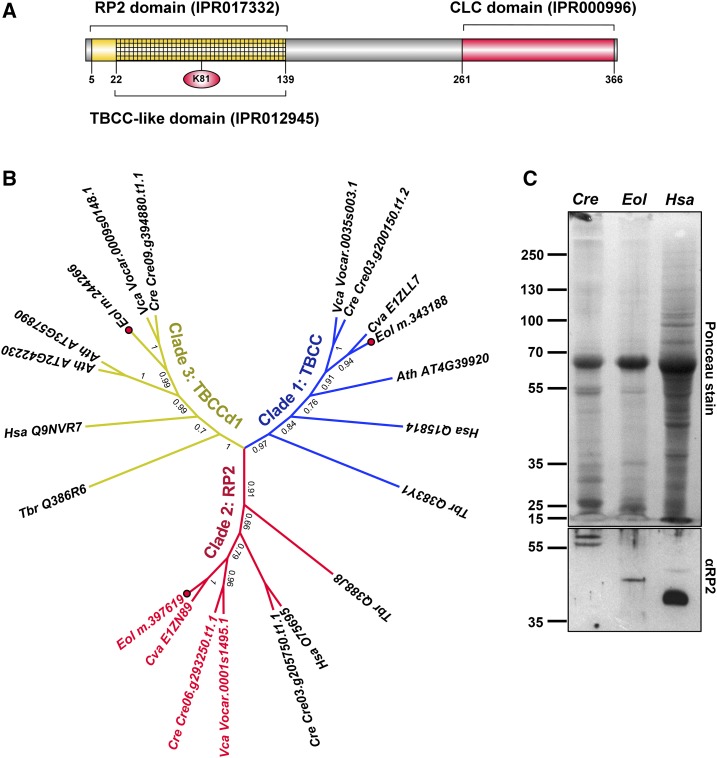
The proteomic analysis of E. oleoabundans membranes resulted in the detection of several unknown proteins. Among them, we identified protein m.397619 (Supplemental Table S9), which presents a domain architecture that has not been described previously. This protein comprises 368 amino acids and contains an N-terminal region corresponding to the Retinitis Pigmentosa Type 2 (RP2) protein family and a C terminus that comprises a Clathrin Light Chain (CLC) domain (Fig. 3A). Accordingly, we named this protein EoRP2-CLC. A survey of proteins with this architecture in the UniProtKB database showed that they are limited to certain unicellular eukaryotes, such as green microalgae from both Trebouxiophyceae and Chlorophyceae classes, several ciliated protozoa, and mold species from the Oomycetes class, but are not present in higher eukaryotes.
Figure 3.
EoRP2-CLC is a membrane TBCC domain-containing protein with a novel domain architecture. A, Protein architecture of EoRP2-CLC. The identified InterPro signatures and the Lys residue (red circle) that may correspond to a homologous substitution of the key Arg residue for GAP activity are indicated. B, ML analysis of TBCC domain-containing proteins. Clade 1/TBCC is in blue, clade 2/RP2 is in red, and clade 3/TBCCd1 is in yellow. RP2-CLC domain architecture proteins are highlighted in red text. Amino acid sequences were aligned with webPRANK. The topology of the ML tree with the highest log likelihood (−8,226.6668) is shown. MLb values are shown next to the branches. Accession numbers are provided: UniProtKB (Hsa, Tbr, and Cva), Phytozome version 10 (Cre and Vca), and The Arabidopsis Information Resource (Ath). Ath, Arabidopsis; Cre, C. reinhardtii; Cva, C. variabilis; Hsa, H. sapiens; Eol, E. oleoabundans (red dots); Tbr, Trypanosoma brucei; Vca, Volvox carteri. C, Immunological detection of RP2-like proteins in microsomes from C. reinhardtii and E. oleoabundans. The 10% (w/v) SDS-PAGE acrylamide gel was loaded with 20 μg of protein per lane. Total protein extracts (10 μL) from human (Hsa) C2BBe1 cells (clone of Caco-2) were analyzed as a positive control, where the 40-kD RP2 human protein was identified.
EoRP2-CLC is a Tubulin-Binding Cofactor C (TBCC) domain-containing protein, as it comprises a predicted TBCC-like domain within the N-terminal RP2 region (Fig. 3A). Three protein families with TBCC domains (Fig. 3B) have been described (Stephan et al., 2007). The first (clade 1/TBCC) is the canonical TBCC, which is essential for de novo native α/β-tubulin heterodimer formation by stimulating GTP hydrolysis in β-tubulin (Lundin et al., 2010); this clade comprises proteins from a diverse range of eukaryotes. The second (clade 2/RP2) contains homologs of human RP2, which are apparently restricted to eukaryotes capable of forming cilium/flagellum (Stephan et al., 2007). The third (clade 3/TBCCd1) comprises noncanonical TBCC domain-containing proteins, which lack a conserved catalytic Arg responsible for GTPase-activating protein (GAP) activity (Bartolini et al., 2002). However, an Arg residue located close to the Arg finger position in TBCC and RP2-like proteins (Supplemental Fig. S5) has been suggested to suffice for GAP activity in TBCCd1 proteins (Feldman and Marshall, 2009). E. oleoabundans has predicted protein homologs for each clade (Fig. 3B); however, only EoRP2-CLC was identified in the membrane proteome. EoRP2-CLC, together with the protein homolog from C. variabilis (UniProtKB no. E1ZN89), which is characterized by autosporic reproduction (Huss et al., 1999), clustered within the RP2 clade (Fig. 3B). Thus, the RP2 clade may not be restricted to cilium/flagellum-forming eukaryotes, as proposed previously (Stephan et al., 2007). Sequence analysis showed that only the TBCC domains from E. oleoabundans and C. variabilis RP2 sequences do not present the conserved catalytic Arg but instead showed a homologous substitution with a Lys residue (Fig. 3A; Supplemental Fig. S5), which may suffice for GAP activity.
The presence of RP2-CLC proteins in the microsomes from both E. oleoabundans and C. reinhardtii was confirmed using a polyclonal antibody raised against Homo sapiens RP2 (HsRP2; Fig. 3C). A single protein band with a molecular mass slightly higher than that predicted for EoRP2-CLC (39 kD) was identified in E. oleoabundans. In C. reinhardtii, two protein bands around the molecular mass predicted for CrRP2-CLC (Cre06.g293250.t1.1; 58 kD) were detected, where one of these bands may correspond to a posttranslationally modified CrRP2-CLC, similar to HsRP2 that is known to be subjected to dual N-terminal acylation (Chapple et al., 2000).
RP2-CLC proteins have a domain architecture that differs from currently characterized RP2 proteins and are present in both flagellated and nonflagellated microalgae (Fig. 3B), suggesting that they may be involved in other noncilia/flagella-specific functions. Contrary to microalgae of the Chlorophyceae class, no obvious homologs to currently known CLC proteins were identified in either the E. oleoabundans in silico-translated protein database or the predicted proteins for C. variabilis (pico-PLAZA; Vandepoele et al., 2013). CLC also has been shown to be absent in other unicellular eukaryotes, such as Cyanidioschyzon merolae (Misumi et al., 2005), Entamoeba histolytica, and Giardia lamblia (Manna et al., 2015); however, this might be due to the high divergence of CLC sequences among eukaryotes (Wang et al., 2003). The existence of NoRP2-CLC as the only candidate for a CLC-harboring protein in E. oleoabundans, together with current evidence that supports a role of HsRP2 in post-Golgi trafficking (Evans et al., 2010), suggests that EoRP2-CLC may play a role in the formation/trafficking of clathrin-coated vesicles in E. oleoabundans cells.
Determining the Subcellular Location of Novel Proteins via FFZE Membrane Fractionation Coupled to Mass Spectrometry-Based Analysis
To assess the subcellular locations of the novel microalgal proteins identified in this work, we employed a membrane fractionation approach. In this study, we avoided traditional fractionation techniques by using FFZE, a liquid-based high-resolution membrane separation technique based on surface charge that has proved useful for subcellular proteome sample preparation (Barkla et al., 2007; Wildgruber et al., 2014; de Michele et al., 2016).
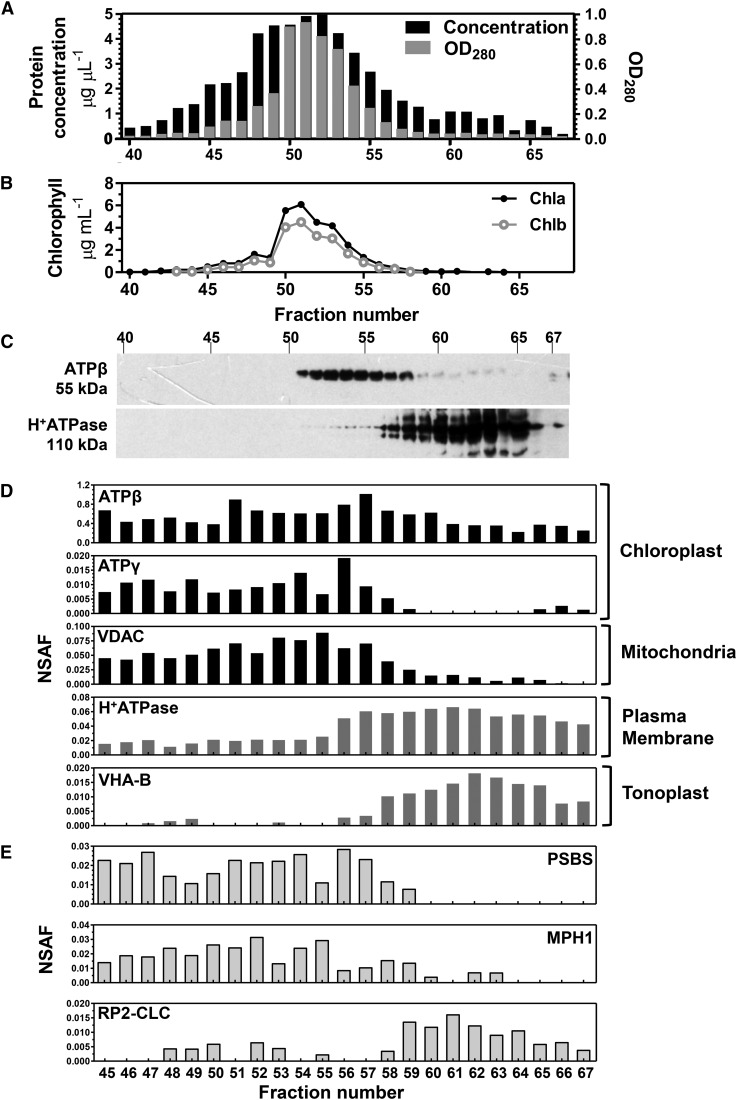
E. oleoabundans microsomes from nitrogen-deprived cultures were separated into 96 individual FFZE fractions (Fig. 4A), and each fraction was subjected to either direct chlorophyll measurements (Fig. 4B) or western-blot analysis against protein markers for both the chloroplast and the plasma membrane (Fig. 4C). Chloroplast membranes were detected in fractions 51 to 58, while more positively charged fractions (56–67) comprised the plasma membrane (Fig. 4C). Individual FFZE fractions, from 45 to 67, were then subjected to shotgun proteomics analysis to gain a more comprehensive overview of the protein profile of each of these fractions. A spectral counting-based quantitative approach, expressed in terms of the normalized spectral abundance factor (NSAF; Zhang et al., 2010), was used to determine the distribution of protein markers specific for different subcellular compartments among the analyzed FFZE fractions (Fig. 4D). Similar to the protein-blot analysis, the mass spectrometry (MS)-based analysis confirmed the presence of two different membrane populations but showed that the chloroplast fractions also were enriched in mitochondrial membranes (fractions 45–58), whereas the plasma membrane fractions comigrated with vacuolar membranes (fractions 56–67; Fig. 4D).
Figure 4.
Subcellular locations of novel proteins from E. oleoabundans via FFZE fractionation coupled to MS-based analysis. Microsomal membranes from nitrogen-deficient cultures were separated by FFZE. A, Protein profile of FFZE fractions. OD280, Optical density at 280 nm. B, Chlorophyll a and b concentrations in FFZE fractions. C, Immunological detection in the respective fractions of ATPβ (a chloroplast marker) and H+-ATPase (a plasma membrane marker). The 10% (w/v) SDS-PAGE acrylamide gel was loaded with 15 μg of protein per lane. The approximate molecular masses of the detected proteins are shown. D, Graphical representation of the normalized spectral count (NSAF values) of protein markers specific for subcellular compartments among the FFZE fractions. Individual FFZE fractions were analyzed by LC-MS/MS. The identification numbers for the surveyed protein markers are as follows: gi|416678 (ATPβ), m.392881 (ATPγ), m.378383 (VDAC), m.363780 (H+-ATPase), and m.395664 (VHA-B). E, Graphical representation of the NSAF values of PSBS, MPH1, and RP2-CLC among the FFZE fractions.
The MS-based analysis of the FFZE fractions was further employed to assess the subcellular location of EoPSBS and EoMPH1. Peptides for these proteins were detected, and their distribution profiles within the FFZE fractions were mapped with those from known marker proteins. These two photosynthesis-related proteins presented similar distributions to that shown by the chloroplast-enriched membrane fractions (Fig. 4E). These results, together with the evidence that both proteins possess a predicted N-terminal CTP (Figs. 1A and 2A), confirmed that EoPSBS and EoMPH1 are chloroplastic membrane proteins, as has been demonstrated for their land plant homologs (Li et al., 2000; Ferro et al., 2010; Liu and Last, 2015b). Similar analysis for the distribution of EoRP2-CLC among the FFZE fractions showed that this protein was more abundant in the plasma membrane-enriched fractions (Fig. 4E), an observation that agrees with the RP2 plasma membrane localization in vertebrates (Chapple et al., 2002; Grayson et al., 2002).
Molecular Phylogenetic Analysis of Identified Proteins Places E. oleoabundans within Trebouxiophycean Algae
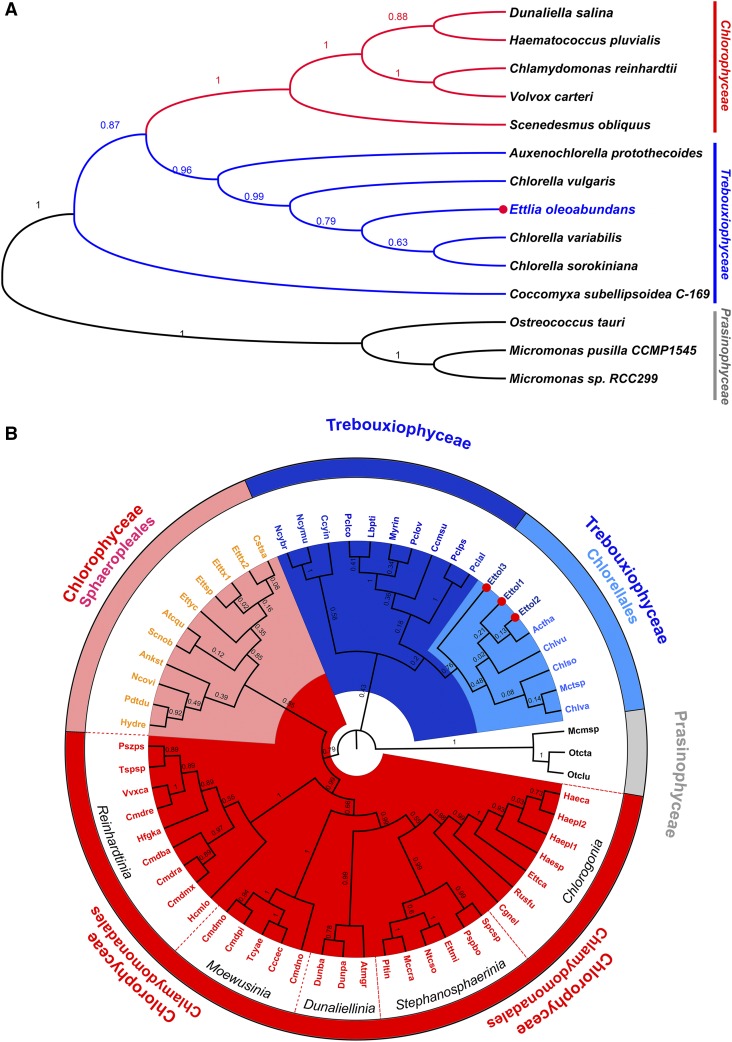
E. oleoabundans is a coccoid green microalga with a complex taxonomic history. It was initially classified within the Neochloris genus (Sphaeropleales) but later reclassified into the Ettlia genus (Chlamydomonadales), which comprised uninucleate cells with thin-walled zoospores (Deason et al., 1991; Guiry and Guiry, 1996). Despite that most researchers commonly refer to this microalga as Neochloris oleoabundans, it is currently classified as Ettlia oleoabundans, which is placed within the Chlorophyceae class according to the classification of the Ettlia genus type species, Ettlia carotinosa (Guiry and Guiry, 1996; Pegg et al., 2015).
The results from this work raised concerns regarding the taxonomic status of E. oleoabundans. Most of the identified proteins (greater than 90%) had best-hit homologs in species from the trebouxiophycean class (Supplemental Fig. S1), and phylogenetic analysis of identified proteins, including PSBS (Fig. 1C), MPH1 (Fig. 2C), TBCC, RP2-CLC (Fig. 3B), and enolase (Supplemental Fig. S7), showed a closer relationship between E. oleoabundans and C. variabilis (Trebouxiophyceae) rather than with chlorophycean species. Moreover, evidence for zoospore formation in several Ettlia spp. is lacking (Yoo et al., 2013), including E. oleoabundans, for which only autosporic reproduction has been observed, similar to Chlorella spp. of the trebouxiophycean class (Huss et al., 1999).
To assess the taxonomy of E. oleoabundans, a molecular phylogenetic analysis based on a multigene approach was performed. This increased the power of discrimination and robustness of the phylogenetic analysis compared with single-gene analysis (Moreira et al., 2000; Gontcharov et al., 2004; Tippery et al., 2012). Six proteins proposed as microalgal phylogenetic markers (Moreira et al., 2000; Tippery et al., 2012; Wei et al., 2013) were chosen, two per genome (Supplemental Table S16): nucleus-encoded Actin and Elongation Factor1-α (EF-1α); plastid-encoded Photosystem II D1 (PSBA) and Rubisco Large Chain (RBCL); and mitochondria-encoded Cytochrome Oxidase Subunit1 (COX1) and COX2. Amino acid sequences representative for the three green microalgae classes, Chlorophyceae, Trebouxiophyceae, and Prasinophyceae (outgroup), were retrieved, aligned, and concatenated. Phylogenetic analysis of the concatenated markers placed E. oleoabundans as a close relative of the Chlorella spp. within the Trebouxiophyceae class (Fig. 5A), a relationship that was strongly supported by the bootstrap value from the maximum likelihood analysis (MLb = 0.79). The relationship of E. oleoabundans with trebouxiophycean algae, rather than with the chlorophycean class, is reinforced by the analysis of E. oleoabundans COX2 (EoCOX2; m.110997; Supplemental Table S2). E. oleoabundans contains an orthodox intact mitochondria-encoded COX2, identified in this study by a single polypeptide, which lacks a predicted N-terminal mitochondrial targeting sequence (PredAlgo). This protein showed around 80% identity to orthodox COX2 homologs from members of the trebouxiophycean class (Supplemental Fig. S6B). Phylogenetic analysis confirmed the close relationship of EoCOX2 with orthodox COX2 from Chlorella spp. rather than with homologs from chlorophycean algae, which clustered into a different clade (Supplemental Fig. S6A). EoCOX2 clearly differs from its homologs in chlorophycean algae, characterized by exhibiting an atypical COX2 heterodimer as a consequence of a lineage-specific fragmentation and nuclear relocation of the mitochondrial COX2 gene (Pérez-Martínez et al., 2001; Rodríguez-Salinas et al., 2012).
Figure 5.
Molecular phylogenetic analysis of E. oleoabundans. A, ML analysis of concatenated nucleus-encoded (EF-1α and Actin), plastid-encoded (PSBA and RBCL), and mitochondria-encoded (COX1 and COX2) amino acid sequences (Supplemental Table S16). The topology of the ML tree with the highest log likelihood (−20,458.0427) is shown. B, ML analysis of 18S rDNA nucleotide sequences (Supplemental Table S17); Ettol2 corresponds to the sequence obtained in this work. The topology of the ML tree with the highest log likelihood (−12,721.9945) is shown. MLb values are shown next to the branches. E. oleoabundans sequences are highlighted (red dots). Green microalgae classes are denoted as follows: Chlorophyceae (red), Trebouxiophyceae (blue), and Prasinophyceae (gray; outgroup). Major taxa represented within these classes are denoted in B.
Due to the reduced availability of completely sequenced microalgal genomes, the multigene approach was performed with a reduced taxon sampling. To improve the accuracy of the analysis, we performed an 18S rDNA phylogenetic analysis with increased taxon sampling, which included three independent 18S partial sequences for E. oleoabundans, one of them obtained in this work (Fig. 5B; Supplemental Table S17). Two major taxa within the Chlorophyceae class, where species of the Neochloris and Ettlia genera are currently classified (Sphaeropleales and Chlamydomonadales), were highly represented. Taxon sampling also was increased for the diverse trebouxiophycean class. The 18S phylogeny confirmed the extremely close relationship between E. oleoabundans and trebouxiophyceaen microalgae, as all three 18S E. oleoabundans sequences clustered together within this class, particularly in the well-defined Chlorellales lineage supported by a robust bootstrapping score (MLb = 0.76; Fig. 5B). Other Ettlia spp. considered in this analysis were still placed in several groups within the chlorophycean class, supporting previous concerns regarding the classification of the species from this genus (Pegg et al., 2015).
Altogether, our results provide compelling evidence for reclassifying E. oleoabundans into the trebouxiophycean class, close to the Chlorellales lineage, and indicate that it is not closely related to chlorophycean microalgae.
Lipid Metabolism Represented in the E. oleoabundans Membrane Proteome
E. oleoabundans has shown potential for biotechnological applications, as its lipid acyl chains are considered an energy-rich feedstock for the production of biofuels and value-added compounds (Hu et al., 2008; Garibay-Hernández et al., 2013; Liu and Benning, 2013). Analysis of the E. oleoabundans membrane proteome under lipid accumulation conditions (nitrogen deprivation) presents an opportunity to study proteins related to lipid metabolism, since many of them are membrane associated (Natter et al., 2005; Joyard et al., 2010; Wang and Benning, 2012). Table I lists the proteins involved in acetyl-CoA synthesis and lipid metabolism identified in this study.
Table I. Acetyl-CoA and lipid metabolism proteins identified in membranes of nitrogen-depleted E. oleoabundans.
Acetyl-CoA and lipid metabolism proteins identified with at least two unique peptides in two or more biological replicates of total microsomal membrane samples are described. Very-low-abundance proteins that were identified exclusively in one biological replicate and/or in FFZE membrane fractions are highlighted with asterisks. The calculated molecular masses are shown, together with the corresponding protein homologs in the model organisms C. reinhardtii (Cr) and Arabidopsis (At) and their corresponding percentage identity values (%ID). Common abbreviations (Abbr.) of the identified proteins are provided. Subcellular localizations were predicted (Pr) using PredAlgo. Curated cell locations (Cu) were established according to the protein homologs. References are provided for protein homologs whose cell locations have been demonstrated experimentally (NA, not available). C, Chloroplast; Cy, cytoplasm; FAE, fatty acid elongase complex; FAS, fatty acid synthase complex; M, mitochondria; O, other; PM, plasma membrane; PX, peroxisome; SP, signal peptide.
| Protein Identifier | Curated Description | Abbr. | Molecular Mass | EC No. | Best Cr Homolog |
Best At Homolog |
Subcellular Localization |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| JGI Version 5.5 Identifier | %ID | The Arabidopsis Information Resource Version 10 Identifier | %ID | Pr | Cu | Reference | |||||
| Acetyl-CoA synthesis | |||||||||||
| m.73526 | Pyruvate dehydrogenase E1 component subunit α, chloroplastic*a | PDH | 47 | 1.2.4.1. | Cre02.g099850.t1.1 | 75 | AT1G01090 | 70 | C | Cb | Terashima et al. (2010) |
| E1α | |||||||||||
| m.371437 | Pyruvate dehydrogenase E1 component subunit β, chloroplastic | PDH | 41 | 1.2.4.1. | Cre03.g194200.t1.2 | 86 | AT1G30120 | 69 | C | Cb | Terashima et al. (2010) |
| E1β | |||||||||||
| m.130266 | Pyruvate dehydrogenase E2 component, chloroplastic | PDH E2 | 50 | 2.3.1.12 | Cre03.g158900.t1.2 | 74 | AT3G25860 | 54 | C | Cb | Terashima et al. (2010) |
| m.324850 | Pyruvate dehydrogenase E3 component, chloroplastic | PDH E3 | 63 | 1.8.1.4. | Cre01.g016514.t1.1 | 72 | AT3G16950 | 60 | C | Cb | Terashima et al. (2010) |
| m.396299 | ATP-citrate synthase α-chain protein*c | ACLA | 47 | 2.3.3.8. | Cre05.g241850.t1.2 | 62 | AT1G10670 | 65 | SP | Cyb | Fatland et al. (2002) |
| m.391431 | ATP-citrate synthase β-chain protein | ACLB | 76 | 2.3.3.8. | Cre02.g088600.t1.2 | 77 | AT5G49460 | 75 | O | Cyb | Fatland et al. (2002) |
| Lipid metabolism | |||||||||||
| Acyl-lipid biosynthesis | |||||||||||
| m.212887 | Acetyl-CoA carboxylase carboxyl transferase subunit α, chloroplastic | ACC | 60 | 6.4.1.2. | Cre12.g519100.t1.2 | 73 | AT2G38040 | 54 | C | Cb | Terashima et al. (2010) |
| α-CT | |||||||||||
| gi|3023244 | Acetyl-CoA carboxylase carboxyl transferase subunit β, chloroplastic*a | ACC | 47 | 6.4.1.2. | Cre12.g484000.t1.1 | 75 | ATCG00500 | 61 | O | Cb | Terashima et al. (2010) |
| β-CT | |||||||||||
| m.390372 | Biotin carboxylase, chloroplastic | ACC BCR | 61 | 6.3.4.14 6.4.1.2. | Cre08.g359350.t1.2 | 80 | AT5G35360 | 74 | C | Cb | Terashima et al. (2010) |
| m.332618 | 3-Hydroxyacyl-[acyl-carrier-protein] dehydratase, chloroplastic*a | FAS HAD | 25 | 4.2.1.59. | Cre03.g208050.t1.2 | 70 | AT5G10160 | 54 | M | Cb | Terashima et al. (2010) |
| m.179214 | Enoyl-[acyl-carrier-protein] reductase [NADH], chloroplastic*c | FAS ENR | 39 | 1.3.1.9. | Cre06.g294950.t1.1 | 82 | AT2G05990 | 76 | C | Cb | Terashima et al. (2010) |
| m.84123 | β-Ketoacyl-CoA reductase*c | FAE KCR | 39 | 1.1.1.330. | Cre09.g392430.t1.1 | 32 | AT1G67730 | 43 | SP | Eb | Beaudoin et al. (2009) |
| m.368848 | Long-chain acyl-CoA synthetase A | LCS | 71 | 6.2.1.3 | Cre13.g566650.t2.1 | 58 | AT4G23850 | 48 | O | LDb | Moellering and Benning (2010) |
| m.224985 | Long-chain acyl-CoA synthetase B | LCS | 76 | 6.2.1.3 | Cre13.g566650.t2.1 | 47 | AT4G11030 | 43 | SP | LDb | Moellering and Benning (2010) |
| m.371326 | Long-chain acyl-CoA synthetase C | LCS | 67 | 6.2.1.3 | Cre12.g507400.t1.2 | 63 | AT5G27600 | 53 | M | Md | NA |
| Isoprenoid biosynthesis via the mevalonate pathway | |||||||||||
| m.149328 | Acetyl-CoA acetyltransferase*a | ACAT | 51 | 2.3.1.9. | Cre02.g146050.t1.2 | 64 | AT5G47720 | 59 | M | Cyb | Carrie et al. (2007) |
| Glycerolipid biosynthesis | |||||||||||
| m.241864 | Glycerol-3-phosphate acyltransferase*e | GPAT | 54 | 2.3.1.15. | Cre06.g273250.t1.2 | 55 | AT5G60620 | 60 | O | Eb, LDb | Gidda et al. (2009); Nguyen et al. (2011) |
| m.357823 | 1-Acyl-sn-glycerol-3-phosphate acyltransferase, chloroplastic*a | LPAAT | 37 | 2.3.1.51. | Cre09.g398289.t1.1 | 63 | AT4G30580 | 56 | C | Cb, LDb | Ferro et al. (2010); Nguyen et al. (2011) |
| m.250190 | Acyltransferase family protein*c | 54 | NA | NA | NA | NA | NA | C | U | NA | |
| Lipid signaling | |||||||||||
| m.34057 | Phosphatidylinositol 4-kinase α*a | PI4Kα | 213 | 2.7.1.67. | Cre05.g245550.t1.1 | 45 | AT1G49340 | 39 | O | PMb, LDb | Nguyen et al. (2011); Zhang and Peck (2011) |
| m.226782 | Sac1p-like phosphoinositide phosphatase*a | SAC1 | 69 | 3.1.1.- | Cre09.g388750.t1.2 | 43 | AT3G51460 | 39 | O | Eb, LDb | Despres et al. (2003); Nguyen et al. (2011) |
| LD structural proteins | |||||||||||
| m.413736 | Major lipid droplet protein | MLDP | 28 | NA | Cre12.g491550.t1.2 | 24 | NA | NA | O | LDb | Davidi et al. (2012) |
| m.392627 | Probable plastid-lipid associated protein A, chloroplastic | PLAP | 59 | NA | Cre07.g325736.t1.1 | 42 | AT5G19940 | 32 | M | Cb | Terashima et al. (2010) |
| m.50827 | Probable plastid-lipid associated protein B, chloroplastic | PLAP | 42 | NA | Cre03.g189300.t1.1 | 44 | AT4G04020 | 48 | M | Cb | Ferro et al. (2010) |
| m.244306 | Probable plastid-lipid associated protein C, chloroplastic | PLAP | 23 | NA | Cre03.g188650.t1.2 | 62 | AT3G26070 | 58 | C | Cb | Terashima et al. (2010) |
| gi|132270 | Rubber elongation factor protein*a | REF | 15 | NA | NA | NA | AT3G05500 | 48 | O | LDb | Horn et al. (2013) |
| Lipid trafficking | |||||||||||
| m.216464 | Membrane-associated 30-kD protein, chloroplastic | VIPP1 | 33 | NA | Cre13.g583550.t1.2 | 54 | AT1G65260 | 54 | C | Cb | Nordhues et al. (2012) |
| m.362261 | Protein trigalactosyldiacylglycerol 2, chloroplastic | TGD2 | 44 | NA | Cre16.g694400.t1.2 | 57 | AT3G20320 | 40 | O | Cb | Terashima et al. (2010) |
| m.116135 | Phospholipid-transporting ATPase*a | ALA | 159 | 3.6.3.1. | Cre16.g656500.t1.1 | 32 | AT1G59820 | 33 | O | Eb, PMb | Poulsen et al. (2008); Mitra et al. (2009) |
| m.417181 | ABC transporter G family member A | ABCG | 70 | 3.6.3.- | Cre07.g313250.t1.2 | 57 | AT3G55100 | 31 | O | PMd | NA |
| m.306564 | ABC transporter G family member C | ABCG | 69 | 3.6.3.- | Cre07.g313250.t1.2 | 55 | AT3G55100 | 29 | O | PMd | NA |
| m.117336 | Chloroplast J-like domain-containing protein | CJD1 | 30 | NA | Cre03.g171100.t1.1 | 34 | AT1G08640 | 26 | C | Cb | Ajjawi et al. (2011) |
| Lipases and fatty acid β-oxidation | |||||||||||
| m.419400 | Putative triacylglycerol lipase*a | TGL | 47 | 3.1.1.3. | Cre07.g348550.t1.1 | 42 | AT5G67050 | 37.3 | SP | Cyd | NA |
| m.225854 | Acyl-CoA oxidase A, peroxisomal*a | ACX | 75 | 1.3.3.6. | Cre05.g232002.t1.1 | 66 | AT5G65110 | 57.9 | O | PXb | Stabenau et al. (1984) |
| m.366023 | Acyl-CoA oxidase B, peroxisomal*e | ACX | 74 | 1.3.3.6. | Cre11.g467350.t1.2 | 62 | AT1G06290 | 36 | O | PXb | Stabenau et al. (1984) |
| m.420224 | Fatty acid β-oxidation multifunctional protein, peroxisomal*c | MFP | 77 | 4.2.1.17. 1.1.1.35. | Cre16.g695050.t1.2 | 66 | AT3G06860 | 55 | SP | PXb | Stabenau et al. (1984) |
| m.356362 | 2,4-Dienoyl-CoA reductase, peroxisomal*c | RED | 35 | 1.3.1.4. | Cre17.g731850.t1.2 | 64 | AT3G12800 | 53 | SP | PXb | Reumann et al. (2009) |
Protein identified exclusively in FFZE membrane fractions bExperimental evidence of subcellular location is available. cProtein identified in one biological replicate of total microsomal samples and in FFZE membrane fractions. dExperimental evidence of subcellular location is not available. eProtein identified in only one biological replicate of total microsomal samples.
Acetyl-CoA Synthesis
The direct carbon precursor for de novo fatty acid synthesis in photosynthetic organisms is plastidic acetyl-CoA, which is synthesized directly by the activity of the chloroplastic pyruvate dehydrogenase (PDH) complex via the oxidative decarboxylation of glycolysis-derived pyruvate (Shtaida et al., 2015). We identified the four subunits of the chloroplastic PDH complex in the membranes of nitrogen-deprived E. oleoabundans (E1α, E1β, E2, and E3; Table I; Fig. 6). Cytoplasmic production of acetyl-CoA also was represented by the identification of the ATP-citrate synthase α- and β-subunits (Table I; Fig. 6), whose cytoplasmic location has been demonstrated in Arabidopsis (Fatland et al., 2002). ATP-citrate synthase has been proposed as a key enzyme for lipid accumulation in mammals, oleaginous yeast, fungi (Courchesne et al., 2009), and C. reinhardtii (Wase et al., 2014).
Figure 6.
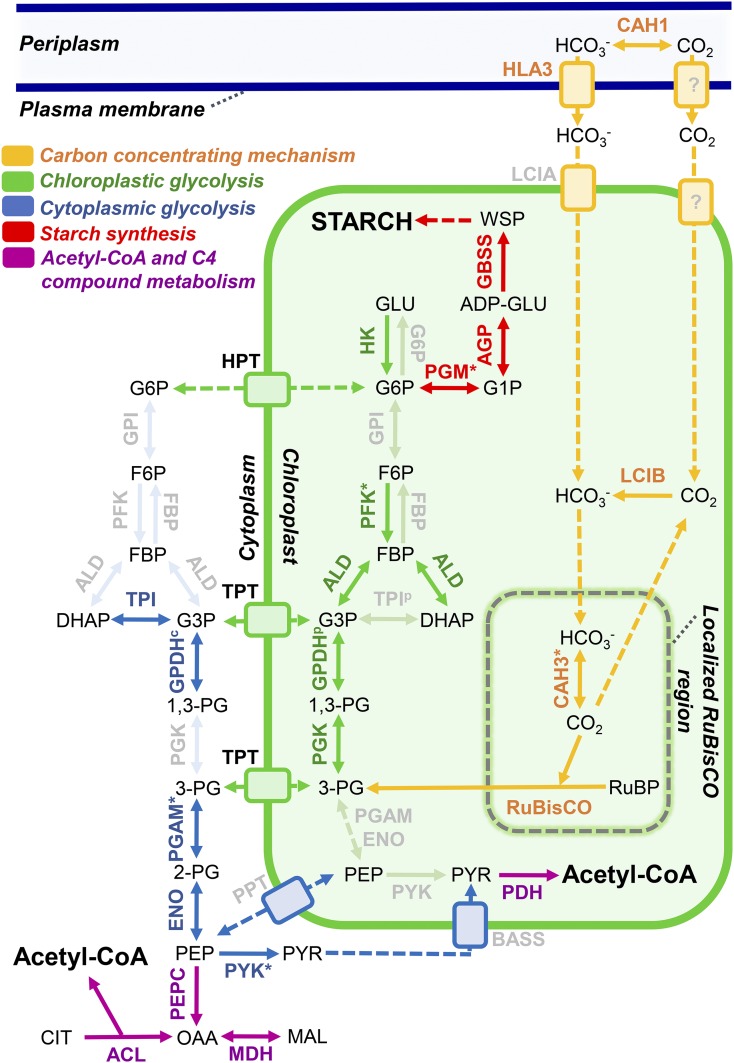
Carbon metabolism in nitrogen-deficient E. oleoabundans. Graphical representation is shown for the carbon metabolism proteins identified by LC-MS/MS in the membrane proteome of E. oleoabundans. All proteins were identified in FFZE fractions except hexokinase, which was identified exclusively in total microsome samples. Very-low-abundance proteins that were identified exclusively in FFZE fractions and not in total microsomes are highlighted with asterisks. Not identified proteins are shown in a clear gray color. Subcellular locations were predicted using PredAlgo together with experimental evidence available for the corresponding homologs. Identified proteins are described in Supplemental Table S4. Protein abbreviations are as follows: ACL, ATP-citrate synthase; AGP, Glc-1-P adenylyltransferase; ALD, aldolase; BASS, Bile Acid:Na+ Symporter, sodium/pyruvate cotransporter; CAH1, carbonic anhydrase, periplasmic; CAH3, carbonic anhydrase, chloroplastic; ENO, enolase; FBP, Fru-1,6-bisphosphatase; G6P, Glc-6-phosphatase; GBSS, granule-bound starch synthase; GPDHc, glyceraldehyde-3-phosphate dehydrogenase, cytosolic; GPDHp, glyceraldehyde-3-phosphate dehydrogenase A, chloroplastic; GPI, Glc-6-P isomerase; HLA3, probable inorganic carbon transporter HLA3; HK, hexokinase; HPT, UhpC-type hexose phosphate translocator; LCIA, putative inorganic carbon transporter LCIA; LCIB, LCIB family protein; MDH, malate dehydrogenase, cytoplasmic; PDH, pyruvate dehydrogenase; PEPC, phosphoenolpyruvate carboxylase; PFK, phosphofructokinase; PGAM, phosphoglycerate mutase; PGK, phosphoglycerate kinase; PGM, phosphoglucomutase; PPT, phosphoenolpyruvate/phosphate translocator; PYK, pyruvate kinase; TPI, triose phosphate isomerase; TPT, triose phosphate/phosphate translocator; RuBisCO, ribulose-1,5-biphosphate carboxylase. Compound abbreviations are as follows: ADP-GLU, ADP-Glc; CIT, citrate; DHAP, dihydroxyacetone phosphate; FBP, Fru-1,6-bisphosphate; F6P, Fru-6-P; G3P, glyceraldehyde-3-phosphate; GLU, Glc; G1P, Glc-1-P; G6P, Glc-6-P; MAL, malate; OAA, oxaloacetate; PEP, phosphoenolpyruvate; 1,3-PG, 1,3-bisphosphoglycerate; 2-PG, 2-phosphoglycerate; 3-PG, 3-phosphoglycerate; PYR, pyruvate; RuBP, ribulose-1,5-biphosphate; WSP, water-soluble polysaccharide.
Lipid Metabolism
The membrane proteome of E. oleoabundans was composed of 3.5% of proteins related to lipid metabolism (Table I). A similar amount of lipid metabolism-related proteins (Table I, proteins highlighted with asterisks) were additionally identified in only one replicate of the total microsomal samples and/or through MS analysis of the FFZE fractions where sample complexity was reduced. This demonstrates that the low abundance of lipid biosynthetic proteins hindered their identification in E. oleoabundans membranes.
Among the proteins identified in E. oleoabundans membranes, the committed step for fatty acid biosynthesis catalyzed by the heteromeric acetyl-CoA carboxylase (ACC; Stern, 2009) was represented. Two ACC chloroplast-targeted components, α-carboxyl transferase and biotin carboxylase (Table I), were identified in all four biological replicates of total microsomal samples, whereas the chloroplast-encoded β-carboxyl transferase subunit (Table I) was detected exclusively through MS analysis of the FFZE fractions. The fourth ACC component, biotin carboxyl carrier protein, was not detected, probably due to its low abundance and low molecular mass (26 kD) predicted from its transcript (Rismani-Yazdi et al., 2012). Incomplete detection of the ACC constituents is more frequent than expected, as only one or two subunits have been identified in several proteomics studies performed in Arabidopsis (Ferro et al., 2003; Froehlich et al., 2003; Kleffmann et al., 2004; Peltier et al., 2006) and C. reinhardtii (Bienvenut et al., 2011; Schmollinger et al., 2014). The chloroplastic fatty acid synthesis also was represented in this work by the identification of 3-hydroxyacyl-ACP dehydratase and enoyl-ACP reductase (Table I), which are components of the multipartite (type II) fatty acid synthase complex (Li-Beisson et al., 2015). In agreement with the existence of very-long-chain fatty acids (acyl chain length beyond 18C) in nitrogen-deprived E. oleoabundans (Tornabene et al., 1983; Garibay-Hernández et al., 2013; Matich et al., 2016), we identified a homolog of the β-ketoacyl-CoA reductase (Table I), a component of the endoplasmic reticulum-bound multienzymatic fatty acid elongase complex (Haslam and Kunst, 2013). Three long-chain acyl-CoA synthetase isoforms (Table I), required for the activation of free fatty acids to acyl-CoA thioesters (Li-Beisson et al., 2015), also were identified.
Glycerolipid metabolism was represented in E. oleoabundans microsomes by glycerol-3-phosphate acyltransferase (GPAT) and 1-acyl-sn-glycerol-3-phosphate acyltransferase (Table I), which catalyze the first two reactions common to glycerolipid synthesis leading to phosphatidic acid formation (Li-Beisson et al., 2015). The GPAT identified in this work is a homolog of a C. reinhardtii LD-associated protein (Nguyen et al., 2011) but also of plant GPAT9 proteins (Shockey et al., 2016) and, thus, may be involved in E. oleoabundans triacylglycerol biosynthesis, as has been demonstrated for its homologs in Parietochloris incisa (Trebouxiophyceae) and Arabidopsis (Iskandarov et al., 2016; Shockey et al., 2016; Singer et al., 2016). Regarding the metabolism of membrane lipids, two phosphoinositide (phosphorylated derivatives of phosphatidylinositol) signaling proteins were identified in E. oleoabundans membranes: phosphatidylinositol 4-kinase-α and Sac1p-like phosphoinositide phosphatase (Table I). These proteins are likely to be involved in the ultrastructural changes required for LD formation in nitrogen-deprived E. oleoabundans (Giovanardi et al., 2013). Evidence shows that phosphatidylinositol levels are responsive to nitrogen depletion in E. oleoabundans (Matich et al., 2016), and changes in phosphoinositide dynamics have been observed in other microalgae species under environmental stress (Einspahr et al., 1988; Heilmann et al., 2001). Moreover, homologs of both phosphoinositide signaling proteins have been identified in C. reinhardtii LDs (Moellering and Benning, 2010; Nguyen et al., 2011), and a link between phosphoinositide signaling and LD homeostasis has been demonstrated in yeast (Saccharomyces cerevisiae; Ren et al., 2014).
In accordance with LD formation in nitrogen-deprived E. oleoabundans (Popovich et al., 2012; Giovanardi et al., 2013), homologs for LD structural proteins were detected in this work (Table I), including members of the probable Plastid-Lipid Associated Protein family (Singh and McNellis, 2011), a rubber (Hevea brasiliensis) elongation factor protein (Horn et al., 2013; Berthelot et al., 2014), and a putative Major Lipid Droplet Protein (MLDP) that shares around 25% identity with currently characterized MLDPs from Haematococcus pluvialis (Peled et al., 2011), C. reinhardtii (Moellering and Benning, 2010), and Dunaliella spp. (Davidi et al., 2012).
We also identified proteins involved in lipid trafficking (Table I), including the membrane-associated 30-kD Vesicle Inducing Protein in Plastids (VIPP1), the trigalactosyldiacylglycerol chloroplastic protein (TG2), and an aminophoshopholipid-transporting ATPase (ALA). VIPP1 has been suggested to play a role in thylakoid membrane formation via membrane vesicles (Nordhues et al., 2012), whereas TG2 is a homolog of the substrate-binding component of a prokaryote-type ATP-binding cassette (ABC) transporter located in the chloroplast envelope that is proposed to participate in the chloroplast import of lipids derived from the endoplasmic reticulum (Awai et al., 2006; Li et al., 2016). ALAs are P4-ATPases implicated in the translocation of specific phospholipids within the two leaflets of biological membranes, a process proposed to generate the local curvature that precedes vesicle budding (Poulsen et al., 2008; Zhou and Graham, 2009). Two homologs of half-sized ABCG transporters also were identified (Table I), which may be involved in lipid trafficking in E. oleoabundans, as ABCG transporters have been related to the transport of lipophilic molecules in other organisms (Verrier et al., 2008; Li et al., 2016).
Our results suggest that lipid degradation is still active in nitrogen-deprived E. oleoabundans despite the massive oil accumulation triggered by this stress condition (Tornabene et al., 1983; Li et al., 2008; Pruvost et al., 2009; Garibay-Hernández et al., 2013). We identified a putative triacylglycerol lipase probably involved in the release of fatty acids from neutral glycerolipids as well as homologs of both core and auxiliary plant peroxisomal proteins that participate in the β-oxidation reactions for the degradation of saturated and unsaturated fatty acids (Table I). In E. oleoabundans, β-oxidation probably takes place in unspecialized peroxisomes (microbodies) lacking the glycolate metabolic enzymes, similar to what has been proposed for Eremosphaera (Stabenau et al., 1984) and Dunaliella (Stabenau et al., 1993) green microalgae species. The identification of lipid catabolism-related proteins in nitrogen-deprived E. oleoabundans supports the idea that fatty acid turnover is constitutive and that a continuous balance between oil synthesis and degradation exists even under nitrogen stress (Li-Beisson et al., 2015).
The coverage of the lipid metabolic pathways in this work was still limited, although additional lipid metabolism-related proteins were identified in the FFZE fractions and/or in only a single total microsomal sample. Incomplete detection of the entire lipid metabolism machinery may be attributed to their low abundance or lack of similarity with current annotated sequences, which prevents their positive identification, but also may reflect their complete absence in the analyzed samples. Our results suggest that the amount of membrane-associated lipid metabolism proteins may be lower than has been proposed (Natter et al., 2005; Joyard et al., 2010; Wang and Benning, 2012), as many have been identified to a major extent in total soluble protein extracts from other microalgae (Guarnieri et al., 2011; Gao et al., 2014).
Carbon Metabolism Proteins in E. oleoabundans Membranes
Additional biological processes were covered almost in their entirety in the E. oleoabundans membrane proteome, including central carbon metabolism and electron transport. A comprehensive description is provided in this study to better understand the major metabolic constraints of carbon partitioning in this microalga. Other biological processes typically related to nitrogen deprivation, including nitrogen acquisition, protein turnover, and oxidative stress responses, also were represented in the E. oleoabundans membrane proteome, as well as processes related to pigment metabolism, transcription regulation, and signaling, all of which are described in Supplemental Information S1.
Carbon metabolism was represented in the membranes of nitrogen-depleted E. oleoabundans by 13% of the total proteome (Supplemental Fig. S2; Supplemental Table S4). Not all carbon metabolism-related proteins were identified in the analysis of total microsomal samples; however, the remainder were identified through MS analysis of the FFZE fractions due to the decrease in sample complexity by fractionation (Fig. 6; Supplemental Table S4, proteins highlighted with asterisks).
Inorganic Carbon Acquisition and Assimilation
The CO2-concentrating mechanism appears to be active in nitrogen-limited E. oleoabundans (Fig. 6, orange; Supplemental Table S4), as we identified proteins involved in active inorganic carbon uptake (High Light Activated3 [HLA3]) as well as in its interconversion (CO2/HCO3−), recapture, and concentration within the cell (α-type carbonic anhydrases, Limiting CO2-Inducible B [LCIB]-like proteins). Key regulators of the Calvin cycle (CP12, Rubisco activase) and almost half (five of 11) of its chloroplastic enzymes were identified (Supplemental Table S4), including phosphoglycerate kinase (PGK), glyceraldehyde-3-phosphate dehydrogenase (GPDH), and aldolase, enzymes that are shared with the chloroplast glycolytic pathway (Fig. 6, green). Photorespiration and one carbon metabolism proteins also were present in membranes of nitrogen-deprived E. oleoabundans (Supplemental Table S4).
Biosynthesis of Photosynthetic Carbon Precursors via a Compartmentalized Glycolytic Pathway
The complete glycolytic pathway, with the exception of Glc-6-P isomerase, was identified in this work (Fig. 6, green and blue; Supplemental Table S4), demonstrating that this pathway is active in nitrogen-deprived E. oleoabundans. This suggests that glycolysis may be the major contributor for pyruvate production, which is presumed to be the primary carbon source for fatty acid biosynthesis (Chapman et al., 2013; Shtaida et al., 2015).
The identification of most of the glycolytic proteins in E. oleoabundans membranes is not as surprising as it may at first appear. The membrane association of some or all of the glycolytic pathway components and their sequestration within different organelles are the most usual forms of glycolytic compartmentalization for regulating central carbon metabolism (Ginger et al., 2010; Johnson and Alric, 2013). Several modes of glycolytic compartmentalization have emerged in microalgae (Ginger et al., 2010; Smith et al., 2012), where a comprehensive view exists only for C. reinhardtii (Klein, 1986; Johnson and Alric, 2013) and some diatoms (Smith et al., 2012). To assess carbon flow compartmentalization in E. oleoabundans, we manually curated the subcellular location of glycolytic proteins (Fig. 6; Supplemental Table S4). Accordingly, the enzymes from the upper part of glycolysis (from hexokinase to PGK; Fig. 6, green) are apparently targeted to the chloroplast, and those from the lower part (from phosphoglycerate mutase to pyruvate kinase; Fig. 6, blue) may be associated with the cytoplasmic face of membranes due to the lack of a predicted target peptide.
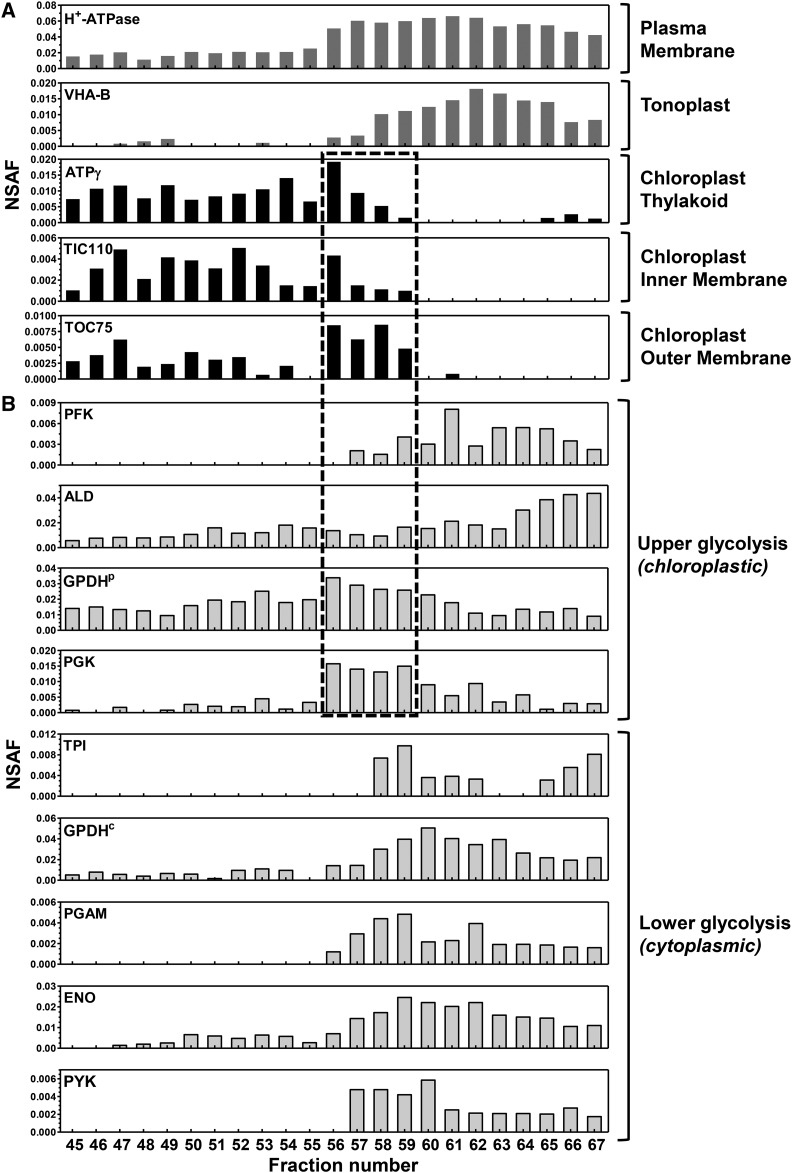
In order to confirm the compartmentalization of the glycolytic pathway, we analyzed the distribution of the glycolytic enzymes in the FFZE fractions using an MS-based approach (Fig. 7). Enzymes from the lower part of glycolysis separated similar to the fractions enriched in tonoplast and plasma membrane (fractions 56–67), indicating their possible association with the cytoplasmic side of these membranes. In contrast, chloroplastic GPDH and PGK, two enzymes of the upper part of glycolysis, were highly abundant in fractions 56 to 59, which also were enriched with the protein marker of the outer chloroplast envelope (TOC75), and to a lesser extent with markers of the thylakoid (ATP synthase subunit γ) and inner chloroplast membranes (TIC110; Fig. 7). This suggests that chloroplastic GPDH and PGK may be associated with the chloroplast envelope, as demonstrated for their counterparts in Arabidopsis (Ferro et al., 2010). The two other enzymes from the upper part of glycolysis, phosphofructokinase and aldolase, showed a distribution profile similar to that from the tonoplast and plasma membrane but still were detected in envelope-enriched fractions (Fig. 7). Thus, we suggest that they may be targeted to both chloroplast and cytoplasmic locations, similar to their C. reinhardtii homologs (Klein, 1986; Johnson and Alric, 2013). Additional targeting of glycolytic proteins to other cell locations such as the tonoplast is possible, as demonstrated for enolase and aldolase in the salt-tolerant plant Mesembryanthemum crystallinum, where they are targeted to the tonoplast to perform moonlighting functions (Barkla et al., 2009).
Figure 7.
FFZE profiles of glycolytic enzymes suggest their targeting to multiple cellular locations. Individual FFZE fractions were analyzed by LC-MS/MS and surveyed for proteins of interest. A, Graphical representation of NSAF values of protein markers specific for subcellular compartments among the FFZE fractions. The surveyed compartment markers are as follows: m.363780 (H+-ATPase), m.392881 (ATPγ), m.227792 (TIC110), and m.134654 (TOC75). B, Graphical representation of NSAF values of glycolytic enzymes among the FFZE fractions. According to their predicted cellular locations, proteins are grouped into upper and lower glycolytic pathway enzymes. These proteins are described in Supplemental Table S4. FFZE fractions enriched with the outer chloroplast membrane are enclosed in the box (fractions 56–59).
Glycolysis compartmentalization in E. oleoabundans is reinforced by the identification of triose phosphate and hexose phosphate translocators that may communicate the two parts of glycolysis across the chloroplast envelope (Fig. 6; Supplemental Table S4). Phosphoenolpyruvate and pyruvate transporters were not identified in this work; however, homologs of a phosphoenolpyruvate/phosphate translocator and a sodium/pyruvate cotransporter (BASS [Bile Acid:Na+ Symporter]; Fig. 6) have been shown to be transcribed in nitrogen-deprived E. oleoabundans (Rismani-Yazdi et al., 2012). Altogether, our results support the compartmentalization of the glycolytic proteins in E. oleoabundans; however, additional experiments to determine their specific localization and dynamics are necessary.
Among the identified glycolytic proteins, it is worth highlighting enolase (EoENO; Supplemental Table S4; Supplemental Fig. S7), whose presence in E. oleoabundans membranes was confirmed by western blot (Supplemental Fig. S7D). Sequence analysis of EoENO and its homologs revealed that enolases from trebouxiophycean and chlorophycean microalgae present an additional N-terminal region (InterPro no. IPR003117) that corresponds to the RIIa domain (Canaves and Taylor, 2002; Supplemental Fig. S7, A and B). Phylogenetic analysis confirmed that RIIa-containing enolases are exclusive to trebouxiophycean and chlorophycean species, clustering separately from enolases of land plants and prasinophyceaen microalgae (Supplemental Fig. S7C). The RIIa domain mediates homodimerization of the regulatory subunit of cAMP-dependent protein kinases (PKA) and high-affinity binding to A-kinase anchoring protein (AKAP) scaffold proteins, required for the integration of signaling pathways and for the subcellular compartmentalization of its components (Newlon et al., 2001). This suggests that, in green microalgae, the RIIa domain may be involved in enolase dimerization and/or anchoring to AKAPs for intracellular targeting and regulatory purposes, similar to the non-PKA RIIa-containing Radial Spoke Protein11 (Yang et al., 2006).
Starch Synthesis
Starch synthesis was represented in the E. oleoabundans membrane proteome by Glc-1-P adenylyltransferase, one of the major rate-controlling enzymes, and by other key enzymes involved in green algal starch metabolism, including the plastidial phosphoglucomutase and the granule-bound starch synthase (Fig. 6, red; Supplemental Table S4). This suggests that starch synthesis may still be active in this microalga after prolonged nitrogen stress, which agrees with the increase in both lipid and starch content that was reported previously for nitrogen-deprived E. oleoabundans (Rismani-Yazdi et al., 2012; Garibay-Hernández et al., 2013).
Photosynthetic and Mitochondrial Electron Transport in E. oleoabundans
Analysis of the E. oleoabundans membrane proteome allowed us to provide a survey of the components of the photosynthetic and respiratory electron transport chains (Fig. 8; Supplemental Tables S1–S3), essential for supplying energy to the processes taking place during nitrogen deprivation.
Figure 8.
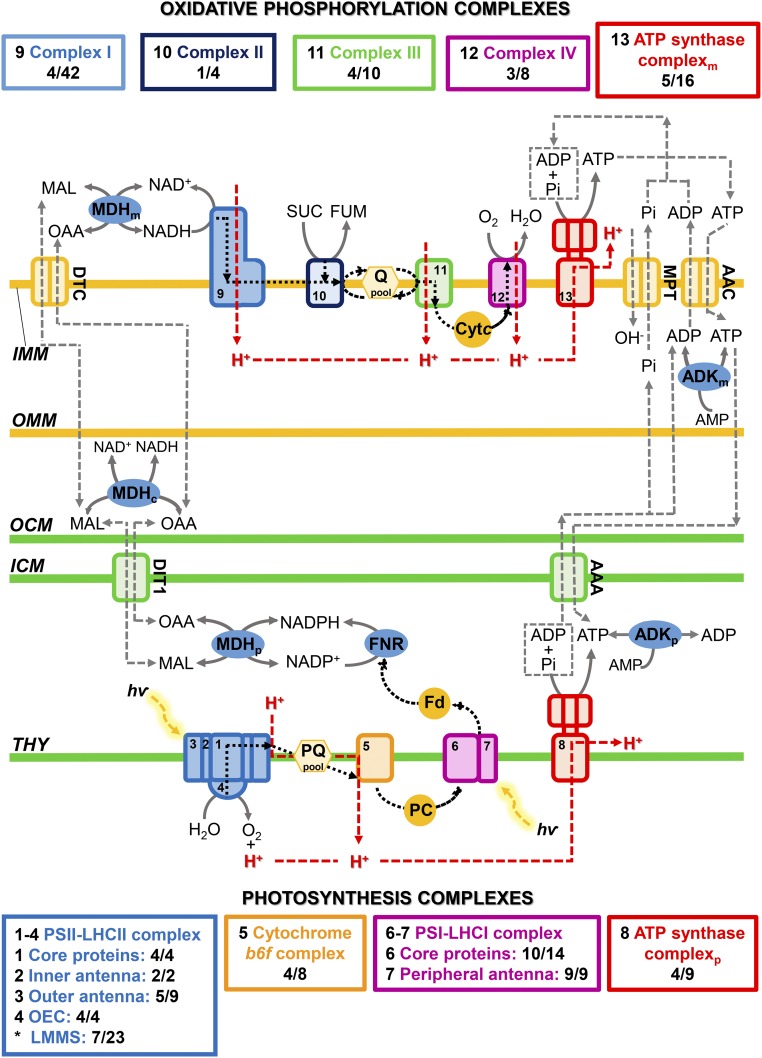
Photosynthesis and oxidative phosphorylation in the membrane proteome of nitrogen-deficient E. oleoabundans. Graphical representation is shown for the proteins identified by LC-MS/MS in the membrane proteome of E. oleoabundans involved in energy conversion and homeostasis. Identified proteins are described in Supplemental Table S1 (photosynthesis), Supplemental Table S2 (oxidative phosphorylation), and Supplemental Table S3 (energy and reducing power homeostasis). The number of proteins detected in E. oleoabundans from each of the complexes of the chloroplastic and mitochondrial electron transfer chains is shown in colored boxes and compared with the number of proteins identified in the complexes of the model alga C. reinhardtii. In complex IV, CrCOX2 is considered as a nonsplit subunit. In the E. oleoabundans ATP synthase mitochondrial complex, the N- and C-terminal peptides of subunit d are considered as a unique nonsplit protein. Black dashed lines indicate electron transfer, and red dashed lines indicate proton translocation. C, Cytoplasmic; M, mitochondrial; P, chloroplastic. Asterisks indicate proteins associated with any part of the PSII-LHCII supercomplex. AAA, ADP/ATP carrier protein, chloroplastic; AAC, ADP/ATP carrier protein, mitochondrial; ADK, adenylate kinase; Cytc, cytochrome c; DIT1, dicarboxylate transporter 1, chloroplastic; DTC, mitochondrial dicarboxylate/tricarboxylate carrier; Fd, ferredoxin; FNR, ferredoxin-NADP reductase; FUM, fumarate; ICM, inner chloroplast membrane; IMM, inner mitochondrial membrane; LHCI, light-harvesting complex of PSI; LHCII, light-harvesting complex of PSII; LMMS, low-molecular-mass subunits; MAL, malate; MDH, malate dehydrogenase; MPT, mitochondrial phosphate carrier protein; OAA, oxaloacetate; OCM, outer chloroplast membrane; OEC, oxygen-evolving complex; OMM, outer mitochondrial membrane; PC, plastocyanin; Pi, inorganic phosphate; PQ, plastoquinone; Q, ubiquinone; SUC, succinate; THY, thylakoid membrane.
The Mitochondrial Respiratory Chain
Each of the five complexes of the mitochondrial respiratory chain were represented in the E. oleoabundans membrane proteome (Fig. 8; Supplemental Table S2). However, comparison of the identified subunits with the oxidative phosphorylation proteome in C. reinhardtii (Stern, 2009) shows that important differences do exist. Contrary to chlorophycean microalgae, E. oleoabundans has an orthodox intact mitochondria-encoded COX2 (Supplemental Fig. S6) and a classical mitochondrial ATP synthase (complex V). The latter is supported by the identification of subunit d (Supplemental Table S2), which is known to be absent from the noncanonical mitochondrial ATP synthases of the chlorophycean lineage (Vázquez-Acevedo et al., 2006, 2016). In addition, sequence analysis of the identified mitochondrial ATPase constituents (α, β, γ, δ, and d; Fig. 8; Supplemental Table S2) suggests that they are encoded by both nuclear and mitochondrial genomes, contrary to the chlorophycean lineage, where all ATPase subunits are nucleus encoded (Vázquez-Acevedo et al., 2006, 2016).
Photosynthesis and Photoprotective Mechanisms
All the photosynthetic complexes, PSI-LHCI, cytochrome b6f, PSII-LHCII, and ATP synthase, were represented in membranes of nitrogen-deprived E. oleoabundans (Fig. 8; Supplemental Table S1). At least 45% of the protein subunits comprising each of the complexes were identified, including several low-molecular-mass PSII proteins (PSBE, PSBH, PSBR, PSB27, PSB29, PSB32, and PSB33; Supplemental Table S1), which have proven difficult to detect owing to their low abundance, small size, and hydrophobicity (Shi and Schröder, 2004; Shi et al., 2012). Compared with glycolysis- and lipid metabolism-related proteins, whose low abundance hindered their detection in E. oleoabundans membranes, the identification of low-molecular-mass PSII proteins in this work can be attributed to the high proportion of chloroplast-localized proteins that constituted the E. oleoabundans membrane proteome (41%; Supplemental Fig. S2A). The identified photosynthesis-related proteins are homologs of the corresponding proteins in the model green alga C. reinhardtii (Stern, 2009; Minagawa and Tokutsu, 2015), suggesting that, contrary to what is observed for the mitochondrial respiratory chain, the composition of the core photosynthetic complexes is highly conserved among green algae.
In agreement with the diminished integrity of the photosynthetic apparatus that has been reported for nitrogen-deprived E. oleoabundans (Benvenuti et al., 2015), proteins involved directly in the synthesis and turnover of the D1 subunit (Filamentous Temperature-Sensitive H [FTSH] ATP-dependent zinc metalloproteases, Low PSII Accumulation1, atypical short-chain dehydrogenase HCF244), as well as in PSI (YCF4) and PSII assembly, stability, and/or repair (peptidyl-prolyl cis-trans-isomerase CYP38, M-Enriched Thylakoid1, MPH1, rubredoxin), were identified in this work (Supplemental Table S1). Additional photoprotective responses appeared to be active in nitrogen-deprived E. oleoabundans, as we were able to identify key molecular effectors of the short-term components of NPQ (Supplemental Table S1): energy-dependent feedback deexcitation quenching (qE; calcium-sensing receptor CAS, PSBS), zeaxanthin-dependent quenching (qZ; violaxanthin deepoxidase), and state transition-dependent quenching (qT; Ser/Thr protein kinase STT7) (Erickson et al., 2015; Minagawa and Tokutsu, 2015). Key components of the two proposed cyclic electron flow pathways (Iwai et al., 2010; Johnson and Alric, 2013), the NADPH dehydrogenase-dependent pathway (Type-II NAD(P)H dehydrogenase) and the ferredoxin-dependent pathway (ferredoxin-NADP reductase, PGR5-like protein1), also were identified. (Supplemental Tables S1 and S3). This supports that alternative electron pathways may be active in nitrogen-deprived E. oleoabundans for photoprotection and for satisfying the varying demand for ATP/NADPH under abiotic stress conditions.
Additional mechanisms known to modulate the redox potential and ATP concentration in different cellular compartments, particularly under fluctuating environmental conditions (Cardol et al., 2003; Johnson and Alric, 2013; Erickson et al., 2015), were represented in nitrogen-deprived E. oleoabundans. We were able to identify the main effectors of the malate shunt (malate dehydrogenase isoforms, dicarboxylate transporter, and mitochondrial dicarboxylate/tricarboxylate carrier) and proteins necessary for regulating ATP concentrations within the chloroplast and the mitochondria (adenylate kinase isoforms, mitochondrial and chloroplastic ADP/ATP carrier proteins, and mitochondrial phosphate carrier protein; Fig. 8; Supplemental Table S3).
CONCLUSION
The results from this work provide a detailed survey of the membrane proteome of an oleaginous microalga. Combining gel-free shotgun proteomics with searching against an organism-specific RNA-Seq-based protein database considerably improved protein identification. This approach overcame both the typical limitations of studying membrane proteins and the difficulty of working with nonsequenced organisms for which the quality and quantity of the data available in reference databases are neither complete nor specific. Although manual annotation may be a time-consuming strategy, we demonstrated its usefulness for analyzing nonsequenced organisms, as it significantly improved the number of identified proteins as well as the accuracy and reliability of their annotations.
This approach allowed the novel identification in E. oleoabundans of the photosynthesis-related proteins MPH1 and PSBS, both thought to be exclusive to higher photosynthetic organisms. These findings suggest that photoprotective mechanisms, including NPQ, are active after prolonged nitrogen deprivation and indicate that, in E. oleoabundans, these mechanisms are more closely related to higher photosynthetic organisms than was proposed previously. The identification of PSBS and the presumed absence of an LHCSR homolog in E. oleoabundans are contrary to what has been observed in C. reinhardtii. In C. reinhardtii, a light-inducible PSBS was identified recently and was demonstrated to be essential for the activation of an LHCSR-dependent qE mechanism to which most of the microalgal NPQ capability has been attributed (Peers et al., 2009; Niyogi and Truong, 2013; Correa-Galvis et al., 2016; Tibiletti et al., 2016). This result questions the conservation of qE mechanisms within green microalgae, where the specific role played by PSBS in E. oleoabundans NPQ must be determined. In addition to the photosynthesis-related proteins, we also detected RP2-CLC, a novel domain architecture protein that is likely involved in the intracellular trafficking of clathrin-coated vesicles in lower eukaryotes, a process that apparently has its own peculiarities in these understudied organisms. Using FFZE fractionation of membranes, we confirmed the chloroplastic location of PSBS and MPH1 together with the enrichment of RP2-CLC in the plasma membrane. Using this strategy also contributed to the identification of very-low-abundance proteins related to E. oleoabundans lipid metabolism, allowing us to identify a detailed list of proteins involved in the major steps of acyl-lipid metabolism, lipid trafficking, lipid signaling, and LD formation in E. oleoabundans. An MS-based analysis of FFZE fractions additionally supported the compartmentalization of glycolytic proteins in E. oleoabundans, which is an important constraint that appears to govern central carbon metabolism and partitioning.
Finally, through molecular phylogenetic approaches, we provide compelling evidence for the phylogenetic grouping of this microalga with the Chlorellales lineage of the trebouxiophycean class of green microalgae rather than with the chlorophycean class in which it is currently classified. Our results provide an important platform for studying E. oleoabundans and underscore the importance of studying nonmodel organisms, as the analysis of specific features in E. oleoabundans demonstrates that its biology differs from that of nonoleoaginous model organisms.
MATERIALS AND METHODS
Microalgae Strains and Culture Conditions
Ettlia oleoabundans UTEX 1185 was grown under phototrophic conditions in 2.8-L Fernbach glass flasks with a working volume of 40% using modified Bold’s Basal Medium (Garibay-Hernández et al., 2013). Axenic cultures with an initial cell density of 1 to 2 × 106 cells mL−1 were maintained for 7 d at 25°C ± 0.5°C under continuous orbital agitation (300 rpm) and white fluorescent light illumination (100 µE m−2 s−1). To induce nitrogen deprivation, 7-d cultures were centrifuged individually for 10 min (10,000g at 4°C), washed once with 200 mL of nitrogen-free modified Bold’s Basal Medium, resuspended in 1.12 L of nitrogen-free modified Bold’s Basal Medium, and transferred into Fernbach flasks. Axenic nitrogen-deprived cultures with an initial cell density of 10 to 15 × 106 cells mL−1 were maintained during 4 d under the aforementioned conditions.
The Chlamydomonas reinhardtii wall-less strain cw15 mt+ was grown under mixotrophic conditions in 0.5-L Erlenmeyer glass flasks with a 40% working volume using Tris-acetate-phosphate medium (Harris, 2009). Axenic cultures with an initial cell density of 1 to 2 × 106 cells mL−1 were maintained for 4 d at 24°C ± 2°C under white fluorescent light illumination (50–100 µE m−2 s−1 and 16/8-h light/dark cycle) and continuous orbital agitation (80 rpm).
Cell density was determined by direct microscopic cell count using a Neubauer chamber. Cultures were tested for the absence of bacteria in Glc-free Luria broth agar (1.5%, w/v) medium plates (Bertani, 1951) incubated at 37°C for at least 24 h.
Microsomal Membrane Isolation
All the operations in this protocol were performed at 4°C. Nitrogen-deprived E. oleoabundans cultures (1.12 L) or C. reinhardtii cultures (0.4 L) were used for microsome isolation. Cultures were centrifuged for 10 min (10,000g at 4°C), washed once with 0.1 m HEPES-KOH, pH 7.5 (50/500 mL of centrifuged culture), and resuspended in 2.5 mL of homogenization medium (400 mm mannitol, 10% [w/v] glycerol, 5% [w/v] polyvinylpyrrolidone-10, 0.5% [w/v] bovine serum albumin, 1 mm phenylmethylsulfonyl fluoride, 30 mm Tris, 2 mm dithiothreitol, 5 mm EGTA, 5 mm MgSO4, 0.5 mm butylated hydroxytoluene, 0.25 mm dibucaine, 1 mm benzamidine, and 26 mm K+-metabisulfite; adjusted to pH 8 with NaOH). Cells were homogenized by passing the cell suspension five times through a French press (Thermo Spectronic; model FA-078) at 20 k.p.s.i. using a mini pressure cell. Microsomal membranes were isolated as described by Barkla et al. (1995). Briefly, the homogenate was centrifuged for 20 min (10,000g at 4°C) and the pellet was discarded. To concentrate the microsomes, the supernatant was centrifuged (80,000g, 50 min, at 4°C; Beckman 45 Ti rotor, L8-M ultracentrifuge). The microsomal pellet was resuspended in suspension medium (400 mm mannitol, 10% [w/v] glycerol, 6 mm Tris/MES, pH 8, and 2 mm dithiothreitol) using a 10-mL glass Teflon homogenizer. Samples were frozen in liquid N2 for storage at −80°C. Microsomes from Arabidopsis (Arabidopsis thaliana) were isolated as described by Barkla et al. (2007).
FFZE
Microsomal membranes from nitrogen-deprived E. oleoabundans were fractionated by FFZE using the BD FFE System (BD Proteomics) as described by Barkla et al. (2007). Briefly, prior to fractionation, the microsomal sample was diluted 2:1 (v/v) in separation medium (10 mm triethanol amine, 10 mm acetic acid, 2 mm KCl, and 250 mm Suc) and centrifuged for 20 min (14,000g at 4°C). The sample was supplemented with MgATP (3 mm final concentration) to enhance membrane separation during FFZE (Barkla et al., 2007). The sample was injected continuously via a peristaltic pump at 1.2 mL h−1 using the anodic sample inlet. Media inlets 2 to 6 and counter-flow inlets C1, C2, and C3 contained separation medium, whereas inlets 1 and 7 contained stabilization medium (40 mm triethanol amine, 40 mm acetic acid, 8 mm KCl, and 180 mm Suc). The cathodic and anionic circuit electrolyte solutions consisted of 100 mm triethanol amine, 100 mm acetic acid, and 20 mm KCl adjusted to pH 7.4 (NaOH); 0.4% (v/v) formaldehyde was added to the anodic solution to prevent the loss of chloride by anodic oxidation.
FFZE was performed in horizontal mode at 5°C and 750 V (150 mA) with media and counter-flow rates of 250 mL h−1. Following separation in the chamber, membrane fractions were collected continually on 96-deep-well microtiter plates (4 mL per well). Fractions from sequential plates corresponding to the same well were pooled; 1.2-mL aliquots were collected per pooled fraction, frozen in liquid N2, and stored at −80°C for LC-MS/MS analysis. The remaining volume of each collected fraction was ultracentrifuged (100,000g, 50 min, at 4°C; Beckman 55.2 Ti rotor, L8-M ultracentrifuge) for membrane concentration. Membrane pellets corresponding to each fraction were resuspended in 25 to 200 µL of suspension buffer (250 mm mannitol, 10% [w/v] glycerol, 10 mm Tris/MES, pH 8, and 2 mm dithiothreitol) and frozen in liquid N2 for storage at −80°C. FFZE separation was monitored by collecting microtiter plates (250 µL per well) at several time points and measuring protein (optical density at 280 nm) using a microplate scanning spectrophotometer (Power WaveX; Bio-Tek Instruments).
Protein and Chlorophyll Concentration Measurements
Protein in microsomal and concentrated FFZE fractions was measured by a modification of the Bradford method (Bradford, 1976). Membrane protein was partially solubilized with 0.5% (v/v) Triton X-100 for 5 min prior to dilution and addition of the dye reagent concentrate; the final Triton X-100 concentration in the assay was 0.05% (w/v). Bovine serum albumin was used as the protein standard.
Chlorophyll in FFZE fractions prior to concentration was measured according to the method of Arnon (1949), with some modifications, using a microplate scanning spectrophotometer (Power WaveX; Bio-Tek Instruments). Absorbance was measured directly at 645 and 663 nm, and calculations were made according to the following equations:
SDS-PAGE, Staining, and Immunoblotting
For protein precipitation, total microsome samples and concentrated FFZE fractions were diluted 50-fold in 1:1 (v/v) ethanol:acetone and incubated overnight at −20°C according to Parry et al. (1989). Samples were then centrifuged for 20 min (14,000g at 4°C). Pellets were air dried, resuspended in Laemmli buffer (Laemmli, 1970), and heated (60°C for 2 min) before loading (15–20 µg of protein per lane) onto 10% to 12.5% (w/v) linear acrylamide gels as indicated. After electrophoresis, SDS-PAGE separated proteins were either fixed and stained with Coomassie Blue R250 or electrophoretically transferred onto nitrocellulose membranes (enhanced chemiluminescence; GE Lifesciences) for immunoblot analysis as described by Vera-Estrella et al. (2004). Digital images were captured using the Gel Doc XR+ System (Bio-Rad). Primary antibodies, either commercially available or custom made, and dilutions employed in this study were as follows: Arabidopsis anti-MPH1 (1:200; Liu and Last, 2015b); C. reinhardtii anti-LHCSR3 (1:1,000; Agrisera; AS14 2766); Homo sapiens anti-enolase (1:1,000; Santa Cruz Biotechnology; sc-7455); H. sapiens anti-RP2 (1:3,000; Chapple et al., 2000); Spinacia oleracea anti-AtpB (1:10,000; McCormac and Barkan, 1999); and global anti-H+-ATPase (1:1,000; Agrisera; AS07 260).
Shotgun Proteomics Analysis
For sample preparation prior to proteomic analysis, total microsome samples (100 μg per replicate; four independent biological replicates) and FFZE fractions (1.2 mL per fraction; 23 individual FFZE fractions) were suspended in TE buffer (10 mm Tris/HCl, pH 7.6, 1 mm EDTA pH 8), and 0.3% [w/v] sodium deoxycholate), precipitated with 72% (w/v) TCA (9% final concentration; 4°C for 1 h), and, after recovering the precipitated protein, submitted to an additional precipitation step with 90% (v/v) acetone (−30°C overnight). The preparation (solubilization, reduction, alkylation, and trypsin digestion) of the vacuum-dried protein extracts and their further manipulation (resolubilization and desalting) prior to LC-MS/MS analysis were performed as described in detail by Barkla et al. (2012).
LC-MS/MS analysis was performed at the proteomics discovery platform of the Institut de Recherches Cliniques de Montréal using the LC-MS/MS equipment described in detail by Barkla et al. (2012). Chromatography buffers were 0.2% (v/v) formic acid (buffer A) and 100% (v/v) acetonitrile/0.2% (v/v) formic acid (buffer B). Peptide samples were loaded on column (600 nL min−1) and eluted (250 nL min−1) with a two-slope gradient: buffer B first increased from 2% to 40% (85 min) and then from 40% to 80% (15 min). LC-MS/MS data acquisition was accomplished using an 11-scan event cycle, composed of a full MS scan for scan event 1 acquired in the Orbitrap. Mass resolution for MS was set to 60,000 (at mass-to-charge ratio of 400) and used to trigger the 10 additional tandem mass spectrometry (MS/MS) events acquired in parallel in the linear ion trap for the top 10 most intense ions. The mass-to-charge ratio range was from 360 to 1,700 for MS scanning with a target value of 1,000,000 charges and from approximately one-third of the parent mass-to-charge ratio to 2,000 for MS/MS scanning with a target value of 10,000 charges. Data-dependent scan events used a maximum ion fill time of 100 ms and one microscan. Target ions already selected for MS/MS were dynamically excluded for 31 s after two counts. Nanospray and S-lens voltages were set to 1.3 to 1.8 kV and 50 V, respectively. Capillary temperature was set to 250°C. MS/MS conditions were as follows: normalized collision energy, 35 V; activation Q, 0.25; activation time, 10 ms.
An E. oleoabundans protein database was generated at the Unidad Universitaria de Apoyo Bioinformático-Universidad Nacional Autónoma de México through in silico six-frame translation of the E. oleoabundans nonredundant transcriptome database (56,550 transcripts; Rismani-Yazdi et al., 2012). TransDecoder (Haas et al., 2013) was used to identify the candidate protein-coding regions based on nucleotide composition and open reading frame length. For database search, peak list files were generated with Proteome Discoverer (version 1.4) using the following parameters: minimum mass, 500 D; maximum mass, 6,000 D; no grouping of MS/MS spectra; precursor charge, auto; minimum number of fragment ions, five. All MS/MS spectra were analyzed with Mascot 2.3 (Matrix Science) against the Viridiplantae (TaxID 33090, unknown version; 677,107 entries) and/or the E. oleoabundans (unknown version; 53,921 entries) protein databases assuming trypsin digestion. The mass tolerances for precursor and fragment ions were set to 10 ppm and 0.6 D, respectively. Cys carbamidomethylation was specified as a fixed modification and Met oxidation as a variable modification.
Scaffold 4.3.4 (Proteome Software) was used to validate MS/MS-based peptide and protein identifications. Scaffold parameters were set to a minimum of two peptides per protein, with minimum probabilities of 99% at the protein level (Protein Prophet algorithm; Nesvizhskii et al., 2003) and 95% at the corresponding peptide level (Scaffold Local FDR algorithm). Proteins containing similar peptides that could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony.
Protein Functional Annotation
Proteins were annotated if they complied with the above-mentioned Scaffold parameters and if they were identified with two or more unique peptides in at least two of four biological replicates, unless stated otherwise. Automatic annotation based on sequence similarity was initially performed using the Blast2GO suite (version 2.8; April 2014) with the default parameters (Götz et al., 2008). To improve protein annotation, each of the identified proteins was individually curated during the period from May 2014 and May 2015. Manual curation was initially based on the results provided by the Blast2GO analysis and additionally supported by the following resources: experimental evidence available in the literature for protein homologs and detailed sequence analysis in the PredAlgo subcellular localization prediction tool (Tardif et al., 2012) and in multiple databases, including ARAMEMNON version 8.0 (Schwacke et al., 2003), InterPro (Mitchell et al., 2015), Phytozome version 10.2 (Goodstein et al., 2012), SUBA3 (Tanz et al., 2013), TCDB (Saier et al., 2014), UniProtKB (SwissProt and TrEMBL entries; UniProt Consortium, 2015), ARALIP (Li-Beisson et al., 2010), AT_CHLORO (Ferro et al., 2010), MEROPS version 9.9 (Rawlings et al., 2014), and PlnTFDB (Pérez-Rodríguez et al., 2010), depending on the requirements. Functional assignments were additionally verified using the IntEnz (Fleischmann et al., 2004) and QuickGO (Binns et al., 2009) databases. A protein sequence from E. oleoabundans was manually annotated as a specific protein only if it contained the domains required for the function that was being assigned or at least the signatures necessary to be allocated to a specific protein family; otherwise, proteins were annotated as unknown even if they were electronically inferred as a known protein.
Genomic DNA Extraction and 18S rDNA Sequencing
Genomic DNA was extracted from 25 mL of nitrogen-sufficient E. oleoabundans cultures as follows. Cells were pelleted by centrifugation (10,000g, 10 min, at room temperature), frozen in liquid N2, and ground with mortar and pestle. Ground cells were suspended in TE buffer to an initial working volume of 600 μL to which 180 μL of lysozyme (50 mg mL−1) was added and incubated for 30 min at room temperature. Then, 120 μL of 10% (w/v) SDS and 3 μL of proteinase K (20 mg mL−1) were added to the sample and incubated for 1 h at 37°C with agitation. After this, 100 μL of 5 m NaCl and 80 μL of cetyl-trimethyl-ammonium bromide/NaCl (10% [w/v]/0.7 m) were added to the sample, which was mixed and incubated for a further 10 min at 65°C with agitation. DNA was extracted with 1 volume of CH3Cl:isoamyl alcohol (24:1, v/v). The upper phase was recovered by centrifugation (14,000g, 5 min, at room temperature) and combined with 1 volume of phenol:CH3Cl:isoamyl alcohol (25:24:1, v/v/v). For DNA precipitation, the upper phase was recovered by centrifugation, combined with 0.6 volume of isopropanol, and incubated for 15 min at −30°C. The precipitate was collected by centrifugation (14,000g, 10 min, at 4°C), washed with 1 mL of cold 75% (v/v) ethanol, dried, and resuspended in 50 μL of TE buffer. Then, genomic DNA was treated with 3 μL of RNase A (20 mg mL−1; 30 min, 37°C, and agitation), taken to a final volume of 300 μL by adding water, and reextracted with 1 volume of phenol:CH3Cl:isoamyl alcohol (25:24:1, v/v/v). The upper phase was recovered by centrifugation, and DNA was precipitated with 0.1 volume of 7.5 m ammonium acetate plus 2.5 volume of absolute ethanol (15 min at −30°C). The precipitate was collected, washed, dried, and dissolved as described above. Genomic DNA quality was assessed by agarose gel electrophoresis and by A260/A280 and A260/A230 ratios (NanoDrop spectrophotometer).
The E. oleoabundans 18S rRNA gene was amplified by PCR (35 cycles; Long PCR Enzyme Mix; ThermoFisher Scientific) using genomic DNA as a template and the oligonucleotide primers (5′-ACCTGGTTGATCCTGCCAG-3′ and 5′-TGATCCTTCYGCAGGTTCAC-3′) according to Moon-van der Staay et al. (2001). A PCR product of approximately 2.5 kb was amplified and purified from an agarose gel using the High Pure PCR Product Purification Kit (Roche Diagnostics), whose quality was verified as described above. A partial 18S rDNA sequence was obtained by sequencing (Applied Biosystems, model 3130xl) and deposited in the NCBI GenBank database under the accession number KX350066.
Molecular Phylogenetic Analysis
To perform a molecular phylogenetic analysis using a multigene approach, two nuclear (Actin and EF-1α), two chloroplastic (PSBA and RBCL), and two mitochondria-encoded (COX1 and COX2) protein markers were selected. The amino acid sequences retrieved for the analysis are described in Supplemental Table S16. Each set of proteins was aligned individually using Clustal Omega (Sievers et al., 2011). The resulting alignments were manually inspected using MEGA7 (Kumar et al., 2016), where all positions with less than 70% site coverage were removed. The verified alignments for each of the six proteins were concatenated using CLC Genomics Workbench 9.0 (Qiagen Bioinformatics). The evolutionary analysis was conducted in MEGA7 using the ML method based on the JTT matrix-based model (Jones et al., 1992). The initial tree was made automatically using the default method (Neighbor Joining/Biological Neighbor Joining). The nodal support was estimated by bootstrap values (Felsenstein, 1985) based on 1,000 replications. Additional phylogenetic analyses of proteins identified in this work were conducted in MEGA7 as described above, where all positions with less than 70% site coverage were eliminated.
Phylogenetic analysis of 18S rDNA data was performed with an increased taxon sampling, mostly based on Pegg et al. (2015), that comprised 62 different 18S rDNA nucleotide sequences (complete and partial) retrieved from the NCBI nucleotide database (Supplemental Table S17). Sequences were aligned using Clustal Omega. The evolutionary analysis was conducted in MEGA7 using the ML method based on the model of Tamura and Nei (1993), where all positions with less than 70% site coverage were eliminated. The initial tree construction and the estimation of nodal support were performed as described above.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number KX350066 [18S ribosomal RNA gene, partial sequence]. The E. oleoabundans protein database and the MS proteomics data have been deposited to the ProteomeXchange Consortium (http://www.proteomexchange.org) via the MassiVE partner repository (http://massive.ucsd.edu) with the MassiVE data set identifier MSV000079977. Additional protein and peptide information from LC-MS/MS analysis of FFZE membrane fractions is provided in Table S18.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. BLASTP best hits for E. oleoabundans membrane proteins.
Supplemental Figure S2. Cellular component and biological process distributions of identified proteins.
Supplemental Figure S3. Sequence analysis of EoPSBS.
Supplemental Figure S4. Sequence analysis of EoMPH1.
Supplemental Figure S5. Sequence analysis of TBCC domain-containing proteins.
Supplemental Figure S6. Sequence analysis of EoCOX2.
Supplemental Figure S7. Analysis of EoENO and its homologs in the green lineage.
Supplemental Tables S1 to S15. Detailed description of proteins identified in the membranes of nitrogen-deprived E. oleoabundans according to the biological process in which they are involved.
Supplemental Table S16. Molecular phylogenetic analysis based on a multigene approach.
Supplemental Table S17. 18S rDNA molecular phylogenetic analysis.
Supplemental Table S18. Proteins surveyed in FFZE membrane fractions via LC-MS/MS analysis.
Supplemental Information S1. Additional biological processes represented in the E. oleoabundans membrane proteome.
Supplementary Material
Acknowledgments
We thank Dr. Denis Faubert and Marguerite Boulos at the Institut de Recherches Cliniques de Montréal-Proteomics Discovery Platform for LC-MS/MS analysis; Dr. Jordan Peccia (Yale University) and Dr. Berat Z. Haznedaroglu (Boğaziçi University) for providing the E. oleoabundans transcriptome; Dr. Alejandro Sanchez-Flores and Karel Estrada at the Unidad Universitaria de Apoyo Bioinformático-Universidad Nacional Autónoma de México (UNAM) for generating the E. oleoabundans protein database; Dr. Diego Gonzalez-Halphen (UNAM) for the gift of the C. reinhardtii strain, Dr. Carlos F. Arias (UNAM) for the gift of C2BBe1 cells, Dr. Robert L. Last (Michigan State University) for the gift of the anti-MPH1 antibody, Dr. Michael Cheetham (University College London) for the gift of the anti-RP2 antibody, and Dr. Patricia Leon (UNAM) for the gift of the anti-AtpB antibody; Luz María Martínez, Mario A. Caro Bermúdez, Mercedes Enzaldo Cruz, and M. Guadalupe Muñoz García for technical assistance; and Gustavo Rodriguez-Alonso for support in phylogenetic analysis.
Glossary
- RNA-Seq
RNA sequencing
- FFZE
free-flow zonal electrophoresis
- rDNA
ribosomal DNA
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- NCBI
National Center for Biotechnology Information
- LD
lipid droplet
- CTP
chloroplast transit peptide
- NPQ
nonphotochemical quenching
- NSAF
normalized spectral abundance factor
- MS
mass spectrometry
- MLb
bootstrap maximum likelihood
- MS/MS
tandem mass spectrometry
- ML
maximum likelihood
Footnotes
This work was supported by CONACyT-Mexico (grant no. 220085 to O.P., grant no. 178232 to R.V.-E., and a scholarship to A.G.-H.) and by the Universidad Nacional Autónoma de México-DGAPA (grant no. IN202514 to R.V.-E. and grant no. IT200312 to A.M.).
References
- Ajjawi I, Coku A, Froehlich JE, Yang Y, Osteryoung KW, Benning C, Last RL (2011) A J-like protein influences fatty acid composition of chloroplast lipids in Arabidopsis. PLoS ONE 6: e25368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alboresi A, Gerotto C, Giacometti GM, Bassi R, Morosinotto T (2010) Physcomitrella patens mutants affected on heat dissipation clarify the evolution of photoprotection mechanisms upon land colonization. Proc Natl Acad Sci USA 107: 11128–11133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon DI. (1949) Copper enzymes in isolated chloroplasts: polyphenoloxidases in Beta vulgaris. Plant Physiol 24: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredondo-Vega BO, Band CJ, Vazquez-Duhalt R (1995) Biochemical composition of Neochloris oleoabundans adapted to marine medium. Cytobios 83: 201–205 [Google Scholar]
- Atteia A, Adrait A, Brugière S, Tardif M, van Lis R, Deusch O, Dagan T, Kuhn L, Gontero B, Martin W, et al. (2009) A proteomic survey of Chlamydomonas reinhardtii mitochondria sheds new light on the metabolic plasticity of the organelle and on the nature of the α-proteobacterial mitochondrial ancestor. Mol Biol Evol 26: 1533–1548 [DOI] [PubMed] [Google Scholar]
- Awai K, Xu C, Tamot B, Benning C (2006) A phosphatidic acid-binding protein of the chloroplast inner envelope membrane involved in lipid trafficking. Proc Natl Acad Sci USA 103: 10817–10822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M, Suzuki I, Shiraiwa Y (2011) Proteomic analysis of high-CO2-inducible extracellular proteins in the unicellular green alga, Chlamydomonas reinhardtii. Plant Cell Physiol 52: 1302–1314 [DOI] [PubMed] [Google Scholar]
- Bagos PG, Liakopoulos TD, Hamodrakas SJ (2004) Finding beta-barrel outer membrane proteins with a Markov chain model. WSEAS Trans Biol Biomed 2: 186–189 [Google Scholar]
- Bailleul B, Rogato A, de Martino A, Coesel S, Cardol P, Bowler C, Falciatore A, Finazzi G (2010) An atypical member of the light-harvesting complex stress-related protein family modulates diatom responses to light. Proc Natl Acad Sci USA 107: 18214–18219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldisserotto C, Ferroni L, Giovanardi M, Boccaletti L, Pantaleoni L, Pancaldi S (2012) Salinity promotes growth of freshwater Neochloris oleoabundans UTEX 1185 (Sphaeropleales, Chlorophyta): morphophysiological aspects. Phycologia 51: 700–710 [Google Scholar]
- Baldisserotto C, Popovich C, Giovanardi M, Sabia A, Ferroni L, Constenla D, Leonardi P, Pancaldi S (2016) Photosynthetic aspects and lipid profiles in the mixotrophic alga Neochloris oleoabundans as useful parameters for biodiesel production. Algal Res 16: 255–265 [Google Scholar]
- Barkla BJ, Vera-Estrella R, Hernández-Coronado M, Pantoja O (2009) Quantitative proteomics of the tonoplast reveals a role for glycolytic enzymes in salt tolerance. Plant Cell 21: 4044–4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkla BJ, Vera-Estrella R, Pantoja O (2007) Enhanced separation of membranes during free flow zonal electrophoresis in plants. Anal Chem 79: 5181–5187 [DOI] [PubMed] [Google Scholar]
- Barkla BJ, Vera-Estrella R, Pantoja O (2012) Protein profiling of epidermal bladder cells from the halophyte Mesembryanthemum crystallinum. Proteomics 12: 2862–2865 [DOI] [PubMed] [Google Scholar]
- Barkla BJ, Zingarelli L, Blumwald E, Smith J (1995) Tonoplast Na+/H+ antiport activity and its energization by the vacuolar H+-ATPase in the halophytic plant Mesembryanthemum crystallinum L. Plant Physiol 109: 549–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini F, Bhamidipati A, Thomas S, Schwahn U, Lewis SA, Cowan NJ (2002) Functional overlap between retinitis pigmentosa 2 protein and the tubulin-specific chaperone cofactor C. J Biol Chem 277: 14629–14634 [DOI] [PubMed] [Google Scholar]
- Beaudoin F, Wu X, Li F, Haslam RP, Markham JE, Zheng H, Napier JA, Kunst L (2009) Functional characterization of the Arabidopsis beta-ketoacyl-coenzyme A reductase candidates of the fatty acid elongase. Plant Physiol 150: 1174–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenuti G, Bosma R, Cuaresma M, Janssen M, Barbosa MJ, Wijffels RH (2015) Selecting microalgae with high lipid productivity and photosynthetic activity under nitrogen starvation. J Appl Phycol 27: 1425–1431 [Google Scholar]
- Bertani G. (1951) Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol 62: 293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthelot K, Lecomte S, Estevez Y, Peruch F (2014) Hevea brasiliensis REF (Hev b 1) and SRPP (Hev b 3): an overview on rubber particle proteins. Biochimie 106: 1–9 [DOI] [PubMed] [Google Scholar]
- Bienvenut WV, Espagne C, Martinez A, Majeran W, Valot B, Zivy M, Vallon O, Adam Z, Meinnel T, Giglione C (2011) Dynamics of post-translational modifications and protein stability in the stroma of Chlamydomonas reinhardtii chloroplasts. Proteomics 11: 1734–1750 [DOI] [PubMed] [Google Scholar]
- Binns D, Dimmer E, Huntley R, Barrell D, O’Donovan C, Apweiler R (2009) QuickGO: a web-based tool for Gene Ontology searching. Bioinformatics 25: 3045–3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldt L, Yellowlees D, Leggat W (2012) Hyperdiversity of genes encoding integral light-harvesting proteins in the dinoflagellate Symbiodinium sp. PLoS ONE 7: e47456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonente G, Passarini F, Cazzaniga S, Mancone C, Buia MC, Tripodi M, Bassi R, Caffarri S (2008) The occurrence of the psbS gene product in Chlamydomonas reinhardtii and in other photosynthetic organisms and its correlation with energy quenching. Photochem Photobiol 84: 1359–1370 [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Canaves JM, Taylor SS (2002) Classification and phylogenetic analysis of the cAMP-dependent protein kinase regulatory subunit family. J Mol Evol 54: 17–29 [DOI] [PubMed] [Google Scholar]
- Cardol P, Gloire G, Havaux M, Remacle C, Matagne R, Franck F (2003) Photosynthesis and state transitions in mitochondrial mutants of Chlamydomonas reinhardtii affected in respiration. Plant Physiol 133: 2010–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrie C, Murcha MW, Millar AH, Smith SM, Whelan J (2007) Nine 3-ketoacyl-CoA thiolases (KATs) and acetoacetyl-CoA thiolases (ACATs) encoded by five genes in Arabidopsis thaliana are targeted either to peroxisomes or cytosol but not to mitochondria. Plant Mol Biol 63: 97–108 [DOI] [PubMed] [Google Scholar]
- Castruita M, Casero D, Karpowicz SJ, Kropat J, Vieler A, Hsieh SI, Yan W, Cokus S, Loo JA, Benning C, et al. (2011) Systems biology approach in Chlamydomonas reveals connections between copper nutrition and multiple metabolic steps. Plant Cell 23: 1273–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantanachat S, Bold HC (1962) Phycological Studies II. Some Algae from Arid Soils. University of Texas, Austin, Texas [Google Scholar]
- Chapman KD, Dyer JM, Mullen RT (2013) Commentary: why don’t plant leaves get fat? Plant Sci 207: 128–134 [DOI] [PubMed] [Google Scholar]
- Chapple JP, Hardcastle AJ, Grayson C, Spackman LA, Willison KR, Cheetham ME (2000) Mutations in the N-terminus of the X-linked retinitis pigmentosa protein RP2 interfere with the normal targeting of the protein to the plasma membrane. Hum Mol Genet 9: 1919–1926 [DOI] [PubMed] [Google Scholar]
- Chapple JP, Hardcastle AJ, Grayson C, Willison KR, Cheetham ME (2002) Delineation of the plasma membrane targeting domain of the X-linked retinitis pigmentosa protein RP2. Invest Ophthalmol Vis Sci 43: 2015–2020 [PubMed] [Google Scholar]
- Chen M, Zhao L, Sun YL, Cui SX, Zhang LF, Yang B, Wang J, Kuang TY, Huang F (2010) Proteomic analysis of hydrogen photoproduction in sulfur-deprived Chlamydomonas cells. J Proteome Res 9: 3854–3866 [DOI] [PubMed] [Google Scholar]
- Correa-Galvis V, Redekop P, Guan K, Griess A, Truong TB, Wakao S, Niyogi KK, Jahns P (2016) Photosystem II subunit PsbS is involved in the induction of LHCSR protein-dependent energy dissipation in Chlamydomonas reinhardtii. J Biol Chem 291: 17478–17487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne NMD, Parisien A, Wang B, Lan CQ (2009) Enhancement of lipid production using biochemical, genetic and transcription factor engineering approaches. J Biotechnol 141: 31–41 [DOI] [PubMed] [Google Scholar]
- Davidi L, Katz A, Pick U (2012) Characterization of major lipid droplet proteins from Dunaliella. Planta 236: 19–33 [DOI] [PubMed] [Google Scholar]
- Davis RW, Volponi JV, Jones HDT, Carvalho BJ, Wu H, Singh S (2012) Multiplex fluorometric assessment of nutrient limitation as a strategy for enhanced lipid enrichment and harvesting of Neochloris oleoabundans. Biotechnol Bioeng 109: 2503–2512 [DOI] [PubMed] [Google Scholar]
- Deason T, Silva PC, Watanabe S, Floyd GL (1991) Taxonomic status of the species of the green algal genus Neochloris. Plant Syst Evol 177: 213–219 [Google Scholar]
- de Michele R, McFarlane HE, Parsons HT, Meents MJ, Lao J, González Fernández-Niño SM, Petzold CJ, Frommer WB, Samuels AL, Heazlewood JL (2016) Free-flow electrophoresis of plasma membrane vesicles enriched by two-phase partitioning enhances the quality of the proteome from Arabidopsis seedlings. J Proteome Res 15: 900–913 [DOI] [PubMed] [Google Scholar]
- Despres B, Bouissonnié F, Wu HJ, Gomord V, Guilleminot J, Grellet F, Berger F, Delseny M, Devic M (2003) Three SAC1-like genes show overlapping patterns of expression in Arabidopsis but are remarkably silent during embryo development. Plant J 34: 293–306 [DOI] [PubMed] [Google Scholar]
- Dittami SM, Michel G, Collén J, Boyen C, Tonon T (2010) Chlorophyll-binding proteins revisited: a multigenic family of light-harvesting and stress proteins from a brown algal perspective. BMC Evol Biol 10: 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HP, Williams E, Wang DZ, Xie ZX, Hsia RC, Jenck A, Halden R, Li J, Chen F, Place AR (2013) Responses of Nannochloropsis oceanica IMET1 to long-term nitrogen starvation and recovery. Plant Physiol 162: 1110–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einspahr KJ, Peeler TC, Thompson GA Jr (1988) Rapid changes in polyphosphoinositide metabolism associated with the response of Dunaliella salina to hypoosmotic shock. J Biol Chem 263: 5775–5779 [PubMed] [Google Scholar]
- Erickson E, Wakao S, Niyogi KK (2015) Light stress and photoprotection in Chlamydomonas reinhardtii. Plant J 82: 449–465 [DOI] [PubMed] [Google Scholar]
- Evans RJ, Schwarz N, Nagel-Wolfrum K, Wolfrum U, Hardcastle AJ, Cheetham ME (2010) The retinitis pigmentosa protein RP2 links pericentriolar vesicle transport between the Golgi and the primary cilium. Hum Mol Genet 19: 1358–1367 [DOI] [PubMed] [Google Scholar]
- Fatland BL, Ke J, Anderson MD, Mentzen WI, Cui LW, Allred CC, Johnston JL, Nikolau BJ, Wurtele ES (2002) Molecular characterization of a heteromeric ATP-citrate lyase that generates cytosolic acetyl-coenzyme A in Arabidopsis. Plant Physiol 130: 740–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Marshall WF (2009) ASQ2 encodes a TBCC-like protein required for mother-daughter centriole linkage and mitotic spindle orientation. Curr Biol 19: 1238–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791 [DOI] [PubMed] [Google Scholar]
- Ferro M, Brugière S, Salvi D, Seigneurin-Berny D, Court M, Moyet L, Ramus C, Miras S, Mellal M, Le Gall S, et al. (2010) AT_CHLORO, a comprehensive chloroplast proteome database with subplastidial localization and curated information on envelope proteins. Mol Cell Proteomics 9: 1063–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro M, Salvi D, Brugière S, Miras S, Kowalski S, Louwagie M, Garin J, Joyard J, Rolland N (2003) Proteomics of the chloroplast envelope membranes from Arabidopsis thaliana. Mol Cell Proteomics 2: 325–345 [DOI] [PubMed] [Google Scholar]
- Fleischmann A, Darsow M, Degtyarenko K, Fleischmann W, Boyce S, Axelsen KB, Bairoch A, Schomburg D, Tipton KF, Apweiler R (2004) IntEnz, the integrated relational enzyme database. Nucleic Acids Res 32: D434–D437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JE, Wilkerson CG, Ray WK, McAndrew RS, Osteryoung KW, Gage DA, Phinney BS (2003) Proteomic study of the Arabidopsis thaliana chloroplastic envelope membrane utilizing alternatives to traditional two-dimensional electrophoresis. J Proteome Res 2: 413–425 [DOI] [PubMed] [Google Scholar]
- Gao C, Wang Y, Shen Y, Yan D, He X, Dai J, Wu Q (2014) Oil accumulation mechanisms of the oleaginous microalga Chlorella protothecoides revealed through its genome, transcriptomes, and proteomes. BMC Genomics 15: 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garibay-Hernández A, Vazquez-Duhalt R, Serrano-Carreón L, Martinez A (2013) Nitrogen limitation in Neochloris oleoabundans: a reassessment of its effect on cell growth and biochemical composition. Appl Biochem Biotechnol 171: 1775–1791 [DOI] [PubMed] [Google Scholar]
- Gerotto C, Alboresi A, Giacometti GM, Bassi R, Morosinotto T (2012) Coexistence of plant and algal energy dissipation mechanisms in the moss Physcomitrella patens. New Phytol 196: 763–773 [DOI] [PubMed] [Google Scholar]
- Gerotto C, Morosinotto T (2013) Evolution of photoprotection mechanisms upon land colonization: evidence of PSBS-dependent NPQ in late Streptophyte algae. Physiol Plant 149: 583–598 [DOI] [PubMed] [Google Scholar]
- Gidda SK, Shockey JM, Rothstein SJ, Dyer JM, Mullen RT (2009) Arabidopsis thaliana GPAT8 and GPAT9 are localized to the ER and possess distinct ER retrieval signals: functional divergence of the dilysine ER retrieval motif in plant cells. Plant Physiol Biochem 47: 867–879 [DOI] [PubMed] [Google Scholar]
- Ginger ML, McFadden GI, Michels PAM (2010) Rewiring and regulation of cross-compartmentalized metabolism in protists. Philos Trans R Soc Lond B Biol Sci 365: 831–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanardi M, Ferroni L, Baldisserotto C, Tedeschi P, Maietti A, Pantaleoni L, Pancaldi S (2013) Morphophysiological analyses of Neochloris oleoabundans (Chlorophyta) grown mixotrophically in a carbon-rich waste product. Protoplasma 250: 161–174 [DOI] [PubMed] [Google Scholar]
- Gontcharov AA, Marin B, Melkonian M (2004) Are combined analyses better than single gene phylogenies? A case study using SSU rDNA and rbcL sequence comparisons in the Zygnematophyceae (Streptophyta). Mol Biol Evol 21: 612–624 [DOI] [PubMed] [Google Scholar]
- Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N, et al. (2012) Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res 40: D1178–D1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz S, García-Gómez JM, Terol J, Williams TD, Nagaraj SH, Nueda MJ, Robles M, Talón M, Dopazo J, Conesa A (2008) High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res 36: 3420–3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia L, Marques AE, da Silva TL, Reis A (2009) Neochloris oleabundans UTEX #1185: a suitable renewable lipid source for biofuel production. J Ind Microbiol Biotechnol 36: 821–826 [DOI] [PubMed] [Google Scholar]
- Grayson C, Bartolini F, Chapple JP, Willison KR, Bhamidipati A, Lewis SA, Luthert PJ, Hardcastle AJ, Cowan NJ, Cheetham ME (2002) Localization in the human retina of the X-linked retinitis pigmentosa protein RP2, its homologue cofactor C and the RP2 interacting protein Arl3. Hum Mol Genet 11: 3065–3074 [DOI] [PubMed] [Google Scholar]
- Guarnieri MT, Nag A, Smolinski SL, Darzins A, Seibert M, Pienkos PT (2011) Examination of triacylglycerol biosynthetic pathways via de novo transcriptomic and proteomic analyses in an unsequenced microalga. PLoS ONE 6: e25851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnieri MT, Nag A, Yang S, Pienkos PT (2013) Proteomic analysis of Chlorella vulgaris: potential targets for enhanced lipid accumulation. J Proteomics 93: 245–253 [DOI] [PubMed] [Google Scholar]
- Guiry MD, Guiry GM (1996) AlgaeBase. http://www.algaebase.org. Accessed May, 2016.
- Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, Couger MB, Eccles D, Li B, Lieber M, et al. (2013) De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc 8: 1494–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EH, editor (2009) The Chlamydomonas Sourcebook: Introduction to Chlamydomonas and Its Laboratory Use, Ed 2 Elsevier Academic Press, Toronto, Ontario, Canada [Google Scholar]
- Haslam TM, Kunst L (2013) Extending the story of very-long-chain fatty acid elongation. Plant Sci 210: 93–107 [DOI] [PubMed] [Google Scholar]
- Heilmann I, Perera IY, Gross W, Boss WF (2001) Plasma membrane phosphatidylinositol 4,5-bisphosphate levels decrease with time in culture. Plant Physiol 126: 1507–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn PJ, James CN, Gidda SK, Kilaru A, Dyer JM, Mullen RT, Ohlrogge JB, Chapman KD (2013) Identification of a new class of lipid droplet-associated proteins in plants. Plant Physiol 162: 1926–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54: 621–639 [DOI] [PubMed] [Google Scholar]
- Huss VAR, Frank C, Hartmann EC, Hirmer M, Kloboucek A, Seidel BM, Wenzeler P, Kessler E (1999) Biochemical taxonomy and molecular phylogeny of the genus Chlorella sensu lato (Chlorophyta). J Phycol 35: 587–598 [Google Scholar]
- Iskandarov U, Sitnik S, Shtaida N, Didi-Cohen S, Leu S, Khozin-Goldberg I, Cohen Z, Boussiba S (2016) Cloning and characterization of a GPAT-like gene from the microalga Lobosphaera incisa (Trebouxiophyceae): overexpression in Chlamydomonas reinhardtii enhances TAG production. J Appl Phycol 28: 907–919 [Google Scholar]
- Iwai M, Takizawa K, Tokutsu R, Okamuro A, Takahashi Y, Minagawa J (2010) Isolation of the elusive supercomplex that drives cyclic electron flow in photosynthesis. Nature 464: 1210–1213 [DOI] [PubMed] [Google Scholar]
- Jinkerson RE, Subramanian V, Posewitz MC (2011) Improving biofuel production in phototrophic microorganisms with systems biology. Biofuels 2: 125–144 [Google Scholar]
- Johnson X, Alric J (2013) Central carbon metabolism and electron transport in Chlamydomonas reinhardtii: metabolic constraints for carbon partitioning between oil and starch. Eukaryot Cell 12: 776–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Taylor WR, Thornton JM (1992) The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci 8: 275–282 [DOI] [PubMed] [Google Scholar]
- Joyard J, Ferro M, Masselon C, Seigneurin-Berny D, Salvi D, Garin J, Rolland N (2010) Chloroplast proteomics highlights the subcellular compartmentation of lipid metabolism. Prog Lipid Res 49: 128–158 [DOI] [PubMed] [Google Scholar]
- Kleffmann T, Russenberger D, von Zychlinski A, Christopher W, Sjölander K, Gruissem W, Baginsky S (2004) The Arabidopsis thaliana chloroplast proteome reveals pathway abundance and novel protein functions. Curr Biol 14: 354–362 [DOI] [PubMed] [Google Scholar]
- Klein U. (1986) Compartmentation of glycolysis and of the oxidative pentose-phosphate pathway in Chlamydomonas reinhardtii. Planta 167: 81–86 [DOI] [PubMed] [Google Scholar]
- Koziol AG, Borza T, Ishida K, Keeling P, Lee RW, Durnford DG (2007) Tracing the evolution of the light-harvesting antennae in chlorophyll a/b-containing organisms. Plant Physiol 143: 1802–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A, Larsson B, von Heijne G, Sonnhammer ELL (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305: 567–580 [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33: 1870–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Levine RB, Costanza-Robinson MS, Spatafora GA (2011) Neochloris oleoabundans grown on anaerobically digested dairy manure for concomitant nutrient removal and biodiesel feedstock production. Biomass Bioenergy 35: 40–49 [Google Scholar]
- Li J, Han D, Wang D, Ning K, Jia J, Wei L, Jing X, Huang S, Chen J, Li Y, et al. (2014) Choreography of transcriptomes and lipidomes of Nannochloropsis reveals the mechanisms of oil synthesis in microalgae. Plant Cell 26: 1645–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Xu C, Li-Beisson Y, Philippar K (2016) Fatty acid and lipid transport in plant cells. Trends Plant Sci 21: 145–158 [DOI] [PubMed] [Google Scholar]
- Li X, Moellering ER, Liu B, Johnny C, Fedewa M, Sears BB, Kuo MH, Benning C (2012) A galactoglycerolipid lipase is required for triacylglycerol accumulation and survival following nitrogen deprivation in Chlamydomonas reinhardtii. Plant Cell 24: 4670–4686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XP, Björkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK (2000) A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403: 391–395 [DOI] [PubMed] [Google Scholar]
- Li XP, Gilmore AM, Caffarri S, Bassi R, Golan T, Kramer D, Niyogi KK (2004) Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J Biol Chem 279: 22866–22874 [DOI] [PubMed] [Google Scholar]
- Li XP, Phippard A, Pasari J, Niyogi KK (2002) Structure-function analysis of photosystem II subunit S (PsbS) in vivo. Funct Plant Biol 29: 1131–1139 [DOI] [PubMed] [Google Scholar]
- Li Y, Horsman M, Wang B, Wu N, Lan CQ (2008) Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol 81: 629–636 [DOI] [PubMed] [Google Scholar]
- Li-Beisson Y, Beisson F, Riekhof W (2015) Metabolism of acyl-lipids in Chlamydomonas reinhardtii. Plant J 82: 504–522 [DOI] [PubMed] [Google Scholar]
- Li-Beisson Y, Shorrosh B, Beisson F, Andersson MX, Arondel V, Bates PD, Baud S, Bird D, Debono A, Durrett TP, et al. (2010) Acyl-lipid metabolism. The Arabidopsis Book e0133, doi/10.1199/tab.0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Benning C (2013) Lipid metabolism in microalgae distinguishes itself. Curr Opin Biotechnol 24: 300–309 [DOI] [PubMed] [Google Scholar]
- Liu J, Last RL (2015a) MPH1 is a thylakoid membrane protein involved in protecting photosystem II from photodamage in land plants. Plant Signal Behav 10: e1076602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Last RL (2015b) A land plant-specific thylakoid membrane protein contributes to photosystem II maintenance in Arabidopsis thaliana. Plant J 82: 731–743 [DOI] [PubMed] [Google Scholar]
- Lundin VF, Leroux MR, Stirling PC (2010) Quality control of cytoskeletal proteins and human disease. Trends Biochem Sci 35: 288–297 [DOI] [PubMed] [Google Scholar]
- Ma Q, Wang J, Lu S, Lv Y, Yuan Y (2013) Quantitative proteomic profiling reveals photosynthesis responsible for inoculum size dependent variation in Chlorella sorokiniana. Biotechnol Bioeng 110: 773–784 [DOI] [PubMed] [Google Scholar]
- Manna PT, Gadelha C, Puttick AE, Field MC (2015) ENTH and ANTH domain proteins participate in AP2-independent clathrin-mediated endocytosis. J Cell Sci 128: 2130–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matich EK, Butryn DM, Ghafari M, del Solar V, Camgoz E, Pfeifer BA, Aga DS, Haznedaroglu BZ, Atilla-Gokcumen GE (2016) Mass spectrometry-based metabolomics of value-added biochemicals from Ettlia oleoabundans. Algal Res 19: 146–154 [Google Scholar]
- McCormac DJ, Barkan A (1999) A nuclear gene in maize required for the translation of the chloroplast atpB/E mRNA. Plant Cell 11: 1709–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R, Wu G, Deshpande RR, Vieler A, Gärtner K, Li X, Moellering ER, Zäuner S, Cornish AJ, Liu B, et al. (2010) Changes in transcript abundance in Chlamydomonas reinhardtii following nitrogen deprivation predict diversion of metabolism. Plant Physiol 154: 1737–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa J, Tokutsu R (2015) Dynamic regulation of photosynthesis in Chlamydomonas reinhardtii. Plant J 82: 413–428 [DOI] [PubMed] [Google Scholar]
- Misumi O, Matsuzaki M, Nozaki H, Miyagishima SY, Mori T, Nishida K, Yagisawa F, Yoshida Y, Kuroiwa H, Kuroiwa T (2005) Cyanidioschyzon merolae genome: a tool for facilitating comparable studies on organelle biogenesis in photosynthetic eukaryotes. Plant Physiol 137: 567–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A, Chang HY, Daugherty L, Fraser M, Hunter S, Lopez R, McAnulla C, McMenamin C, Nuka G, Pesseat S, et al. (2015) The InterPro protein families database: the classification resource after 15 years. Nucleic Acids Res 43: D213–D221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SK, Walters BT, Clouse SD, Goshe MB (2009) An efficient organic solvent based extraction method for the proteomic analysis of Arabidopsis plasma membranes. J Proteome Res 8: 2752–2767 [DOI] [PubMed] [Google Scholar]
- Moellering ER, Benning C (2010) RNA interference silencing of a major lipid droplet protein affects lipid droplet size in Chlamydomonas reinhardtii. Eukaryot Cell 9: 97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon-van der Staay SY, De Wachter R, Vaulot D (2001) Oceanic 18S rDNA sequences from picoplankton reveal unsuspected eukaryotic diversity. Nature 409: 607–610 [DOI] [PubMed] [Google Scholar]
- Morales-Sánchez D, Tinoco-Valencia R, Kyndt J, Martinez A (2013) Heterotrophic growth of Neochloris oleoabundans using glucose as a carbon source. Biotechnol Biofuels 6: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira D, Le Guyader H, Philippe H (2000) The origin of red algae and the evolution of chloroplasts. Nature 405: 69–72 [DOI] [PubMed] [Google Scholar]
- Mou S, Zhang X, Dong M, Fan X, Xu J, Cao S, Xu D, Wang W, Ye N (2013) Photoprotection in the green tidal alga Ulva prolifera: role of LHCSR and PsbS proteins in response to high light stress. Plant Biol (Stuttg) 15: 1033–1039 [DOI] [PubMed] [Google Scholar]
- Mühlhaus T, Weiss J, Hemme D, Sommer F, Schroda M (2011) Quantitative shotgun proteomics using a uniform 15N-labeled standard to monitor proteome dynamics in time course experiments reveals new insights into the heat stress response of Chlamydomonas reinhardtii. Mol Cell Proteomics 10: M110.004739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natter K, Leitner P, Faschinger A, Wolinski H, McCraith S, Fields S, Kohlwein SD (2005) The spatial organization of lipid synthesis in the yeast Saccharomyces cerevisiae derived from large scale green fluorescent protein tagging and high resolution microscopy. Mol Cell Proteomics 4: 662–672 [DOI] [PubMed] [Google Scholar]
- Ndimba BK, Ndimba RJ, Johnson TS, Waditee-Sirisattha R, Baba M, Sirisattha S, Shiraiwa Y, Agrawal GK, Rakwal R (2013) Biofuels as a sustainable energy source: an update of the applications of proteomics in bioenergy crops and algae. J Proteomics 93: 234–244 [DOI] [PubMed] [Google Scholar]
- Nesvizhskii AI, Keller A, Kolker E, Aebersold R (2003) A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 75: 4646–4658 [DOI] [PubMed] [Google Scholar]
- Newlon MG, Roy M, Morikis D, Carr DW, Westphal R, Scott JD, Jennings PA (2001) A novel mechanism of PKA anchoring revealed by solution structures of anchoring complexes. EMBO J 20: 1651–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HM, Baudet M, Cuiné S, Adriano JM, Barthe D, Billon E, Bruley C, Beisson F, Peltier G, Ferro M, et al. (2011) Proteomic profiling of oil bodies isolated from the unicellular green microalga Chlamydomonas reinhardtii: with focus on proteins involved in lipid metabolism. Proteomics 11: 4266–4273 [DOI] [PubMed] [Google Scholar]
- Niyogi KK, Truong TB (2013) Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr Opin Plant Biol 16: 307–314 [DOI] [PubMed] [Google Scholar]
- Nordhues A, Schöttler MA, Unger AK, Geimer S, Schönfelder S, Schmollinger S, Rütgers M, Finazzi G, Soppa B, Sommer F, et al. (2012) Evidence for a role of VIPP1 in the structural organization of the photosynthetic apparatus in Chlamydomonas. Plant Cell 24: 637–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olguín EJ, Castillo OS, Mendoza A, Tapia K, González-Portela RE, Hernández-Landa VJ (2015) Dual purpose system that treats anaerobic effluents from pig waste and produce Neochloris oleoabundans as lipid rich biomass. N Biotechnol 32: 387–395 [DOI] [PubMed] [Google Scholar]
- Ou YY, Gromiha MM, Chen SA, Suwa M (2008) TMBETADISC-RBF: discrimination of beta-barrel membrane proteins using RBF networks and PSSM profiles. Comput Biol Chem 32: 227–231 [DOI] [PubMed] [Google Scholar]
- Parry RV, Turner JC, Rea PA (1989) High purity preparations of higher plant vacuolar H+-ATPase reveal additional subunits: revised subunit composition. J Biol Chem 264: 20025–20032 [PubMed] [Google Scholar]
- Peers G, Truong TB, Ostendorf E, Busch A, Elrad D, Grossman AR, Hippler M, Niyogi KK (2009) An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature 462: 518–521 [DOI] [PubMed] [Google Scholar]
- Pegg C, Wolf M, Alanagreh L, Portman R, Buchheim MA (2015) Morphological diversity masks phylogenetic similarity of Ettlia and Haematococcus (Chlorophyceae). Phycologia 54: 385–397 [Google Scholar]
- Peled E, Leu S, Zarka A, Weiss M, Pick U, Khozin-Goldberg I, Boussiba S (2011) Isolation of a novel oil globule protein from the green alga Haematococcus pluvialis (Chlorophyceae). Lipids 46: 851–861 [DOI] [PubMed] [Google Scholar]
- Peltier JB, Cai Y, Sun Q, Zabrouskov V, Giacomelli L, Rudella A, Ytterberg AJ, Rutschow H, van Wijk KJ (2006) The oligomeric stromal proteome of Arabidopsis thaliana chloroplasts. Mol Cell Proteomics 5: 114–133 [DOI] [PubMed] [Google Scholar]
- Pérez-Martínez X, Antaramian A, Vázquez-Acevedo M, Funes S, Tolkunova E, d’Alayer J, Claros MG, Davidson E, King MP, González-Halphen D (2001) Subunit II of cytochrome c oxidase in chlamydomonad algae is a heterodimer encoded by two independent nuclear genes. J Biol Chem 276: 11302–11309 [DOI] [PubMed] [Google Scholar]
- Pérez-Rodríguez P, Riaño-Pachón DM, Corrêa LGG, Rensing SA, Kersten B, Mueller-Roeber B (2010) PlnTFDB: updated content and new features of the plant transcription factor database. Nucleic Acids Res 38: D822–D827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovich CA, Damiani C, Constenla D, Martínez AM, Freije H, Giovanardi M, Pancaldi S, Leonardi PI (2012) Neochloris oleoabundans grown in enriched natural seawater for biodiesel feedstock: evaluation of its growth and biochemical composition. Bioresour Technol 114: 287–293 [DOI] [PubMed] [Google Scholar]
- Poulsen LR, López-Marqués RL, McDowell SC, Okkeri J, Licht D, Schulz A, Pomorski T, Harper JF, Palmgren MG (2008) The Arabidopsis P4-ATPase ALA3 localizes to the Golgi and requires a β-subunit to function in lipid translocation and secretory vesicle formation. Plant Cell 20: 658–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruvost J, Van Vooren G, Cogne G, Legrand J (2009) Investigation of biomass and lipids production with Neochloris oleoabundans in photobioreactor. Bioresour Technol 100: 5988–5995 [DOI] [PubMed] [Google Scholar]
- Rawlings ND, Waller M, Barrett AJ, Bateman A (2014) MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res 42: D503–D509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Pei-Chen Lin C, Pathak MC, Temple BRS, Nile AH, Mousley CJ, Duncan MC, Eckert DM, Leiker TJ, Ivanova PT, et al. (2014) A phosphatidylinositol transfer protein integrates phosphoinositide signaling with lipid droplet metabolism to regulate a developmental program of nutrient stress-induced membrane biogenesis. Mol Biol Cell 25: 712–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reumann S, Quan S, Aung K, Yang P, Manandhar-Shrestha K, Holbrook D, Linka N, Switzenberg R, Wilkerson CG, Weber APM, et al. (2009) In-depth proteome analysis of Arabidopsis leaf peroxisomes combined with in vivo subcellular targeting verification indicates novel metabolic and regulatory functions of peroxisomes. Plant Physiol 150: 125–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rismani-Yazdi H, Haznedaroglu BZ, Hsin C, Peccia J (2012) Transcriptomic analysis of the oleaginous microalga Neochloris oleoabundans reveals metabolic insights into triacylglyceride accumulation. Biotechnol Biofuels 5: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Salinas E, Riveros-Rosas H, Li Z, Fucíková K, Brand JJ, Lewis LA, González-Halphen D (2012) Lineage-specific fragmentation and nuclear relocation of the mitochondrial cox2 gene in chlorophycean green algae (Chlorophyta). Mol Phylogenet Evol 64: 166–176 [DOI] [PubMed] [Google Scholar]
- Saier MH Jr, Reddy VS, Tamang DG, Västermark A (2014) The transporter classification database. Nucleic Acids Res 42: D251–D258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Gessner G, Luff M, Heiland I, Wagner V, Kaminski M, Geimer S, Eitzinger N, Reissenweber T, Voytsekh O, et al. (2006) Proteomic analysis of the eyespot of Chlamydomonas reinhardtii provides novel insights into its components and tactic movements. Plant Cell 18: 1908–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmollinger S, Mühlhaus T, Boyle NR, Blaby IK, Casero D, Mettler T, Moseley JL, Kropat J, Sommer F, Strenkert D, et al. (2014) Nitrogen-sparing mechanisms in Chlamydomonas affect the transcriptome, the proteome, and photosynthetic metabolism. Plant Cell 26: 1410–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoes AM, Brown SD, Dodevski I, Babbitt PC (2009) Annotation error in public databases: misannotation of molecular function in enzyme superfamilies. PLOS Comput Biol 5: e1000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwacke R, Schneider A, van der Graaff E, Fischer K, Catoni E, Desimone M, Frommer WB, Flügge UI, Kunze R (2003) ARAMEMNON, a novel database for Arabidopsis integral membrane proteins. Plant Physiol 131: 16–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi LX, Hall M, Funk C, Schröder WP (2012) Photosystem II, a growing complex: updates on newly discovered components and low molecular mass proteins. Biochim Biophys Acta 1817: 13–25 [DOI] [PubMed] [Google Scholar]
- Shi LX, Schröder WP (2004) The low molecular mass subunits of the photosynthetic supracomplex, photosystem II. Biochim Biophys Acta 1608: 75–96 [DOI] [PubMed] [Google Scholar]
- Shockey J, Regmi A, Cotton K, Adhikari N, Browse J, Bates PD (2016) Identification of Arabidopsis GPAT9 (At5g60620) as an essential gene involved in triacylglycerol biosynthesis. Plant Physiol 170: 163–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtaida N, Khozin-Goldberg I, Boussiba S (2015) The role of pyruvate hub enzymes in supplying carbon precursors for fatty acid synthesis in photosynthetic microalgae. Photosynth Res 125: 407–422 [DOI] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, et al. (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7: 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer SD, Chen G, Mietkiewska E, Tomasi P, Jayawardhane K, Dyer JM, Weselake RJ (2016) Arabidopsis GPAT9 contributes to synthesis of intracellular glycerolipids but not surface lipids. J Exp Bot 67: 4627–4638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh DK, McNellis TW (2011) Fibrillin protein function: the tip of the iceberg? Trends Plant Sci 16: 432–441 [DOI] [PubMed] [Google Scholar]
- Škunca N, Altenhoff A, Dessimoz C (2012) Quality of computationally inferred Gene Ontology annotations. PLOS Comput Biol 8: e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SR, Abbriano RM, Hildebrand M (2012) Comparative analysis of diatom genomes reveals substantial differences in the organization of carbon partitioning pathways. Algal Res 1: 2–16 [Google Scholar]
- Song P, Li L, Liu J (2013) Proteomic analysis in nitrogen-deprived Isochrysis galbana during lipid accumulation. PLoS ONE 8: e82188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabenau H, Winkler U, Säftel W (1984) Enzymes of β-oxidation in different types of algal microbodies. Plant Physiol 75: 531–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabenau H, Winkler U, Säftel W (1993) Localization of glycolate dehydrogenase in two species of Dunaliella. Planta 191: 362–364 [Google Scholar]
- Stephan A, Vaughan S, Shaw MK, Gull K, McKean PG (2007) An essential quality control mechanism at the eukaryotic basal body prior to intraflagellar transport. Traffic 8: 1323–1330 [DOI] [PubMed] [Google Scholar]
- Stern D, editor (2009) The Chlamydomonas Sourcebook: Organellar and Metabolic Processes, Ed 2 Elsevier Academic Press, Toronto, Ontario, Canada [Google Scholar]
- Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10: 512–526 [DOI] [PubMed] [Google Scholar]
- Tan S, Tan HT, Chung MCM (2008) Membrane proteins and membrane proteomics. Proteomics 8: 3924–3932 [DOI] [PubMed] [Google Scholar]
- Tanz SK, Castleden I, Hooper CM, Vacher M, Small I, Millar HA (2013) SUBA3: a database for integrating experimentation and prediction to define the SUBcellular location of proteins in Arabidopsis. Nucleic Acids Res 41: D1185–D1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif M, Atteia A, Specht M, Cogne G, Rolland N, Brugière S, Hippler M, Ferro M, Bruley C, Peltier G, et al. (2012) PredAlgo: a new subcellular localization prediction tool dedicated to green algae. Mol Biol Evol 29: 3625–3639 [DOI] [PubMed] [Google Scholar]
- Terashima M, Specht M, Naumann B, Hippler M (2010) Characterizing the anaerobic response of Chlamydomonas reinhardtii by quantitative proteomics. Mol Cell Proteomics 9: 1514–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibiletti T, Auroy P, Peltier G, Caffarri S (2016) Chlamydomonas reinhardtii PsbS protein is functional and accumulates rapidly and transiently under high light. Plant Physiol 171: 2717–2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tippery NP, Fučíková K, Lewis PO, Lewis LA (2012) Probing the monophyly of the Sphaeropleales (Chlorophyceae) using data from five genes. J Phycol 48: 1482–1493 [DOI] [PubMed] [Google Scholar]
- Tornabene TG, Holzer G, Lien S, Burris N (1983) Lipid composition of the nitrogen starved green alga Neochloris oleoabundans. Enzyme Microb Technol 5: 435–440 [Google Scholar]
- Tusnády GE, Simon I (2001) The HMMTOP transmembrane topology prediction server. Bioinformatics 17: 849–850 [DOI] [PubMed] [Google Scholar]
- UniProt Consortium (2015) UniProt: a hub for protein information. Nucleic Acids Res 43: D204–D212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valledor L, Furuhashi T, Recuenco-Muñoz L, Wienkoop S, Weckwerth W (2014) System-level network analysis of nitrogen starvation and recovery in Chlamydomonas reinhardtii reveals potential new targets for increased lipid accumulation. Biotechnol Biofuels 7: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandepoele K, Van Bel M, Richard G, Van Landeghem S, Verhelst B, Moreau H, Van de Peer Y, Grimsley N, Piganeau G (2013) pico-PLAZA, a genome database of microbial photosynthetic eukaryotes. Environ Microbiol 15: 2147–2153 [DOI] [PubMed] [Google Scholar]
- Vázquez-Acevedo M, Cardol P, Cano-Estrada A, Lapaille M, Remacle C, González-Halphen D (2006) The mitochondrial ATP synthase of chlorophycean algae contains eight subunits of unknown origin involved in the formation of an atypical stator-stalk and in the dimerization of the complex. J Bioenerg Biomembr 38: 271–282 [DOI] [PubMed] [Google Scholar]
- Vázquez-Acevedo M, Vega-deLuna F, Sánchez-Vásquez L, Colina-Tenorio L, Remacle C, Cardol P, Miranda-Astudillo H, González-Halphen D (2016) Dissecting the peripheral stalk of the mitochondrial ATP synthase of chlorophycean algae. Biochim Biophys Acta 1857: 1183–1190 [DOI] [PubMed] [Google Scholar]
- Vera-Estrella R, Barkla BJ, Bohnert HJ, Pantoja O (2004) Novel regulation of aquaporins during osmotic stress. Plant Physiol 135: 2318–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrier PJ, Bird D, Burla B, Dassa E, Forestier C, Geisler M, Klein M, Kolukisaoglu U, Lee Y, Martinoia E, et al. (2008) Plant ABC proteins: a unified nomenclature and updated inventory. Trends Plant Sci 13: 151–159 [DOI] [PubMed] [Google Scholar]
- Wang B, Lan CQ (2011) Biomass production and nitrogen and phosphorus removal by the green alga Neochloris oleoabundans in simulated wastewater and secondary municipal wastewater effluent. Bioresour Technol 102: 5639–5644 [DOI] [PubMed] [Google Scholar]
- Wang J, Virta VC, Riddelle-Spencer K, O’Halloran TJ (2003) Compromise of clathrin function and membrane association by clathrin light chain deletion. Traffic 4: 891–901 [DOI] [PubMed] [Google Scholar]
- Wang X, Liu Q, Zhang B (2014) Leveraging the complementary nature of RNA-Seq and shotgun proteomics data. Proteomics 14: 2676–2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Benning C (2012) Chloroplast lipid synthesis and lipid trafficking through ER-plastid membrane contact sites. Biochem Soc Trans 40: 457–463 [DOI] [PubMed] [Google Scholar]
- Wase N, Black PN, Stanley BA, DiRusso CC (2014) Integrated quantitative analysis of nitrogen stress response in Chlamydomonas reinhardtii using metabolite and protein profiling. J Proteome Res 13: 1373–1396 [DOI] [PubMed] [Google Scholar]
- Wei L, Xin Y, Wang D, Jing X, Zhou Q, Su X, Jia J, Ning K, Chen F, Hu Q, et al. (2013) Nannochloropsis plastid and mitochondrial phylogenomes reveal organelle diversification mechanism and intragenus phylotyping strategy in microalgae. BMC Genomics 14: 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildgruber R, Weber G, Wise P, Grimm D, Bauer J (2014) Free-flow electrophoresis in proteome sample preparation. Proteomics 14: 629–636 [DOI] [PubMed] [Google Scholar]
- Wu N, Li Y, Lan CQ (2011) Production and rheological studies of microalgal extracellular biopolymer from lactose using the green alga Neochloris oleoabundans. J Polym Env 19: 935–942 [Google Scholar]
- Yang P, Diener DR, Yang C, Kohno T, Pazour GJ, Dienes JM, Agrin NS, King SM, Sale WS, Kamiya R, et al. (2006) Radial spoke proteins of Chlamydomonas flagella. J Cell Sci 119: 1165–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Mininberg B, Tarbet A, Weathers P (2013) At high temperature lipid production in Ettlia oleoabundans occurs before nitrate depletion. Appl Microbiol Biotechnol 97: 2263–2273 [DOI] [PubMed] [Google Scholar]
- Yang Y, Xu J, Vail D, Weathers P (2011) Ettlia oleoabundans growth and oil production on agricultural anaerobic waste effluents. Bioresour Technol 102: 5076–5082 [DOI] [PubMed] [Google Scholar]
- Yoo C, Choi GG, Kim SC, Oh HM (2013) Ettlia sp. YC001 showing high growth rate and lipid content under high CO2. Bioresour Technol 127: 482–488 [DOI] [PubMed] [Google Scholar]
- Yoon K, Han D, Li Y, Sommerfeld M, Hu Q (2012) Phospholipid:diacylglycerol acyltransferase is a multifunctional enzyme involved in membrane lipid turnover and degradation while synthesizing triacylglycerol in the unicellular green microalga Chlamydomonas reinhardtii. Plant Cell 24: 3708–3724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ye N, Mou S, Xu D, Fan X (2013) Occurrence of the PsbS and LhcSR products in the green alga Ulva linza and their correlation with excitation pressure. Plant Physiol Biochem 70: 336–341 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wen Z, Washburn MP, Florens L (2010) Refinements to label free proteome quantitation: how to deal with peptides shared by multiple proteins. Anal Chem 82: 2272–2281 [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Peck SC (2011) Simplified enrichment of plasma membrane proteins for proteomic analyses in Arabidopsis thaliana. Proteomics 11: 1780–1788 [DOI] [PubMed] [Google Scholar]
- Zhou X, Graham TR (2009) Reconstitution of phospholipid translocase activity with purified Drs2p, a type-IV P-type ATPase from budding yeast. Proc Natl Acad Sci USA 106: 16586–16591 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.