ABSTRACT
Rabies remains a public health threat in most parts of the world, and approximately 99% of the cases are transmitted by dogs. There is an urgent need to develop an efficacious and affordable vaccine to control canine-transmitted rabies in developing countries. Our previous studies demonstrate that overexpression of chemokines/cytokines such as CCL-3 (MIP-1α) and granulocyte-macrophage colony-stimulating factor (GM-CSF) can enhance the immunogenicity of rabies vaccines. In the present study, the chemokine CXCL13 was inserted into the genome of the recombinant rabies virus (rRABV) strain LBNSE, and the effect of the chemokine CXCL13 on the immunogenicity of RABV was investigated. It was found that LBNSE-CXCL13 recruited follicular helper T (Tfh) and germinal center (GC) B cells, promoted the formation of GCs, and increased the population of plasma cells in immunized mice. Further studies showed that mice immunized with LBNSE-CXCL13 produced more rabies virus-neutralizing antibodies (VNAs) and developed better protection than those immunized with the parent virus LBNSE or the GM-CSF-expressing RABV (LBNSE-GM-CSF). Collectively, these findings provide a better understanding of the role of CXCL13 expression in the immunogenicity of the RABV, which may help in designing more-efficacious rabies vaccines.
IMPORTANCE Rabies is endemic in most parts of the world, and more effort is needed to develop affordable and effective vaccines to control or eliminate this disease. The chemokine CXCL13 recruits both Tfh and B cells, which is essential for the homing of Tfh cells and the development of B cell follicles. In this study, the effect of the overexpression of CXCL13 on the immunogenicity of the RABV was evaluated in a mouse model. We found that CXCL13 expression promoted humoral immunity by recruiting Tfh and GC B cells, facilitating the formation of GCs, and increasing the number of plasma cells. As expected, the overexpression of CXCL13 resulted in enhanced virus-neutralizing antibody (VNA) production and protection against a virulent RABV challenge. These findings provide a better understanding of the role of CXCL13 in RABV-induced immune responses, which will help in designing more efficacious rabies vaccines.
KEYWORDS: CXCL13, rabies virus, T follicular helper cells, germinal center B cells, humoral immunity
INTRODUCTION
Rabies, a fatal and ancient neurological disease that occurs in both humans and warm-blooded animals (1, 2), still causes more than 59,000 human deaths every year and poses a potential threat to more than 3.3 billion people (3, 4). Its pathogen, the rabies virus (RABV), belongs to the genus Lyssavirus within the family Rhabdoviridae and has a single-stranded RNA genome with a negative-sense orientation that encodes five structural proteins: nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), and RNA polymerase (L) (1, 2). RABV particles from the saliva of infected animals enter the peripheral nervous system via sensory nerves through nerve spindles or via neuromuscular junctions (NMJs). The virus then reaches cell bodies in the spinal cord, brainstem, or sensory ganglia and moves along the spinal cord to the brain. Once the virus invades the brain, it replicates extensively, and the clinical disease appears quickly (5).
Although almost all warm-blooded animals are reservoirs of rabies, dogs account for more than 99% of the human deaths associated with this disease (6, 7). Vaccination of domestic dogs provides a cost-effective method to prevent and eliminate human rabies (4). The mass vaccination of domestic dogs (>70%) has nearly eliminated cases of human rabies in developed countries. However, due to financial, logistical, and other challenges, thousands of people in developing countries die of rabies each year (6). The availability of more-affordable vaccines for domestic animals may help resolve this problem. Previous studies have shown that a single intramuscular (i.m.) application of the live recombinant RABV (rRABV) variant TriGAS, expressing triple G proteins, induces robust and sustained virus-neutralizing antibody (VNA) production, which is required for the clearance of RABV infection (8, 9). Our previous studies have shown that an attenuated RABV expressing chemokines or cytokines enhances innate and adaptive immune responses by recruiting and/or activating dendritic cells (DCs) (10–14). A single dose of such vaccines can provide effective protection for animals against a rabies challenge. Therefore, promoting DC activation is an effective strategy to enhance the immune responses of the host.
After antigen uptake, DCs migrate to T-cell areas of secondary lymphoid organs and become fully stimulatory DCs. The clustering of DCs with T and B cells is essential for the induction of an immune response (15). After antigen stimulation, CD4+ naive T cells differentiate into helper T type 1 (Th1), type 2 (Th2), interleukin 17 (IL-17)-producing helper T (Th17), inducible regulatory T (iTReg), or follicular helper T (Tfh) cells (16, 17). Tfh cells are defined as CD4+ T cells that express chemokine C-X-C motif receptor 5 (CXCR5), inducible T-cell costimulator (ICOS), programmed cell death protein-1 (PD-1), B-cell lymphoma 6 (BCL-6), and IL-21. Tfh cells have the ability to home to B cell areas in secondary lymphoid tissues via interactions mediated by CXCR5 and its ligand, CXCL13 (18). Tfh cells provide both costimulation and stimulatory signals to B cells to mediate the positive selection of high-affinity B cells and the differentiation of plasma cells within the germinal centers (GCs), thereby determining which B cells exit GCs as memory B cells and long-lived plasma cells (16, 19). Due to their crucial role in the production of humoral immunity, Tfh cells have recently been identified as potential targets for rational vaccine design (16).
CXCL13, a CXC and homeostatic chemokine, is highly expressed in the lymphoid follicles of all secondary lymphoid organs, including the spleen, lymph nodes, and Peyer's patches (20). CXCL13 is expressed by both GC Tfh cells (21–23) and follicular DCs (FDCs) (24) in B-cell follicles. CXCL13 recruits both Tfh cells and B lymphocytes, so it is essential for the homing of Tfh cells and B lymphocytes into secondary lymphoid organs and for the development of B-cell follicles (25–36). Its receptor, CXCR5, is highly expressed on B cells and is largely responsible for B-cell movement into CXCL13-rich follicular areas in lymph nodes (25, 27). In this study, the effect of the overexpression of CXCL13 in RABV immunogenicity was evaluated in a mouse model. Our results demonstrate that the expression of CXCL13 enhances RABV-induced humoral immunity by recruiting Tfh and GC B cells, which sheds light on potential strategies for developing future rabies vaccines.
RESULTS
Construction and characterization of rRABV expressing CXCL13.
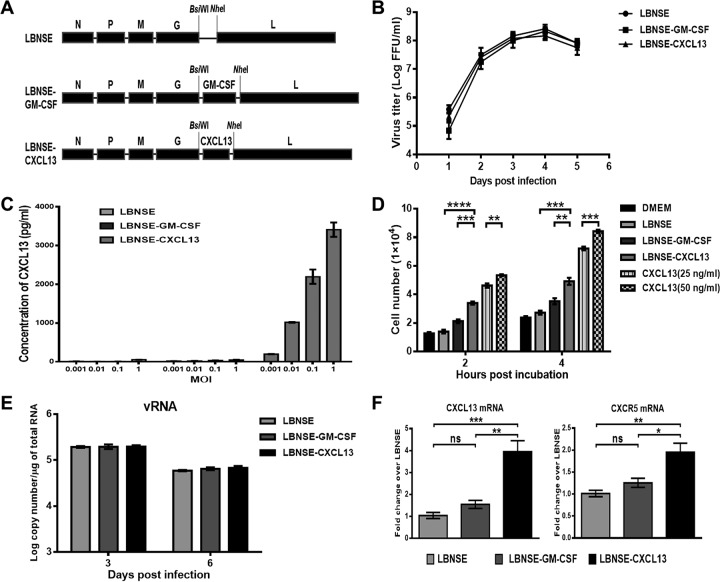
The CXCL13 gene was amplified from cDNA extracted from mouse peripheral blood mononuclear cells (PBMCs) and then cloned into the rRABV strain LBNSE genome, resulting in LBNSE-CXCL13 (Fig. 1A). The rRABV LBNSE-CXCL13 was rescued in BSR cells, and the insertion of CXCL13 was confirmed at the nucleotide level via reverse transcription (RT)-PCR and sequencing. To characterize LBNSE-CXCL13 in vitro, the viral growth kinetics was examined in BSR cells. A multistep growth pattern of LBNSE-CXCL13 was generated in BSR cells (Fig. 1B), and there was no significant difference compared with the growth kinetics of the LBNSE parent virus or the rRABV expressing granulocyte-macrophage colony-stimulating factor (GM-CSF) (LBNSE-GM-CSF) constructed previously (10), indicating that viral replication in BSR cells was not affected by the insertion of the CXCL13 gene into the LBNSE genome. The expression of CXCL13 in BSR cells was measured via enzyme-linked immunosorbent assay (ELISA), and it was found that CXCL13 was produced in a dose-dependent manner (Fig. 1C). To determine if CXCL13 has any biological activity in vitro, a transwell model was performed to test the ability to recruit splenocytes, with a recombinant CXCL13 protein (25 or 50 ng/ml) used as a positive control (36, 37). It was found that LBNSE-CXCL13 was more effective in recruiting splenocytes than LBNSE and LBNSE-GM-CSF at 2 and 4 h (Fig. 1D).
FIG 1.
Construction and characterization of the rRABV expressing CXCL13. (A) Schematic diagram for the construction of LBNSE, LBNSE-GM-CSF, and LBNSE-CXCL13. The mouse CXCL13 gene was cloned and inserted into the RABV genome in place of the deleted long noncoding region, and rRABVs were rescued according to the method described in Materials and Methods. (B) A multistep growth curve was generated in BSR cells. Cells were infected with LBNSE, LBNSE-GM-CSF, or LBNSE-CXCL13 at a multiplicity of infection (MOI) of 0.01 FFU and incubated at 37°C. Viruses were harvested at 1, 2, 3, 4, and 5 dpi, and viral titers were determined. All titrations were carried out in quadruplicate, and the data are presented as the means ± standard deviations (SD). (C) Production of CXCL13 in BSR cells. Cells were infected with different viruses at MOIs of 0.001, 0.01, 0.1, and 1. After incubation at 37°C for 24 h, the culture supernatants were collected, and the CXCL13 concentrations produced by the indicated rRABVs were determined with a commercial ELISA kit. (D) Chemotactic effects of cultured medium from BSR cells infected with rRABVs at an MOI of 1 on mouse splenocytes. Splenocytes (5 × 105) were applied to the upper wells of chemotaxis chambers. Two and 4 h later, the cells migrating to the bottom chamber were counted. (E and F) BALB/c mice were inoculated via i.m. injection of 1 × 106 FFU of rRABVs. The muscles from the hind legs of mice (n = 3) were harvested at 3 and 6 dpi. Total RNA was extracted, and viral genomic RNA (vRNA) (E) and CXCL13 mRNA and CXCR5 mRNA (F) were analyzed via qRT-PCR. All the data are expressed as the means ± SD. Asterisks indicate significant differences between the indicated experimental groups.
To confirm the expression of CXCL13 in vivo, mice were immunized via i.m. injections of 1 × 106 fluorescent focus units (FFU) of each rRABV. Muscle tissues at the inoculation sites were extracted, and the expression levels of viral genomic RNA (vRNA) and of CXCL13 mRNA and the mRNA for its receptor, CXCR5, were quantified via quantitative real-time RT-PCR (qRT-PCR). The replication of LBNSE-CXCL13 showed no significant difference compared with that of LBNSE or LBNSE-GM-CSF at the injection site (Fig. 1E). As expected, LBNSE-CXCL13 produced more CXCL13 mRNA at 3 days postinfection (dpi), which resulted in greater recruitment of CXCR5-expressing cells at the injection site than occurred in mice injected with the LBNSE parent virus or with LBNSE-GM-CSF (Fig. 1F). Collectively, these data indicate that CXCL13 was successfully cloned into the RABV genome, was highly expressed during RABV replication, and possessed biological function both in vitro and in vivo.
Recruitment and/or activation of DCs stimulated by CXCL13.
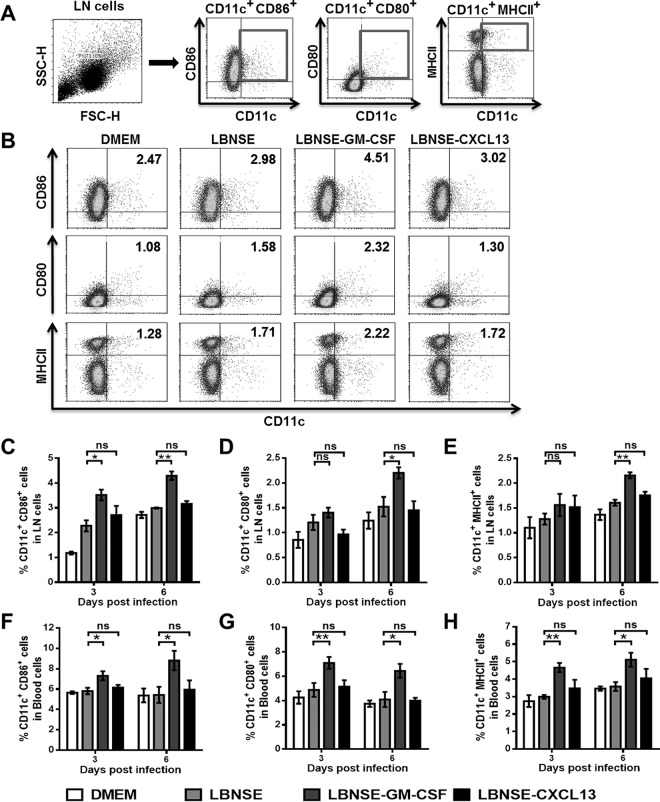
To investigate whether CXCL13 expression recruits and/or activates DCs in vivo, mice were immunized via i.m. injections of 1 × 106 FFU of each rRABV. Flow cytometry was performed to quantify the activated DC cells (CD11c+ CD86+, CD11c+ CD80+, or CD11c+ MHCII+) in the draining (inguinal) lymph nodes and blood at 3 and 6 dpi. LBNSE-GM-CSF, which has been reported to recruit and/or activate DCs in vivo (10, 14), was used as a positive control. The gating strategies for analyzing the DCs (Fig. 2A) and representative flow cytometric plots for measuring activated DCs (Fig. 2B) are presented. A significantly greater number of activated DCs were detected in the draining lymph nodes (Fig. 2C to E) and blood (Fig. 2F to H) from mice immunized with LBNSE-GM-CSF than in those from mice immunized with LBNSE or LBNSE-CXCL13 at 3 and 6 dpi. However, similar quantities of activated DCs were detected in the draining lymph nodes (Fig. 2C to E) and blood (Fig. 2F to H) of mice immunized with LBNSE-CXCL13 and those immunized with LBNSE at 3 and 6 dpi. Together, these data suggest that the expression of CXCL13, unlike the expression of GM-CSF, does not facilitate the recruitment and/or activation of DCs in vivo.
FIG 2.
Recruitment and/or activation of DCs in mice postinfection with rRABVs. BALB/c mice (n = 3) were infected via i.m. injection of 1 × 106 FFU of different rRABVs, and the draining (inguinal) lymph nodes (LNs) and blood were harvested at 3 and 6 dpi. Single-cell suspensions were prepared, stained with antibodies against DCs and DC activation markers, and analyzed via flow cytometry. (A and B) Representative gating strategies for the detection of DCs (A) and representative flow cytometric plots of DCs (B) from the draining LNs are shown. (C to H) Analyses for activated DCs (CD11c+ CD86+, CD11c+ CD80+, or CD11c+ MHCII+) from the draining LNs (C, D, E) and the blood (F, G, H) at 3 and 6 dpi are presented. All the data are expressed as the means ± standard errors of the means (SEM) (n = 3). Asterisks indicate significant differences between the indicated experimental groups.
Recruitment of Tfh and GC B cells by CXCL13.
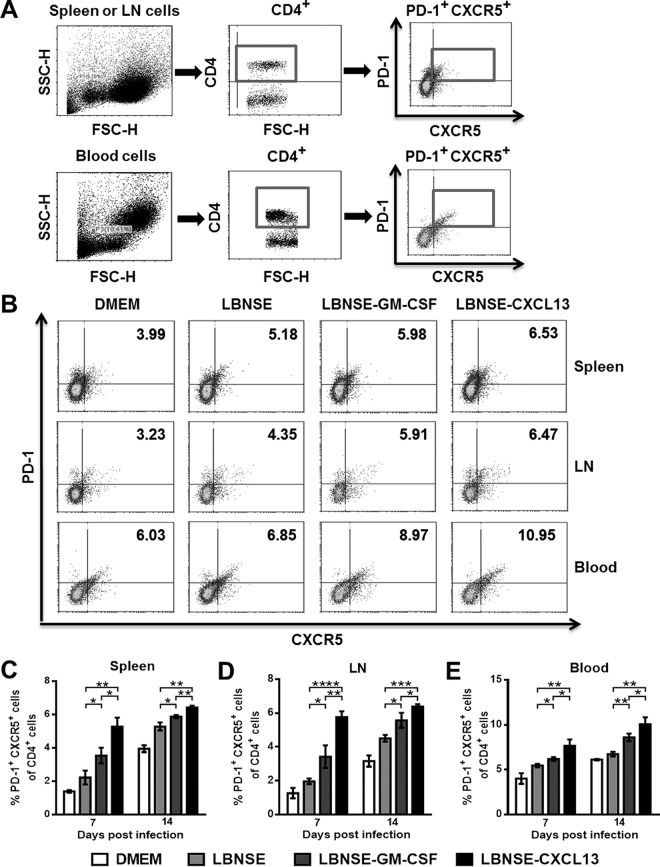
To investigate whether the overexpression of CXCL13 recruits Tfh cells in vivo, mice were immunized via i.m. injections of 1 × 106 FFU of each rRABV, and flow cytometry was performed to quantify the Tfh cells (CD4+ PD1+ CXCR5+) in the spleen, draining lymph nodes, and blood at 7 and 14 dpi. The gating strategies for the detection of Tfh cells are shown in Fig. 3A, and representative flow cytometric plots for analyzing Tfh cells at 14 dpi are shown in Fig. 3B. Significantly more Tfh cells were detected in the spleens (Fig. 3C), draining lymph nodes (Fig. 3D), and blood (Fig. 3E) of mice immunized with LBNSE-CXCL13 than in those of mice immunized with LBNSE or LBNSE-GM-CSF at 7 and 14 dpi. These data demonstrate that overexpression of CXCL13 recruits Tfh cells in vivo.
FIG 3.
Recruitment of Tfh cells by CXCL13. BALB/c mice (n = 3) were infected via i.m. injection of 1 × 106 FFU of different rRABVs, and the spleens, draining LNs, and blood were harvested at 7 and 14 dpi. Single-cell suspensions were prepared, stained with antibodies against Tfh cells and Tfh cell activation markers, and analyzed via flow cytometry. (A and B) Representative gating strategies for the detection of Tfh cells (A) and representative flow cytometric plots of Tfh cells (B) are shown. (C to E) The results of a detailed analysis for activated Tfh cells (CD4+ CXCR5+ PD-1+) at 7 and 14 dpi are presented for the spleen (C), the draining LNs (D), and the blood (E). Data are expressed as the means ± SEM (n = 3). Asterisks indicate significant differences between the indicated experimental groups.
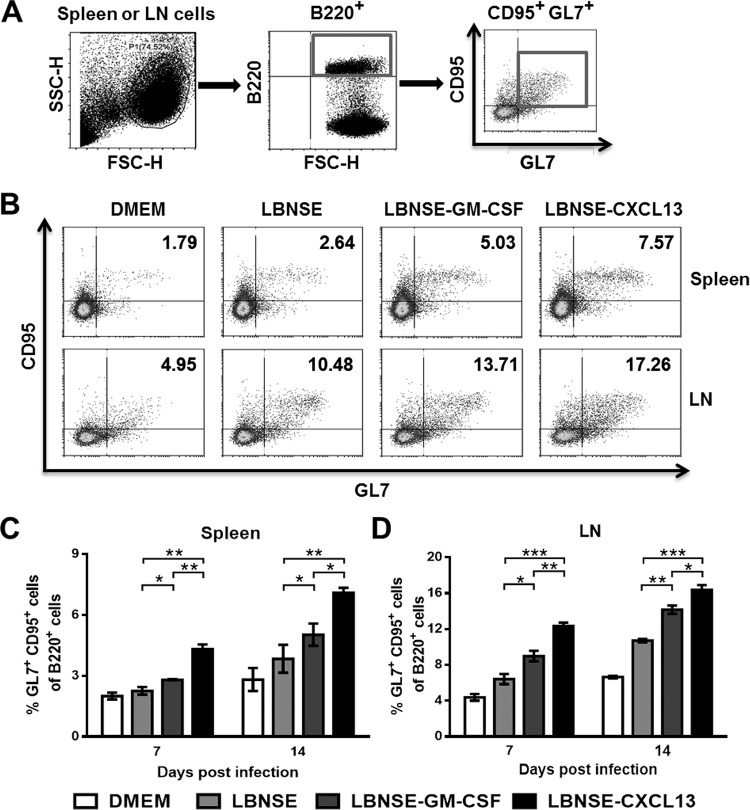
Since the expression of CXCL13 facilitates recruitment of GC B cells, the GC B cells (B220+ CD95+ GL7+) in the spleen and draining lymph nodes were quantified at 7 and 14 dpi. The gating strategies (Fig. 4A) and representative flow cytometric plots for the detection of GC B cells at 14 dpi (Fig. 4B) are shown. Significantly more GC B cells were detected in the spleens (Fig. 4C) and draining lymph nodes (Fig. 4D) of mice immunized with LBNSE-CXCL13 than in those from mice immunized with LBNSE or LBNSE-GM-CSF at 7 and 14 dpi. Together, the above data indicate that CXCL13 expression promotes the recruitment of GC B cells in vivo.
FIG 4.
Recruitment of GC B cells by CXCL13. BALB/c mice (n = 3) were infected via i.m. injection of 1 × 106 FFU of different rRABVs, and the spleens and draining LNs were harvested at 7 and 14 dpi. Single-cell suspensions were prepared, stained with antibodies against GC B cells and GC B cell activation markers, and analyzed via flow cytometry. (A and B) Representative gating strategies for the detection of GC B cells (A) and representative flow cytometric plots of GC B cells (B) are shown. (C and D) The results of a detailed analysis for activated GC B cells (B220+ CD95+ GL7+) at 7 and 14 dpi are presented for the spleen (C) and the draining LNs (D). Data are presented as the means ± SEM (n = 3). Asterisks indicate significant differences between the indicated experimental groups.
Expression of CXCL13 facilitates the formation of GCs.
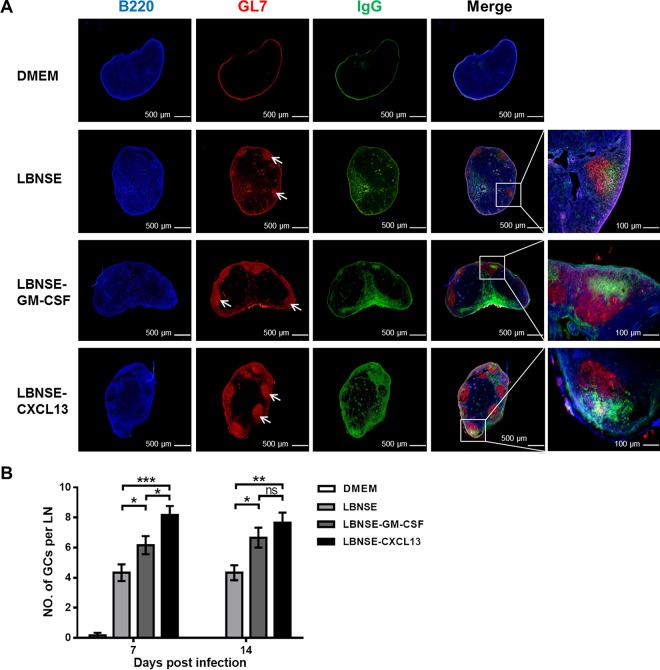
GCs are the primary area where high-affinity antibodies are produced via the interaction between Tfh and B cells (38). To investigate if CXCL13 expression promotes the formation of GCs, mice were immunized via i.m. injection of 1 × 106 FFU of each rRABV. Draining lymph nodes were harvested at 7 and 14 dpi, and the formation of GCs was measured via histology and immunofluorescence. Representative results for GC formation at 7 dpi after immunizations with each rRABV are shown (Fig. 5A). Significantly more and larger GCs were found in mice immunized with LBNSE-CXCL13 than in mice immunized with LBNSE (P < 0.001) or LBNSE-GM-CSF (P < 0.05) at 7 dpi. In addition, significantly more GCs were found in mice immunized with LBNSE-CXCL13 than in mice immunized with LBNSE (P < 0.01) at 14 dpi (Fig. 5B). Collectively, these data demonstrate that CXCL13 expression promotes the formation of GCs.
FIG 5.
Expression of CXCL13 facilitates the formation of GCs. BALB/c mice (n = 3) were infected via i.m. injection of 1 × 106 FFU of different rRABVs, and the draining LNs were collected at 7 and 14 dpi. Then, the draining LNs were excised, and tissue sections were prepared and stained for germinal centers (GL7, red; B220, blue; and IgG, green). Scale bars, 500 μm or 100 μm (rightmost column only). (A) Representative results are shown. (B) The numbers of GCs formed at 7 and 14 dpi were calculated. All the data are expressed as the means ± SEM (n = 3). Asterisks indicate significant differences between the indicated experimental groups; ns, not significant.
Plasma CXCL13 levels correlate with GC activity and VNA titers in mice.
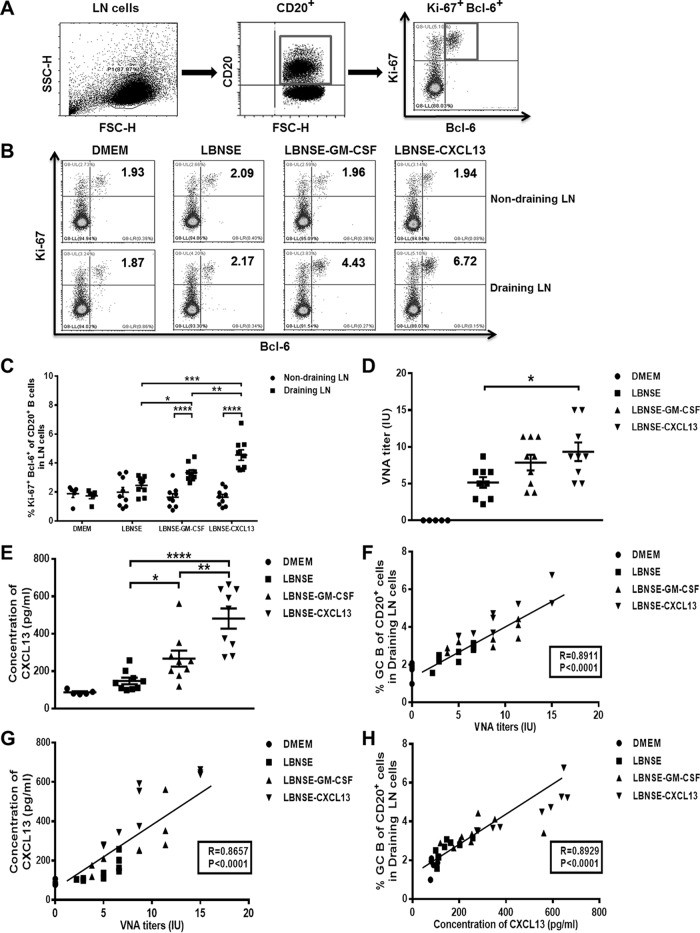
Next, the relationship between plasma CXCL13 levels, GC activity, and VNA titers was examined (23, 39–42). CD20, a B-lymphocyte-specific surface molecule, is widely expressed during B-lymphocyte development, from the early pre-B-cell stage until final differentiation into plasma cells (43). Ki-67 is a cell proliferation marker that is highly expressed in GC B cells (44). BCL-6, a transcription factor, is expressed in GC B cells but not in other types of B lymphocytes (45). Thus, GC B cells can also be identified as Ki-67+ Bcl-6+ when gating on CD20+ cells as described previously (23). Mice were immunized via i.m. injection of 1 × 106 FFU of each rRABV. At 7 dpi, draining (inguinal) and nondraining (cervical) lymph nodes were collected and then used to quantify the frequency of GC B cells (CD20+ Ki-67+ Bcl-6+). Serum samples were obtained and used to measure plasma CXCL13 concentrations and VNA titers. The gating strategies (Fig. 6A), representative flow cytometric plots (Fig. 6B), and analysis of GC B cells (Fig. 6C) at the indicated time points are shown. VNA titers in plasma samples were determined using fluorescent antibody virus neutralization (FAVN) tests (Fig. 6D), and plasma CXCL13 concentrations were measured via ELISA (Fig. 6E). The frequency of vaccine-induced GC B cells was found to correlate with VNA titers (r = 0.8911; P < 0.001) (Fig. 6F). In addition, plasma CXCL13 levels correlated with VNA titers (r = 0.8657; P < 0.001) (Fig. 6G) and with the frequency of vaccine-induced GC B cells (r = 0.8929; P < 0.001) (Fig. 6H). In summary, these data indicate that there is a significant and strong correlation among plasma CXCL13 levels, GC activity, and VNA titers.
FIG 6.
Plasma CXCL13 levels correlate with GC activity and VNA titers in mice. BALB/c mice were infected via i.m. injection of 1 × 106 FFU of DMEM (n = 5), LBNSE (n = 9), LBNSE-GM-CSF (n = 9), or LBNSE-CXCL13 (n = 9), and then draining (inguinal) and nondraining (cervical) LNs were collected at 7 dpi. Single-cell suspensions were prepared, stained with antibodies against GC B cells and GC B cell activation markers, and analyzed via flow cytometry. (A and B) Representative gating strategies for the detection of GC B cells (A) and representative flow cytometric plots of plasma B cells (B) are shown. (C) The results of a detailed analysis of activated GC B cells (CD20+ Ki-67+ Bcl-6+) at 7 dpi are presented for the draining and nondraining LNs. (D) Serum samples were harvested at 7 dpi, and RABV VNA titers were measured via FAVN tests as described in Materials and Methods. (E) The concentration of plasma CXCL13 was determined using a commercial ELISA kit. (F) Correlations of GC B cell activity in the draining LNs and RABV VNA titers in mice 7 days after immunization were determined. (G) Correlations of plasma CXCL13 concentrations and RABV VNA titers in mice 7 days after immunization were determined. (H) Correlations of GC B cell activity in the draining LNs and plasma CXCL13 concentrations in mice 7 days after immunization were determined. All the data are expressed as the means ± SEM (n = 3). Asterisks indicate significant differences between the indicated experimental groups.
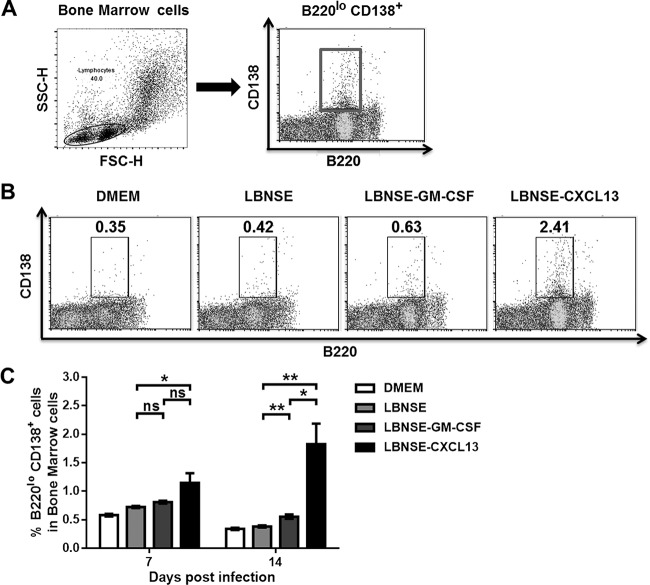
Expression of CXCL13 increases the population of plasma cells.
After differentiating into plasma cells, GC B cells lose the capacity to express CXCR5 and thus leave secondary lymphoid organs (46). GC B cells can differentiate into long-lived plasma cells that reside mainly in bone marrow (47–50). To investigate whether LBNSE-CXCL13 could promote the production of plasma cells in vivo, mice were immunized via i.m. injection of 1 × 106 FFU of each rRABV. At 7 and 14 dpi, bone marrow was harvested and flow cytometry was performed to quantify the plasma cells (B220low CD138+). The gating strategies for analyzing the plasma cells (Fig. 7A) and representative flow cytometric plots at 14 dpi (Fig. 7B) are shown. Significantly more plasma cells were found in mice immunized with LBNSE-CXCL13 than in mice immunized with LBNSE (P < 0.05) at 7 dpi or than were found in mice immunized with LBNSE (P < 0.01) or LBNSE-GM-CSF (P < 0.05) at 14 dpi (Fig. 7C). These data indicate that CXCL13 expression promotes an increase in the population of plasma cells in immunized mice.
FIG 7.
Expression of CXCL13 promotes an increase in the quantity of plasma cells. BALB/c mice (n = 3) were infected via i.m. injection of 1 × 106 FFU of different rRABVs, and the bone marrow samples were harvested at 7 and 14 dpi. Single-cell suspensions were prepared, stained with antibodies against plasma B cells and plasma B cell activation markers, and analyzed via flow cytometry. (A and B) Representative gating strategies for the detection of plasma B cells (A) and representative flow cytometric plots of plasma B cells (B) are shown. (C) The results of a detailed analysis of activated plasma B cells (B220low CD138+) at 7 and 14 dpi are presented for the bone marrow samples. All the data are expressed as the means ± SEM (n = 3). Asterisks indicate significant differences between the indicated experimental groups.
Pathogenicity and immunogenicity of LBNSE-CXCL13.
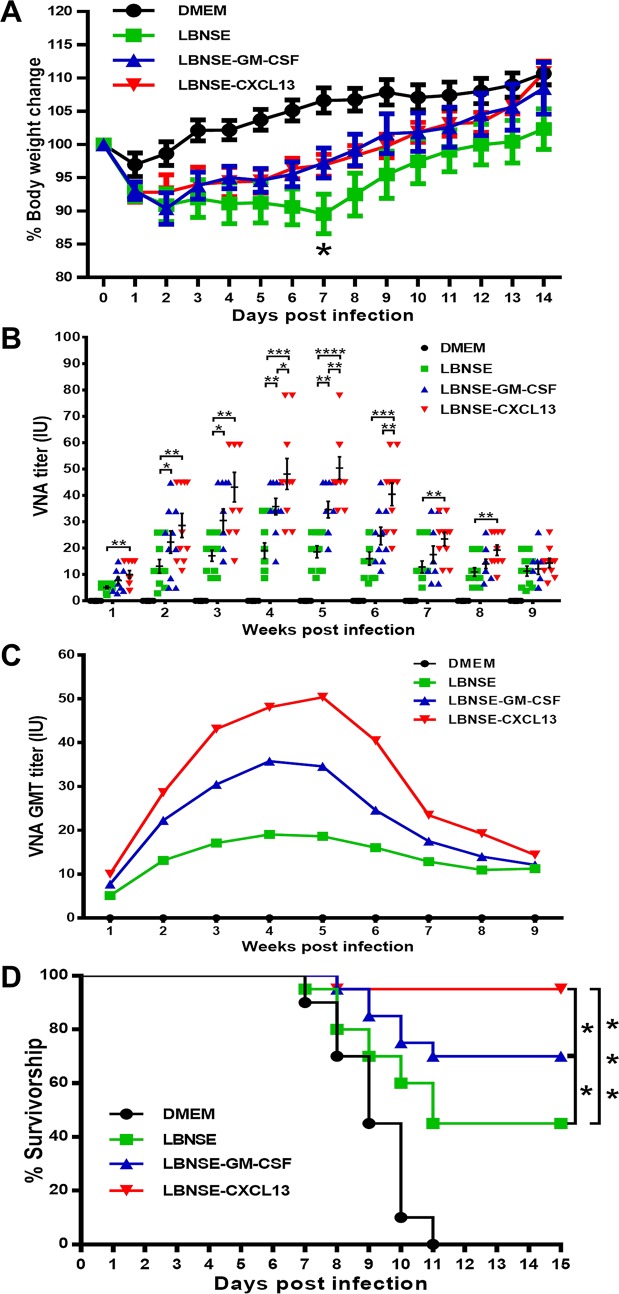
To determine whether the overexpression of CXCL13 has adverse effects in animals, ICR mice were infected intracerebrally (i.c.) with a high dose (4 × 106 FFU) of rRABVs. Infected mice were monitored daily for 2 weeks to assess weight loss and disease development. No significant neurological symptoms were found during the 2-week observation. Mice immunized with LBNSE-CXCL13 (92.83%) showed weight losses in amounts similar to those of mice immunized with LBNSE-GM-CSF (90.36%) or LBNSE (90.87%) at 2 dpi. The trend in weight loss for LBNSE-CXCL13-immunized mice was similar to the trend in LBNSE-GM-CSF-immunized mice: both groups reached their lowest weights at 2 dpi. However, the weight loss induced in LBNSE-immunized mice (89.54%) reached the lowest point at 7 dpi, which was significantly lower than that induced by LBNSE-CXCL13 (96.88%) or LBNSE-GM-CSF (97.22%) (Fig. 8A). Collectively, these data illustrate that the expression of CXCL13 has no adverse effect on the pathogenicity of RABV in vivo.
FIG 8.
Pathogenicity and immunogenicity of rRABVs in mice. (A) ICR mice were infected i.c. with 1 × 106 FFU of different rRABVs or DMEM (mock infection), and their body weights were monitored daily for 2 weeks. Data are presented as the mean values ± SEM (n = 9 or 10). (B and C) Groups of ICR mice (n = 10) were immunized via i.m. injection of 1 × 106 FFU of rRABVs. At indicated time points postimmunization, blood samples were collected and VNA titers were measured via FAVN tests. Titers were normalized to international units based on the WHO standard and are expressed as mathematic mean titers (B) and geometric mean titers (C). (D) Groups of ICR mice (n = 20) were immunized i.m. with 1 × 106 FFU of rRABVs. Two weeks after immunization, the mice were challenged i.c. with 50 LD50 of pathogenic RABV strain CVS-24 and observed for another 2 weeks, and survivorship was recorded. Asterisks indicate significant differences between the indicated experimental groups as determined from one-way analysis of variance (ANOVA) (A and B) or Fisher's exact tests (× 2).
To determine if the recruitment of Tfh, GC B cells, and plasma cells in the spleen, draining lymph nodes, blood, or bone marrow increases the immunogenicity of LBNSE-CXCL13, mice were i.m. immunized via a single injection of 1 × 106 FFU of the different rRABVs. Blood samples were collected weekly, and sera were used for the quantification of VNAs via FAVN tests. At 1 week postimmunization (wpi), significantly higher VNA titers were detected in mice immunized with LBNSE-CXCL13 (10.02 IU) than in mice immunized with LBNSE (5.09 IU) (P < 0.01), and this significant difference lasted until 8 wpi. In addition, significantly higher VNA titers were detected in mice immunized with LBNSE-CXCL13 (48.12 IU) than in mice immunized with LBNSE-GM-CSF (35.78 IU) (P < 0.05) at 4 wpi, and this significant difference lasted until 6 wpi (Fig. 8B). Geometric mean titers (GMTs) reflect the dynamics of VNA induction, and the result indicates that LBNSE-CXCL13 induces more-rapid and more-robust VNA production than LBNSE or LBNSE-GM-CSF (Fig. 8C). To investigate if the higher VNA titers correlated with better protection, mice were challenged with 50 times the 50% lethal dose (LD50) of pathogenic RABV strain CVS-24 through an i.c. route at 14 dpi and then monitored for the development of rabies for another 2 weeks. Significantly more survivors were observed among the mice immunized with LBNSE-CXCL13 (95%) than among those immunized with LBNSE (45%) or LBNSE-GM-CSF (70%) (Fig. 8D). Taken together, these data demonstrate that LBNSE-CXCL13 increases VNA production and provides better protection than LBNSE or LBNSE-GM-CSF.
DISCUSSION
Our previous endeavor to develop more-efficacious rabies vaccines focused on enhancing the recruitment and/or activation of DCs, the major antigen-presenting cells. Following this rationale, the rRABV expressing GM-CSF (LBNSE-GM-CSF) developed in our previous studies demonstrated the capacity to upregulate DC and B-cell activation both in vitro and in vivo, resulting in enhanced humoral immune responses and protection against rabies in both murine and canine models (10, 13, 14). Compared with LBNSE-GM-CSF, the rRABV expressing CXCL13 (LBNSE-CXCL13) developed in this study does not promote the activation of DCs in RABV-immunized mice. Instead, the expression of CXCL13 significantly upregulated the recruitment of Tfh and GC B cells, resulting in enhanced humoral immune responses.
Tfh cells are universally defined by the expression of the chemokine receptor CXCR5, which causes them to migrate to B cell follicles following CXCL13 gradients (51). CXCR5 is also highly expressed on B cells and is largely responsible for B cell movement into CXCL13-rich follicular areas in lymph nodes (26, 28). The CXCL13-CXCR5 chemokine axis plays a major role in organizing both B-cell follicles and GCs (25, 52). Notably, recent studies have indicated that the fusion of an antigen to CXCL13 significantly enhances the potency of DNA vaccines. CXCL13 fused with green fluorescent protein (GFP) was found to enhance the level of GFP antibody detected and to elicit higher levels of specific IgG1 antibodies than of IgG2a antibodies, which indicates a Th2 response and a contribution factor to humoral immunity (37). Recent studies have also shown that CXCL13 recruits B cells to nonlymphoid organs, contributing to the organization of local immune responses against influenza and hepatitis B virus infections (53, 54). In our present study, CXCL13 was inserted into the genome of the RABV and expressed in a considerable quantity both in vitro and in vivo, which was shown to have strong biological activity in a transwell model and to help recruit the cells expressing CXCR5 to the injection sites in vivo. As a consequence of this activity, a large number of Tfh and GC B cells were attracted to the draining lymph nodes of immunized mice.
High-affinity antibodies and long-lived memory B cells are the hallmarks of the humoral response. Activated B cells undergo affinity maturation and differentiation in GCs and are dependent on signals provided by Tfh cells. The accumulation of T-cell-derived signals over time results in B cells expressing high-affinity immunoglobulin receptors and in their differentiation into antibody-secreting cells (ASCs). In our work, the expression of CXCL13 significantly increased the recruitment of Tfh cells, resulting in an increased number of activated GC B cells. However, the complete differentiation of Tfh cells is thought to require a signal derived from antigen-specific B cells in the follicles (51). The interaction between Tfh and B cells is likely to be important for complete Tfh cell differentiation (55). We found that the expression of CXCL13 resulted in the recruitment of an increased number of B cells into draining lymph nodes, which might enhance antigen presentation and the interaction between Tfh and B cells, promoting the differentiation of Tfh cells and thus enhancing humoral immune responses.
The GC response is a critical immune mechanism by which antibody (Ab) affinity occurs, memory B cells develop, and long-lived plasma cells are produced (38). Tfh cells regulate the size of GCs, limit the entry of low-affinity B cells into GCs, promote high-affinity B-cell occupancy in the GCs, and select high-affinity B cells during affinity maturation (16). Thus, the most important function of Tfh cells is their critical role in GC development and function (16). Mice lacking CXCL13 show major aberrations in their follicular architecture and reduced numbers of lymph nodes and Peyer's patches (25, 27). In this study, it was found that the overexpression of CXCL13 increased the number of Tfh cells, which facilitated the development of draining lymph nodes and the formation of GCs. GCs are also the site of differentiation of long-lived memory B cells and long-lived plasma cells. The ASCs exported from GCs persist in bone marrow niches that support their longevity. Not surprisingly, more plasma cells were found in the bone marrow from LBNSE-CXCL13-immunized mice than from LBNSE-immunized mice.
Although serological analyses of VNA titers provide important information about the efficiency of vaccines, outcomes are measured at time points long after the initial immunizations (23). One critical parameter for assessing the efficiency of vaccines is their ability to generate GC responses. Currently, the preferred means of quantifying GC responses is by analyzing Tfh and B cells in GCs. However, lymphoid tissue is rarely available from immunized dogs or humans, making the monitoring of GC activity via the direct assessment of Tfh and B cells in GCs problematic. A recent study reported that CXCL13 is a plasma biomarker of GC activity and that plasma CXCL13 concentrations correlate with GC activity (23). Consistent with this report, it was found that there was a strong correlation among plasma CXCL13 concentrations, GC activity, and RABV VNA titers. Therefore, plasma CXCL13 concentrations may be a potential indicator for assessing the efficiency of rabies vaccine in future studies.
In summary, our study demonstrates that the rRABV expressing CXCL13 recruits more Tfh and GC B cells than the parent virus, promotes the formation of GCs, and enhances VNA production. In addition, the overexpression of CXCL13 has no significant adverse effects on mice. These findings provide a better understanding of the role of CXCL13 in RABV-induced humoral immune responses and provide some clues for the development of novel rabies vaccines.
MATERIALS AND METHODS
Cells, viruses, antibodies, recombinant proteins, and animals.
BSR cells (a clone derived from BHK-21 cells) were cultured in Dulbecco's modified Eagle's medium (Mediatech) containing 10% fetal bovine serum (FBS; Gibco). LBNSE is an rRABV that was constructed from the SAD-B19 strain (56, 57) through a mutation of the G at amino acid positions 194 and 333 (10). Recombinant RABV strains were propagated in BSR cells. The challenge virus CVS-24 was propagated in the brains of suckling mice. Fluorescein isothiocyanate (FITC)-conjugated antibodies against the RABV N protein were purchased from FujiRab (Melvin, PA). The antibodies used for flow cytometric analyses included FITC anti-mouse CD11c (clone N418), APC anti-mouse CD80 (clone 16-10A1), PE anti-mouse CD86 (clone GL-1), PE/Cy7 anti-mouse I-A/I-E (MHCII) (clone M5/114.15.2), FITC anti-mouse CD4 (clone GK1.5), APC anti-mouse CD185 (CXCR5) (clone L138D7), PE anti-mouse CD279 (PD-1) (clone RMP1-30), FITC anti-mouse/human CD45R/B220 (clone RA3-6B2), Alexa Fluor 647 anti-mouse/human GL7 (clone GL7), APC anti-mouse CD138 (Syndecan-1) (clone 281-2), FITC anti-mouse CD20 (clone SA275A11), PE anti-mouse Ki-67 (clone 16A8), and APC anti-human/mouse Bcl-6 (clone 7D1), all purchased from BioLegend (San Diego, CA), and PE anti-mouse CD95 (APO-1/Fas) (clone 15A7) antibodies from eBioscience (San Diego, CA). Antibodies used for immunofluorescence included Alexa Fluor 647 anti-mouse/human CD45R/B220 (clone RA3-6B2), Alexa Fluor 647 anti-mouse IgG (clone Poly4053), and Alexa Fluor 594 streptavidin, all purchased from BioLegend (San Diego, CA), and anti-human/mouse GL7 biotin (clone GL7) antibodies from eBioscience (San Diego, CA). Recombinant mouse CXCL13 proteins were purchased from R&D Systems (USA). Recombinant mouse IL-4 and recombinant mouse GM-CSF were purchased from Novoprotein Scientific, Inc. (Shanghai, China). Biotinylated secondary antibodies were purchased from Vector Laboratories (Burlingame, CA). Female BALB/c mice and ICR mice (6 weeks old) were purchased from the Center for Disease Control and Prevention of Hubei Province, China. All animal experiments were carried out as approved by the Scientific Ethics Committee of Huazhong Agricultural University (permit number HZAUMO-2015-016).
Construction of rRABV clones.
Mouse CXCL13 cDNA was amplified from the total RNA extracted from RABV-infected mouse peripheral blood mononuclear cells (PBMCs) using the SuperScript III One-Step reverse transcription (RT)-PCR system with Platinum Taq DNA polymerase (Invitrogen Life Technology). The primer sets used for PCR assays were designed by Primer 6 (Premier Biosoft International) (Table 1). The PCR products were digested with BsiWI and NheI (New England BioLabs) and then ligated into the rRABV vector LBNSE, which had been described previously (10).
TABLE 1.
qRT-PCR primers used in this study
| Primer | Sequence (5′–3′) |
|---|---|
| vRNA-F | TCATCTGCCAGTGCTACGTC |
| vRNA-R | GAGGAATTCTTCGGGAAAGG |
| CXCL13-F | TCTGGAAGCCCATTACACAA |
| CXCL13-R | TTTGTAACCATTTGGCACGA |
| CXCR5-F | GGTGCTGGTAATCCTGGAGA |
| CXCR5-R | GATCTTGTGCAGAGCGATCA |
Rescue of rRABVs.
Recombinant RABVs were rescued as described previously (58) and then propagated in BSR cells. Briefly, BSR cells were transfected with 2.0 μg of a fully infectious clone, 0.5 μg of pH-N, 0.25 μg of pH-P, 0.15 μg of pH-G, and 0.1 μg of pH-L using the SuperFect transfection reagent (Qiagen) according to the manufacturer's protocol. After incubating for 4 days, the culture medium was harvested and then examined for the presence of rescued viruses using FITC-conjugated anti-RABV N Abs.
Virus titration.
Viruses were titrated via direct fluorescent antibody assays in BSR cells as described previously (11). Briefly, BSR cells seeded in 96-well plates were inoculated with serial 10-fold dilutions of the virus and incubated at 37°C for 3 days. The culture supernatant was removed, and the cells were washed 3 times with phosphate-buffered saline (PBS). The cells were fixed with 80% ice-cold acetone at −20°C for 30 min. After 3 washes with PBS, the cells were stained with FITC-conjugated anti-RABV N protein antibodies for 1 h at home temperature. After 3 washes with PBS, antigen-positive foci on the cells were counted under an Olympus IX51 fluorescence microscope, and viral titers were calculated as fluorescent focus units per milliliter. All titrations were carried out in quadruplicate.
Virus-neutralizing antibody tests.
Serum from each mouse was collected for the measurement of VNAs using the fluorescent antibody virus neutralization (FAVN) tests described previously (58). Briefly, 50 μl of serial 3-fold dilutions of test and standard serum samples was added to 96-well microplates in 100-μl volumes. Each sample was added to four duplicate chambers. A 50-μl volume of a rabies challenge virus, CVS-11, suspension containing 50 to 200 FFU was added to each chamber. The microplates were then incubated at 37°C for 1 h in an incubator with 5% CO2. Then, 50 μl of BSR cells (5 × 105 cells/ml) was added into each chamber, and the microplates were incubated at 34°C in an incubator with 5% CO2 for 3 days. The plates were fixed in 80% ice-cold acetone at −20°C for 30 min and then air dried. Cells were stained with FITC-conjugated anti-RABV N Abs for 45 min at 37°C and then washed three times with PBS. The results were assessed using an Olympus IX51 fluorescence microscope. VNA titers were expressed in international units per milliliter based on comparisons with the titer of a reference serum sample obtained from the National Institute for Biological Standards and Control (Herts, UK) and included in each test.
Chemotaxis assay.
Splenocytes from BALB/c mice were obtained via Ficoll-Hypaque (Solarbio Life Sciences, China) density gradient centrifugation. The chemotactic responses of mouse splenocytes to rRABVs were detected using the chemotaxis microchamber technique (36, 37). Briefly, BSR cells were inoculated with rRABVs at a multiplicity of infection (MOI) of 1, and after 24 h, the cultured supernatants were collected and added into the lower wells of chemotaxis chambers (Corning, USA) with membranes with 5-μm pores. Recombinant mouse CXCL13 peptide (25 or 50 ng/ml) dissolved in RPMI 1640 medium was used as a positive control. A total of 5 × 105 cells in 400 μl of RPMI 1640 medium was added to the upper wells of the chemotaxis chambers. Chemotaxis was allowed to proceed for 2 or 4 h, and then the membranes were removed. Migrated cells in the bottom chambers were counted in a counting chamber.
Quantitative real-time RT-PCR.
The hair on the lateral region of the left hind thigh was cut, and a total volume of 100 μl different viruses or Dulbecco's modified Eagle medium (DMEM) was injected into the muscles of each mouse. The injection site was labeled with a permanent ink marker. At indicated time points postimmunization, the muscle tissue around the injection site was collected on ice at the indicated time points and homogenized with TRIzol (Invitrogen). Total RNA was extracted, and first-strand cDNA synthesis was performed using a First-Strand cDNA synthesis kit (Toyobo) and oligo(dT) primers (TaKaRa). Each qRT-PCR was carried out in duplicate with approximately 1 μg of DNase-treated RNA and 10 nM primer pair solutions using a one-step SYBR green qRT-PCR mix kit (Toyobo). For the absolute quantification of viral genomic RNA (vRNA), a plasmid expressing the RABV N protein was used as a standard (12). For the relative quantification of CXCL13 or CXCR5, mRNA copy numbers were normalized to those of the housekeeping gene β-actin. The primers used are listed in Table 1.
Flow cytometry.
Flow cytometry was carried out to quantify the immune cells in the spleen, lymph nodes (draining or nondraining), bone marrow, and peripheral blood. Briefly, the spleens, lymph nodes, and bone marrow of mice were collected, pressed through a 40-μm nylon filter, and washed with PBS. Red blood cells were lysed with ACK lysis buffer (BioSource International, Inc., Camarillo, CA) for 1 min at room temperature. Single-cell suspensions (at 106 cells) were prepared in 0.2% bovine serum albumin (BSA) and stained for flow cytometric antibody analysis. After incubation on ice for 30 min, cells were washed twice in PBS containing 0.2% BSA. Then, the cells were resuspended in 0.2% BSA. For CD20, Ki-67, and Bcl-6 identification, cells were first stained for surface antigen (CD20) and then were permeabilized, fixed, and stained (Ki-67 and Bcl-6) using the Mouse Regulatory T Cell staining kit according to the manufacturer's protocol (eBioscience). Data collection and analysis were performed using a BD LSR-II flow cytometer, BD FACS-Diva software (BD Pharmingen), and FlowJo software (TreeStar, San Carlos, CA).
Histology and immunofluorescence.
Draining inguinal lymph nodes were isolated and fixed for 24 h in 4% paraformaldehyde. They were then dehydrated in 30% sucrose, snap-frozen in molds containing OCT medium, and cut into 30-μm-thick sections. Sections were fluorescently stained with Alexa Fluor 647 anti-mouse/human CD45R/B220 (Biolegend), Alexa Fluor 488 goat anti-mouse IgG (Biolegend), or anti-human/mouse GL7 (Biolegend), followed by Alexa Fluor 594 streptavidin (eBioscience) (59). Fluorescent images were captured under an Olympus IX51 fluorescence microscope.
Statistical analysis.
All data were analyzed using GraphPad Prism software (GraphPad Software, lnc, CA). For the percent survival experiments, Kaplan-Meier survival curves were analyzed using the log rank test. The ozone correlation test was used for all correlation analyses. An unpaired two-tailed t test was used to determine statistical significance for the other data analyzed. For all tests, the following notations are used to indicate significant differences between groups: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
ACKNOWLEDGMENTS
This work was partially supported by the National Program on Key Research Project of China (2016YFD0500400), the National Natural Science Foundation of China (31372419, 31522057), the European Union's Seventh Framework Programme LinkTADs (No. 613804, to L.Z.), the Ministry of Science and Technology of China (863 program, number 2011AA10A212), and the Ministry of Agriculture of China (special fund for Agro-scientific Research in the Public Interest, 201303042, to Z.F.F.).
REFERENCES
- 1.Davis BM, Rall GF, Schnell MJ. 2015. Everything you always wanted to know about rabies virus (but were afraid to ask). Annu Rev Virol 2:451–471. doi: 10.1146/annurev-virology-100114-055157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albertini AA, Ruigrok RW, Blondel D. 2011. Rabies virus transcription and replication. Adv Virus Res 79:1–22. doi: 10.1016/B978-0-12-387040-7.00001-9. [DOI] [PubMed] [Google Scholar]
- 3.Lankester F, Hampson K, Lembo T, Palmer G, Taylor L, Cleaveland S. 2014. Infectious disease. Implementing Pasteur's vision for rabies elimination. Science 345:1562–1564. doi: 10.1126/science.1256306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wunner WH, Briggs DJ. 2010. Rabies in the 21 century. PLoS Negl Trop Dis 4:e591. doi: 10.1371/journal.pntd.0000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fooks AR, Banyard AC, Horton DL, Johnson N, McElhinney LM, Jackson AC. 2014. Current status of rabies and prospects for elimination. Lancet 384:1389–1399. doi: 10.1016/S0140-6736(13)62707-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arya JM, Dewitt K, Scott-Garrard M, Chiang YW, Prausnitz MR. 2016. Rabies vaccination in dogs using a dissolving microneedle patch. J Control Release 239:19–26. doi: 10.1016/j.jconrel.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Franka R, Smith TG, Dyer JL, Wu XF, Niezgoda M, Rupprecht CE. 2013. Current and future tools for global canine rabies elimination. Antiviral Res 100:220–225. doi: 10.1016/j.antiviral.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Hooper DC, Morimoto K, Bette M, Weihe E, Koprowski H, Dietzschold B. 1998. Collaboration of antibody and inflammation in clearance of rabies virus from the central nervous system. J Virol 72:3711–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schutsky K, Curtis D, Bongiorno EK, Barkhouse DA, Kean RB, Dietzschold B, Hooper DC, Faber M. 2013. Intramuscular inoculation of mice with the live-attenuated recombinant rabies virus TriGAS results in a transient infection of the draining lymph nodes and a robust, long-lasting protective immune response against rabies. J Virol 87:1834–1841. doi: 10.1128/JVI.02589-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen Y, Wang H, Wu H, Yang F, Tripp RA, Hogan RJ, Fu ZF. 2011. Rabies virus expressing dendritic cell-activating molecules enhances the innate and adaptive immune response to vaccination. J Virol 85:1634–1644. doi: 10.1128/JVI.01552-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao L, Toriumi H, Kuang Y, Chen H, Fu ZF. 2009. The roles of chemokines in rabies virus infection: overexpression may not always be beneficial. J Virol 83:11808–11818. doi: 10.1128/JVI.01346-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao L, Toriumi H, Wang H, Kuang Y, Guo X, Morimoto K, Fu ZF. 2010. Expression of MIP-1alpha (CCL3) by a recombinant rabies virus enhances its immunogenicity by inducing innate immunity and recruiting dendritic cells and B cells. J Virol 84:9642–9648. doi: 10.1128/JVI.00326-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou M, Wang L, Zhou S, Wang Z, Ruan J, Tang L, Jia Z, Cui M, Zhao L, Fu ZF. 2015. Recombinant rabies virus expressing dog GM-CSF is an efficacious oral rabies vaccine for dogs. Oncotarget 6:38504–38516. doi: 10.18632/oncotarget.5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou M, Zhang G, Ren G, Gnanadurai CW, Li Z, Chai Q, Yang Y, Leyson CM, Wu W, Cui M, Fu ZF. 2013. Recombinant rabies viruses expressing GM-CSF or flagellin are effective vaccines for both intramuscular and oral immunizations. PLoS One 8:e63384. doi: 10.1371/journal.pone.0063384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brilot F, Strowig T, Munz C. 2008. NK cells interactions with dendritic cells shape innate and adaptive immunity. Front Biosci 13:6443–6454. [DOI] [PubMed] [Google Scholar]
- 16.Crotty S. 2014. T follicular helper cell differentiation, function, and roles in disease. Immunity 41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ueno H, Banchereau J, Vinuesa CG. 2015. Pathophysiology of T follicular helper cells in humans and mice. Nat Immunol 16:142–152. doi: 10.1038/ni.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mesquita D Jr, Cruvinel WM, Resende LS, Mesquita FV, Silva NP, Camara NO, Andrade LE. 2016. Follicular helper T cell in immunity and autoimmunity. Braz J Med Biol Res 49:e5209. doi: 10.1590/1414-431X20165209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sage PT, Sharpe AH. 2016. T follicular regulatory cells. Immunol Rev 271:246–259. doi: 10.1111/imr.12411. [DOI] [PubMed] [Google Scholar]
- 20.Cyster JG. 1999. Chemokines and cell migration in secondary lymphoid organs. Science 286:2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 21.Kroenke MA, Eto D, Locci M, Cho M, Davidson T, Haddad EK, Crotty S. 2012. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. J Immunol 188:3734–3744. doi: 10.4049/jimmunol.1103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasheed AU, Rahn HP, Sallusto F, Lipp M, Muller G. 2006. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. Eur J Immunol 36:1892–1903. doi: 10.1002/eji.200636136. [DOI] [PubMed] [Google Scholar]
- 23.Havenar-Daughton C, Lindqvist M, Heit A, Wu JE, Reiss SM, Kendric K, Belanger S, Kasturi SP, Landais E, Akondy RS, McGuire HM, Bothwell M, Vagefi PA, Scully E, Tomaras GD, Davis MM, Poignard P, Ahmed R, Walker BD, Pulendran B, McElrath MJ, Kaufmann DE, Crotty S. 2016. CXCL13 is a plasma biomarker of germinal center activity. Proc Natl Acad Sci U S A 113:2702–2707. doi: 10.1073/pnas.1520112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang XM, Cho BY, Suzuki K, Xu Y, Green JA, An JP, Cyster JG. 2011. Follicular dendritic cells help establish follicle identity and promote B cell retention in germinal centers. J Exp Med 208:2497–2510. doi: 10.1084/jem.20111449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ansel KM, Ngo VN, Hyman PL, Luther SA, Forster R, Sedgwick JD, Browning JL, Lipp M, Cyster JG. 2000. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 26.Campbell DJ, Kim CH, Butcher EC. 2003. Chemokines in the systemic organization of immunity. Immunol Rev 195:58–71. doi: 10.1034/j.1600-065X.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 27.Forster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. 1996. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell 87:1037–1047. doi: 10.1016/S0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- 28.Legler DF, Loetscher M, Roos RS, Clark-Lewis I, Baggiolini M, Moser B. 1998. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J Exp Med 187:655–660. doi: 10.1084/jem.187.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moser B, Schaerli P, Loetscher P. 2002. CXCR5(+) T cells: follicular homing takes center stage in T-helper-cell responses. Trends Immunol 23:250–254. doi: 10.1016/S1471-4906(02)02218-4. [DOI] [PubMed] [Google Scholar]
- 30.Mueller SN, Hosiawa-Meagher KA, Konieczny BT, Sullivan BM, Bachmann MF, Locksley RM, Ahmed R, Matloubian M. 2007. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science 317:670–674. doi: 10.1126/science.1144830. [DOI] [PubMed] [Google Scholar]
- 31.Crotty S. 2012. The 1-1-1 fallacy. Immunol Rev 247:133–142. doi: 10.1111/j.1600-065X.2012.01117.x. [DOI] [PubMed] [Google Scholar]
- 32.Victora GD, Nussenzweig MC. 2012. Germinal centers. Annu Rev Immunol 30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 33.Zotos D, Tarlinton DM. 2012. Determining germinal centre B cell fate. Trends Immunol 33:281–288. doi: 10.1016/j.it.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. 2009. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haynes NM, Allen CDC, Lesley R, Ansel KM, Killeen N, Cyster JG. 2007. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1(High) germinal center-associated subpopulation. J Immunol 179:5099–5108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- 36.Allen CDC, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N, Cyster JG. 2004. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol 5:943–952. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- 37.Guo JH, Fan MW, Sun JH, Jia R. 2009. Fusion of antigen to chemokine CCL20 or CXCL13 strategy to enhance DNA vaccine potency. Int Immunopharmacol 9:925–930. doi: 10.1016/j.intimp.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 38.King C, Tangye SG, Mackay CR. 2008. T follicular helper (T(FH)) cells in normal and dysregulated immune responses. Annu Rev Immunol 26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 39.Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S, Godzik A, Kaufmann DE, McElrath MJ, Nussenzweig MC, Pulendran B, Scanlan CN, Schief WR, Silvestri G, Streeck H, Walker BD, Walker LM, Ward AB, Wilson IA, Wyatt R. 2012. A blueprint for HIV vaccine discovery. Cell Host Microbe 12:396–407. doi: 10.1016/j.chom.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanders RW, van Gils MJ, Derking R, Sok D, Ketas TJ, Burger JA, Ozorowski G, Cupo A, Simonich C, Goo L, Arendt H, Kim HJ, Lee JH, Pugach P, Williams M, Debnath G, Moldt B, van Breemen MJ, Isik G, Medina-Ramirez M, Back JW, Koff WC, Julien JP, Rakasz EG, Seaman MS, Guttman M, Lee KK, Klasse PJ, LaBranche C, Schief WR, Wilson IA, Overbaugh J, Burton DR, Ward AB, Montefiori DC, Dean H, Moore JP. 2015. HIV-1 vaccines. HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science 349:aac4223. doi: 10.1126/science.aac4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jardine JG, Ota T, Sok D, Pauthner M, Kulp DW, Kalyuzhniy O, Skog PD, Thinnes TC, Bhullar D, Briney B, Menis S, Jones M, Kubitz M, Spencer S, Adachi Y, Burton DR, Schief WR, Nemazee D. 2015. Priming a broadly neutralizing antibody response to HIV-1 using a germline-targeting immunogen. Science 349:156–161. doi: 10.1126/science.aac5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dosenovic P, von Boehmer L, Escolano A, Jardine J, Freund NT, Gitlin AD, McGuire AT, Kulp DW, Oliveira T, Scharf L, Pietzsch J, Gray MD, Cupo A, van Gils MJ, Yao KH, Liu C, Gazumyan A, Seaman MS, Bjorkman PJ, Sanders RW, Moore JP, Stamatatos L, Schief WR, Nussenzweig MC. 2015. Immunization for HIV-1 broadly neutralizing antibodies in human Ig knockin mice. Cell 161:1505–1515. doi: 10.1016/j.cell.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tedder TF, Engel P. 1994. CD20: a regulator of cell-cycle progression of B lymphocytes. Immunol Today 15:450–454. doi: 10.1016/0167-5699(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 44.Norrback KF, Dahlenborg K, Carlsson R, Roos G. 1996. Telomerase activation in normal B lymphocytes and non-Hodgkin's lymphomas. Blood 88:222–229. [PubMed] [Google Scholar]
- 45.Cattoretti G, Chang CC, Cechova K, Zhang J, Ye BH, Falini B, Louie DC, Offit K, Chaganti RS, Dalla-Favera R. 1995. BCL-6 protein is expressed in germinal-center B cells. Blood 86:45–53. [PubMed] [Google Scholar]
- 46.Hargreaves DC, Hyman PL, Lu TT, Ngo VN, Bidgol A, Suzuki G, Zou YR, Littman DR, Cyster JG. 2001. A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med 194:45–56. doi: 10.1084/jem.194.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manz RA, Hauser AE, Hiepe F, Radbruch A. 2005. Maintenance of serum antibody levels. Annu Rev Immunol 23:367–386. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- 48.Shapiro-Shelef M, Calame K. 2005. Regulation of plasma-cell development. Nat Rev Immunol 5:230–242. doi: 10.1038/nri1572. [DOI] [PubMed] [Google Scholar]
- 49.Slifka MK, Antia R, Whitmire JK, Ahmed R. 1998. Humoral immunity due to long-lived plasma cells. Immunity 8:363–372. doi: 10.1016/S1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 50.Tokoyoda K, Hauser AE, Nakayama T, Radbruch A. 2010. Organization of immunological memory by bone marrow stroma. Nat Rev Immunol 10:193–200. doi: 10.1038/nri2727. [DOI] [PubMed] [Google Scholar]
- 51.Crotty S. 2011. Follicular helper CD4 T cells (T-FH). Annu Rev Immunol 29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 52.Voigt I, Camacho SA, de Boer BA, Lipp M, Forster R, Berek C. 2000. CXCR5-deficient mice develop functional germinal centers in the splenic T cell zone. Eur J Immunol 30:560–567. [DOI] [PubMed] [Google Scholar]
- 53.Rangel-Moreno J, Moyron-Quiroz JE, Hartson L, Kusser K, Randall TD. 2007. Pulmonary expression of CXC chemokine ligand 13, CC chemokine ligand 19, and CC chemokine ligand 21 is essential for local immunity to influenza. Proc Natl Acad Sci U S A 104:10577–10582. doi: 10.1073/pnas.0700591104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Publicover J, Gaggar A, Nishimura S, Van Horn CM, Goodsell A, Muench MO, Reinhardt RL, van Rooijen N, Wakil AE, Peters M, Cyster JG, Erle DJ, Rosenthal P, Cooper S, Baron JL. 2013. Age-dependent hepatic lymphoid organization directs successful immunity to hepatitis B. J Clin Invest 123:3728–3739. doi: 10.1172/JCI68182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu D, Vinuesa CG. 2010. The elusive identity of T follicular helper cells. Trends Immunol 31:377–383. doi: 10.1016/j.it.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 56.Rasalingam P, Rossiter JP, Mebatsion T, Jackson AC. 2005. Comparative pathogenesis of the SAD-L16 strain of rabies virus and a mutant modifying the dynein light chain binding site of the rabies virus phosphoprotein in young mice. Virus Res 111:55–60. doi: 10.1016/j.virusres.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 57.Conzelmann KK, Cox JH, Schneider LG, Thiel HJ. 1990. Molecular cloning and complete nucleotide sequence of the attenuated rabies virus SAD B19. Virology 175:485–499. doi: 10.1016/0042-6822(90)90433-R. [DOI] [PubMed] [Google Scholar]
- 58.Tian DY, Luo ZC, Zhou M, Li MM, Yu L, Wang C, Yuan JL, Li F, Tian B, Sui BK, Chen HC, Fu ZF, Zhao L. 2015. Critical role of K1685 and K1829 in the large protein of rabies virus in viral pathogenicity and immune evasion. J Virol 90:232–244. doi: 10.1128/JVI.02050-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, Garcia-Sastre A, Compans R, Pulendran B. 2011. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]