Abstract
Lynch syndrome (LS) and familial adenomatous polyposis (FAP) are major sources of hereditary colorectal cancer (CRC) and are associated with other malignancies. There is some heterogeneity in management strategies in Japan. We undertook a survey of management of hereditary CRC in hospitals that are members of the Japan Society of Colorectal Cancer Research. One hundred and ninety departments responded, of which 127 were from designated cancer care hospitals (DCCHs) according to the Japanese government. There were 25 488 operations for CRC in these departments in 2015. The DCCHs performed better with regard to usage of Japan Society of Colorectal Cancer Research guidelines, referring new CRC patients for LS screening, and having in‐house genetic counselors and knowledge of treatment for LS. There were 174 patients diagnosed with LS and 602 undergoing follow‐up in 2011–2015, which is fewer than the number expected from CRC operations in 2015. These numbers were not affected by whether the institution was a DCCH. Universal screening for LS was carried out in 8% of the departments. In contrast, 541 patients were diagnosed with FAP and 273 received preventive proctocolectomy/colectomy in 2011–2015. The DCCH departments undertook more surgery than non‐DCCH departments, although most of the management, including surgical procedures and use of non‐steroidal anti‐inflammatory drugs, was similar. Management of desmoid tumor in the abdominal cavity differed according to the number of patients treated. In conclusion, there was heterogeneity in management of LS but not FAP. Most patients with LS may be overlooked and universal screening for LS is not common in Japan.
Keywords: Disease management, familial adenomatous polyposis, Japanese, Lynch syndrome, screening
Colorectal cancer is the second most common cancer in Japan, the third most common cancer in the USA, and one of the most common malignancies worldwide.1, 2, 3 The hereditary forms of colorectal cancer (CRC) are considered to comprise 5% of all cases. Therefore, genetic testing and appropriate management are recommended.4, 5
Lynch syndrome (LS), which is also known as hereditary non‐polyposis CRC, is the most common form of hereditary CRC and is an autosomal dominant disorder caused by DNA MMR genes including MLH1, MSH2, MSH6, and PMS2.6, 7 Most LS patients have MSI‐H.8 Lynch syndrome accounts for 2–4% of all CRCs in Western countries, although there are few data for LS in Japan.4, 5, 9, 10, 11, 12 The JSCCR published its guidelines in 2012 for the clinical management of hereditary CRC.11 Diagnosis of LS is important because of the high risk of CRC and endometrial cancer, and increased risk of gastric, ovarian, urinary tract, and small bowel cancer in probands and their relatives because of chromosomal dominant heredity.4, 5, 12 In the USA, the necessity of LS diagnosis has already been established and concern has shifted to THE cost‐effectiveness of diagnosis.13, 14 At first, LS was screened by family history using the Amsterdam II and Revised Bethesda guidelines.7, 15 Then, many algorithms were proposed as screening methods to save time and money, although universal screening (MSI test and/or immunohistochemistry for MMR gene proteins) is considered the gold standard for diagnosis of LS.16, 17 Microsatellite instability is considered to be a useful biomarker for programmed death (PD)‐1 antibody therapy in patients with advanced CRC and endometrial cancer.18
Familial adenomatous polyposis is a hereditary disease caused by mutations of APC.4, 5, 10, 18 All patients with FAP are considered to have had CRC at some point during their lifetime, unless they have received any treatment for adenomatous polyposis.4, 5, 10, 18 Therefore, follow‐up by colonoscopy from the teenage years onwards and preventive proctocolectomy are recommended to patients and relatives with FAP.4, 5, 11, 19 The rare desmoid tumor is one of the common diseases that accompanies FAP.5, 19, 20, 21, 22 Abdominal desmoid tumor is especially intractable because of its high recurrence rate after surgery.
Data about hereditary CRC in Japan are limited.10, 11 Our department has developed the surgical procedure of proctocolectomy with hand‐sewn ileal J‐pouch anal anastomosis, and reported effective pharmacological management of advanced desmoid tumor using dacarbazine and doxorubicin.23, 24, 25 Therefore, we undertook a questionnaire survey associated with hereditary CRC to establish the current situation for management of LS and FAP in Japan.
We also assessed the differences in management and knowledge of hereditary CRC by DCCHs and non‐DCCHs to evaluate heterogeneity among the departments. Three hundred and ninety‐nine DCCHs have been certified by the Japanese government in an attempt to eliminate cancer care disparities in Japan. Unlike university hospitals and cancer center hospitals located in big cities, DCCHs are distributed widely, even in local areas, and we considered them suitable to assess heterogeneity in the management of hereditary CRC in Japan.
Materials and Methods
Questionnaire
The questionnaire consisted of three sections. The first section was concerned with medical care systems for hereditary CRC (Table 1). The questions included: type of hospital, number of surgical procedures carried out for CRC in 2015, collection of family history details at first visit, the persons who collect the family history, presence of a genetic counselor in or near the hospital, and use of the JSCCR guidelines. The second section consisted of questions about LS (Table 2), including: consideration of new CRC patients to have LS, the number of LS patients undergoing follow‐up, the number of LS patients with CRC undergoing follow‐up, resection area (segmental or prophylactic colectomy/proctocolectomy),26, 27 simultaneous resection of uterus and bilateral ovaries and fallopian tubes during colorectal surgery in postmenopausal patients (preventive gynecological surgery),28 use of 5‐FU‐based adjuvant chemotherapy,29 prophylactic treatment with aspirin,30 the number of patients with suspected LS in 2011–2015 who received counseling, MSI testing, or MMR gene sequencing, and were finally diagnosed with LS, knowledge about the usefulness of PD‐1 antibody in clinical trials for treatment of advanced cancer in patients with MSI‐H,18 and practice of universal screening of new CRC patients. The last section consisted of questions regarding FAP (Table 3), including: number of FAP patients undergoing follow‐up, number of FAP patients diagnosed in 2011–2015, number of patients receiving preventive surgery in 2011–2015 overall or by laparoscopic procedure,31 whether the operation was carried out in their own hospital or elsewhere, surgical procedures, such as proctocolectomy or colectomy, proctocolectomy with hand‐sewn or stapled ileal–anal anastomosis,32, 33 construction of pouch or not, type of pouch in case of pouch construction,23, 24, 34 timing of operation, use of NSAIDs such as sulindac,35 and number of patients with desmoid tumor in 2011–2015, type of desmoid tumor, and management.20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36
Table 1.
Medical care systems for patients with heriditary colorectal cancer in designated cancer care hospitals (DCCHs) and non‐DCCHs in Japan
| Number of departments | P‐value | |||
|---|---|---|---|---|
| Total (%) | DCCH | Non‐DCCH | ||
| (n = 127) | (n = 63) | |||
| Type of hospital | ||||
| University hospital | 88 (46) | 73 | 15 | <0.0001 |
| Public hospital | 69 (36) | 46 | 23 | |
| Private hospital | 33 (17) | 8 | 25 | |
| Family history collection at first visit | ||||
| Yes | 184 (97) | 123 | 61 | 0.9900 |
| No | 6 (3) | 4 | 2 | |
| Who collects family history? | ||||
| Doctor | 110 (58) | 72 | 38 | 0.7100 |
| Doctor and other staff | 50 (26) | 35 | 15 | |
| Staff other than doctor | 28 (15) | 18 | 10 | |
| Paper | 2 (1) | 2 | 0 | |
| Existence of genetic counselor in hospital | ||||
| Yes | 65 (34) | 61 | 4 | <0.0001 |
| No | 125 (66) | 66 | 59 | |
| Existence of genetic counselor near hospital | ||||
| Yes | 136 (72) | 94 | 42 | 0.2900 |
| No | 54 (28) | 33 | 21 | |
| Use of guidelines edited by JSCCR | ||||
| Yes | 150 (79) | 109 | 41 | 0.0010 |
| No | 40 (21) | 18 | 22 | |
Bold values indicate significance. JSCCR, Japan Society of Colorectal Cancer Research.
Table 2.
Management associated with patients with Lynch syndrome (LS) in designated cancer care hospitals (DCCHs) and non‐DCCHs in Japan
| Questions about LS | Number of departments | P‐value | ||
|---|---|---|---|---|
| Total (%) | DCCH | Non‐DCCH | ||
| (n = 127) | (n = 63) | |||
| Consideration of LS to new CRC patients | ||||
| Yes | 164 (86) | 116 | 48 | 0.004 |
| No | 26 (14) | 11 | 15 | |
| Surgical procedure | ||||
| Same as sporadic CRC | 108 (58) | 74 | 34 | 0.790 |
| Preventive proctocolectomy or colectomy | 19 (9) | 13 | 6 | |
| No comment | 63 (33) | 40 | 23 | |
| Recommendation of preventive gynecological surgery | ||||
| Yes | 35 (18) | 19 | 16 | 0.007 |
| No | 109 (57) | 83 | 26 | |
| No comment | 46 (24) | 25 | 21 | |
| Adjuvant setting by 5‐fluorouracil | ||||
| Yes | 125 (66) | 91 | 34 | 0.009 |
| No | 21 (11) | 15 | 6 | |
| No comment | 44 (23) | 21 | 23 | |
| Chemical prevention by aspirin | ||||
| Yes | 6 (3) | 4 | 2 | 0.030 |
| No | 140 (74) | 101 | 39 | |
| No comment | 44 (23) | 22 | 22 | |
| Practice of universal screening for LS | ||||
| Yes | 15 (8) | 14 | 1 | 0.020 |
| No | 175 (92) | 113 | 62 | |
| Usefulness of PD‐1 antibody | ||||
| Known | 127 (67) | 91 | 36 | 0.046 |
| Not known | 63 (33) | 36 | 27 | |
Bold values indicate significance. CRC, colorectal cancer; PD‐1, programmed death‐1.
Table 3.
Management associated with patients with familial adenomatous polyposis (FAP) in designated cancer care hospitals (DCCHs) and non‐DCCHs in Japan
| Questions about FAP | Number of departments | P‐value | ||
|---|---|---|---|---|
| Total (%) | DCCH | Non‐DCCH | ||
| (n = 127) | (n = 63) | |||
| Place of surgery | ||||
| Own hospital | 164 (86) | 117 | 47 | 0.0009 |
| Another hospital | 26 (14) | 10 | 16 | |
| Resection area | ||||
| Proctocolectomy | 136 (72) | 97 | 39 | 0.0600 |
| Colectomy | 27 (14) | 17 | 10 | |
| No reply | 27 (14) | 13 | 14 | |
| Anastomosis in case of proctocolectomy | ||||
| Handsewn ileal–anal anastomosis | 95 (50) | 63 | 32 | 0.1900 |
| Stapled ileal–anal anastomosis | 64 (34) | 47 | 17 | |
| No reply | 31 (16) | 17 | 14 | |
| Pouch construction in case of proctocolectomy | ||||
| Yes | 152 (80) | 106 | 46 | 0.1700 |
| No | 9 (5) | 6 | 3 | |
| No reply | 29 (15) | 15 | 14 | |
| Type of pouch in case of pouch construction | ||||
| J | 150 (99) | 104 | 46 | 0.6400 |
| J or W | 1 (1) | 1 | 0 | |
| W | 1 (1) | 1 | 0 | |
| Recommendation of operation at diagnosis | ||||
| Yes | 44 (23) | 29 | 15 | 0.8800 |
| No | 146 (77) | 98 | 48 | |
| Timing of operation depending on patient's lifestyle | ||||
| Yes | 184 (97) | 123 | 61 | 0.9900 |
| No | 6 (3) | 4 | 2 | |
| Use of NSAID as chemoprevention drug | ||||
| Yes | 81 (43) | 58 | 23 | 0.2000 |
| No | 108 (57) | 69 | 39 | |
| No reply | 1 (1) | 0 | 1 | |
| Main treatment for desmoid in abdominal wall | ||||
| Resection | 125 (66) | 91 | 34 | 0.0460 |
| No resection, drug | 27 (14) | 16 | 11 | |
| Introduction to the other hospitals | 17 (9) | 7 | 10 | |
| No reply | 21 (11) | 13 | 8 | |
| Main treatment for desmoid in abdominal cavity | ||||
| Resection | 118 (62) | 82 | 36 | 0.0300 |
| No resection, drug | 33 (17) | 25 | 8 | |
| Introduction to other hospitals | 16 (8) | 6 | 10 | |
| No reply | 23 (12) | 14 | 9 | |
Bold values indicate significance. NSAID, non‐steroidal anti‐inflammatory drug.
Data collection
We asked all departments that belonged to JSCCR to reply to the questionnaire from April 7 to May 13, 2016 using e‐mail and letter. Questions could be answered using a website or by letter to the JSCCR office. Data submitted to the website were automatically recorded in Excel files and data received by post were inputted to the same files. One hundred and ninety departments (177 hospitals) out of 568 in JSCCR responded to the questionnaire. In detail, 184 of 418 surgical departments, six of 96 medical departments, and 0 of 39 pathology, seven radiology, and eight basic research departments responded.
Data analysis
The responses to the questions were counted or categorized depending on the type of questions. The results were further analyzed by χ2‐test or t‐test to evaluate if the type of hospital (DCCH or not) influenced the answers. Responses related to desmoid tumor treatment were analyzed by logistic test to evaluate whether the number of patients influenced the answers. Statistical analysis was carried out using JMP version 11 (SAS Japan, Tokyo, Japan). P < 0.05 was considered significantly different.
Results
Medical care systems for hereditary CRC
The results of questions about medical care systems are listed in Table 1. Eighty‐eight departments (46%) belonged to university hospitals and 127 (67%) departments were categorized as belonging to DCCHs. There were 25 488 surgical procedures for CRC in 2015 (Table 4). Family history details were collected at the first visit by most departments (97%) and collected by doctors alone (58%), doctors and other staff (26%), or staff other than doctors (15%). A counselor was present in only 34% of the hospitals. However, 72% of departments could consult genetic counselors near the hospital if necessary. Seventy‐nine percent of departments used the JSCCR guidelines.
Table 4.
Number of patients assessed in this study
| Number of patients | P‐value | |||
|---|---|---|---|---|
| Total | DCCH | Non‐DCCH | ||
| CRC surgery in 2015 | ||||
| Median (range) | 120 | 135 (2–663) | 90 (0–229) | <0.0001 |
| Total | 25 488 | 19 362 | 6126 | |
| LS | ||||
| Under follow‐up | ||||
| Median (range) | 0 | 0 (0–114) | 0 (0–20) | 0.1900 |
| Total | 601 | 493 | 108 | |
| With CRC under follow‐up | ||||
| Median (range) | 0 | 0 (0–89) | 0 (0–18) | 0.2100 |
| Total | 464 | 382 | 82 | |
| Suspected in 2011–2015 | ||||
| Median (range) | 1 | 1 (0–465) | 0 (0–44) | 0.1400 |
| Total | 1634 | 1443 | 191 | |
| Receiving genetic counselling | ||||
| Median (range) | 0 | 0 (0–459) | 0 (0–18) | 0.2500 |
| Total | 925 | 880 | 45 | |
| Receiving MSI test | ||||
| Median (range) | 0 | 0 (0–278) | 0 (0–17) | 0.1900 |
| Total | 732 | 676 | 56 | |
| Receiving sequencing | ||||
| Median (range) | 0 | 0 (0–48) | 0 (0–4) | 0.0260 |
| Total | 326 | 302 | 24 | |
| Diagnosed in 2011–2015 | ||||
| Median (range) | 0 | 0 (0–30) | 0 (0–7) | 0.4300 |
| Total | 174 | 138 | 36 | |
| FAP | ||||
| Under follow‐up | ||||
| Median (range) | 2 | 3 (0–118) | 0 (0–150) | 0.0900 |
| Total | 1232 | 968 | 264 | |
| Diagnosed in 2011–2015 | ||||
| Median (range) | 1 | 2 (0–46) | 0 (0–14) | 0.0030 |
| Total | 541 | 462 | 79 | |
| Diagnosed by sequencing | ||||
| Median (range) | 0 | 0 (0–22) | 0 (0–5) | 0.0390 |
| Total | 152 | 131 | 21 | |
| Preventive surgery in 2011–2015 | ||||
| Median (range) | 0 | 1 (0–16) | 0 (0–9) | 0.0090 |
| Total | 273 | 223 | 50 | |
| By laparoscopic surgery | ||||
| Median (range) | 0 | 0 (0–12) | 0 (0–5) | 0.0020 |
| Total | 215 | 184 | 31 | |
| Desmoid in all areas | ||||
| Median (range) | 0 | 0 (0–7) | 0 (0–5) | 0.1400 |
| Total | 129 | 99 | 30 | |
| Desmoid in abdominal wall | ||||
| Median (range) | 0 | 0 (0–4) | 0 (0–3) | 0.3500 |
| Total | 46 | 11 | 35 | |
| Desmoid in abdominal cavity | ||||
| Median (range) | 0 | 0 (0–7) | 0 (0–4) | 0.0380 |
| Total | 104 | 83 | 21 | |
Bold values indicate significance. CRC, colorectal cancer; DCCH, designated cancer care hospital; FAP, familial adenomatous polyposis; LS, Lynch syndrome; MSI, microsatellite instability.
We analyzed the differences in medical care systems in DCCHs or non‐DCCHs to evaluate the heterogeneity among the departments. There were significant differences between DCCHs and non‐DCCHs for type of hospital (P < 0.0001), number of operations carried out in 2015 (P < 0.0001), presence of a genetic counselor (P < 0.0001), and use of JSCCR guidelines (P = 0.001). However, there was no significant difference in collection of family history details and presence of a genetic counselor near the hospital. These results indicated that most of the departments could consult a genetic counselor if necessary.
Management of patients with LS
The results of questions about LS are listed in Tables 2,4. New CRC patients were considered to have LS in 86% of the departments. There were 602 LS patients undergoing follow‐up, 464 LS patients with CRC undergoing follow‐up, 1443 suspected LS cases in 2011–2015, and 174 patients diagnosed with LS in 2011–2015 (Table 4). The medians of these numbers were 0, except for suspected LS patients, for which the median was 1 (Table 4). These numbers seemed low compared with the number of surgical procedures for CRC. Nine percent of departments undertook preventive proctocolectomy/colectomy and 18% of departments recommended preventive gynecological surgery with CRC surgery to postmenopausal women.26, 27, 28 Eleven percent of departments did not use 5‐FU‐based adjuvant chemotherapy, which implies knowledge about the ineffectiveness of 5‐FU‐based adjuvant chemotherapy for CRC in patients with MSI‐H.29 Three percent of departments used aspirin for prevention of CRC, which implies knowledge of the report by Burn et al.,30 although the recommendation for chemoprophylaxis has not been certified yet. Universal screening was carried out in only 8% of the departments. The usefulness of PD‐1 antibody in clinical trials against CRC in patients with MSI‐H, including LS, was known to 67% of departments.18
We analyzed the difference in management and knowledge of LS between DCCHs and non‐DCCHs. There was a significant difference between DCCHs and non‐DCCHs in considering that new CRC patients had LS (P = 0.004). However, there was no significant difference between DCCHs and non‐DCCHs in the number of patients assessed for LS, except for the number receiving MMR gene sequencing (Table 4). There were significant differences between DCCHs and non‐DCCHs in recommendation of preventive gynecological surgery (P = 0.007), knowledge of 5‐FU‐based adjuvant chemotherapy (P = 0.009), aspirin chemoprophylaxis (P = 0.03), and usefulness of PD‐1 antibody for MSI‐H patients, including those with LS (P = 0.046), and universal screening (P = 0.02). In 2011–2015, only 174 of 1635 suspected patients were diagnosed with LS, although the method of diagnosis of LS differed between DCCHs and non‐DCCHs (Fig. 1a). In DCCHs, 126 of 138 patients were diagnosed with LS by sequencing of MMR genes. However, in non‐DCCHs, 26 of 36 patients were diagnosed with LS by methods other than sequencing of MMR genes.
Figure 1.
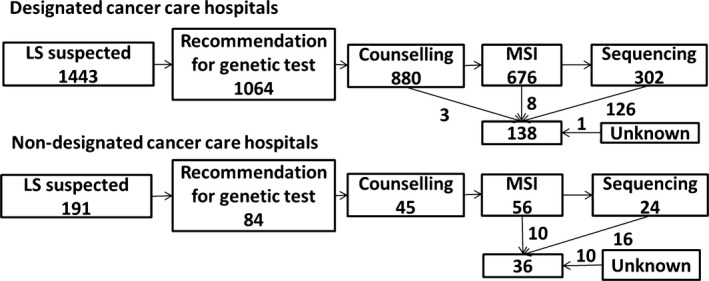
Flow chart from suspected Lynch syndrome (LS) to diagnosis of LS in designated cancer care hospitals (DCCHs; upper chart) or non‐DCCHs (lower chart) in Japan. Upper chart, 138 patients were diagnosed with LS in 2011–2015, mainly by mismatch repair gene sequencing in DCCHs. Lower chart, in non‐DCCHs in 2011–2015, 36 patients were diagnosed with LS by methods other than sequencing. MSI, microsatellite instability.
FAP management
The results of questions about FAP are listed in Tables 3,4. A total of 1232 FAP patients were followed up, 541 were newly diagnosed as FAP, and 273 received preventive proctocolectomy/colectomy in 2011–2015. Medians of these patients were 2, 1, and 0, respectively. However, 86% of departments replied that they carried out preventive surgery in their own hospitals. The responses to questions about surgical procedures were as follows: proctocolectomy (72%) or colectomy (14%), proctocolectomy with hand‐sewn (50%) or stapled (34%) ileal–anal anastomosis, ileal pouch construction (80%) or not (5%) in case of proctocolectomy, and J‐pouch construction in cases of pouch construction (99%). Lack of response to questions about these surgical procedures indicated departments where the surgery was undertaken elsewhere. Although 23% of departments recommended proctocolectomy soon after diagnosis of FAP, the operation was usually postponed to meet the patients' requirements. Non‐steroidal anti‐inflammatory drugs were used for chemoprophylaxis in 43% of departments.
One hundred and twenty‐nine FAP patients had desmoid tumors. Forty‐six patients had desmoid tumors in the abdominal wall and 104 had desmoid tumors in the abdominal cavity. Seventy‐four percent and 71% of departments managed desmoid tumor by resection, with or without other treatment in cases involving the abdominal wall and abdominal cavity, respectively.
We analyzed the difference in management of FAP between DCCHs and non‐DCCHs. There were significant differences between DCCHs and non‐DCCHs for the numbers of patients who were diagnosed with FAP (P = 0.003), received preventive surgery (P = 0.009), received preventive laparoscopic surgery (P = 0.002), and were diagnosed by sequencing of the APC gene in 2011–2015 (P = 0.039; Table 4). There was a significant difference between DCCHs and non‐DCCHs in terms of performing surgeries in their own hospital or elsewhere (Table 3; P < 0.0009). However, there was no significant difference between the hospitals for surgical procedures, including proctocolectomy or colectomy, proctocolectomy with hand‐sewn or stapled ileal–anal anastomosis, and pouch construction, or chemoprophylactic use of NSAIDs. There was no significant difference in the number of desmoid tumors, regardless of the location, although there was a significant difference in the treatment of desmoid tumors (P = 0.046 in abdominal wall, P = 0.03 in abdominal cavity) (Table 3). We further analyzed the treatment selection according to the number of patients (Fig. 2). Although there was no significant difference in the treatment of abdominal wall desmoid tumors (Fig. 2a,b), there was a significant association between the number of patients and treatment of abdominal cavity desmoid tumors (Fig. 2c,d). This difference was shown in DCCHs (P < 0.0001) but not in non‐DCCHs (P = 0.36) (Fig. 2c,d).
Figure 2.
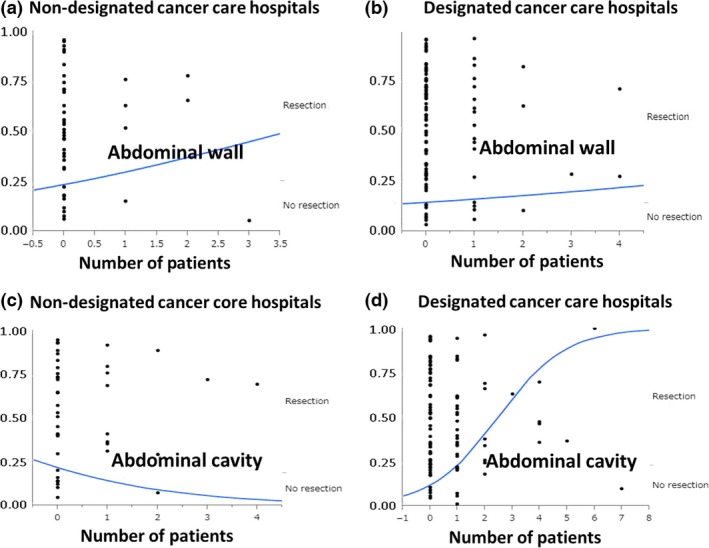
Logistic analysis between desmoid tumor treatment (resection or no resection) and number of desmoid tumor patients with familial adenomatous polyposis treated at designated cancer care hospitals (DCCHs) or non‐DCCHs in Japan. (a,b) Analysis of treatment decisions for abdominal wall desmoid tumor at non‐DCCHs (a) and DCCHs (b). (c,d) Analysis of treatment decisions for abdominal cavity desmoid tumor at non‐DCCHs (c) and DCCHs (d).
Discussion
To the best of our knowledge, this is the first report regarding the status of hereditary CRC management in Japanese hospitals. Although this was a retrospective study by questionnaire, our data indicate the problems of management of hereditary CRC in Japan.
The JSCCR consists of doctors who specialize in CRC, including surgeons, physicians, pathologists, radiologists, and basic researchers. We found that 25 488 CRC patients underwent surgery in 2015. This corresponded to ~20% of the total number of CRC patients in Japan, which is estimated at 130 000.
The presence of a genetic counselor was not common, even in DCCHs (Table 1). However, if necessary, the patients could be introduced to a counselor near the hospital and the JSCCR guidelines seemed to be used.
The number of CRC patients with LS seemed to be lower than would have been expected if the ratio in Japan were similar to that in Western countries.4, 5, 9, 12 One hundred and seventy‐four CRC patients were diagnosed with LS in 2011–2015, which corresponded to only 0.17% if the hospitals that responded to the questionnaire were considered to treat 100 000 CRC patients in 2011–2015. This incidence is lower than that reported by Kumamoto et al.10 The diagnosis of LS depended on MSI‐H alone or methods other than genetic testing in some cases, especially in non‐DCCHs. These results suggest a lower incidence of LS in Japan than in Western countries, or that many LS patients were overlooked in the clinic. Universal screening for LS was carried out in only 8% of departments in the present study, although most hospitals perform universal screening in the USA.37 Data about the incidence of LS in Japan will soon be available from the departments that perform universal screening. The differences in management and knowledge of LS between DCCHs and non‐DCCHs seem reasonable because insufficient data about LS have been collected to show any advantage in LS diagnosis in CRC patients in Japan.
The numbers of FAP patients diagnosed and undergoing follow‐up in 2011–2015 were higher than the corresponding numbers of LS patients. The incidence of FAP should be lower than that of LS, even if the number of cases of LS is lower in Japan than in Western countries. This difference should be related to the method of diagnosis. Familial adenomatous polyposis was diagnosed from the clinical features of colon polyposis and family history. Unlike for LS, genetic testing (sequencing) is not indispensable for diagnosis of FAP in most cases. The number of patients diagnosed with FAP and the number who received preventive surgery in 2011–2015 differed significantly between DCCHs and non‐DCCHs. However, there was no difference in the surgical procedures and timing or use of NSAIDs for chemoprophylaxis between DCCHs and non‐DCCHs. These results indicate that most management of FAP was undertaken similarly in departments belonging to JSCCR, although the number of patients differed according to the size of the hospitals.
Treatment for desmoid tumor in the abdominal cavity, but not in the abdominal wall, differed significantly when data were analyzed by the number of patients. Patients with desmoid tumors were rare in most departments. There were differences in follow‐up after preventive surgery depending on the number of patients.
The present study had several limitations. We investigated the issues associated with management of LS and FAP in Japan; however, we did not collect data about patient sex, age, economic status, details of follow‐up, or other malignancies, except for desmoid tumor. We focused on the departments belonging to JSCCR and DCCHs and non‐DCCHs to evaluate the heterogeneity of management for LS and FAP. Therefore, our results do not strictly reflect the heterogeneity in Japan because we collected data from specialized hospitals for CRC management. Further studies about treatment and care of patients with LS and FAP in Japan are required to resolve the limitations in this study.
In conclusion, there were differences in the management of LS and FAP in Japan. The low incidence of LS in this study indicated that many patients were overlooked, or that there really was a low incidence of LS in Japan. Therefore, the ratio of LS in newly diagnosed CRC patients should be investigated as soon as possible before the introduction of universal screening in Japan. Compared with LS, surgical management of FAP patients seemed appropriate both in the DCCHs and non‐DCCHs.
Disclosure Statement
The authors have no conflict of interest.
Abbreviations
- 5‐FU
5‐fluorouracil
- CRC
colorectal cancer
- DCCH
designated cancer care hospital
- FAP
familial adenomatous polyposis
- JSCCR
Japan Society of Colorectal Cancer Research
- LS
Lynch syndrome
- MMR
mismatch repair
- MSI‐H
high‐frequency microsatellite instability
- NSAID
nonsteroidal anti‐inflammatory drug
- PD‐1
programmed death‐1
Acknowledgments
This study was supported by the Japan Society of Colorectal Cancer Research (JSCCR). We are grateful to the members of JSCCR for replying to the questionnaire. We also thank the staff of JSCCR for data collection and Edanz for editing the manuscript.
Cancer Sci 108 (2017) 243–249
Funding Information
Japan Society of Colorectal Cancer Research
References
- 1. http://ganjoho.jp/reg_stat/statistics/stat/summary.html.
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29. [DOI] [PubMed] [Google Scholar]
- 3. Arnold M, Sierra MS, Laversanne M et al Global patterns and trends in colorectal cancer incidence and mortality. Gut 2016. doi:10.1136/gutjnl‐2015‐310912.x. [DOI] [PubMed] [Google Scholar]
- 4. Stoffel EM, Mangu PB, Gruber SB et al Hereditary colorectal cancer syndromes: American Society of Clinical Oncology clinical practice guideline endorsement of the familial risk‐colorectal cancer: European Society for Medical Oncology clinical practice guidelines. J Clin Oncol 2015; 33: 209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Syngal S, Brand RE, Church JM et al ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 2015; 110: 223–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lynch HT, Shaw MW, Magnuson CW et al Hereditary factors in cancer. Study of two large midwestern kindreds. Arch Intern Med 1966; 117: 206–12. [PubMed] [Google Scholar]
- 7. Umar A, Boland CR, Terdiman JP et al Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004; 96: 261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aaltonen LA, Peltomäki P, Mecklin JP et al Replication errors in benign and malignant tumors from hereditary nonpolyposis colorectal cancer patients. Cancer Res 1994; 54: 1645–8. [PubMed] [Google Scholar]
- 9. Moreira L, Balaguer F, Lindor N et al Identification of Lynch syndrome among patients with colorectal cancer. JAMA 2012; 308: 1555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kumamoto K, Ishida H, Suzuki O et al Lower prevalence of Lynch syndrome in colorectal cancer patients in a Japanese hospital‐based population. Surg Today 2016; 46: 713–20. [DOI] [PubMed] [Google Scholar]
- 11. Japanese Society for Cancer of the Colon and Rectum . JSCCR Guidelines 2012 for the Clinical Practice of Hereditary Colorectal Cancer. Tokyo, Japan: Kanehara Shuppan, 2012. (in Japanese). [Google Scholar]
- 12. Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group . Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med 2009; 11: 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mvundura M, Grosse SD, Hampel H et al The cost‐effectiveness of genetic testing strategies for Lynch syndrome among newly diagnosed patients with colorectal cancer. Genet Med 2010; 12: 93–104. [DOI] [PubMed] [Google Scholar]
- 14. Snowsill T, Huxley N, Hoyle M et al A model‐based assessment of the cost‐utility of strategies to identify Lynch syndrome in early‐onset colorectal cancer patients. BMC Cancer 2015; 15: 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park JG, Vasen HF, Park KJ et al Suspected hereditary nonpolyposis colorectal cancer: International Collaborative Group on Hereditary Non‐Polyposis Colorectal Cancer (ICG‐HNPCC) criteria and results of genetic diagnosis. Dis Colon Rectum 1999; 42: 710–5. [DOI] [PubMed] [Google Scholar]
- 16. Bellcross CA, Bedrosian SR, Daniels E et al Implementing screening for Lynch syndrome among patients with newly diagnosed colorectal cancer: summary of a public health/clinical collaborative meeting. Genet Med 2012; 14: 152–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cohen SA, Laurino M, Bowen DJ et al Initiation of universal tumor screening for Lynch syndrome in colorectal cancer patients as a model for the implementation of genetic information into clinical oncology practice. Cancer 2016; 122: 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Le DT, Uram JN, Wang H et al PD‐1 blockade in tumors with mismatch‐repair deficiency. N Engl J Med 2015; 372: 2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vasen HF, Möslein G, Alonso A et al Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut 2008; 57: 704–13. [DOI] [PubMed] [Google Scholar]
- 20. Ghert M, Yao X, Corbett T et al Treatment and follow‐up strategies in desmoid tumours: a practice guideline. Curr Oncol 2014; 21: e642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Joglekar SB, Rose PS, Sim F et al Current perspectives on desmoid tumors: the mayo clinic approach. Cancers (Basel) 2011; 3: 3143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. von Mehren M, Randall RL, Benjamin RS et al Soft Tissue Sarcoma, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016; 14: 758–86. [DOI] [PubMed] [Google Scholar]
- 23. Utsunomiya J, Iwama T, Imajo M et al Total colectomy, mucosal proctectomy, and ileoanal anastomosis. Dis Colon Rectum 1980; 23: 459–66. [DOI] [PubMed] [Google Scholar]
- 24. Fujita S, Kusunoki M, Shoji Y et al Quality of life after total proctocolectomy and ileal J‐pouch‐anal anastomosis. Dis Colon Rectum 1992; 35: 1030–9. [DOI] [PubMed] [Google Scholar]
- 25. Gega M, Yanagi H, Yoshikawa R et al Successful chemotherapeutic modality of doxorubicin plus dacarbazine for the treatment of desmoid tumors in association with familial adenomatous polyposis. J Clin Oncol 2006; 24: 102–5. [DOI] [PubMed] [Google Scholar]
- 26. Maeda T, Cannom RR, Beart RW Jr et al Decision model of segmental compared with total abdominal colectomy for colon cancer in hereditary nonpolyposis colorectal cancer. J Clin Oncol 2010; 28: 1175–80. [DOI] [PubMed] [Google Scholar]
- 27. Natarajan N, Watson P, Silva‐Lopez E et al Comparison of extended colectomy and limited resection in patients with Lynch syndrome. Dis Colon Rectum 2010; 53: 77–82. [DOI] [PubMed] [Google Scholar]
- 28. Karamurzin Y, Soslow RA, Garg K. Histologic evaluation of prophylactic hysterectomy and oophorectomy in Lynch syndrome. Am J Surg Pathol 2013; 37: 579–85. [DOI] [PubMed] [Google Scholar]
- 29. Ribic CM, Sargent DJ, Moore MJ et al Tumor microsatellite‐instability status as a predictor of benefit from fluorouracil‐based adjuvant chemotherapy for colon cancer. N Engl J Med 2003; 349: 247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burn J, Gerdes AM, Macrae F et al Long‐term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 2011; 378: 2081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Konishi T, Ishida H, Ueno H et al Feasibility of laparoscopic total proctocolectomy with ileal pouch‐anal anastomosis and total colectomy with ileorectal anastomosis for familial adenomatous polyposis: results of a nationwide multicenter study. Int J Clin Oncol 2016; 21: 953–961. doi:10.1007/s10147‐016‐0977‐x. [DOI] [PubMed] [Google Scholar]
- 32. Kartheuser A, Stangherlin P, Brandt D et al Restorative proctocolectomy and ileal pouch‐anal anastomosis for familial adenomatous polyposis revisited. Fam Cancer 2006; 5: 241–60. [DOI] [PubMed] [Google Scholar]
- 33. Church J. In which patients do I perform IRA, and why? Fam Cancer 2006; 5: 237–40. [DOI] [PubMed] [Google Scholar]
- 34. Aziz O, Athanasiou T, Fazio VW et al Meta‐analysis of observational studies of ileorectal versus ileal pouch‐anal anastomosis for familial adenomatous polyposis. Br J Surg 2006; 93: 407–17. [DOI] [PubMed] [Google Scholar]
- 35. Kim B, Giardiello FM. Chemoprevention in familial adenomatous polyposis. Best Pract Res Clin Gastroenterol 2011; 25: 607–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Church J, Lynch C, Neary P et al A desmoid tumor‐staging system separates patients with intra‐abdominal, familial adenomatous polyposis‐associated desmoid disease by behavior and prognosis. Dis Colon Rectum 2008; 51: 897–901. [DOI] [PubMed] [Google Scholar]
- 37. Beamer LC, Grant ML, Espenschied CR et al Reflex immunohistochemistry and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow‐up of abnormal results. J Clin Oncol 2012; 30: 1058–63. [DOI] [PMC free article] [PubMed] [Google Scholar]


