Abstract
Background
Roux-en-Y gastric bypass (RYGB) is the most effective method for the treatment of obesity and metabolic disease Roux-en-Y gastric bypass (RYGB) may reduce body weight by altering the feeding responses evoked by the short term satiety peptides.
Materials and Methods
Here, we measured meal size (MS, chow), intermeal interval (IMI) length and satiety ratio (SR, IMI/MS; food consumed per a unit of time) by the small and the large forms of gastrin releasing peptide (GRP) in rats, GRP-10 and GRP-29 (0, 0.1, 0.5 nmol/kg) infused in the celiac artery (CA, supplies stomach and upper duodenum) and the cranial mesenteric artery (CMA, supplies small and large intestine) in a RYGB rat model.
Results
GRP-10 reduced MS, prolonged the IMI and increased the SR only in the RYGB group, whereas GRP-29 evoked these responses by both routes and in both groups.
Conclusion
The RYGB procedure augments the feeding responses evoked by exogenous GRP, possibly by decreasing total food intake, increasing latency to the first meal, decreasing number of meals or altering the sites of action regulating MS and IMI length by the two peptides.
Keywords: GRP, Roux-en-Y, Food Intake, Celiac Artery, Cranial Mesenteric Artery
Introduction
Roux-en-Y gastric bypass (RYGB) is the most effective method for the treatment of obesity and metabolic disease (1, 2). Following this surgery, plasma levels of the orexigenic hormone ghrelin decrease, whereas plasma levels of the anorexigenic peptides glucagon like peptide-1 (GLP-1) and peptide tyrosine tyrosine (PYY) increase (3–6). Although these effects are thought to reduce appetite and improve glucose homeostasis, the extent to which these or other gut peptides are actually involved in RYGB remains in doubt. As such, the current work tested the hypothesis that a modified RYGB procedure, in which part of the stomach is removed, alters the feeding responses evoked by another anorexigenic peptide gastrin releasing peptides (GRP), namely meal size (MS), intermeal interval (IMI) length and satiety ratio (SR, IMI/MS, or amount of food consumed per a unit of time) and changes the gastrointestinal sites that regulate them. We have shown that GRP alters the previous feeding behaviors, i.e. MS, IMI and SR, (7–12) through sites supplied by the celiac artery (CA, supplies stomach and upper duodenum) (13, 14).
This hypothesis is based on three facts. First, the primary source of GRP is the myenteric neurons of the stomach (15–18), which are reduced in number and in size by the RYGB procedure. Second, the distribution of GRP receptors are in the distal stomach > distal duodenum > proximal stomach > colon = jejunum, esophagus, pylorus, proximal duodenum, ileum, rectum (Figure 5)(9). RYGB changes the distribution of these receptors and the amount and type of chyme contacting them. Such changes may also alter the feeding responses evoked by the various satiety gut peptides such as exogenous and endogenous GRP. Third, the site of action regulating reduction of MS and prolongation of the IMI by GRP in normal rats resides in the area supplies by the celiac artery (CA), i.e., stomach and upper duodenum (13, 14). However, because the RYGB surgery alters the architecture of the gastrointestinal tract, the site of action regulating these feeding responses by GRP may change.
Figure 5. Distribution of Gastrin Releasing Peptide Receptors.
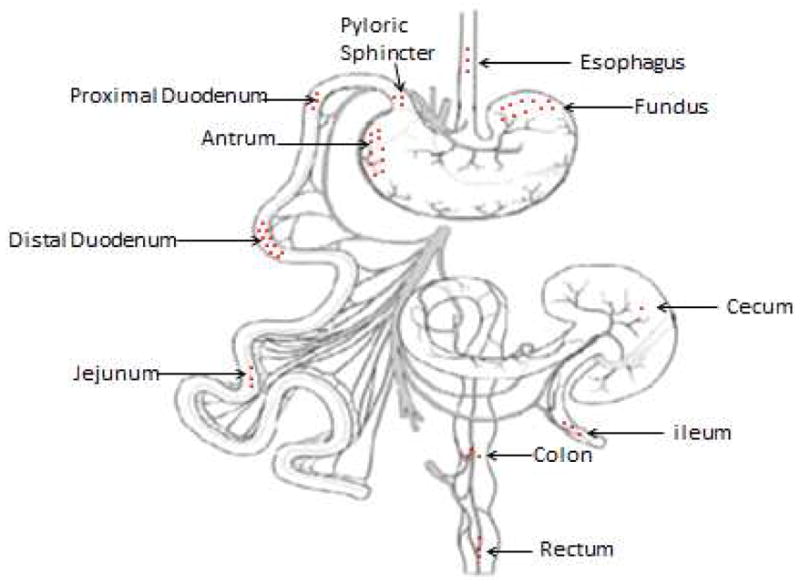
This schematic presentation indicates the distribution GRP in the gut. The highest secretion of GRP is shown to be primarily in the distal stomach, distal duodenum, proximal stomach and intermediately secreted in the colon, moderately secreted in the jejunum esophagus, pyloric sphincter, proximal duodenum, ileum and rectum (3 dots), and the lowest secretion was in the cecum.
The current work measured MS, IMI, SR by exogenous GRP-10 and GRP-29, the small and the large form of GRP in the rat respectively, and the gastrointestinal site of action regulating them in a modified RYGB rat model. Based on our earlier work in normal rats (13, 14), here, we tested two doses of GRP-10 and GRP-29, 0.1 and 0.5 nmol/kg, infused prior to the onset of the dark cycle in the CA and in the cranial mesenteric artery (CMA, supplies small and part of the large intestine) in near spontaneously free feeding rats. Our findings suggest that indeed RYGB alters the feeding responses evoked by exogenous GRP and the sites of action regulating them in the rat.
Materials and Methods
The Tuskegee University Animal Care and Use Committee approved the animal protocol for this experiment. Adult male Sprague Dawley rats weighing between 400 – 450 g (n=12 sham and 16 RYGB divided equally into CA and CMA groups) were individually housed in the BioDAQ E2 system (Research Diets, New Brunswick, NJ) in a controlled environment (12 h dark/12 h light cycle – lights off at 1800 h, 21.5° C), with water and pelleted rodent chow (Teklad, Madison, WI) available ad libitum.
Surgical Procedures
Modified Roux-en-Y Gastric Bypass (Figure 1)
Figure 1. Roux-en-Y gastric bypass.
The surgery is performed in two steps. First, a gastric pouch is created by removing the fundus and closing the antrum. Second, the jejunum is cut 40 cm distal to the pyloric sphincter and (A) is anastomosed (end to side) to the newly created gastric pouch (A′), and the proximal end of the jejunum (B) is anastomosed by an end to side anastomosis with the jejunum 10 cm distal to the original cut.
The procedure has been described previously (19) and is illustrated here in Figure 1. Briefly, following midline celiotomy, the jejunum was exposed and transected 40 cm distal to the pyloric sphincter. The fundus, the proximal upper third of the stomach, was removed, and the wall of the body of the stomach (the middle part of the stomach) was closed using a 5-0 PDS suture material. The antrum, the distal lower third of the stomach, was separated from the body of the stomach, and each compartment was closed using a 5-0 PDS suture material. An opening was created in the pouch formed by the closed body of the stomach and the distal segment of the severed jejunum was anastomosed by an end to side anastomosis in the newly created gastric pouch using an 8-0 Ethilon suture material. The proximal end of the jejunum was anastomosed using an end to side anastomosis, with the jejunum at a point located 10 cm distal to the first transection point using a 7-0 vicryl suture material. This procedure created a 40 cm biliopancreatic limb and a 10 cm Roux, alimentary limb. Sham surgery was achieved by performing the same cuts and anastomosed back together without changing the architecture of the gastrointestinal tract
Vascular Catheterization
One catheter was implanted in each rat, as described previously (13, 14, 20–22). Catheters (Micro-Renathane R-ITC-SP 9.5, Braintree Scientific, Braintree MA) were 24 cm long. The intravascular portion of the catheter was 0.25 mm OD × 0.12 mm ID, and the size of the remaining part was 0.84 mm OD × 0.36 mm ID. Catheterizations were performed using a surgical microscope (Carl Zeiss Opmi 160 12.5x/18B, 1×250, Monument, CO). General anesthesia, indicated by the absence of a pedal withdrawal reflex, was achieved with intramuscular injection of 1 ml/kg body weight of a mixture of 5.0 ml of Ketaset [100 mg/kg], 2.5 ml of Rompun® [xylazine 20 mg/kg], Bayer, Shawnee Mission, KS, 1.0 ml of acepromazine maleate® [10 mg/kg], Bayer, Shawnee Mission, KS and 1.5 ml of saline. The abdominal wall was clipped and cleaned with three alternating betadine solution and alcohol swabs. A ventral midline celiotomy was performed.
The CA was exposed and a temporary ligation was placed near the branch point from the aorta to prevent bleeding. The CA was punctured with a sterile 30 gauge needle 1–2 mm distal to this ligature, and the catheter was threaded into the artery and fixed in place using cyanoacrylate glue. The temporary ligation was removed, and the catheter was threaded out of the abdominal cavity subcutaneously, exteriorized between the scapulae and secured with sutures and cyanoacrylate glue. The CMA was similarly catheterized. The femoral artery (FA) was exposed on the medial aspect of the right thigh, freed from the surrounding fat and connective tissue, clamped (MC6 double clamp 0.9 cm, Microsurgery Instruments, Inc. Bellaire, TX), and catheterized similarly. The portal vein (PV) was located and exposed on the ventral aspect of the liver and similarly catheterized.
The muscles of the abdominal wall were closed using a polydioxanone II (4-0) absorbable suture in a simple continuous pattern, and the skin was closed using surgical staples. Postoperative care included Metacam® (Meloxicam® [1.1 mg/kg]) subcutaneously for pain control, Boehringer Ingelheim, St. Joseph, MO and Baytril® (Enrofloxacin® [0.05 ml], Bayer, Shawnee Mission, KS) intramuscularly as an anti-bacterial medication, each given daily for 5 d. Rats were allowed two weeks of recovery time. The criteria for complete recovery following surgery included the absence of clinical signs (e.g., signs of pain, porphyria secretion, cold extremities, lethargy) and the return of food intake to pre-operative levels. Catheters were flushed twice daily (0900 h and 1700 h) with 0.3 ml heparinized saline.
The patency of CA and CMA catheters was confirmed during surgery, first, by injecting 0.5 ml sterile saline into the catheters and verifying pallor in the perfused tissue, and, second, by injecting 0.5 ml methylene blue and verifying dye in the perfused tissues. In addition, at the end of the experiment, all rats were sacrificed with an overdose of pentobarbital, and the catheters were infused with latex, whose distribution was verified. Verification of the PV and FA were done by injecting latex only.
Meal Patterns
The BioDAQ E2 Food and Water Intake system detects brief episodes of food intake while minimizing food spillage and hoarding and generates a computerized data stream including times of the initiation of intake activity, the period of the activity, and the weight consumed. The criterion for a meal was consumption of ≥ 0.2 g, and the criterion for intermeal interval (IMI) was no feeding activity for ≥ 15 min (14, 20–22).
After two weeks of recovery from surgery, rats were habituated to the laboratory environment and the experimental design daily for two weeks. For the dose-response experiment, at 1700 h, 1 h before lights off, feeder gates were closed each rat was weighed, handled for a few minutes and received a 0.3 ml infusion of heparinized saline into its catheter. At 1800 h, lights were off, feeder gates were opened. First MS, IMI and SR were determined and formed individual baselines for each of the rats. These were compared later with the experimental data. If these did not match within two standard deviations, they were not included in the statistical analysis. All rats were included in the analysis.
On Mondays, Wednesdays and Fridays at 1800 h rats received a heparinized saline infusion. On Tuesdays, Thursdays and Saturdays, the rats received infusions of GRP-10 or GRP-29 (0, 0.1, 0.5 nmol/kg; Bachem, Torrance, CA, USA) at 1800 h. Sundays were reserved for the maintenance, but the catheters were flushed with 0.3 ml of the heparinized saline solution twice daily including Sundays. Treatments were done in random order.
Statistical Analysis
For each peptide MS, IMI, SR, total intake, latency to the first meal, duration of the first meal and number of meals were analyzed individually using three-way analyses of variance (route × surgery × treatment), with repeated measures on treatment, followed by Bonferroni-corrected t-tests for pairwise comparisons. Results were considered significant if p < 0.05. Data are displayed as the mean ± standard error of the mean (SEM).
Results
GRP-10
Meal Size: ANOVA revealed a main effect of surgery (F1, 24=4.55, p=0.043). Meal size was lower in the RYGB group compared to the sham group (p=0.04) (Figure 2).
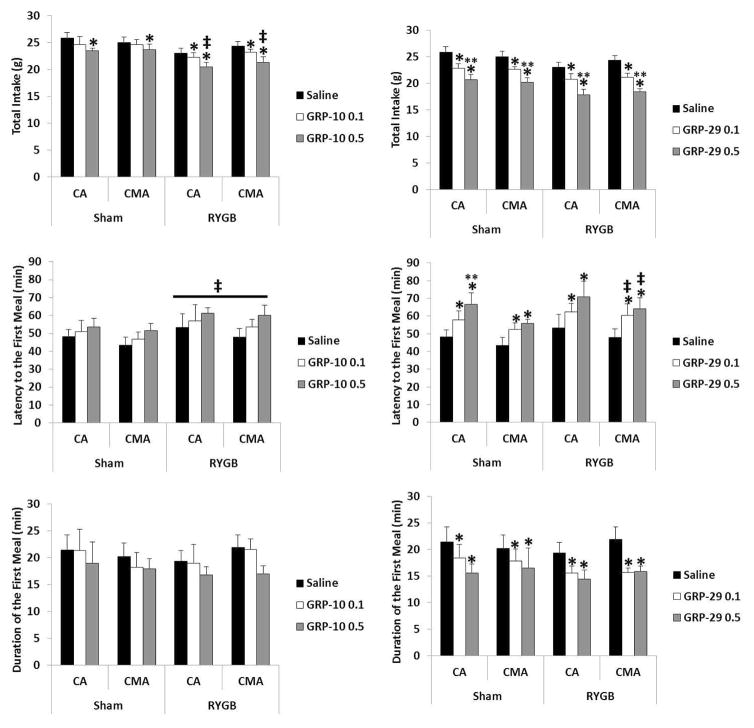
Figure 2. Effect of gastrin releasing peptide-10 and 29 on the first meal size, intermeal interval length and satiety ratio in Roux-en-Y gastric bypass rats.
Gastrin releasing peptide-10 and 29(GRP-10, 0, 0.1, 0.5 nmol/kg, GRP-29, 0, 0.1, 0.5 nmol/kg) was infused in the celiac artery (CA) and cranial mesenteric artery (CMA) in sham-operated rats and in Roux-en-Y gastric bypass (RYGB) –operated, near spontaneously free feeding rats prior to the onset of the dark cycle and the first meal size (MS, normal rat chow), intermeal interval (IMI) and satiety ratio (SR = IMI/MS) were determined. GRP-10 lowered meal size, prolonged the IMI was prolonged and increased the SR was increased in the RYGB group more than in the sham group (‡, p<0.05). GRP-29 given by both routes reduced MS relative to saline vehicle (*, p<0.05) and more so by the 0.5 nmol/kg dose compared to the 0.1 nmol/kg dose (**, p<0.05). In the RYGB group both doses of GRP-29 and by both routes prolonged the IMI relative to saline vehicle (*, p<0.05), and there was more prolongation by the 0.5nmol/kg in the CMA than in the sham group (‡, p<0.05). Only GRP-29 given by the CA prolonged the IMI in the sham group (*, p<0.05). GRP-29 0.1 and 0.5nmol/kg increased the SR relative to saline control (*, p<0.05) and GRP-29 0.5nmol/kg increased it more than 0.1nmol/kg (**, p<0.05.).
IMI: ANOVA revealed a main effect of surgery (F1, 24=47.16, p<0.001). The IMI was prolonged more in the RYGB group compared to the sham group (p=0.04) (Figure 2).
SR: ANOVA revealed a main effect of surgery (F1, 24=45.55, p<0.001). The SR was increased more in the RYGB group more than in the sham group (p=0.03) (Figure 2).
Total Intake: ANOVA revealed a main effect of surgery (F1, 24=14.386, p = 0.001) and treatment (F2, 48=5.89, p = 0.005). Total intake was lower in the RYGB group by GRP-10 0.1 and 0.5nmol/kg relative to saline vehicle (p= 0.05 and 0.014 respectively), whereas in the sham group only GRP-10 0.5nmol/kg lowered total intake relative to saline vehicle (p=0.05). The reduction in total intake by GRP-10 0.5nmol/kg in the RYGB group was more than the reduction by the same dose in the sham group (Figure 3).
Figure 3. Effect of gastrin releasing peptide-10 and 29 on various feeding behaviors in Roux-en-Y gastric bypass rats.
Gastrin releasing peptide-10 and 29(GRP-10, 0, 0.1, 0.5 nmol/kg, GRP-29, 0, 0.1, 0.5 nmol/kg) was infused in the celiac artery (CA) and cranial mesenteric artery (CMA) in sham-operated and in Roux-en-Y gastric bypass (RYGB) –operated, near spontaneously free feeding rats prior to the onset of the dark cycle and total intake (24-hr intake, chow), latency to first meal and duration of first meal were recorded. GRP-10 reduced total intake relative to saline vehicle (*, p<0.05) and there was more reduction in total intake and prolongation in the latency to the first meal in the RYGB group than the sham group (‡, p<0.05). Both doses of GRP-29 reduced total intake in both groups relative to saline control (*, p<0.05) and 0.5nmol/kg reduced it more than 0.1nmol/kg (**, p<0.001). In both groups and by both routes GRP-29 increased the latency to the first meal relative to saline control (*, p<0.05) and more so in the RYGB group than the sham group (‡, p<0.05). GRP-29 0.5nmol/kg given by the CA route increased the latency to the first meal more than 0.1nmol/kg in the sham group (**, p<0.05). Both GRP-29 0.1 and 0.5nmol/kg reduced the duration of the first meal relative to saline vehicle (*, p<0.05).
Latency to the first meal: ANOVA revealed a main effect of surgery (F1, 24=6.103, p = 0.021). The latency to the first meal in the RYGB group was increased compared to the sham group (Figure 3).
Duration of the first meal: ANOVA revealed no main effect of treatment, surgery or route or any interaction between them (Figure 3).
Number of Meals: ANOVA revealed a main effect of surgery (F1, 24=12.365, p < 0.001). The number of meals in the RYGB group was less compared to the number of meals in the sham group (Figure 4).
Figure 4. Effect of gastrin releasing peptide-10 and -29 on the number of meals in Roux-en-Y gastric bypass rats.
Gastrin releasing peptide-10 and -29(GRP-10, 0, 0.1, 0.5 nmol/kg, GRP-29, 0, 0.1, 0.5 nmol/kg) were infused individually in the celiac artery (CA) and cranial mesenteric artery (CMA) in sham-operated and in Roux-en-Y gastric bypass (RYGB) –operated, near spontaneously free feeding rats prior to the onset of the dark cycle and the number of meals were counted during the dark cycle. The number of meals in the RYGB group was less than the number of meals in the sham group (‡, p<0.05) in those rats given GRP-10.The number of meals were reduced by GRP-29 relative to saline vehicle (*, p<0.05) and there was more reduction in the RYGB group (‡, p<0.05).
GRP-29
MS: ANOVA revealed a main effect of routes (F1, 24=22.3, p<0.001), a main effect of treatments (F1, 24=138.2, p<0.001) and an interaction between treatments and routes (F1, 24=9.14, p<0.001). Meal size was reduced by both doses of GRP-29 given in the CMA relative to saline vehicle (p<0.001 each) and the 0.5nmol/kg produced greater reduction than 0.1nmol/kg (p = 0.003). Meal size was reduced by both doses of GRP-29 given in the CA relative to saline vehicle (p<0.001 each) but there was no difference between them (p=0.24) (Figure 2).
IMI: ANOVA revealed a main effect of route (F1, 24=23.0, p<0.001), a main effect of surgery (F1, 24=88.6, p<0.001), an interaction between route and surgery (F1, 24=41.8, p<0.001), a main effect of treatment (F1, 24=67.0, p<0.001), interaction between treatment and route (F1, 24=8.7, p=0.001), interaction between treatment and surgery (F1, 24=6.4, p=0.004) and interaction between treatment, route and surgery (F1, 24=9.5, p<0.001). The IMI was prolonged by both doses of GRP-29 given by both routes in the RYGB group whereas in the sham group GRP-29 given only by the CA prolonged the IMI relative to saline vehicle (Figure 2).
SR: ANOVA revealed a main effect of route (F1, 24 = 56.8, p<0.001), main effect of surgery (F1, 24=88.6, p<0.001), an interaction between route and surgery (F1, 24=20.6, p<0.001), a main effect of treatment (F1, 24=47.9, p<0.001) and an interaction between treatment and route (F1, 24=11.1, p=0.001). The SR increased by both doses of GRP-29 (p<0. 001 each) given by both routes and in both groups of rats relative to saline control. In the RYGB group the SR was increased by GRP-29 0.5nmol/kg given in both routes more than 0.1nmol/kg (p=0.046) (Figure 2).
Total Intake: ANOVA revealed a main effect of surgeries (F1, 24 =8.1, p=0.009) and treatment (F1, 24=47.2, p<0.001). Total intake was reduced by both doses of GRP-29 in both groups of rats and 0.5nmol/kg reduced it more than 0.1nmol/kg in both groups (p<0.001) (Figure 3).
Latency to the First Meal: ANOVA revealed a main effect of route (F1, 24 =6.1, p=0.021), surgery (F1, 24 =4.5, p=0.044) and treatment (F1, 24=8.2, p=0.001). The latency to the first meal was prolonged by both doses of GRP-29 given in both routes (p=0. 026 and 0.004 respectively). The latency was longer by GRP-29 given in the CMA in the RYGB group compared to the sham group (p=0.05). In both groups GRP-29 0.5nmol/kg given in the CA prolonged the latency to the first meal more so than 0.1nmol/kg (p=0.014) (Figure 3).
Duration of the first meal: ANOVA revealed a main effect of treatment (F1, 24=5.8, p=0.005). The duration of the first meal was shortened by both doses of GRP-29 0.1 (p=0.039, and 0.001 respectively) relative to saline vehicle (Figure 3).
Number of Meals: ANOVA revealed a main effect of surgery (F1, 24=18.0, p<0.001) and treatment (F2, 48=15.9, p<0.001). The number of meals was reduced by both doses of GRP-29 in RYGB group (p=0.002 and <0. 001 respectively) relative to saline vehicle whereas only 0.5nmol reduced it in the sham group (p= 0.033) (Figure 4).
Discussion
Our overall goal is to elucidate possible mechanisms by which RYGB reduces body weight. Here, we hypothesized that this surgery potentiates the satiety/satiation effects evoked by GRP-10 and GRP-29. In support of our hypothesis, the feeding responses by both peptides were increased in the RYGB rats compared to sham rats. The RYGB surgery created a favorable milieu for GRP to evoke its feeding responses in these rats compared to sham-operated rats.
Prior to this study, it has been shown that in rats RYGB causes increase in plasma levels of satiety peptides such as PYY and GLP-1 (3, 23, 24). Recently, Abegg et al. demonstrated that in rats, subcutaneous injections of the GLP-1 antagonist Exendin (9–39) increased food intake in RYGB rats compared to sham-operated male rats and the GLP-1 agonist Exendin-4 decreased it (25). RYGB did not, however increase the effect of Exendin (9–39) in female rats (26). The current work is the first to test the effect of GRP on food intake in RYGB rats. Our results show that in rats the RYGB surgery potentiates the feeding responses evoked by exogenous GRP.
Gastrin releasing peptide is a mammalian homologue of the amphibian skin peptide bombesin (Bn) (9). In mammals there are two forms of GRP, GRP-10 and GRP-27. However, in rats the large molecular form of GRP is GRP-29 (7). The current study examined the feeding responses evoked by both peptides in RYGB rats.
Using an identical experimental design to the one utilized here (14), we have shown that GRP-10 given by the CA, CMA, portal vein (PV) and femoral artery (FA) failed to reduce MS and prolong the IMI length in normal Sprague Dawley rats. The current study confirms this finding in sham rats. However, in the RYGB group MS was reduced, IMI was prolonged and SR was increased (Figure 2). These results suggest that the change in the architecture of the gut, as a result of the surgery, whether by increasing receptor numbers, receptor activity or receptor distribution, may have created a favorable environment for GRP-10 to evoke its feeding responses in the RYGB group. In support of this claim we have shown that GRP receptors are mostly distributed in the stomach and upper gut, i.e. the area supplied by the CA and to a less extent in the lower gut, i.e. the area supplied by the CMA (9). However, as shown here GRP-10 was able to reduce MS by the CMA route in the RYGB rats. Again, any combination of the following possibilities may explain this result, change in GRP receptor numbers, change in GRP receptor activity and/or change in GRP receptor distribution in the gastrointestinal tract as a result of the RYGB surgery.
Reduction of MS by GRP-10 was also accompanied by reduction in the average total food intake during the dark cycle, reduction in total number of meals and an increased latency to the first meal in the RYGB group. Based on this finding RYGB surgery may contribute to the reduction of body weight. However, this prediction requires measurement of body weight, which we have not done in this study.
The current work found that the IMI length was increased in the RYGB group more than in sham rats, a result which was also reflected by the increase in the satiety ratio, IMI divided by MS or the amount of food consumed per a unit of time. These results suggest that the surgery, either by increasing the number, activity or distribution of the GRP receptors in the gut, increased the feeding responses evoked by GRP-10, a result which has not been seen in sham rats or in our previous study in normal rats (14). Again, such results may contribute to the reduction of body weight resulted following the RYGB surgery.
In normal rats, we have shown that GRP-29 infused through the CA reduces MS and prolongs the IMI (14). The current finding in sham rats confirms these results. In addition, in the RYGB rats both feeding responses by GRP-29 were augmented, i.e. both doses and both routes produced feeding responses, and there was a difference between the RYGB rats compared to the sham group for the IMI. Similar to GRP-10 these responses by GRP-29 maybe due to increased number, activity or distribution of the BB2 receptors, which mediates reduction of food intake by GRP, as a result of the surgery. These possibilities require further testing.
Finally, in the RYGB group reduction of MS and prolongation of the IMI by GRP-29 was accompanied by reduction of total food intake during the dark cycle, reduction in meal numbers during the dark period and increased latency to the first meal. Collectively these findings may suggest that GRP-29 participate in the reduction of body weight by the RYGB surgery. As in the case with GRP-10 this possibility has not been tested in the current work.
In conclusion, this study found that a modified RYGB surgery augments the feeding responses, namely meal size and intermeal interval length, evoked by exogenous GRP-10 and GRP-29, the small and the large GRP forms in rats, which in turn may contribute to the reduction of body weight resulted following this surgery. Further studies are required to determine the mechanisms by which GRP reduces MS and prolongs IMI length in the RYGB rat model.
Acknowledgments
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Supported by grant 1SC1DK094972-01A1
Footnotes
Authors Contribution: Ayman Sayegh writing and design, Martha Washington design, writing, surgery and statistical analysis, Thaer Mhalhal writing help with surgery, design, and statistical analysis. Jose Berger and Randy Seeley helped with surgery
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, et al. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012. JAMA surgery. 2014;149:275–287. doi: 10.1001/jamasurg.2013.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher BL, Schauer P. Medical and surgical options in the treatment of severe obesity. American journal of surgery. 2002;184:9S–16S. doi: 10.1016/s0002-9610(02)01173-x. [DOI] [PubMed] [Google Scholar]
- 3.le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Annals of surgery. 2006;243:108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings DE. Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. International journal of obesity. 2009;33(Suppl 1):S33–40. doi: 10.1038/ijo.2009.15. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Liu J. Plasma ghrelin modulation in gastric band operation and sleeve gastrectomy. Obesity surgery. 2009;19:357–362. doi: 10.1007/s11695-008-9688-3. [DOI] [PubMed] [Google Scholar]
- 6.Stenstrom B, Furnes MW, Tommeras K, Syversen U, Zhao CM, et al. Mechanism of gastric bypass-induced body weight loss: one-year follow-up after micro-gastric bypass in rats. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2006;10:1384–1391. doi: 10.1016/j.gassur.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Reeve JR, Jr, Washington MC, Park KH, Johnson T, Hunt J, et al. Sequence analysis and feeding responses evoked by the large molecular form of gastrin releasing peptide (GRP) in the rat GRP-29. Peptides. 2014;59:1–8. doi: 10.1016/j.peptides.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Washington MC, Salyer S, Aglan AH, Sayegh AI. Intravenous infusion of gastrin-releasing peptide-27 and bombesin in rats reveals differential effects on meal size and intermeal interval length. Peptides. 2014;51:145–149. doi: 10.1016/j.peptides.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sayegh AI. The role of bombesin and bombesin-related peptides in the short-term control of food intake. Progress in molecular biology and translational science. 2013;114:343–370. doi: 10.1016/B978-0-12-386933-3.00010-8. [DOI] [PubMed] [Google Scholar]
- 10.Wright SA, Washington MC, Garcia C, Sayegh AI. Gastrin releasing peptide-29 requires vagal and splanchnic neurons to evoke satiation and satiety. Peptides. 2012;33:125–131. doi: 10.1016/j.peptides.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Washington MC, Sayegh AI. Gastrin releasing peptides increase Fos-like immunoreactivity in the enteric nervous system and the dorsal vagal complex. Peptides. 2011;32:1600–1605. doi: 10.1016/j.peptides.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 12.Washington MC, Wright SA, Sayegh AI. Gastrin releasing peptide-29 evokes feeding responses in the rat. Peptides. 2011;32:241–245. doi: 10.1016/j.peptides.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Washington MC, Mhalhal TR, Sayegh AI. The BB2 receptor antagonist BW2258U89 attenuates the feeding responses evoked by exogenous gastrin releasing peptide-29. Hormones and behavior. 2016;85:1–4. doi: 10.1016/j.yhbeh.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Washington MC, Aglan AH, Sayegh AI. The stomach and/or upper duodenum contain sites of action that control meal size and intermeal interval length by exogenous rat gastrin releasing peptide. Peptides. 2014;55:41–46. doi: 10.1016/j.peptides.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Dockray GJ, Vaillant C, Walsh JH. The neuronal origin of bombesin-like immunoreactivity in the rat gastrointestinal tract. Neuroscience. 1979;4:1561–1568. doi: 10.1016/0306-4522(79)90019-8. [DOI] [PubMed] [Google Scholar]
- 16.Ekblad E, Ekelund M, Graffner H, Hakanson R, Sundler F. Peptide-containing nerve fibers in the stomach wall of rat and mouse. Gastroenterology. 1985;89:73–85. doi: 10.1016/0016-5085(85)90747-4. [DOI] [PubMed] [Google Scholar]
- 17.Costa M, Furness JB, Yanaihara N, Yanaihara C, Moody TW. Distribution and projections of neurons with immunoreactivity for both gastrin-releasing peptide and bombesin in the guinea-pig small intestine. Cell and tissue research. 1984;235:285–293. doi: 10.1007/BF00217852. [DOI] [PubMed] [Google Scholar]
- 18.Moghimzadeh E, Ekman R, Hakanson R, Yanaihara N, Sundler F. Neuronal gastrin-releasing peptide in the mammalian gut and pancreas. Neuroscience. 1983;10:553–563. doi: 10.1016/0306-4522(83)90152-5. [DOI] [PubMed] [Google Scholar]
- 19.Meguid MM, Ramos EJ, Suzuki S, Xu Y, George ZM, et al. A surgical rat model of human Roux-en-Y gastric bypass. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2004;8:621–630. doi: 10.1016/j.gassur.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Washington MC, Williams K, Sayegh AI. The feeding responses evoked by endogenous cholecystokinin are regulated by different gastrointestinal sites. Hormones and behavior. 2016;78:79–85. doi: 10.1016/j.yhbeh.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 21.Williams KE, Washington MC, Johnson-Rouse T, Johnson RE, Freeman C, et al. Exogenous glucagon-like peptide-1 acts in sites supplied by the cranial mesenteric artery to reduce meal size and prolong the intermeal interval in rats. Appetite. 2016;96:254–259. doi: 10.1016/j.appet.2015.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sayegh AI, Washington MC, Johnson RE, Johnson-Rouse T, Freeman C, et al. Celiac and the cranial mesenteric arteries supply gastrointestinal sites that regulate meal size and intermeal interval length via cholecystokinin-58 in male rats. Hormones and behavior. 2015;67:48–53. doi: 10.1016/j.yhbeh.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 23.le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Annals of surgery. 2007;246:780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki S, Ramos EJ, Goncalves CG, Chen C, Meguid MM. Changes in GI hormones and their effect on gastric emptying and transit times after Roux-en-Y gastric bypass in rat model. Surgery. 2005;138:283–290. doi: 10.1016/j.surg.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Abegg K, Gehring N, Wagner CA, Liesegang A, Schiesser M, et al. Roux-en-Y gastric bypass surgery reduces bone mineral density and induces metabolic acidosis in rats. American journal of physiology Regulatory, integrative and comparative physiology. 2013;305:R999–R1009. doi: 10.1152/ajpregu.00038.2013. [DOI] [PubMed] [Google Scholar]
- 26.Asarian L, Abegg K, Geary N, Schiesser M, Lutz TA, et al. Estradiol increases body weight loss and gut-peptide satiation after Roux-en-Y gastric bypass in ovariectomized rats. Gastroenterology. 2012;143:325–327. e322. doi: 10.1053/j.gastro.2012.05.008. [DOI] [PubMed] [Google Scholar]