Abstract
We often think of the lentiginoses, phacomatoses and other neurocutaneous syndromes as conditions that affect the skin and also predispose to a variety of tumors. However, we rarely think of Peutz-Jeghers syndrome (PJS), Carney complex (CNC), Cowden disease (CD), neurofibromatosis type-1 (NF-1) or tuberous sclerosis (TSC) as conditions that are multiple endocrine neoplasias (MEN). Indeed, all of these conditions predispose to a variety of endocrine tumors, in additions to many other neoplasms. On the other hand, the classic MENs, type 1 and 2 (MEN-1 and MEN-2, respectively) are almost never thought in terms of their skin manifestations. In this review, we present extensively the MEN-1, MEN-2 and PJS syndromes, and briefly refer to CD, NF-1, and TSC. CNC is discussed in another article in this journal issue.
Keywords: Multiple endocrine neoplasia, hereditary tumor syndrome, Peutz-Jeghers syndrome, Cowden disease, neurofibromatosis type 1, tuberous sclerosis
MULTIPLE ENDOCRINE NEOPLASIA TYPE 1 (MEN-1)
Etiology/Pathophysiology
Familial MEN-1 is an autosomal dominant disorder with variable penetrance characterized by tumors of the parathyroids, anterior pituitary, pancreas, and other locations of the gastrointestinal tract, and other tissues. The MEN1 gene which codes for the protein menin is located on chromosome 11q13 and was first identified in 1997 (1). Individuals affected by MEN-1 inherit a menin inactivated allele (first hit); tumorigenesis in specific tissues follows inactivation of the remaining normal allele (second hit). MEN-1 is regarded as an endocrinopathy presenting in young adulthood. (2) As with many hereditary tumors, MEN-1 typically presents earlier than sporadic tumors of the same tissue type. The age disparity at presentation is striking for MEN-1-related hyperparathyroidism (third versus sixth decade in sporadic cases) but less pronounced for gastrinoma and insulinoma (fourth versus fifth decade in each). (3) By contrast, there has been no apparent age difference for the onset of MEN-1-associated and sporadic prolactinoma (fourth decade) (4–5). The age of clinical onset of treatable MEN-1-related tumors is an important factor in formulating biochemical and DNA screening recommendations.
Clinical Presentation
Hyperparathyroidism is usually the earliest and most common endocrine manifestation of MEN-1, with hypercalcemia being an almost universal finding at the time of MEN-1 diagnosis; clinically evident hyperparathyroidism in MEN-1 has been reported at ages 5 and 7 years and several times at age 8 years (1–4). However, no morbidity has been reported from early hyperparathyroidism in MEN-1. MEN-1-associated prolactinomas have been reported previously in children aged 10–13 years and growth hormone (GH)–producing adenomas in MEN-1 have been described as early as in a 5-year-old boy (5). Gastrinoma, the other defining feature of MEN-1 has not been seen earlier than age 12, whereas MEN-1-related insulinoma has been described as early as age 6 (1–4).
Cutaneous manifestations of MEN-1
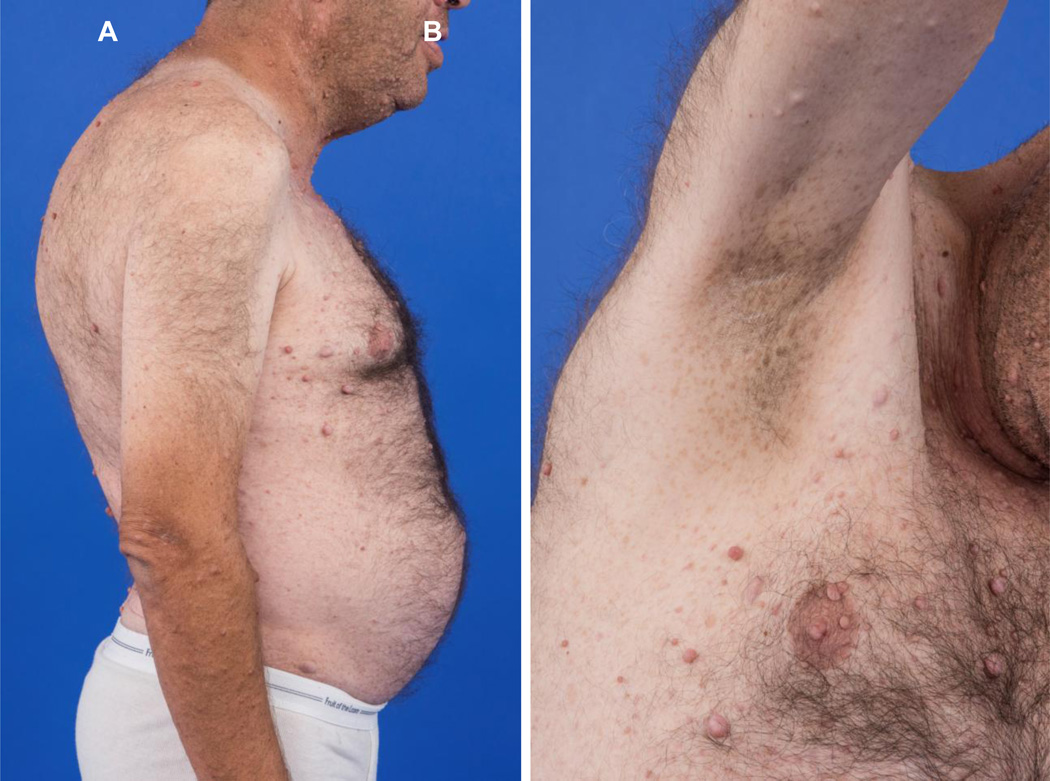
Patients with MEN-1 have angiofibormas, collagenomas, lipomas and various macules (6–12). Facial angiofibromas are diagnostic of MEN-1 at an early age and may be used for the detection of carriers at an early age within a family that already known to be affected with MEN-1 (6, 11). Melanoma and squamous cell carcinoma may occur coincidentally (13).
Diagnosis
A diagnosis of MEN-1 is made when either: 1. A patient has 2 or more MEN-1-associated lesions, or 2. A patient has only one lesion, but there exists a family history of relatives with MEN1; or 3. A patient has a positive genetic test for an abnormal MEN1 sequence, a mutation or a defect that has previously been associated with the disease in other individuals (14–16).
As mentioned above, there are three main types of lesions associated with MEN-1: Parathyroid glands hyperplasia or adenomas, tumors of the endocrine pancreas and gut (duodenum), and pituitary adenomas. Roughly 80% (8 in 10) of patients with MEN1 will have developed at least one of these tumors by the age of 50, and around 40% (4 in 10) by the age of 20 years. The condition varies greatly even within families; not everyone will have the same tumors and they will not occur at the same age.
Screening/Monitoring
Patients with known MEN-1 should be screened annually, starting in childhood (after the age of 5 years), for the main manifestations of the disorder (15, 16). Careful monitoring of the growth rate and annual screening of plasma ionized calcium and serum intact parathyroid hormone (i-PTH) in children of families with MEN-1, who are known mutation carriers is recommended. If the growth rate is increased as in acromegaly, or decreased as in prolactinomas, Cushing disease, or Cushing syndrome, appropriate tests are ordered. The latter tumors can also be associated with an increase in weight gain. Routine screening for pituitary tumors in the absence of symptoms is not necessary until late childhood or early adolescence (ages 10–15 years) because they occur only rarely before these ages. Similarly, fasting gastrin, C-peptide, and plasma insulin determinations and serial prolactin measurements (obtained every 20 minutes for 1 hour through an indwelling catheter that has been inserted an hour before the sampling to avoid stress-related, falsely elevated levels) and pituitary or abdominal imaging are not necessary until adolescence. Although such biochemical testing may be done annually thereafter, imaging may be done only as needed and when the presence of a tumor is suggested by clinical symptoms or laboratory testing; clinical symptoms such as hypoglycemia, delayed puberty, irregular menses, gynecomastia, galactorrhea, weight gain, moon facies, hirsutism, abnormal visual fields, decrease in school performance, suggest a pituitary tumor and should be followed by an MRI of the pituitary with gadolinium. Depending on the size of the pituitary tumor, symptoms of secondary adrenal insufficiency may also be present. If hypoglycemia or peptic ulcer disease are the presenting symptoms, abdominal imaging is necessary to screen for insulinoma and gastrinoma, respectively; however insulinomas and gastrinomas are extraordinarily rare in children with MEN-1. In young adults with MEN-1, in addition to the annual biochemical testing prescribed earlier, annual imaging of the abdomen (and the pancreas in particular) may be obtained; pituitary imaging may be obtained in the absence of symptoms or suggestive biochemical testing no more frequently than every 5 years to avoid false-positive studies and unnecessary follow-up testing.
Molecular Genetics and Recommendations for Screening
The identification of the MEN1 gene led to mutation testing that is now available at several centers (1–4, 14–16). The main candidates for MEN1 mutational analysis include the index cases with MEN-1, their unaffected relatives, and some cases with features atypical for MEN-1. There have been several hundreds of mutations in menin; they are spread almost equally along the length of the gene without any apparent genotype–phenotype correlation. Some mutations occur more frequently than others, but there are no obvious “hot spots” in the nine coding exons of the gene (14–16). The mutations that are more frequent involve exons 83, 84, 209–211, and 514–516, which contain unstable DNA sequences (e.g., dinucleotide repeats or poly(C) tracts). A small number of MEN-1 kindreds do not have mutations in the MEN1 gene but rather 11q13 deletions; others have mutations in other genes. The recent recognition of significant morbidity from MEN-1 at young ages (5), the need for carrier testing in large families with known mutations, and advances in molecular testing will probably lead to more frequent genetic testing in the future.
Treatment
The treatment of tumors associated with MEN-1 in children is mainly surgical, with the exception of prolactinomas and the medical management of Zollinger–Ellison syndrome (ZES) (1–4,14–16). Gigantism may be treated by somatostatin analogues and dopamine agonists as in adult patients with similar tumors, but the primary approach would be by transsphenoidal surgery (TSS). Orally administered dopamine agonists are the main treatment for prolactinomas, and only occasionally may a macroprolactinoma that is not responsive to medical treatment and causes symptoms or continues to grow need to be excised by TSS. Medical gastrectomy by omeprazole or ranitidine and other proton-pump inhibitors or H2-blockers, respectively, is all that is required for the treatment of hypergastrinemia. Surgery should be avoided as the primary treatment of ZES. Hyperparathyroidism is treated surgically if a serum calcium level consistently above 12 mg/dL or when nephrolithiasis or bone loss is evident. Other tumors associated with MEN-1 that are symptomatic or have the potential of being malignant, such as insulinoma or the rare case of a malignant gastrinoma, are also approached surgically.
MULTIPLE ENDOCRINE NEOPLASIA TYPE 2
Etiology/Pathophysiology
The MEN-2 syndromes are the phenotypic expressions of variants of activating mutations in the RET proto-oncogene, a tyrosine kinase receptor (17–27). All clinical manifestations of the MEN-2 syndromes reflect the inappropriate transduction of this growth- and survival-promoting signal in the neural crest–derived tissues that naturally express RET (17, 18). The primary and most common lesion of the MEN-2 syndromes is medullary thyroid cancer (MTC) (22); it is present in up to 95% of patients with MEN-2 and is preceded by hyperplasia of the calcitonin-secreting thyroid parafollicular C cells (20). Histological evidence of MTC in more than 95% of obligate gene carriers is present by age 35 years. Calcitonin provocative testing is not as specific for MTC as was believed initially because a small fraction of persons with normal calcitonin provocative testing were shown to be obligate noncarriers of their kindred’s characteristic RET mutation. Conversely, those classified as being affected on the basis of C cell hyperplasia alone should be reevaluated with RET testing to allow accurate counseling about their children’s risk for MEN-2 (17–23).
Clinical Presentation
Pheochromocytoma typically affects 50% to 60% of MEN-2A kindreds with a high rate of bilaterality but a low rate of extra-adrenal sites or malignancy (17–27). The tumor is commonly present in MEN-2B and absent by definition in familial medullary thyroid cancer (FMTC). A yearly program for pheochromocytoma that uses urinary catecholamines and metabolites to screen can effectively identify tumors at an early stage (<2 cm), before the development of hypertension or other adverse sequelae. Laparoscopic adrenalectomy is now the method of choice for the surgical removal of a pheochromocytoma.
Parathyroid disease is detected clinically in 10% to 15% of patients with MEN-2A (17–27). Recently, two variants of MEN-2A with distinctive nonendocrine manifestations have been recognized. MEN-2A with cutaneous lichen amyloidosis, which is characterized by pruritic lesions composed of subepidermal keratin deposits over the scapular region147, and MEN-2A with partial or extensive Hirschsprung syndrome, which represents another distinct clinical syndrome. Patients with this disease exhibit evidence of both RET hyperfunction and hypofunction in a tissue-specific fashion.
Expanding the clinical spectrum of the MEN-2 syndromes are the distinctive skeletal findings found in virtually all MEN-2B patients: elongated facies with a long, relatively thin nose, proliferation of corneal nerves, mucosal neuromas of the lips and tongue, and gastrointestinal ganglioneuromas (22, 23). In addition, these patients frequently have aggressive tumors, both MTC and pheochromocytoma. These manifestations all appear to stem from abnormal proliferation of neural crest elements during fetal and postnatal life and may be orchestrated by a hyperfunctioning RET tyrosine kinase receptor.
Cutaneous manifestations of MEN-2
Patients with MEN-2 may preset with lichen amyloidosis (Figure 1) (28, 29). Patients with MEN2B have a distinct Marfanoid habitus and mucosal neuromas are often obvious in the oral area and the lips (22).
Figure 1.
Lichen amyloidosis in a patient with MEN2A
Diagnosis
Diagnosis of MTC is made by genetic testing (in the familial setting, see below), a palpable neck ormass (or masses) and/or elevation of calcitonin levels (20–23, 30, 31). MTC is frequently the first neoplastic manifestation in MEN2A patients, with MTC occurring as early as in the first 5 years of life. In families without an established diagnosis of MEN2A, patients typically present with a neck mass between the ages of 15 and 20 years. MEN2A accounts for 70% to 80% of individuals with hereditary MTC. Occasionally, a patient may present with one of the two rare variants of MEN2A, the one with Hirschsprung’s disease (24) and the other with cutaneous lichen amyloidosis and MTC (26) is found in the work-up. The second inherited subtype of MTC, MEN2B, accounts for only 5% of hereditary MTC cases. MEN 2B is characterized by clinically aggressive MTC, pheochromocytoma, a Marfanoid body habitus, mucosal (and other) neuromas, and intestinal tumors (mostly ganglioneuromas); these patients typically do not manifest hyperparathyroidism. The third inherited subtype of MTC is familial MTC (FMTC). This subtype accounts for 10% to 20% of hereditary MTC cases; only the thyroid gland is affected typically in these patients, although rare large families with both MEN2A and FMTC also exist. In general, FMTC patients present later with MTC than those with MEN2A or MEN2B, usually between 20 and 40 years of age.
Screening/Monitoring
In known carriers of a RET mutation, other than prophylactic thyroidectomy (see below), annual screening for pheochromocytoma should be performed after age 5, along with a serum ionized calcium measurement; usually hyperparathyroidism in these patients does not develop until later childhood or adolescence, so, if necessary, one can limit the screening to that for pheochromocytoma only between the ages 5 and 10 years. Plasma catecholamines are the best screening test for pheochromocytoma (see below). An ionized calcium level that is elevated should be followed by measurements of parathyroid hormone.
Molecular Genetics and Recommendations for Screening
Analyzing the RET gene in the MEN-2A and FMTC syndromes revealed that most of the patients had mutated one of five cysteine codons in exons 10 and 11; however, isolated FMTC also was caused by mutations in the first tyrosine kinase domain of the receptor (18, 20). The cysteine-to-arginine change in codon 634 of the RET protein appears to be the most common mutation in the MEN-2 syndromes and is also found in the few kindreds with MEN-2A that have pheochromocytoma and hyperparathyroidism but no other clinical manifestations. In MEN-2A/Hirschsprung disease, most kindreds have mutations in codons 609, 618, and 620. In MEN-2B, the mutations are in the tyrosine kinase domain.
Over the past 35 years, there has been a substantial improvement in survival in MEN-2 families, an improvement largely attributable to the success of family screening programs, first provocative biochemical tests, and more recently, detection of RET gene mutations (30–33). RET gene analysis has superseded older methods because of its high sensitivity and specificity, utility in younger children, and much higher degree of patient acceptance. A typical screening program for known MEN-2A and FMTC kindreds is to initiate testing for RET mutations at 2 years of age. In the case of MEN-2B, it is generally possible to recognize the characteristic mucosal neuroma phenotype within the first 2 years of life. Prophylactic thyroidectomy is now recommended for all patients who test positive for most disease-causing mutations of the RET gene. In addition to established MEN-2 kindreds, 6% to 8% of MTC patients with no apparent family history of the disorder harbor germline RET mutations and thus may have offspring at risk. On the basis of this figure, it appears prudent to offer germline RET analysis to everyone with apparent sporadic MTC.
Treatment
Other than prophylactic thyroidectomy, most other tumors associated with MEN-2 are also treated surgically (30–32). As in MEN-1, hyperparathyroidism is treated surgically when necessary (see above) by near-total parathyroidectomy (or by total parathyroidectomy and reimplantation of parathyroid tissue in the nondominant forearm). The rate of recurrent hypercalcemia is less in MEN-2 that in MEN-1 after these procedures but does not differ substantially between them. Pheochromocytomas are removed preferably laparoscopically today.
PEUTZ-JEGHERS SYNDROME
Etiology/Pathophysiology
The hallmark of this syndrome is the presence of pigmented spots on the lips, which are first present in early childhood (34–39). These lesions are associated with gastrointestinal hamartomatous polyps (38, 39). Gastrointestinal cancers are frequent in Peutz-Jeghers syndrome (PJS) (35–37); they may arise from genetic changes known to occur in colorectal carcinoma and other tumors because the PJS-responsible gene, STK1/LKB1, functions as a tumor suppressor (37). PJS patients are also at an increased risk for breast, ovarian, testicular, uterine, and cervical cancers, as well as nonmalignant lesions in these tissues; the overall incidence of carcinoma in patients with PJS varies from 20% to 50%, and it appears at a relatively early age (36, 37, 41).
Endocrine tumors are also frequent in PJS (42, 43). These include thyroid nodules and cancer and genital tract neoplasms. The latter include, in female patients with PJS, ovarian neoplasms from both the epithelium and stromal cells and adenoma malignum of the cervix and adenocarcinoma of the endometrium. Male patients with PJS often have Leydig cell tumors or a Sertoli cell tumor that is uniquely found in PJS and in Carney complex (CNC) (44, 45): large cell calcifying Sertoli cell tumor (LCCSCT). LCCSCT in PJS, as in CNC, may be associated with increased aromatization of adrenal or testicular androgens, which produces estradiol and other estrogens (estrone, in particular) that may lead to precocious puberty and prepubertal or peripubertal gynecomastia (45).
Clinical presentation
Most patients with PJS present in childhood with complications of polyps (i.e, bowel obstruction). Peri-oral and mucosal pigmentation may also attract attention and lead to diagnosis at an early age. Both polyps and pigmented spots and other skin pigmentation defects become apparent before puberty, typically after the age of 5 years. Occasionally, the first manifestation in boys is gynecomastia due to LCCSCT.
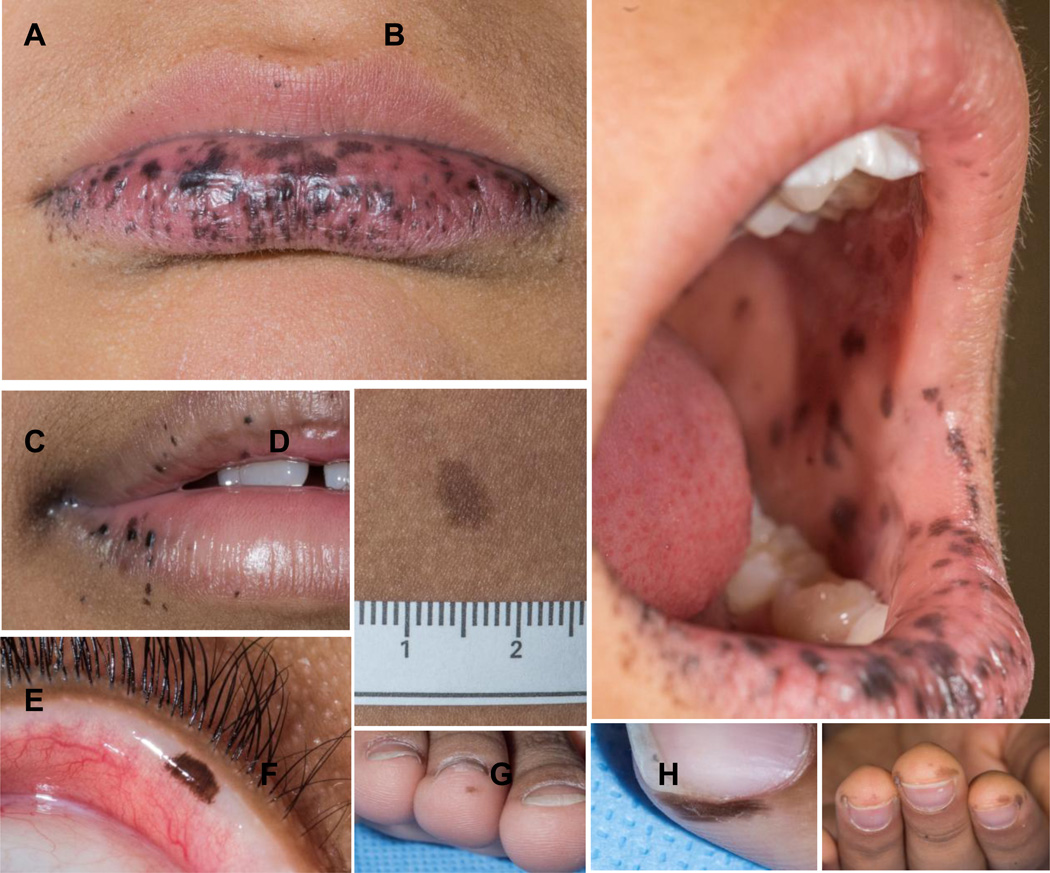
Cutaneous manifestations of PJS
Patients with PJS may preset with multiple lentigines with intense coverage of the lips and the peri-oral area (34, 38); a variety of other pigmented lesions are common (Figure 2).
Figure 2.
A, B, C: Lip, oral mucosa, and peri-oral pigmentation in a patient with PJS; D: Other pigmented lesions, such as café-au-lait spots, are also frequent in patients with PJS; E, F, G, H: some times, pigmented spots and nevi are found in unusual spots in patients with PJS.
Diagnosis
Diagnosis of PJS is made when at least one of the classic stigmata of the disease is present and/or if there is family history (34). Diagnosis is confirmed by molecular testing for STK11/LKB1 mutations and/or deletions (see below).
Molecular Genetics
Most PJS families have been linked to 19p13.3; the PJS gene at 19p13.3 is STK11 (for serine threonine kinase 11) also known as LKB1 (34–37, 40). More than half the families with PJS have mutations in this gene, although the percentage varies greatly from one study to the next. STK11/LKB1 is a novel serine–threonine kinase containing nine exons; The kinase domain of the STK11/LKB1 gene is highly conserved between mouse and human (34). Although it has been suggested that mouse Lkb1 is a nuclear protein, wild-type STK11/LKB1 shows both nuclear and cytoplasmic localization. A number of recent studies have elucidated the effects of inherited STK11/LKB1 mutations in PJS kindreds based on the functional domains of the protein; in most cases, elimination of the kinase activity underlies the molecular cause of the phenotype (36, 37).
Treatment & Prognosis
Tumors associated with PJS are surgically removed, as needed. Patients need to be surveyed for the development of colon cancer, and they are predisposed to other malignancies, as well. LCCSCTs do not need to removed, as they are typically benign, but gynecomastia in boys may be surgically treated (44, 45). Mild gynecomastia may successfully respond to treatment with aromatase inhibitors (45). Patients with PJS typically do well, as long as they are enrolled in regular clinical. Significant morbidity is caused by the development of polyps and the risk for colon and other malignancies.
COWDEN DISEASE
Cowden Disease (CD) is associated with hamartomas and tumors of ecto-, meso- and endodermal origin affecting multiple organs (46–48). Thyroid and breast masses, hamartomatous polyps, and mucocutaneous lesions (e.g., oral papillomatosis, acral keratosis, and multiple fibromas) occur consistently in this syndrome, which is also associated with anomalies of the skeletal and nervous systems (43). CD was first mapped to chromosome 10; mutations in the tumor-suppressor gene PTEN on 10q were found shortly after that (46). CD is allelic to Ruvalcaba–Myhre–Smith, Bannayan–Zonana, or Bannayan–Riley–Ruvalcaba syndrome, which is also associated with hamartomatous intestinal polyposis, lentiginosis of the genitalia and developmental defects (e.g., macrocephaly and eye and skeletal anomalies), and myopathy (46–48). Both syndromes are due to mutations of the PTEN gene on chromosome 10, although genetic heterogeneity may exist (49).
NEUROFIBROMATOSIS Type 1 (NF-1)
NF-1 affects about 1 in 4000 individuals worldwide. NF-1 is clinically characterized by multiple café-au-lait spots and associated cutaneous neurofibromas (50, 51). The National Institutes of Health Consensus Conference developed diagnostic criteria that require at least two of main clinical features in order to diagnose NF-1 (52). These features include the cutaneous findings of café-au-lait macules, neurofibromas, and axillary freckling (Figure 3), and optic gliomas, iris hamartomas, bony abnormalities and other tumors (53–55)). Patients with NF1 are at an approximately 2-4 fold higher risk of developing tumors than the general population with a risk of malignancy estimated at between 5 and 15 percent (50). The gastrointestinal tract may be involved in NF-1 and includes mucosal and myenteric nerve hyperplasia, gastrointestinal stromal tumors (GIST), carcinoids, pheochromocytomas, paragangliomas, as well as pancreatic neuroendocrine tumors (50, 53–55).
Figure 3.
A,: Multiple neurofibromas and pigmented skin lesions, as well as skeletal deformities in a patient with NF-1; B: Axillary freckling in the same patient.
TUBEROUS SCLEROSIS
Tuberous sclerosis (TSC) is an autosomal dominant neurocutaneous disorder with an incidence of approximately 1 in 5,000 to 10,000 live births (56); approximately 70–80% of TSC patients represent sporadic cases (57). TSC is a multisystem disorder with involvement of the brain, eyes, skin, heart, lungs and kidneys with characteristic hamartomatous lesions (50, 57). The classic TSC triad, known as Vogt’s triad, includes mental retardation, seizures, and facial angiofibromas; the diagnostic criteria for TSC include the presence of two major features, or one major and two minor features (57). TSC is associated with an increased risk of benign as well as malignant tumors, especially in the kidneys, brain, and soft tissues. The relative risk of malignancy in children with TSC is approximately 18-fold higher than the general population. Although neuroendocrine tumors have been reported in patients with TSC, they are not currently considered one of the major features TSC (58, 59). A number of case reports have documented endocrine tumors in patients with TSC, and a recent systematic review summarized the available articles on this association (50). Pituitary tumors have been reported in four patients with TSC, including adrenocorticotropic hormone, growth hormone, and prolactin secreting tumors (50, 58, 59). Both parathyroid adenoma and parathyroid hyperplasia have been reported. Rarely, pancreatic endocrine tumors including gastrinoma and insulinomas have also been described in TSC; there is also a report of a pheochromocytoma and a carcinoid occurring together with TSC (50). Molecular testing is widely available for TSC today (50, 60) as well as therapeutic trials of using TSC molecular pathway inhibitors (61).
CONCLUSION
Hereditary disorders associated with endocrine tumors range from the classic MEN syndromes to the neurocutaneous and phacomatoses conditions. In each of these disorders, skin manifestations are essential in diagnosing the disorder.
Acknowledgments
ACKNOWLEDGEMENTS/Funding
This review was supported by the research project Z01-HD008920 (Principal Investigator: Dr. Constantine A Stratakis) of the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), National Institutes of Health (NIH), Bethesda, MD, USA.
Footnotes
Compliance with ethical standards
Conflict of interest
The author declares that he has no conflict of interest.
REFERENCES
- 1.Chandrasekharappa SC, Guru SC, Manickam P, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997 Apr 18;276(5311):404–407. doi: 10.1126/science.276.5311.404. [DOI] [PubMed] [Google Scholar]
- 2.Marx SJ, Agarwal SK, Kester MB, et al. Multiple endocrine neoplasia type 1: clinical and genetic features of the hereditary endocrine neoplasias. Recent progress in hormone research. 1999;54:397–438. discussion 438-399. [PubMed] [Google Scholar]
- 3.Vasen HF, Lamers CB, Lips CJ. Screening for the multiple endocrine neoplasia syndrome type I. A study of 11 kindreds in The Netherlands. Archives of internal medicine. 1989 Dec;149(12):2717–2722. [PubMed] [Google Scholar]
- 4.Marx S, Spiegel AM, Skarulis MC, Doppman JL, Collins FS, Liotta LA. Multiple endocrine neoplasia type 1: clinical and genetic topics. Annals of internal medicine. 1998 Sep 15;129(6):484–494. doi: 10.7326/0003-4819-129-6-199809150-00011. [DOI] [PubMed] [Google Scholar]
- 5.Stratakis CA, Schussheim DH, Freedman SM, et al. Pituitary macroadenoma in a 5-year-old: an early expression of multiple endocrine neoplasia type 1. The Journal of clinical endocrinology and metabolism. 2000 Dec;85(12):4776–4780. doi: 10.1210/jcem.85.12.7064. [DOI] [PubMed] [Google Scholar]
- 6.Vashi N, Hunt R, Fischer M, Meehan S, Pomeranz MK. Angiofibromas in multiple endocrine neoplasia type 1. Dermatol Online J. 2012 Dec 15;18(12):20. [PubMed] [Google Scholar]
- 7.Saggini A, Brandi ML. Skin lesions in hereditary endocrine tumor syndromes. Endocr Pract. 2011 Jul-Aug;17(Suppl 3):47–57. doi: 10.4158/EP11055.RA. [DOI] [PubMed] [Google Scholar]
- 8.Asgharian B, Turner ML, Gibril F, Entsuah LK, Serrano J, Jensen RT. Cutaneous tumors in patients with multiple endocrine neoplasm type 1 (MEN1) and gastrinomas: prospective study of frequency and development of criteria with high sensitivity and specificity for MEN1. J Clin Endocrinol Metab. 2004 Nov;89(11):5328–5336. doi: 10.1210/jc.2004-0218. [DOI] [PubMed] [Google Scholar]
- 9.Sakurai A, Matsumoto K, Ikeo Y, Nishio SI, Kakizawa T, Arakura F, Ishihara Y, Saida T, Hashizume K. Frequency of facial angiofibromas in Japanese patients with multiple endocrine neoplasia type 1. Endocr J. 2000 Oct;47(5):569–573. doi: 10.1507/endocrj.47.569. [DOI] [PubMed] [Google Scholar]
- 10.Darling TN, Skarulis MC, Steinberg SM, Marx SJ, Spiegel AM, Turner M. Multiple facial angiofibromas and collagenomas in patients with multiple endocrine neoplasia type 1. Arch Dermatol. 1997 Jul;133(7):853–857. [PubMed] [Google Scholar]
- 11.Pack S, Turner ML, Zhuang Z, Vortmeyer AO, Böni R, Skarulis M, Marx SJ, Darling TN. Cutaneous tumors in patients with multiple endocrine neoplasia type 1 show allelic deletion of the MEN1 gene. J Invest Dermatol. 1998 Apr;110(4):438–440. doi: 10.1046/j.1523-1747.1998.00140.x. [DOI] [PubMed] [Google Scholar]
- 12.Darling TN, Skarulis MC, Steinberg SM, Marx SJ, Spiegel AM, Turner M. Multiple facial angiofibromas and collagenomas in patients with multiple endocrine neoplasia type 1. Arch Dermatol. 1997 Jul;133(7):853–857. [PubMed] [Google Scholar]
- 13.Baldauf C, Vortmeyer AO, Koch CA, Sticherling M. Combination of multiple skin malignancies with multiple endocrine neoplasia type 1: coincidental or pathogenetically related? Dermatology. 2009;219(4):365–367. doi: 10.1159/000193058. [DOI] [PubMed] [Google Scholar]
- 14.Thakker RV. Multiple endocrine neoplasia type 1 (MEN1) Best Pract Res Clin Endocrinol Metab. 2010 Jun;24(3):355–370. doi: 10.1016/j.beem.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Almeida MQ, Stratakis CA. Solid tumors associated with multiple endocrine neoplasias. Cancer Genet Cytogenet. 2010 Nov;203(1):30–36. doi: 10.1016/j.cancergencyto.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schernthaner-Reiter MH, Trivellin G, Stratakis CA. MEN1, MEN4, and Carney Complex: Pathology and Molecular Genetics. Neuroendocrinology. 2016;103(1):18–31. doi: 10.1159/000371819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gagel RF. Ret protooncogene mutations and endocrine neoplasia--a story intertwined with neural crest differentiation. Endocrinology. 1996 May;137(5):1509–1511. doi: 10.1210/endo.137.5.8612478. [DOI] [PubMed] [Google Scholar]
- 18.Eng C, Clayton D, Schuffenecker I, et al. The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. JAMA. 1996 Nov 20;276(19):1575–1579. [PubMed] [Google Scholar]
- 19.Edery P, Eng C, Munnich A, Lyonnet S. RET in human development and oncogenesis. BioEssays : news and reviews in molecular, cellular and developmental biology. 1997 May;19(5):389–395. doi: 10.1002/bies.950190506. [DOI] [PubMed] [Google Scholar]
- 20.Gagel RF, Levy ML, Donovan DT, Alford BR, Wheeler T, Tschen JA. Multiple endocrine neoplasia type 2a associated with cutaneous lichen amyloidosis. Annals of internal medicine. 1989 Nov 15;111(10):802–806. doi: 10.7326/0003-4819-111-10-802. [DOI] [PubMed] [Google Scholar]
- 21.Borrego S, Eng C, Sanchez B, Saez ME, Navarro E, Antinolo G. Molecular analysis of the ret and GDNF genes in a family with multiple endocrine neoplasia type 2A and Hirschsprung disease. The Journal of clinical endocrinology and metabolism. 1998 Sep;83(9):3361–3364. doi: 10.1210/jcem.83.9.5093. [DOI] [PubMed] [Google Scholar]
- 22.Eng C, Mulligan LM. Mutations of the RET proto-oncogene in the multiple endocrine neoplasia type 2 syndromes, related sporadic tumours, and hirschsprung disease. Human mutation. 1997;9(2):97–109. doi: 10.1002/(SICI)1098-1004(1997)9:2<97::AID-HUMU1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 23.Hoff AO, Cote GJ, Gagel RF. Multiple endocrine neoplasias. Annu Rev Physiol. 2000;62:377–411. doi: 10.1146/annurev.physiol.62.1.377. [DOI] [PubMed] [Google Scholar]
- 24.Angrist M, Bolk S, Halushka M, Lapchak PA, Chakravarti A. Germline mutations in glial cell line-derived neurotrophic factor (GDNF) and RET in a Hirschsprung disease patient. Nat Genet. 1996 Nov;14(3):341–344. doi: 10.1038/ng1196-341. [DOI] [PubMed] [Google Scholar]
- 25.Decker RA, Peacock ML, Watson P. Hirschsprung disease in MEN 2A: increased spectrum of RET exon 10 genotypes and strong genotype-phenotype correlation. Hum Mol Genet. 1998 Jan;7(1):129–134. doi: 10.1093/hmg/7.1.129. [DOI] [PubMed] [Google Scholar]
- 26.Koch CA. Molecular pathogenesis of MEN2-associated tumors. Fam Cancer. 2005;4(1):3–7. doi: 10.1007/s10689-004-7022-3. [DOI] [PubMed] [Google Scholar]
- 27.Brauer VF, Scholz GH, Neumann S, Lohmann T, Paschke R, Koch CA. RET germline mutation in codon 791 in a family representing 3 generations from age 5 to age 70 years: should thyroidectomy be performed? Endocr Pract. 2004 Jan-Feb;10(1):5–9. doi: 10.4158/EP.10.1.5. [DOI] [PubMed] [Google Scholar]
- 28.Scapineli JO, Ceolin L, Puñales MK, Dora JM, Maia AL. MEN 2A-related cutaneous lichen amyloidosis: report of three kindred and systematic literature review of clinical, biochemical and molecular characteristics. Fam Cancer. 2016 doi: 10.1007/s10689-016-9892-6. [DOI] [PubMed] [Google Scholar]; Rodriguez FJ, Stratakis CA, Evans DG. Genetic predisposition to peripheral nerve neoplasia: diagnostic criteria and pathogenesis of neurofibromatoses, Carney complex, and related syndromes. Acta Neuropathol. 2012 Mar;123(3):349–367. doi: 10.1007/s00401-011-0935-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verga U, Fugazzola L, Cambiaghi S, Pritelli C, Alessi E, Cortelazzi D, Gangi E, Beck-Peccoz P. Frequent association between MEN 2A and cutaneous lichen amyloidosis. Clin Endocrinol (Oxf) 2003 Aug;59(2):156–161. doi: 10.1046/j.1365-2265.2003.01782.x. [DOI] [PubMed] [Google Scholar]
- 30.Lodish MB, Stratakis CA. RET oncogene in MEN2, MEN2B, MTC and other forms of thyroid cancer. Expert Rev Anticancer Ther. 2008 Apr;8(4):625–632. doi: 10.1586/14737140.8.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boikos SA, Stratakis CA. Molecular mechanisms of medullary thyroid carcinoma: current approaches in diagnosis and treatment. Histol Histopathol. 2008 Jan;23(1):109–116. doi: 10.14670/HH-23.109. [DOI] [PubMed] [Google Scholar]
- 32.Lodish M, Dagalakis U, Chen CC, Sinaii N, Whitcomb P, Aikin A, Dombi E, Marcus L, Widemann B, Fox E, Chuk M, Balis F, Wells S, Jr, Stratakis CA. (111)In-octreotide scintigraphy for identification of metastatic medullary thyroid carcinoma in children and adolescents. J Clin Endocrinol Metab. 2012 Feb;97(2):E207–E212. doi: 10.1210/jc.2011-2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nella AA, Lodish MB, Fox E, Balis FM, Quezado MM, Whitcomb PO, Derdak J, Kebebew E, Widemann BC, Stratakis CA. Vandetanib successfully controls medullary thyroid cancer-related Cushing syndrome in an adolescent patient. J Clin Endocrinol Metab. 2014 Sep;99(9):3055–3059. doi: 10.1210/jc.2013-4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lodish MB, Stratakis CA. The differential diagnosis of familial lentiginosis syndromes. Fam Cancer. 2011 Sep;10(3):481–490. doi: 10.1007/s10689-011-9446-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sammour T, Hayes IP, Hill AG, Macrae FA, Winter DC. Familial colorectal cancer syndromes: an overview of clinical management. Expert Rev Gastroenterol Hepatol. 2015 Jun;9(6):757–764. doi: 10.1586/17474124.2015.1026328. [DOI] [PubMed] [Google Scholar]
- 36.Beggs AD, Latchford AR, Vasen HF, Moslein G, Alonso A, Aretz S, Bertario L, Blanco I, Bülow S, Burn J, Capella G, Colas C, Friedl W, Møller P, Hes FJ, Järvinen H, Mecklin JP, Nagengast FM, Parc Y, Phillips RK, Hyer W, Ponz de Leon M, Renkonen-Sinisalo L, Sampson JR, Stormorken A, Tejpar S, Thomas HJ, Wijnen JT, Clark SK, Hodgson SV. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010 Jul;59(7):975–986. doi: 10.1136/gut.2009.198499. [DOI] [PubMed] [Google Scholar]
- 37.van Lier MG, Westerman AM, Wagner A, Looman CW, Wilson JH, de Rooij FW, Lemmens VE, Kuipers EJ, Mathus-Vliegen EM, van Leerdam ME. High cancer risk and increased mortality in patients with Peutz-Jeghers syndrome. Gut. 2011 Feb;60(2):141–147. doi: 10.1136/gut.2010.223750. [DOI] [PubMed] [Google Scholar]
- 38.Gondak RO, da Silva-Jorge R, Jorge J, Lopes MA, Vargas PA. Oral pigmented lesions: Clinicopathologic features and review of the literature. Med Oral Patol Oral Cir Bucal. 2012 Nov 1;17(6):e919–e924. doi: 10.4317/medoral.17679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richey JD, Bradish JR, Lacy SR, Warren S. Carney syndrome in a patient previously considered to have Peutz-Jeghers syndrome. J Am Acad Dermatol. 2014 Feb;70(2):e44–e46. doi: 10.1016/j.jaad.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 40.Bauer AJ, Stratakis CA. The lentiginoses: cutaneous markers of systemic disease and a window to new aspects of tumourigenesis. J Med Genet. 2005 Nov;42(11):801–810. doi: 10.1136/jmg.2003.017806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hearle N, Schumacher V, Menko FH, Olschwang S, Boardman LA, Gille JJ, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res. 2006 May 15;12(10):3209–3215. doi: 10.1158/1078-0432.CCR-06-0083. [DOI] [PubMed] [Google Scholar]
- 42.Stratakis CA. Genetics of Carney complex and related familial lentiginoses, and other multiple tumor syndromes. Front Biosci. 2000 Mar 1;5:D353–D366. doi: 10.2741/stratakis. [DOI] [PubMed] [Google Scholar]
- 43.Winterfield L, Schultz J, Stratakis CA, Cowen EW. Gynecomastia and mucosal lentigines in an 8-year-old boy. J Am Acad Dermatol. 2005 Oct;53(4):660–662. doi: 10.1016/j.jaad.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 44.Gourgari E, Saloustros E, Stratakis CA. Large-cell calcifying Sertoli cell tumors of the testes in pediatrics. Curr Opin Pediatr. 2012 Aug;24(4):518–522. doi: 10.1097/MOP.0b013e328355a279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crocker MK, Gourgari E, Lodish M, Stratakis CA. Use of aromatase inhibitors in large cell calcifying sertoli cell tumors: effects on gynecomastia, growth velocity, and bone age. J Clin Endocrinol Metab. 2014 Dec;99(12):E2673–E2680. doi: 10.1210/jc.2014-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mester J, Eng C. Cowden syndrome: recognizing and managing a not-so-rare hereditary cancer syndrome. J Surg Oncol. 2015 Jan;111(1):125–130. doi: 10.1002/jso.23735. [DOI] [PubMed] [Google Scholar]
- 47.Pilarski R. Cowden syndrome: a critical review of the clinical literature. J Genet Couns. 2009 Feb;18(1):13–27. doi: 10.1007/s10897-008-9187-7. [DOI] [PubMed] [Google Scholar]
- 48.Farooq A, Walker LJ, Bowling J, Audisio RA. Cowden syndrome. Cancer Treat Rev. 2010 Dec;36(8):577–583. doi: 10.1016/j.ctrv.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 49.Yin Y, Shen WH. PTEN: a new guardian of the genome. Oncogene. 2008 Sep 18;27(41):5443–5453. doi: 10.1038/onc.2008.241. [DOI] [PubMed] [Google Scholar]
- 50.Lodish MB, Stratakis CA. Endocrine tumours in neurofibromatosis type 1, tuberous sclerosis and related syndromes. Best Pract Res Clin Endocrinol Metab. 2010 Jun;24(3):439–449. doi: 10.1016/j.beem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rasmussen SA, Friedman JM. NF1 gene and neurofibromatosis 1. Am J Epidemiol. 2000 Jan 1;151(1):33–40. doi: 10.1093/oxfordjournals.aje.a010118. [DOI] [PubMed] [Google Scholar]
- 52.Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol. 1988 May;45(5):575–578. [PubMed] [Google Scholar]
- 53.Zoller ME, Rembeck B, Oden A, Samuelsson M, Angervall L. Malignant and benign tumors in patients with neurofibromatosis type 1 in a defined Swedish population. Cancer. 1997 Jun 1;79(11):2125–2131. [PubMed] [Google Scholar]
- 54.Mao C, Shah A, Hanson DJ, Howard JM. Von Recklinghausen's disease associated with duodenal somatostatinoma: contrast of duodenal versus pancreatic somatostatinomas. J Surg Oncol. 1995 May;59(1):67–73. doi: 10.1002/jso.2930590116. [DOI] [PubMed] [Google Scholar]
- 55.Hersh JH. Health supervision for children with neurofibromatosis. Pediatrics. 2008 Mar;121(3):633–642. doi: 10.1542/peds.2007-3364. [DOI] [PubMed] [Google Scholar]
- 56.Osborne JP, Fryer A, Webb D. Epidemiology of tuberous sclerosis. Ann N Y Acad Sci. 1991;615:125–127. doi: 10.1111/j.1749-6632.1991.tb37754.x. [DOI] [PubMed] [Google Scholar]
- 57.Rosser T, Panigrahy A, McClintock W. The diverse clinical manifestations of tuberous sclerosis complex: a review. Semin Pediatr Neurol. 2006 Mar;13(1):27–36. doi: 10.1016/j.spen.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 58.Dworakowska D, Grossman AB. Are neuroendocrine tumours a feature of tuberous sclerosis? A systematic review. Endocr Relat Cancer. 2009 Mar;16(1):45–58. doi: 10.1677/ERC-08-0142. [DOI] [PubMed] [Google Scholar]
- 59.Nandagopal R, Vortmeyer A, Oldfield EH, Keil MF, Stratakis CA. Cushing's syndrome due to a pituitary corticotropinoma in a child with tuberous sclerosis: an association or a coincidence? Clin Endocrinol (Oxf) 2007 Oct;67(4):639–641. doi: 10.1111/j.1365-2265.2007.02941.x. [DOI] [PubMed] [Google Scholar]
- 60.Roach ES, Sparagana SP. Diagnosis of tuberous sclerosis complex. J Child Neurol. 2004 Sep;19(9):643–649. doi: 10.1177/08830738040190090301. [DOI] [PubMed] [Google Scholar]
- 61.Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008 Jan 10;358(2):140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]