Abstract
Carbohydrates are complex macromolecules in biological metabolism. Enzymatic synthesis of carbohydrates is recognized as a powerful tool to overcome the problems associated with large scale synthesis of carbohydrates. Novel enzymes with significant transglycosylation ability are still in great demand in glycobiology studies. Here we report a novel glycoside hydrolase family 16 “elongating” β-transglycosylase from Paecilomyces thermophila (PtBgt16A), which efficiently catalyzes the synthesis of higher polymeric oligosaccharides using β-1,3/1,4-oligosaccharides as donor/acceptor substrates. Further structural information reveals that PtBgt16A has a binding pocket around the −1 subsite. The catalytic mechanism of PtBgt16A is partly similar to an exo-glycoside hydrolase, which cleaves the substrate from the non-reducing end one by one. However, PtBgt16A releases the reducing end product and uses the remainder glucosyl as a transglycosylation donor. This catalytic mechanism has similarity with the catalytic mode of amylosucrase, which catalyzes the transglycosylation products gradually extend by one glucose unit. PtBgt16A thus has the potential to be a tool enzyme for the enzymatic synthesis of new β-oligosaccharides and glycoconjugates.
Keywords: enzyme catalysis, enzyme mechanism, enzyme structure, glycoside hydrolase, protein crystallization
Introduction
Carbohydrates and their derivatives are ubiquitous in nature and play vital roles in many biological systems (1, 2). Therefore, the synthesis of carbohydrate-based compounds is of considerable interest for both research and commercial purposes (3–6). In organisms, glycosidic linkages are mainly synthesized by Leloir glycosyltransferases. Leloir glycosyltransferases have experienced notable progress as synthetic tools but are still far from being a general preparative methodology due to the difficulties of recombinant expression and purification of these often membrane proteins and their limited stability in cell-free systems (7). Advantageously for chemists, other kinds of synthetic tools named non-Leloir transglycosylases are readily available and stable and have been demonstrated to be effective catalysts for carbohydrate synthesis (8). Nevertheless, non-Leloir transglycosylases are unusual glycoside hydrolases (GHs)3 as they efficiently catalyze the formation of glycosidic bonds, whereas most GHs favor the mechanistically related hydrolysis of oligo- and polysaccharides (3, 8).
A large number of retaining GHs catalyze both hydrolysis and transglycosylation reactions, but little is known about how the balance between these two activities (transglycosylation/hydrolysis ratio) is determined (9). Some of them display interesting transglycosylation (10, 11). The beneficial properties of transglycosylation by GHs have encouraged researchers to improve these properties via different strategies (12, 13). One of these strategies is based on the basis of substitution of the catalytic nucleophile with a neutral amino acid, which forms new enzymes called glycosynthases (14). They are inactive GHs mutants but efficiently catalyze glycoside bond formation with glycosyl fluoride donors and an acceptor. Another strategy is directed evolution or molecular modification, which modulates the function of transglycosylation of GHs to improve the activity or specificity of transglycosylation (9, 15, 16). Thus, the original starting non-Leloir transglycosylases are particularly important. Novel non-Leloir transglycosylases with high transglycosylation ability and desirable properties are still in great demand.
β-1,3-Glucan derivatives (oligo-, polysaccharides, and glycoconjugates) are important carbohydrates in immunization, cellular metabolism, and other physiological functions (17, 18). Thus enzymatic synthesis of β-1,3-glucan derivatives is valuable in glycobiology (13, 19). GH family 16 enzymes are retaining enzymes. They utilize a covalent glycosyl-enzyme intermediate, which is broken-down by glycosyl transfer to water or a carbohydrate acceptor substrate in hydrolysis or transglycosylation reaction, respectively (3). Many GH family 16 members are active toward β-1,4- or β-1,3-glycosidic bonds in various glucans (20–22). Some GH family 16 members indeed are transglycosylases toward β-1,3-glycosidic bonds (named chitin β-1,3/1,6-glucanosyltransferase) (23). PtBgt16A is a novel GH family 16 member from Paecilomyces thermophila. Sequence alignment predicted that PtBgt16A may be active toward β-glucan. However, an enzymatic assay showed that PtBgt16A is a novel “elongating” β-transglycosylase, which exhibited transglycosylation activity to synthesize higher polymeric oligosaccharides. The unique elongating catalytic mechanism was further revealed by structural and functional experiments. To our knowledge, the catalytic properties of PtBgt16A are different from any other transglycosylases in glycoside hydrolases. Thus, these results provide new information about non-Leloir transglycosylases from GHs.
Results
Gene Cloning and Sequence Analysis
The PtBgt16A-full protein was predicted to be anchored in the outer membrane because of the presence of a lipoprotein signal peptide and a transmembrane architecture (Fig. 1A). In the N-terminal region of PtBgt16A-full protein, the signal peptide is followed by a GH family 16 catalytic module (PtBgt16A). The C-terminal region of PtBgt16A-full protein is composed of a transmembrane region (Fig. 1A). Within the GH family 16 members, two conserved glutamates in the pattern EXDX(X)E play the role of the catalytic residues (20–22). In PtBgt16A, the equivalent to the nucleophile is Glu117, whereas the general acid/base is Glu122 (Fig. 1B). To study the enzymatic properties of this GH family 16 catalytic module without potential interference from the signal peptide and the transmembrane region and to facilitate crystallization assays, we decided to clone the nucleotide sequence corresponding to the GH family 16 catalytic module. The recombinant protein (PtBgt16A) was purified by one step of immobilized metal ion affinity chromatography. The recombinant PtBgt16A migrated as a single band with molecular mass of 32 kDa on SDS-PAGE (Fig. 2).
FIGURE 1.
Sequence analysis of PtBgt16A. A, domain analysis of PtBgt16A-full full-length protein. B, structural sequence alignment of some GH family 16 members. Identical residues are shown in white on a red background, and conservative residues are shown in red on a white background. Two catalytic glutamic acid residues, Glu117 and Glu122, are marked by red dots. The key residue, Trp112, of PtBgt16A is marked by a red star. Four disulfide bonds of PtBgt16A are marked by green numbers underneath the relative residues. The sequences of PtBgt16A, P. thermophila β-1,3-1,4-glucanase (PtLic16A; Protein Data Bank code 3WDT), P. chrysosporium β-1,3(4)-glucanase (PcLam16A; Protein Data Bank code 2CL2), Zobellia galactanivorans β-1,3-glucanase (ZgLamCGH16; Protein Data Bank code 4CRQ), Rhodothermus marinus β-1,3-glucanase (RmLamR; Protein Data Bank code 3ILN), and Nocardiopsis sp. β-1,3-glucanase (BglF; Protein Data Bank code 2HYK) were aligned using T-Coffee (43), and the figure was produced using ESPript (44).
FIGURE 2.

SDS-PAGE of proteins during purification of the recombinant PtBgt16A by nickel-iminodiacetic acid. Lane M, standard protein molecular weight markers; lane 1, supernatant of lysate cells; lane 2, purified enzyme.
Enzymatic Identification
As PtBgt16A showed about 40% amino acid sequence identity with several GH family 16 β-glucanases (22), we first considered that PtBgt16A was a β-1,3-glucanase or β-1,3-1,4-glucanase. The hydrolytic activity of the purified PtBgt16A was screened by the dinitrosalicylic acid method using different β-glucan substrates, viz. β-1,3-glucans (laminarin, curdlan, and yeast β-glucan), β-1,3-1,4-glucans (lichenin, barley β-glucan, and oat β-glucan), and β-1,4-glucan (carboxymethylcellulose). However, no activity was detected toward all tested β-glucan substrates, which indicated that PtBgt16A was not a β-glucanase. To verify the catalytic ability of PtBgt16A, some natural polysaccharides (locust bean gum, birchwood xylan, pullulan, chitin, and soluble starch) and artificial substrates (pNP-β-xylopyranoside, oNP-β-galactopyranoside, pNP-α-galactopyranoside, pNP-α-glucopyranoside, and pNP-β-glucopyranoside) were further determined. Similarly, no activity was detected toward all tested substrates. In consideration of the sequence similarity, the potential activity of PtBgt16A toward different β-1,3-oligosaccharides (degree of polymerization (DP), 2–6) and β-1,4-oligosaccharides (DP, 2–5) was further investigated (Fig. 3, A and B). Surprisingly, significant activity was detected in the presence of PtBgt16A toward β-1,3-oligosaccharides and β-1,4-oligosaccharides. Thin-layer chromatography (TLC) analysis indicated that PtBgt16A could cleave the β-1,3/1,4 linkage in the oligosaccharides and meanwhile synthesize a series of new higher polymeric oligosaccharides. The amount of newly generated oligosaccharide was higher than glucose, which implied that PtBgt16A is a transglycosylase rather than a hydrolase.
FIGURE 3.
Catalytic ability of PtBgt16A. TLC analysis of products hydrolyzed by PtBgt16A toward laminarioligosaccharides (A) and cello-oligosaccharides (B) is shown. M, marker; G, glucose; L2–L6, laminaribiose, laminaritriose, laminaritetraose, laminaripentaose, and laminarihexaose, respectively; C2–C5, cellobiose, cellotriose, cellotetraose, and cellopentose, respectively. Left, before reaction; right, after reaction. MALDI-TOF MS analysis of transglycosylation reaction products by PtBgt16A toward laminaritriose (C) and cellotriose (D) is shown. The peaks in the spectra correspond to the monoisotopic masses of sodium adducts [M + Na]+ of the oligosaccharides. a.u., arbitrary units.
Catalytic Property of PtBgt16A
To determine the molecular masses of transglycosidation products, the products from laminaritetraose and cellotetraose were analyzed by matrix-assisted laser desorption ionization/time-of-flight mass spectrometry (MALDI-TOF MS) (Fig. 3, C and D). Results indicated that PtBgt16A catalyzed the transglycosylation of laminaritetraose and cellotetraose to form a series of oligosaccharides. The main components were hexaose, heptaose and octaose, and the highest DPs of oligosaccharides detected were 16 and 21. The DP of oligosaccharides was continuous, which implied that the transglycosylation products by PtBgt16A may elongate by one glucose residue.
The transglycosylation reaction courses of PtBgt16A were further investigated by analyzing the products from the β-1,3-oligosaccharides, including laminaritriose, laminaritetraose, laminaripentaose, and laminarihexaose (Fig. 4). TLC analysis suggested that PtBgt16A could cleave the β-1,3 linkage in β-1, 3-oligosaccharides and form the new linkage at the same time. Whereas the content of substrates decreased quickly, the hydrolytic products were not accumulated. Most of the β-1,3-oligosaccharides substrates were formed into various higher polymeric oligosaccharides after 2-h incubation. The above mentioned results suggested that PtBgt16A was an elongating transglycosylase but not a hydrolase. The unique catalytic property of PtBgt16A is different from the known GH family 16 members and other glycoside hydrolases.
FIGURE 4.

TLC analysis of transglycosylation reaction course by PtBgt16A. Purified PtBgt16A (1 unit/ml) was added to 1% (w/v) laminarioligosaccharides in 50 mm sodium acetate buffer, pH 5.5, and then incubated at 50 °C for 2 h. M, marker sugars; G1, glucose; L2–L6, laminaribiose, laminaritriose, laminaritetraose, laminaripentaose, and laminarihexaose, respectively.
Characterization and Substrate Specificity of PtBgt16A
The effect of pH and temperature on the transglycosylase activity of PtBgt16A was determined using laminaritriose. The enzyme displayed maximal transglycosylase activity at pH 4.5 in 50 mm sodium acetate buffer (Fig. 5A). It was stable within the range of pH 4.5–8.0, retaining more than 80% of its initial activity (Fig. 5B). PtBgt16A exhibited optimal activity at 60 °C and retained over 50% of its initial activity between 55 and 70 °C (Fig. 5C). The enzyme was stable up to 65 °C (Fig. 5D).
FIGURE 5.
The effects of temperature and pH on activity and stability of the purified PtBgt16A. A, optimal pH. B, pH stability. C, optimal temperature. D, thermostability. The optimal pH was determined in different buffers, including McIlvaine buffer (■), sodium acetate buffer (●), Tris-HCl buffer (▴), and glycine-NaOH buffer (▾). Laminaritriose was used as substrate to determine the enzymatic characterization of PtBgt16A.
The specific activity of PtBgt16A toward different substrates was determined by high performance anion exchange chromatography (HPAEC) (Table 1). PtBgt16A showed significantly higher transglycosylase activity toward the β-1,3-oligosaccharides than other tested oligosaccharides. Among the β-1,3-oligosaccharides tested, PtBgt16A exhibited highest specific activity toward laminaripentaose (776.2 units/mg) but showed no activities toward laminaribiose. PtBgt16A also exhibited low activity toward β-1,4-oligosaccharides (6.2–27.9 units/mg) except cellobiose. No activity was detected toward manno-oligosaccharides, xylo-oligosaccharides, malto-oligosaccharides, chitin oligosaccharides, raffinose, and stachyose. These results suggested that PtBgt16A was a β-transglycosylase. The appropriate substrates were β-1,3/1,4-linked gluco-oligosaccharides with a minimum DP of 3.
TABLE 1.
Substrate specificity of PtBgt16A
| Specific activitya | Relative activityb | |
|---|---|---|
| units/mg | % | |
| Laminaribiose | —c | 0 |
| Laminaritriose | 106.7 ± 2.3 | 13.7 |
| Laminaritetraose | 456.7 ± 15.2 | 58.8 |
| Laminaripentaose | 776.2 ± 26.1 | 100 |
| Laminarihexaose | 574.3 ± 16.8 | 74.0 |
| Cellobiose | — | 0 |
| Cellotriose | 6.2 ± 0.16 | 0.8 |
| Cellotetrose | 24.9 ± 0.77 | 3.2 |
| Cellopentose | 27.9 ± 2.02 | 3.6 |
| Manno-oligosaccharides | — | 0 |
| Xylo-oligosaccharides | — | 0 |
| Malto-oligosaccharides | — | 0 |
| Chitin oligosaccharides | — | 0 |
| Raffinose | — | 0 |
| Stachyose | — | 0 |
a Data represent the means ± S.D. of three independent experiments (n = 3).
b Relative activity indicates the ratio between specific activity and highest activity.
c No activity was detected.
NMR Analysis of the Reaction Products
TLC analysis showed that the transglycosylation products by PtBgt16A are a series of mixed oligosaccharides. To confirm the generated glycosidic bonds of the transglycosylation products, the reaction products by PtBgt16A toward laminaritriose and cellotriose were analyzed by two-dimensional NMR spectroscopy (HSQC and HMBC) (Fig. 6). The well resolved anomeric correlations (C1/H1: 101.88–103.11 ppm/4.64–4.72 ppm) of the associated carbohydrates were detected in the HSQC spectrum of cellotriose transglycosylation products. The above 1H chemical shift of H1 was then identified in HMBC spectrum cross-signals (C/H1: 83.38–84.52 ppm/4.64–4.70 ppm). The corresponding 13C NMR spectrum of associated carbohydrates displayed a characteristic chemical shift at 84 ppm (Fig. 6A), which indicated that transglycosylation products of cellotriose contain β-1,3 linkages. In the meantime, the characteristic chemical shift at C/H of 78.00–78.86 ppm/4.40–4.47 ppm (Fig. 6A) was identified in the HMBC spectrum, which indicated that β-1,4 linkages also existed in the transglycosylation products of cellotriose by PtBgt16A. Two-dimensional NMR spectroscopy (HSQC and HMBC) of transglycosylation products by PtBgt16A toward laminaritriose showed an analogous phenomenon. The well resolved characteristic chemical shift at C/H of 78.03–78.91 ppm/4.40–4.47 ppm indicated that β-1,4 linkages were produced in the transglycosylation products of laminaritriose by PtBgt16A, although β-1,3 linkages (C/H: 83.38.00–85.11 ppm/4.63–4.69 ppm) also existed in the transglycosylation products of laminaritriose by PtBgt16A (Fig. 6B). These results suggested that the transglycosylation products by PtBgt16A were mixed oligosaccharides containing β-1,3 linkages and β-1,4 linkages. Thus, the glycosidic bonds that formed during the transglycosylation reaction by PtBgt16A were mixed β-1,3 linkages and β-1,4 linkages.
FIGURE 6.
Two-dimensional NMR data (HMBC) of transglycosylation products by PtBgt16A. A, the enlarged picture of HMBC spectra of cellotriose before (1) and after (2) reaction. B, the enlarged picture of HMBC spectra of laminaritriose before (1) and after (2) reaction. Characteristic chemical shifts occurred at 84 and 78 ppm, indicating a β-1,3 and β-1,4 linkage in the transglycosylation products, respectively. Transglycosylation reaction (30 ml) was performed with PtBgt16A (1 unit/ml) and 1% (w/v) laminaritriose or cellotriose in 50 mm sodium acetate buffer, pH 5.5, at 50 °C for 2 h.
Crystal Structure of PtBgt16A
To understand the elongating catalytic mechanism of PtBgt16A, the crystal structure of PtBgt16A was determined. The crystal structure of PtBgt16A was determined at 1.59-Å resolution in space group P1211. The Rwork/Rfree was 14.87%/17.89% (Table 2). The asymmetric unit contained two monomer models. The protein monomer, with approximate dimensions of 50 × 445 × 35 Å, exhibited single domain architecture and consisted of residues 3–299. The overall structure of PtBgt16A is presented in Fig. 7A. The overall fold of PtBgt16A could be defined as a classical sandwich-like β-jelly roll structure in which all the strands are connected by loops and α-helices. The sandwich was formed by the face-to-face packing of two antiparallel sheets containing seven and eight strands in the order of β1-β18-β8-β14-β15-β16 and β2-β7-β17-β9-β10-β11-β13-β12, respectively. Both β-sheets were twisted and bent, forming a convex and a concave side of the molecule. The protein featured a roughly V-shaped groove with structural homology to other GH family 16 members (22, 24). The catalytic cleft contained the catalytic proton donor and nucleophile (Glu122 and Glu117, respectively) as in previous studies (20, 22). Some hydrophobic residues were found arranged along the inner catalytic cleft. This central groove, ∼15 Å in length and 5 Å in depth, lies between α2 and α3 at the N terminus of the protein and between α5 and α6 at its C terminus.
TABLE 2.
X-ray data collection and refinement statistics of PtBgt16A
Values in parentheses represent the data at the highest resolution shell. r.m.s.d., root mean square deviation; SSRF, Shanghai Synchrotron Radiation Facility.
| PtBgt16A | |
|---|---|
| Data collection statistics | |
| Radiation source | SSRF-BL19U1 |
| Wavelength (Å) | 0.9792 |
| Temperature of measurements (K) | 100 |
| Resolution (Å) | 37.17–1.59 (1.65–1.59) |
| Space group | P1211 |
| Unit cell parameters | |
| a, b, c (Å) | a = 45.3, b = 91.5, c = 59.5 |
| α, β, γ (°) | α = 90, β = 93.8, γ = 90 |
| Protein molecules in asymmetric unit | 2 |
| Unique reflections | 62,905 (6,124) |
| Completeness (%) | 96.6 (94.1) |
| Rmergea (%) | 9.0 (30.3) |
| Mean I/σ(I) | 17.93 (6.11) |
| Wilson B-factor (Å2) | 14.51 |
| Refinement statistics | |
| Resolution (Å) | 1.59 |
| Rworkb (%) | 14.87 (15.34) |
| Rfreeb (%) | 17.89 (22.75) |
| No. residues | 594 |
| No. water molecules | 699 |
| No. atoms | 5,297 |
| r.m.s.d. | |
| Bond lengths (Å) | 0.007 |
| Bond angles (°) | 1.17 |
| Average B-factors (Å2) | 17.50 |
| Macromolecules | 15.60 |
| Solvent | 25.20 |
| Ramachandran | |
| Most favored regions (%) | 97.17 |
| Additional allowed regions (%) | 2.83 |
| Disallowed regions (%) | 0 |
| Clashscore | 2.25 |
| Protein Data Bank code | 5JVV |
a ΣhklΣi|Ii(hkl) − 〈I(hkl)〉|/ΣhklΣiIi(hkl) where Ii(hkl) is the ith observation of reflection hkl and 〈I(hkl)〉 is the weighted average intensity for all observations i of reflection hkl.
b Σhkl‖Fobs| − k|Fcalc‖/Σhkl|Fobs|; around 1,000–1,200 reflections were used for Rfree calculation.
FIGURE 7.
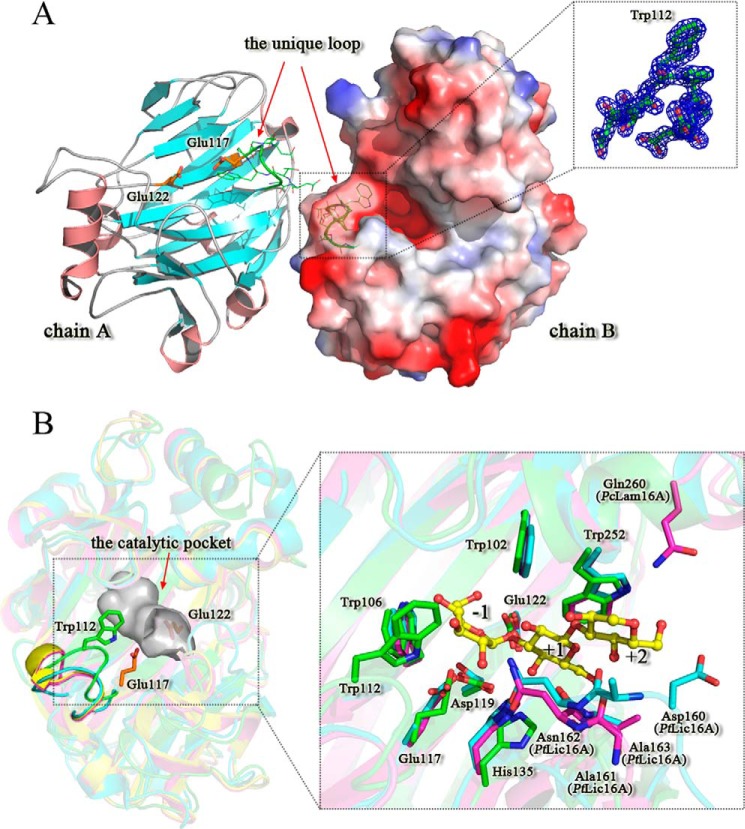
Crystal structure of PtBgt16A. A, overall structure and the unique catalytic groove of PtBgt16A. The two PtBgt16A molecules from the dimer are shown, one as a ribbon diagram and the other by electrostatic potential surface. The unique loop is highlighted in green. The electron densities of the unique loop are shown as the σA-weighted mFo − DFc omit map contoured at the 3.0 σ level in an amplified view. B, substrate interactions of PtBgt16A. Left, superposition of PtBgt16A on other GH family 16 enzymes is shown as a ribbon diagram with ribbons colored according to each enzyme: PtBgt16A in green, PtLic16A (Protein Data Bank code 3WDT) in blue, PcLam16A (Protein Data Bank code 2W39) in purple, and PcLam16A (Protein Data Bank code 2WLQ) in yellow. The binding pocket of PtBgt16A is shown in surface representation. Right, substrate interactions of PtBgt16A by ligands superposed. The −1 site glucose residue is from PcLam16A-laminaribiose complex (Protein Data Bank code 2W39), and the +1 and +2 site glucose residues are from PcLam16A-laminariheptaose complex (Protein Data Bank code 2WLQ). All of the superposed ligands are shown in stick representation and colored in yellow. The amino residues from PtBgt16A, PtLic16A, and PcLam16A are colored green, blue, and purple, respectively.
The Unique Binding Pocket and the Active Site
The active site of PtBgt16A was located in a long deep channel at the concave side, allowing binding of oligosaccharides. An inserted loop (109GDTWPDDG116) was found to be present in the one end of the catalytic groove (Fig. 7). Some strong hydrogen bonds are involved in the stability of this loop: Nϵ1 atom of Trp112 was stabilized by Oϵ2 atom of Glu117 (2.86 Å), Oδ2 of Asp115 was directly hydrogen-bonded to the nitrogen atom of Tyr111 (2.96 Å), oxygen atom of Tyr111 was directly hydrogen-bonded to the nitrogen atom of Asp115 (3.07 Å), and oxygen atom of Pro113 was directly hydrogen-bonded to the nitrogen atom of Gly116 (3.04 Å). This inserted loop formed a bulge region across the catalytic groove of PtBgt16A that blocked the one end of the catalytic groove and thus formed a pocket around the active center (Fig. 7B). It was precisely because of the presence of this unique loop that the catalytic groove of PtBgt16A could only provide −1 site in the non-reducing end. To explore the substrate binding of PtBgt16A, laminaritriose ligand was modeled into the putative subsites −1 to +2 according to the structure superposition of complex structures of Phanerochaete chrysosporium GH family 16 laminarinase (PcLam16A) PcLam16A-L2 (Protein Data Bank code 2W39) and PcLam16A-L7 (Protein Data Bank code 2WLQ) (Fig. 7B). According to the superposed complex structures, several key residues were identified to form the active site of PtBgt16A (Fig. 8A). The Nϵ1 atom of Trp102 formed a direct hydrogen bond to the O6 hydroxyls of the −1 glucose residue. Aromatic residues Trp106 stacked against the glucose units at subsites −1, forming a hydrophobic sugar-binding platform. The O4 hydroxyl of the +1 glucose residue was directly hydrogen-bonded to the side chains of residues Asp119 and His135. Trp252 was involved in hydrophobic interactions with subsite +1. Two strictly conserved catalytic glutamate residues, Glu117 and Glu122, lay at either end of the core β-sheet (β10), acting as a nucleophile and a proton donor, respectively (Fig. 8A). The importance of these two conserved catalytic residues was corroborated by site-directed mutagenesis in which substitution of each of these residues reduced the activity of the enzyme (Fig. 8C). It is noteworthy that residue Trp112 from the inserted loop played a key role in the formation of the −1 site of binding pocket. It not only provides steric hindrance but also forms a direct hydrogen bond to the O3 hydroxyls of the −1 glucose residue. The importance of residue Trp112 for substrate binding was corroborated by site-directed mutagenesis (Fig. 8C): the mutant W112A exhibited a specific activity of 12.11 units/mg (only 1.88% of the wild type transglycosylation activity).
FIGURE 8.
Catalytic mechanism of PtBgt16A transglycosylation. A, schematic representation of substrate interactions of PtBgt16A. The oligosaccharide is drawn as sticks. The hydrogen bonding interactions are shown as dotted lines. B, schematic mechanism of the catalysis reaction by PtBgt16A. C, enzyme activity of PtBgt16A mutants. Data represent the mean ± S.D. of three independent experiments (n = 3).
Structure superposition indicated that PtBgt16A, PcLam16A (25), and a P. thermophila GH family 16 β-1,3-1,4-glucanase (PtLic16A) (22) have a similar architecture of active sites at −1 and +1 sites (Fig. 7B). Some identified key residues above (Trp102, Trp106, Glu117, Asp119, His135, and Trp252) were conserved in all three enzymes. However, PtBgt16A showed distinct architecture of active sites at +2 and +3 sites. The +2 sites of PtLic16A and PcLam16A have a strict conserved “-161ANA163-” motif (PtLic16A numbering). The main chain of residue Ala and the side chain of residue Asp could form a direct hydrogen bond to the +1 and +2 glucose residues, which take part in the process of subtract recognition. Furthermore, Gln260 in PcLam16A (Asp160 in PtLic16A) in the +3 binding site of this enzyme could form a direct hydrogen bond to the +3 glucose residue; however, the “-ANA-” motif and the +3 binding site are completely absent in PtBgt16A. The catalytic groove of PtBgt16A thus presents an open architecture in +2 site, and the reducing end of the substrate is localized in the solvent region.
Discussion
GH family 16 is a huge glycoside hydrolase family that often has activity toward β-1,4- or β-1,3-glycosidic bonds in various glucans and galactans (26). Most GH family 16 members exhibit glycoside hydrolase activity toward plant and marine polysaccharides, including endo-1,3-β-glucanases (EC 3.2.1.39), licheninases (EC 3.2.1.73), β-agarases (EC 3.2.1.81), β-porphyranases (EC 3.2.1.178), κ-carrageenases (EC 3.2.1.83), and xyloglucan-specific endo-β-1,4-glucanase (EC 3.2.1.151). Previous studies revealed that some GH family 16 enzymes could catalyze the hydrolysis of mixed linked β-1,3- and β-1,4-glucan substrates (β-1,3-1,4-glucan). One typical enzyme is licheninase. It is also known as “β-1,3-1,4-glucanase,” which strictly cleaves a β-1,4-glycosidic linkage adjacent to a C3 substituted glucose residue in mixed linked β-1,3-1,4-glucans but is inactive against β-1,4-glucans or β-1,3-glucans (27). Another well known enzyme is β-1,3(4)-glucanase (EC 3.2.1.6), which catalyzes the hydrolysis of a β-1,4- or β-1,3-glycosidic linkage in β-glucans when the glucose residue whose reducing group is involved in the linkage to be hydrolyzed is itself substituted at C3 (28). These two typical β-1,3/1,4-hydrolases both follow an endo-type catalytic mode and often show specificity toward a given substrate motif of β-1,3 and β-1,4 bonds in the vicinity of the bond to be cleaved in mixed linked β-glucan substrates. PtBgt16A is a GH family 16 member that shows low identity (∼40%) with PtLic16A (22) (Fig. 1B). Catalytic properties indicated that PtBgt16A adopts an entirely different catalytic mode compared with known β-1,3/1,4-glycoside hydrolases. It is noteworthy that some GH family 16 members indeed are transglycosylases, such as xyloglucan:xyloglucosyltransferases (EC 2.4.1.207) (29) and chitin β-1,3/1,6-glucanosyltransferase (EC 2.4.1.-) (23). However, PtBgt16A displayed unique transglycosylase activity that is different from all reported GH family 16 members. The suitable substrates of PtBgt16A were β-1,3/1,4-linked gluco-oligosaccharides, and the minimum DP of substrate was 3. PtBgt16A could cleave the β-1,3/1,4 linkage in the oligosaccharides and meanwhile synthesize a large number of higher polymeric oligosaccharides. Moreover, the transglycosidation products were a series of mixed oligosaccharides. There was almost no glucose generating in this reaction.
The overall structure of PtBgt16A exhibited a classical GH family 16 sandwich-like β-jelly roll fold (21, 24). However, an inserted loop (109GDTWPDDG116) near the catalytic groove formed a binding pocket around the active center (Fig. 7). All eight residues of this inserted loop were well defined in the electron density maps, and some strong hydrogen bonds are involved in the stability of the loop. Moreover, the B-factors of the atoms composing this additional loop (average B-factors, 20.06 Å2) were not significantly different from the B-factors of other protein atoms (average B-factors, 17.50 Å2) (Fig. 9A). When considering all these elements, this additional loop seems to be stable and not a flexible region. Several glycoside hydrolases go through an open-closed-open conformational transition upon substrate binding and product release by a flexible loop. There is no evidence showing that PtBgt16A may follow this mode, and no GH family 16 member exhibited this mode before (26). Sequence alignment showed that this loop region was not conserved in different GH family 16 members (Fig. 9B). In contrast to other GH family 16 hydrolases that have the active site in the bottom of a large cleft, the active site of PtBgt16A is at the bottom of a pocket. The −2 subsite is not found in PtBgt16A as it is blocked by the additional loop. Thus, this binding pocket may be the structural basis for the unique transglycosylation activity by PtBgt16A.
FIGURE 9.

B-factor putty (A) and sequence alignment (B) of the unique loop of PtBgt16A. The key residue, Tyr112, of PtBgt16A is marked by a red star. The sequences of PtBgt16A (Protein Data Bank code 5JVV), PtLic16A (Protein Data Bank code 3WDT), PcLam16A (Protein Data Bank code 2CL2), ZgLamCGH16 (Protein Data Bank code 4CRQ), RmLamR (Protein Data Bank code 3ILN), and BglF (Protein Data Bank code 2HYK) were aligned using T-Coffee (43), and the figure was produced in ESPript (44).
Based on the superposed structures of PtBgt16A, PcLam16A, and PcLam16A, the plausible substrate binding and several key residues of PtBgt16A were identified. The detailed transglycosidation catalytic mechanism of PtBgt16A was further speculated. The catalytic mechanism of PtBgt16A consists of a retaining reaction: a general acid/base catalyst (proton donor; Glu122) works first as an acid and then as a base in two steps (Fig. 8B). In the first step, one oligosaccharide (β-1,3 linkage or β-1,4 linkage; DP ≥ 3) occupies binding sites −1 to +2, and a proton donor (Glu122) facilitates departure of the leaving group by donating a proton to the oxygen atom between glycosyl −1 and +1. As for the unique binding pocket of PtBgt16A, the nucleophile (Glu117) only could form an enzyme-sequestered covalent intermediate toward the −1 glucose residue. The other part of the oligosaccharide is then released from the binding pocket as the leaving group. In the second step, another oligosaccharide as acceptor occupies binding sites +1 and +2, and the deprotonated proton donor (Glu122) acts as a general base to activate the C3 or C4 hydroxyl of the acceptor, which then carries out a nucleophilic attack on the glucosyl-enzyme intermediate, leading to the formation of a new transglycosylation product, which adopts a β-1,3 linkage or β-1,4 linkage with the reducing end of glucosyl. The open form in binding sites +3 could lead the acceptor recognition region accepting both β-1,3-linked and β-1,4-linked oligosaccharides. Thus, the formed glycosidic bonds of transglycosylation products were mixed β-1,3 linkage and β-1,4 linkage.
The catalytic mechanism of PtBgt16A is partly similar to an exo-glycoside hydrolase, which cleaves the substrate from the non-reducing end one by one (11). However, PtBgt16A released the reducing end product and used the remaining glucosyl as a transglycosylation donor. This characteristic results in the transglycosylation products being gradually extended by one glucose unit. The catalytic property of PtBgt16A may be similar to a member of GH family 13, amylosucrase (EC 2.4.1.4), which catalyzes the synthesis of amylose-like polymers from sucrose (30). The amylosucrase catalyzes the following elongating chemical reaction: sucrose + (1,4-α-d-glucosyl)n → d-fructose + (1,4-α-d-glucosyl)n + 1, which generates α-1,4-glucan in a stepwise fashion, releasing fructose from sucrose at each step of extension. The active sites of amylosucrase are also located in the bottom of a pocket, which exhibits an exo-acting enzyme mode (30, 31). Reported β-1,3-transglycosylases, often named β-1,3-glucanosyltransferases (EC 2.4.1.-), catalyze the transfer of a glycosyl group from a β-1,3-linked carbohydrate donor to a suitable (carbohydrate) acceptor (32, 33). β-1,3-Glucanosyltransferases are grouped into three GH families: 16, 17, and 72, which function as cross-linking enzymes, branching enzymes, and elongation enzymes, respectively (34). However, to our knowledge, the catalytic modes of PtBgt16A are different from all three of these β-1,3-glucanosyltransferases and any other non-Leloir transglycosylases in glycoside hydrolases. Because of its novel elongating catalytic mechanism, PtBgt16A should be named β-transglycosylase. β-1,3-Glucan is a major structural component of fungi that forms a fibrillary network responsible for the mechanical strength of the cell wall. Sequence alignment by NCBI Blast shows that the homologous sequences of PtBgt16A are only found in the filamentous fungi. Moreover, the PtBgt16A-full protein was predicted to be anchored in the outer membrane because of the presence of a lipoprotein signal peptide and a transmembrane architecture. Thus, the biological significance of PtBgt16A is most likely involvement in fungal β-glucan metabolism and cell wall rearrangement.
Oligosaccharides, polysaccharides, and glycoconjugates are a relevant part of the bioactive components of the natural products exploited in therapeutics, diagnostics, food additives, and biomaterials (1, 2, 4). Therefore, methods for glycosidic synthesis and modification are urgently needed. Enzymatic synthesis of carbohydrates is still challenging at present due to the low efficiency of glycosyltransferases and complexity of carbohydrates. The catalytic mechanism of non-Leloir transglycosylases is similar to their hydrolytic counterparts, and it is unclear how these enzymes overcome the ubiquity of water, thus avoiding the hydrolytic reaction (3). It is still difficult to create novel non-Leloir transglycosylases using the vast diversity of glycoside hydrolases as protein templates by protein engineering. Although the exact biological role of PtBgt16A is still elusive, we noticed that PtBgt16A has its own unique advantages in enzymatic synthesis of carbohydrates. It has high transglycosylation activity (776.2 units/mg toward laminaripentaose) but nearly no hydrolytic activity. PtBgt16A cleaves the substrate from the non-reducing end by a glucose unit like an exo-glycoside hydrolase and uses this glucosyl as a transglycosylation donor. This unique catalytic mechanism gives PtBgt16A the ability to synthesize glycoconjugates and long-chain oligosaccharides, opening a novel pathway for non-Leloir transglycosylases in glycoside synthesis and modification. Moreover, it provides an excellent protein template for further structure-based protein engineering study in transglycosylases.
Experimental Procedures
Cloning, Expression, and Purification
The thermophilic fungus P. thermophila J18 was used in this study. The strain has been deposited in the China General Microbiological Culture Center under accession number AS3.6885 (35). Cells were grown as described in a previous study to isolate genomic DNA and total RNA (36). The procedure to clone the target gene from P. thermophila J18 was as described in a previous study with some modifications (36). Degenerate primers DP1 and DP2 (supplemental Table S1) were designed on the basis of the conserved sequences (GEIDIIEGV and DTTFCGDWA) of known GH family 16 β-glucanases. The full-length cDNA sequence of the target gene was obtained by 5′ and 3′ rapid amplification of cDNA ends (RACE) using a SMART RACE cDNA amplification kit (Clontech). The obtained PCR product was purified, cloned, and sequenced (designated as PtBgt16A-full). The PtBgt16A-full gene is predicted to be a membrane-anchored protein composed of a GH family 16 catalytic module (PtBgt16A) and a C-terminal transmembrane region. To study the enzymatic properties of this GH family 16 catalytic module, the gene fragments of GH family 16 catalytic module were amplified by PCR using the primers PtBgt16A-NheI and PtBgt16A-XhoI (supplemental Table S1). The NheI and XhoI sites (underlined) were added to the forward and reverse primers, respectively. The sequenced PtBgt16A cDNA sequence was deposited in the GenBank nucleotide sequence database under accession number KX234714. The purified PCR products were digested with NheI and XhoI and subcloned into the pET-28a(+) vector (Novagen). Mutants E117A, E122A, and W112A were generated using the Fast Mutagenesis System site-directed mutagenesis kit (TransGen Biotech, China). The primers used for site-directed mutagenesis are shown in supplemental Table S1. All recombinant plasmids encoding these mutations were sequenced and verified.
The recombinant plasmids were transformed into Escherichia coli BL21(DE3) competent cells for gene expression. Seed cultures of E. coli BL21(DE3) harboring PtBgt16A in the pET-28a(+) vector were prepared by incubation in LB medium containing 50 μg/ml kanamycin at 37 °C on a rotary shaker at 200 rpm for 4 h. When the absorbance of the culture broth at 600 nm reached 0.6–0.8, overexpression of the protein was induced by addition of 1 mm isopropyl β-d-thiogalactopyranoside. The cultures were further grown at 30 °C for 12 h, and the cells were harvested by centrifugation at 10,000 × g for 10 min at 4 °C. The recombinant proteins were purified using a nickel-iminodiacetic acid column (1 × 5 cm) and Sephacryl S-100 HR column (GE Healthcare) as described previously (10). The purified protein fractions were combined and concentrated for subsequent experiments. All mutants were expressed and purified in an identical manner.
Enzyme Assay
Transglycosylation activity was quantitated by HPAEC using different oligosaccharides as the substrates (10). Namely, 2 μl of suitably diluted enzyme and 8 μl of each 10% (w/v) oligosaccharide were mixed with 30 μl of 50 mm sodium acetate buffer, pH 5.5. The reaction mixture was incubated at 50 °C for 5 min and then terminated by boiling for 5 min. The mixture was diluted 500-fold with 100 mm NaOH and analyzed by HPAEC (ICS-5000+, Thermo) with pulsed amperometric detection on a CarboPac PA10 (4 × 250-mm) preparative column (Thermo) with a 0–350 mm sodium acetate gradient in 100 mm NaOH (20 min) at a flow rate of 1 ml/min. One unit of enzyme activity was defined as the amount of enzyme required to consume 1 μmol of oligosaccharides/min. Substrate specificity of PtBgt16A was determined by measuring the activity of the enzyme in the presence of different substrates such as laminarioligosaccharides, cello-oligosaccharides, manno-oligosaccharides, xylo-oligosaccharides, malto-oligosaccharides, chitin oligosaccharides, raffinose, and stachyose. The enzyme activity was determined under standard conditions. Raffinose and stachyose were purchased from Aladdin (China). Other oligosaccharides were purchased from Megazyme (Ireland).
A hydrolytic assay was performed according to the method of Yang et al. (35). Namely, 0.1 ml of properly diluted enzyme solution was added into 0.9 ml of 1% (w/v) polysaccharides (prepared in 50 mm sodium acetate buffer, pH 5.5). The reaction mixture was incubated at 50 °C in a water bath for 10 min. The liberated reducing sugars were quantified using the dinitrosalicylic acid method. One unit of hydrolytic activity was defined as the amount of enzyme liberating 1 μmol of monosaccharide-equivalent reducing sugars/min under the above assay conditions.
Transglycosylation Properties of PtBgt16A
Transglycosylation properties of PtBgt16A were investigated by analyzing the products from the laminarioligosaccharides (Megazyme), viz. laminaritriose, laminaritetraose, laminaripentaose, and laminarihexaose. Purified PtBgt16A (1 unit/ml) was added to 1% (w/v) laminarioligosaccharides in 50 mm sodium acetate buffer, pH 5.5, and then incubated at 50 °C for 2 h. Samples withdrawn at different times were immediately boiled for 5 min and then analyzed by TLC (16). Samples were spotted on a TLC plate (Kieselgel 60, Merck), developed in butan-1-ol:acetic acid: water (2:1:1, v/v/v) as solvent, and sprayed with a methanol-sulfuric acid mixture (95:5, v/v). The hydrolysis products were visualized after heating the plate at 130 °C in an oven for a few minutes.
Characterization of the PtBgt16A
The optimal pH of purified PtBgt16A was determined by measuring the activity from pH 2.0 to 11.0 using various buffers at 50 mm: McIlvaine buffer (pH 2.0–7.5), sodium acetate buffer (pH 3.5–6.0), Tris-HCl buffer (pH 7.0–9.5), and glycine-NaOH buffer (pH 9.0–11.0). To determine pH stability, residual activity was measured after incubation of the enzyme at 30 °C for 30 min in the aforementioned buffers.
The optimal temperature was determined at 40–70 °C in 50 mm sodium acetate buffer, pH 5.5. Enzymatic activity was determined under standard conditions. Thermostability of the enzyme was determined by measuring residual activity after incubation of the enzyme at different temperatures (40–70 °C) for 30 min in 50 mm sodium acetate buffer, pH 5.5.
Analysis of Transglycosylation Products
To determine the degree of polymerization of transglycosylation products, reaction mixtures derived from laminaritriose and cellotriose were analyzed by MALDI-TOF MS. Enzyme (1 unit/ml) was incubated with 1% (w/v) laminaritriose or cellotriose in 50 mm sodium acetate buffer, pH 5.5, at 50 °C for 2 h (50-μl assay volume). The sample was diluted 100-fold prior to mixing with an equal volume of matrix (2,5-dihydroxybenzoic acid; 10 mg/ml in water). Sample (1 μl) was then spotted onto the MALDI plate and analyzed in an AB SCIEX TOF/TOFTM 5800 system operated in positive ion mode.
For structural analysis of transglycosylation products, a transglycosylation reaction (30 ml) was performed with PtBgt16A (1 unit/ml) and 1% (w/v) laminaritriose or cellotriose in 50 mm sodium acetate buffer, pH 5.5, at 50 °C for 2 h. The reaction mixtures were deionized by ion exchange resin and then concentrated by rotary evaporation. The purified transglycosylation products (∼10 mg) were freeze-dried and dissolved in deuterium oxide (500 μl) prior to recording spectroscopy on a Bruker Avance 500 NMR spectrometer. Two-dimensional HSQC and HMBC spectra were acquired using standard pulse sequences. For the blank control, the same NMR analysis was performed using purified laminaritriose and cellotriose.
Crystallization and Data Collection
PtBgt16A was concentrated to 10 mg/ml in crystallographic buffer (20 mm Tris-HCl, pH 8.0, containing 100 mm NaCl). Crystallization experiments were performed in 48-well plates by the sitting drop vapor diffusion method at 20 °C, and each sitting drop was prepared by mixing 1 μl each of protein solution and reservoir solution. Optimized crystals suitable for diffraction were grown in drops containing 1 μl of protein solution and 1 μl of reservoir solution (35% PEG 3350, 0.1 m sodium citrate, pH 4.4) at 20 °C. The rhabdoid crystals were obtained 10 days later.
Crystals were soaked in reservoir solution supplemented with 20% glycerol and then vitrified in liquid nitrogen. Diffraction data for PtBgt16A was collected at 100 K using beamline BL19U1 at Shanghai Synchrotron Radiation Facility (Shanghai, China). All diffraction data were indexed, integrated, and scaled using the program HKL-3000 (37). The X-ray data collection statistics are presented in Table 2.
Phase Determination, Model Building, and Refinement
The structure of PtBgt16A was determined by molecular replacement using the coordinates of P. thermophila GH family 16 β-1,3-1,4-glucanase (Protein Data Bank code 3WDT) as the search model (22) according to their protein sequence alignments. Thereafter, model building and refinement were performed using Coot (38) and Phenix.refine (39). The final models were analyzed and validated with MolProbity (40). Structural homologs of PtBgt16A were identified using the DALI server (41). The refinement statistics are shown in Table 2. The secondary structural elements were identified with DSSP (42). The schematic depictions of the structures were prepared in PyMOL (version 1.3; Schrödinger LLC). The sequence alignments were created with T-Coffee (43) and ESPript (44). The coordinates and structure factors of PtBgt16A have been deposited in the Protein Data Bank under accession code 5JVV.
Author Contributions
Z. J. designed and supervised the research and revised the manuscript. Z. Q. designed the research, performed experiments, analyzed data, and wrote the manuscript. S. Y., L. Z., and Q. Y. analyzed data and revised the manuscript. X. Y. performed site-directed mutagenesis and measurement of enzymatic activity. All the authors read and approved the manuscript.
Supplementary Material
Acknowledgments
We are grateful to the staff of the National Center for Protein Science, Shanghai, China and Shanghai Synchrotron Radiation Facility for assistance in X-ray data collection.
This work was supported by National Science Fund for Distinguished Young Scholars Grant 31325021 and Program for Changjiang Scholars Grant T2014055. The authors declare that they have no conflicts of interest with the contents of this article.
The nucleotide sequence(s) reported in this paper has been submitted to the GenBankTM/EBI Data Bank with accession number(s) KX234714.
The atomic coordinates and structure factors (code 5JVV) have been deposited in the Protein Data Bank (http://www.pdb.org/).

This article contains supplemental Table S1.
- GH
- glycoside hydrolase
- DP
- degree of polymerization
- HPAEC
- high performance anion exchange chromatography
- Pt
- P. thermophila
- pNP
- p-nitrophenyl
- oNP
- o-nitrophenyl
- HSQC
- heteronuclear single quantum correlation
- HMBC
- heteronuclear multiple bond correlation
- Pc
- P. chrysosporium
- Zg
- Z. galactanivorans
- Rm
- R. marinus.
References
- 1.Cobucci-Ponzano B., Strazzulli A., Rossi M., and Moracci M. (2011) Glycosynthases in biocatalysis. Adv. Synth. Catal. 353, 2284–2300 [Google Scholar]
- 2.Díez-Municio M., Herrero M., Olano A., and Moreno F. J. (2014) Synthesis of novel bioactive lactose-derived oligosaccharides by microbial glycoside hydrolases. Microb. Biotechnol. 7, 315–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bissaro B., Monsan P., Fauré R., and O'Donohue M. J. (2015) Glycosynthesis in a waterworld: new insight into the molecular basis of transglycosylation in retaining glycoside hydrolases. Biochem. J. 467, 17–35 [DOI] [PubMed] [Google Scholar]
- 4.Cobucci-Ponzano B., and Moracci M. (2012) Glycosynthases as tools for the production of glycan analogs of natural products. Nat. Prod. Rep. 29, 697–709 [DOI] [PubMed] [Google Scholar]
- 5.Spadiut O., Ibatullin F. M., Peart J., Gullfot F., Martinez-Fleites C., Ruda M., Xu C., Sundqvist G., Davies G. J., and Brumer H. (2011) Building custom polysaccharides in vitro with an efficient, broad-specificity xyloglucan glycosynthase and a fucosyltransferase. J. Am. Chem. Soc. 133, 10892–10900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li T., Tong X., Yang Q., Giddens J. P., and Wang L. X. (2016) Glycosynthase mutants of endoglycosidase S2 show potent transglycosylation activity and remarkably relaxed substrate specificity for antibody glycosylation remodeling. J. Biol. Chem. 291, 16508–16518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pérez X., Faijes M., and Planas A. (2011) Artificial mixed-linked β-glucans produced by glycosynthase-catalyzed polymerization: tuning morphology and degree of polymerization. Biomacromolecules 12, 494–501 [DOI] [PubMed] [Google Scholar]
- 8.Seibel J., Beine R., Moraru R., Behringer C., and Buchholz K. (2006) A new pathway for the synthesis of oligosaccharides by the use of non-Leloir glycosyltransferases. Biocatal. Biotransformation 24, 157–165 [Google Scholar]
- 9.Teze D., Hendrickx J., Czjzek M., Ropartz D., Sanejouand Y. H., Tran V., Tellier C., and Dion M. (2014) Semi-rational approach for converting a GH1 β-glycosidase into a β-transglycosidase. Protein Eng. Des. Sel. 27, 13–19 [DOI] [PubMed] [Google Scholar]
- 10.Qin Z., Yan Q., Lei J., Yang S., Jiang Z., and Wu S. (2015) The first crystal structure of a glycoside hydrolase family 17 β-1,3-glucanosyltransferase displays a unique catalytic cleft. Acta Crystallogr. D Biol. Crystallogr. 71, 1714–1724 [DOI] [PubMed] [Google Scholar]
- 11.Matsuzawa T., Jo T., Uchiyama T., Manninen J. A., Arakawa T., Miyazaki K., Fushinobu S., and Yaoi K. (2016) Crystal structure and identification of a key amino acid for glucose tolerance, substrate specificity, and transglycosylation activity of metagenomics β-glucosidase Td2F2. FEBS J. 283, 2340–2353 [DOI] [PubMed] [Google Scholar]
- 12.Lundemo P., Karlsson E. N., and Adlercreutz P. (2016) Eliminating hydrolytic activity without affecting the transglycosylation of a GH1 β-glucosidase. Appl. Microbiol. Biotechnol. 10.1007/s00253-016-7833-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hrmova M., Imai T., Rutten S. J., Fairweather J. K., Pelosi L., Bulone V., Driguez H., and Fincher G. B. (2002) Mutated barley (1,3)-β-d-glucan endohydrolases synthesize crystalline (1,3)-β-d-glucans. J. Biol. Chem. 277, 30102–30111 [DOI] [PubMed] [Google Scholar]
- 14.Wei J., Lv X., Lu Y., Yang G., Fu L., Yang L., Wang J., Gao J., Cheng S., Duan Q., Jin C., and Li X. (2013) Glycosynthase with broad substrate specificity—an efficient biocatalyst for the construction of oligosaccharide library. Eur. J. Org. Chem. 12, 2414–2419 [Google Scholar]
- 15.Feng H. Y., Drone J., Hoffmann L., Tran V., Tellier C., Rabiller C., and Dion M. (2005) Converting a β-glycosidase into a β-transglycosidase by directed evolution. J. Biol. Chem. 280, 37088–37097 [DOI] [PubMed] [Google Scholar]
- 16.Qin Z., Yan Q., Yang S., and Jiang Z. (2016) Modulating the function of a β-1,3-glucanosyltransferase to that of an endo-β-1,3-glucanase by structure-based protein engineering. Appl. Microbiol. Biotechnol. 100, 1765–1776 [DOI] [PubMed] [Google Scholar]
- 17.Goodridge H. S., Wolf A. J., and Underhill D. M. (2009) β-Glucan recognition by the innate immune system. Immunol. Rev. 230, 38–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magee A. S., Langeslay R. R., Will P. M., Danielson M. E., Wurst L. R., and Iiams V. A. (2015) Modification of the degree of branching of a β-(1,3)-glucan affects aggregation behavior and activity in an oxidative burst assay. Biopolymers 103, 665–674 [DOI] [PubMed] [Google Scholar]
- 19.Vasur J., Kawai R., Jonsson K. H., Widmalm G., Engström A., Frank M., Andersson E., Hansson H., Forsberg Z., Igarashi K., Samejima M., Sandgren M., and Ståhlberg J. (2010) Synthesis of cyclic β-glucan using laminarinase 16A glycosynthase mutant from the basidiomycete Phanerochaete chrysosporium. J. Am. Chem. Soc. 132, 1724–1730 [DOI] [PubMed] [Google Scholar]
- 20.Labourel A., Jam M., Jeudy A., Hehemann J. H., Czjzek M., and Michel G. (2014) The β-glucanase ZgLamA from Zobellia galactanivorans evolved a bent active site adapted for efficient degradation of algal laminarin. J. Biol. Chem. 289, 2027–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeng W. Y., Wang N. C., Lin C. T., Shyur L. F., and Wang A. H. (2011) Crystal structures of the laminarinase catalytic domain from Thermotoga maritima MSB8 in complex with inhibitors: essential residues for β-1,3- and β-1,4-glucan selection. J. Biol. Chem. 286, 45030–45040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng Y. S., Huang C. H., Chen C. C., Huang T. Y., Ko T. P., Huang J. W., Wu T. H., Liu J. R., and Guo R. T. (2014) Structural and mutagenetic analyses of a 1,3-1,4-β-glucanase from Paecilomyces thermophila. Biochim. Biophys. Acta 1844, 366–373 [DOI] [PubMed] [Google Scholar]
- 23.Blanco N., Sanz A. B., Rodríguez-Peña J. M., Nombela C., Farkaš V., Hurtado-Guerrero R., and Arroyo J. (2015) Structural and functional analysis of yeast Crh1 and Crh2 transglycosylases. FEBS J. 282, 715–731 [DOI] [PubMed] [Google Scholar]
- 24.Ilari A., Fiorillo A., Angelaccio S., Florio R., Chiaraluce R., van der Oost J., and Consalvi V. (2009) Crystal structure of a family 16 endoglucanase from the hyperthermophile Pyrococcus furiosus—structural basis of substrate recognition. FEBS J. 276, 1048–1058 [DOI] [PubMed] [Google Scholar]
- 25.Vasur J., Kawai R., Andersson E., Igarashi K., Sandgren M., Samejima M., and Ståhlberg J. (2009) X-ray crystal structures of Phanerochaete chrysosporium laminarinase 16A in complex with products from lichenin and laminarin hydrolysis. FEBS J. 276, 3858–3869 [DOI] [PubMed] [Google Scholar]
- 26.Lombard V., Golaconda Ramulu H., Drula E., Coutinho P. M., and Henrissat B. (2014) The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 42, D490–D495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furtado G. P., Ribeiro L. F., Santos C. R., Tonoli C. C., Souza A. R. D., Oliveira R. R., and Ward R. J. (2011) Biochemical and structural characterization of a β-1,3-1,4-glucanase from Bacillus subtilis 168. Process Biochem. 46, 1202–1206 [Google Scholar]
- 28.Meng D. D., Wang B., Ma X. Q., Ji S. Q., Lu M., and Li F. L. (2016) Characterization of a thermostable endo-1,3(4)-β-glucanase from Caldicellulosiruptor sp. strain F32 and its application for yeast lysis. Appl. Microbiol. Biotechnol. 100, 4923–4934 [DOI] [PubMed] [Google Scholar]
- 29.Campbell P., and Braam J. (1999) In vitro activities of four xyloglucan endotransglycosylases from Arabidopsis. Plant J. 18, 371–382 [DOI] [PubMed] [Google Scholar]
- 30.Skov L. K., Mirza O., Henriksen A., De Montalk G. P., Remaud-Simeon M., Sarçabal P., Willemot R. M., Monsan P., and Gajhede M. (2001) Amylosucrase, a glucan-synthesizing enzyme from the α-amylase family. J. Biol. Chem. 276, 25273–25278 [DOI] [PubMed] [Google Scholar]
- 31.Skov L. K., Mirza O., Sprogøe D., Dar I., Remaud-Simeon M., Albenne C., Monsan P., and Gajhede M. (2002) Oligosaccharide and sucrose complexes of amylosucrase. Structural implications for the polymerase activity. J. Biol. Chem. 277, 47741–47747 [DOI] [PubMed] [Google Scholar]
- 32.Dobruchowska J. M., Jonsson J. O., Fridjonsson O. H., Aevarsson A., Kristjansson J. K., Altenbuchner J., Watzlawick H., Gerwig G. J., Dijkhuizen L., Kamerling J. P., and Hreggvidsson G. O. (2016) Modification of linear (β1→3)-linked gluco-oligosaccharides with a novel recombinant β-glucosyltransferase (trans-β-glucosidase) enzyme from Bradyrhizobium diazoefficiens. Glycobiology 26, 1157–1170 [DOI] [PubMed] [Google Scholar]
- 33.Mouyna I., Hartland R. P., Fontaine T., Diaquin M., Simenel C., Delepierre M., Henrissat B., and Latgé J. P. (1998) A 1,3-β-glucanosyltransferase isolated from the cell wall of Aspergillus fumigatus is a homologue of the yeast Bgl2p. Microbiology 144, 3171–31809846753 [Google Scholar]
- 34.Mouyna I., Hartl L., and Latgé J. P. (2013) β-1,3-Glucan modifying enzymes in Aspergillus fumigatus. Front. Microbiol. 4, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang S., Qiaojuan Y., Jiang Z., Fan G., and Wang L. (2008) Biochemical characterization of a novel thermostable β-1,3-1,4-glucanase (lichenase) from Paecilomyces thermophila. J. Agric. Food Chem. 56, 5345–5351 [DOI] [PubMed] [Google Scholar]
- 36.Hua C., Yan Q., Jiang Z., Li Y., and Katrolia P. (2010) High-level expression of a specific β-1,3-1,4-glucanase from the thermophilic fungus Paecilomyces thermophila in Pichia pastoris. Appl. Microbiol. Biotechnol. 88, 509–518 [DOI] [PubMed] [Google Scholar]
- 37.Minor W., Cymborowski M., Otwinowski Z., and Chruszcz M. (2006) HKL-3000: the integration of data reduction and structure solution-from diffraction images to an initial model in minutes. Acta Crystallogr. D Biol. Crystallogr. 62, 859–866 [DOI] [PubMed] [Google Scholar]
- 38.Emsley P., and Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 39.Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., et al. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen V. B., Arendall W. B. 3rd, Headd J. J., Keedy D. A., Immormino R. M., Kapral G. J., Murray L. W., Richardson J. S., and Richardson D. C. (2010) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holm L., and Rosenström P. (2010) Dali server: conservation mapping in 3D. Nucleic Acids Res. 38, W545–W549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kabsch W., and Sander C. (1983) Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22, 2577–2637 [DOI] [PubMed] [Google Scholar]
- 43.Notredame C., Higgins D. G., and Heringa J. (2000) T-Coffee: a novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 302, 205–217 [DOI] [PubMed] [Google Scholar]
- 44.Robert X., and Gouet P. (2014) Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 42, W320–W324 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.