Abstract
Objectives
Deep Brain Stimulation (DBS) has been either approved or is currently under investigation for a number of psychiatric disorders.
Materials and Methods
We review clinical and preclinical concepts as well as the neurocircuitry that may be of relevance for the implementation of DBS in posttraumatic stress disorder (PTSD).
Results
PTSD is a chronic and debilitating illness associated with dysfunction in well-established neural circuits, including the amygdala and prefrontal cortex. Though most patients often improve with medications and/or psychotherapy, approximately 20–30% are considered to be refractory to conventional treatments. In other psychiatric disorders, DBS has been investigated in treatment-refractory patients.
To date, preclinical work suggests that stimulation at high frequency delivered at particular timeframes to different targets, including the amygdala, ventral striatum, hippocampus and prefrontal cortex may improve fear extinction and anxiety-like behavior in rodents. In the only clinical report published so far, a patient implanted with electrodes in the amygdala has shown striking improvements in PTSD symptoms.
Conclusions
Neuroimaging, preclinical and preliminary clinical data suggest that the use of DBS for the treatment of PTSD may be practical but the field requires further investigation.
Keywords: Amygdala, Anxiety, Deep Brain Stimulation, Fear extinction, Prefrontal cortex, Post-traumatic stress disorder
INTRODUCTION
Described anecdotally for millennia and having carried a multitude of names throughout history, including shell shock, battle fatigue, soldier heart, and accident neurosis, the term Posttraumatic Stress Disorder (PTSD) as it is called today appeared in the third edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-III). Although the core symptoms and etiology of PTSD have remained in the description of the disorder, notable changes have been incorporated into the DSM-V diagnostic criteria. Until the latest edition, PTSD was classified as a fear and anxiety disorder. In the DSM-V (1), PTSD is no longer described as such, but as a separate entity that has expanded to include negative cognitions and alterations in mood (e.g. persistent negative emotional states, persistent blame), as well as arousal and reactivity symptoms (e.g. anger, impulsiveness, recklessness and self-destructiveness).
PTSD can develop after a traumatic event is witnessed or experienced and usually involves the threat of death, serious injury or a serious threat to a person’s physical integrity, in combination with intense feelings of fear and helplessness. The most common types of trauma resulting in PTSD include sexual assault, military combat, serious accidents and the unexpected death of a loved one. The disorder is characterized by four clusters of symptoms: intrusive or re-experiencing symptoms, persistent avoidance of stimuli associated with the trauma, alterations in mood, and persistent symptoms of hyperarousal. For a diagnosis of PTSD, the described symptoms must persist for over one month following the traumatic event and result in clinically significant distress or impairment in day-to-day functioning.
Despite variations in methodology and discrepancies in reported figures, most epidemiological studies indicate that posttraumatic stress disorder is a major health concern worldwide. In the United States, the estimated lifetime prevalence of PTSD is around 5–8%(2). Overall, women are more than twice as likely as men to develop PTSD (3). In war veterans, the prevalence is higher than average with rates reaching between 11–17% for soldiers deployed in the Gulf War and the Iraq-Afghanistan wars(4, 5).
PTSD causes considerable distress and can significantly interfere with social and occupational functioning. It is not uncommon for individuals suffering with the disease to lose their jobs, experience difficulties in relationships, and develop secondary psychological disorders and health complications. The most common co-morbidities include mood and anxiety disorders and substance abuse. PTSD has also been shown to increase the risk for suicidality (i.e., suicidal ideation, intent and/or plan, and attempt). In fact, the lifetime incidence of suicide attempts and ideation in PTSD was second only to that of individuals with depression(6). Taken together, the multifaceted issues associated with PTSD translate to a substantial economic burden through lost productivity and healthcare costs on the order of billions of dollars.
In North America, the lifetime cumulative exposure to any traumatic event sufficient to cause PTSD is estimated at 60–80%(7). Yet only a minority of trauma victims (10%–30%) subsequently meet criteria for PTSD(8). This consistent finding brings about questions regarding resiliency and vulnerability factors, chiefly, why are some individuals able recover and rebound from a psychological trauma and others go on to develop and suffer from PTSD?
Twin studies have shown that the development of PTSD following a trauma is heritable, and that genetic risk factors may account for up to 30–40% of this heritability(9, 10). Many factors relating to subjective experience of the traumatic event and post-trauma variables such as low social support, fear intensity during trauma, perceived threat to life, social withdrawal, a comorbid psychological problem, and poor family functioning have been shown to be strong predictors of PTSD development(11). Pre-trauma variables such as previous traumatic experiences, general childhood adversity, psychiatric history, reported childhood abuse, family psychiatric history and personality traits such as high negative emotionality have also been consistently reported to increase the risk for PTSD(12). Other risk factors and biological correlates involved in the pathophysiology of PTSD include genetic polymorphism(13), endocrine dysregulation(14), reduced levels of neurotrophic factors(15) as well as abnormal monoamine(16) and neuropeptide levels(17).
PRECLINICAL MODELS: GENERAL ASPECTS
For an animal model to adequately resemble a human disorder, it must meet several criteria: the behavior observed must parallel that in humans; the model must be able to test predictions regarding the mechanisms and etiology of the disorder; and it should share neural mechanisms that are comparable to those found in human patients(18, 19). Most animal models proposed to date are more suited to study short-term fear and anxiety-like responses to stress/trauma than the long-term responses observed in PTSD(20). In this review, we will focus mainly on fear conditioning and extinction paradigms as they share common mechanisms and neurocircuitry with PTSD.
Fear Conditioning
Pavlovian fear conditioning is a learning paradigm whereby an animal learns to associate a neutral cue (the conditioned stimulus; CS; often a tone or a light), with an aversive stimulus, (the unconditioned stimulus; US; often a foot shock or high volume sound). After repeat CS-US pairings, presentation of the CS in the absence of the aversive stimulus elicits a conditioned response (CR). This is commonly measured as the time the animals spend in a “freezing state,” in which only respiratory movements are observed. In fear conditioning studies, in addition to US and CS, freezing can also be measured in relation to environment or context.
In humans, fear conditioning has been postulated to play a key role in the pathogenesis of anxiety disorders(21). In particular, it has been proposed that patients or individuals susceptible to anxiety disorders are more sensitive to aversive stimuli and therefore acquire stronger fear learning than controls, such that a CS is more robustly reinforced and elicits a stronger CR(22). Consistent with the aversive hypersensitivity aspect of the conditioning model, PTSD patients show increased autonomic and electromyographic responses to visually presented and imagined threat stimuli(23). Furthermore, they present larger skin conductance levels, heart rate increases and electromyogram responses to images conditioned with aversive electrical shocks than non-PTSD trauma-exposed controls (24, 25). These results suggest that PTSD is associated with higher sympathetic nervous system arousal during conditioning and that individuals with PTSD may be more ‘conditionable’ than trauma-exposed individuals without PTSD.
Neurocircuitry of Fear Conditioning
Structures largely implicated in the mechanisms of fear conditioning are the amygdala, prefrontal cortex and the hippocampus.
The amygdala consists of multiple nuclei, including the basolateral complex (BLA), consisting of the lateral nucleus (LA), the basal nucleus (BA) and the accessory basal nucleus; the cortical nucleus; the central nucleus (CE), and intercalated cell clusters (ITC) (Figure 1). The LA receives stimuli from various sensory modalities including auditory, visual, and somatic(26). These inputs convey a fast signal for danger or aversive stimuli(27), which is in contrast to the slower sensory signal delivered from cortical association areas(27, 28) or hippocampal afferents(29). As a central relay of sensory information, the LA has been suggested to integrate aspects of US and CS during fear conditioning(30). From the LA, the signal is propagated to the central nucleus (CE), which in turn projects to multiple brainstem and hypothalamic areas. These are involved in different aspects of the fear response, including freezing behavior(31), autonomic responses (e.g. changes in heart rate and blood pressure)(32), and the modulation of the hypothalamic-pituitary adrenal axis(33). Overall, these structures and their connectivity in rodents correlate well with the anatomy of the human amygdala (34).
Figure. 1.
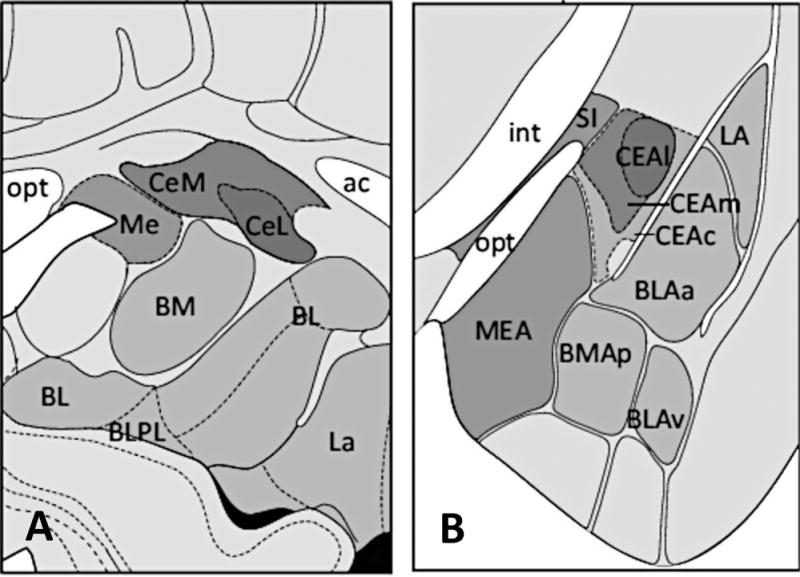
Schematic representation of coronal sections showing the nuclear subdividion of the amygdala in humans (left) and rodents (right). Although nomenclature varies, many regions are thought to have cross-species homology. Anterior commissure (ac); Basomedial nucleus (BM; BMA); Basolateral nucleus (BL; BLA); Basolateral nucleus paralaminar part (BLPL); Central nucleus (Ce, CEA); Central nucleus lateral part (CeL; CEAl); Central nucleus medial part (CeM; CEAm); Central nucleus capsular part (CEAc); Internal capsule (int); Lateral nucleus (La; LA); Medial nucleus (Me; MEA); Optic tract (opt); Substantia innominata (SI). Figure modified and reprinted from references (104 and 106) with permission from Elsevier and reference (105) by permission from Macmillan Publishers Ltd.
The homology of prefrontal cortical structures in rodents and humans is fairly controversial(18, 35). In rodents, the PFC includes two topologically different areas: the medial prefrontal cortex (mPFC) and the orbital prefrontal cortex (oPFC). The former may be subdivided in anterior cingulate cortex, the prelimbic cortex (PL), and infralimbic cortex (IL; Figure 2)(18, 35). In fear conditioning, the PL has been suggested to play a role in maintaining freezing behavior beyond the initial rapid response processed within the amygdala (36).
Figure. 2.
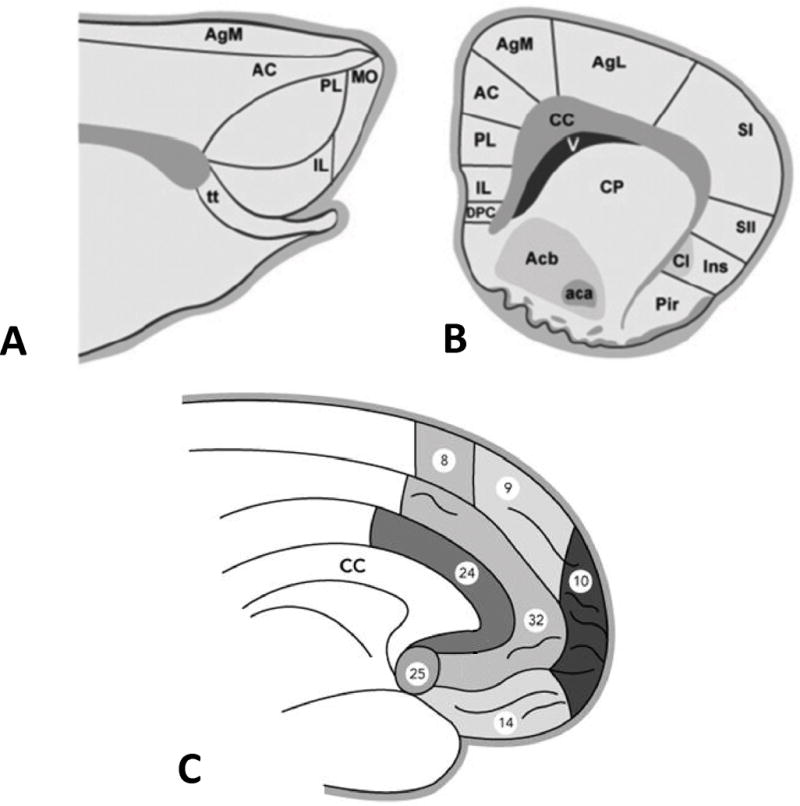
Prefrontal cortex in rodents and humans. In rodents the medial surface of the frontal cortex may be divided in frontal, anterior cingulate (AC), prelimbic (PL), and infralimbic cortices (IL) (A sagittal plane; B coronal plane). In C, cytoarchitectonic subdivision of the medial surface of the prefrontal cortex in humans. Numbers in C represent Brodmann areas. Aca, anterior commissure; Acb, nucleus accumbens; AgM, agranular cortex medial; AgL, agranular cortex lateral; CC, corpus callosum; Cl, claustrum; CP, caudate putamen; DPC, dorsal peduncular cortex; Ins, insula; MO, medial orbital cortex; Pir, piriform cortex; SI, primary somatosensory cortex; SII, somatosensory cortex; V, ventricle; tt, tenia tecta. Reprinted from references (18, 107) with permission from Elsevier.
In rodents, the hippocampus is divided in ventral (vHPC) and dorsal (dHPC). The former corresponds to the anterior and the latter to the posterior hippocampus in primates(37). In general, the hippocampus does not play a major role in the initial acquisition of conditioned fear responses, except when these are context-dependent (e.g. spatial orientation and color of the testing chamber)(38). It projects to both PL and IL, and has reciprocal connections with the BLA, providing contextual modulation over fear responses(39).
Fear Extinction
Fear extinction is an important learning phenomenon that refers to the gradual reduction of conditioned responses upon repeated presentations of the neutral CS. Once extinction has occurred, a way of testing whether the new memory has been learned is to present the animals once more with a reminder CS, a phenomenon sometimes known as ‘extinction recall’. Rather than a memory deletion, fear extinction is theorized to involve the acquisition of new memories that serve to suppress the initial conditioned fear memory (40).
In the clinic, PTSD has been commonly described as a disorder of fear extinction impairment(39). Most people that experience a traumatic event will likely undergo emotional changes and physical symptoms in the aftermath(41). For example, hypervigilance, anxiety, and avoidance of trauma-related stimuli are common after a traumatic event but usually resolve and dissipate after a period of a couple of months in the majority of individuals. Over time, healthy subjects are able to learn that stimuli reminding them of the traumatic event do not predict the re-occurrence of the trauma (i.e. they are able to extinguish the association between the trauma-related stimuli and the aversive nature of the traumatic experience). In individuals that suffer from PTSD, the conditioned fear fails to extinguish and trauma reminders continue to elicit pathological responses long after the danger has passed.
Another important notion is that the extinction paradigm may play a valuable role in the treatment of anxiety disorders such as phobias and PTSD. In exposure therapy, PTSD patients are exposed to trauma-related cues or stimuli that gradually increase in intensity within the context of a safe environment(42). In most patients, multiple sessions effectively reduce symptoms, allowing them to learn to inhibit fear responses in light of new safety cues(43). However, in more severe cases of PTSD and other anxiety disorders, exposure therapy is not as effective, suggesting that these refractory patients may have deficits in extinction learning(44, 45). Furthermore, clinical follow-up data show that benefits from cognitive therapy or imagination-based exposure are not maintained in up to 40% of individuals treated for PTSD at 12-months(46).
Neurocircuitry of Fear Extinction
Studies demonstrating a spontaneous recovery of a conditioned fear following extinction indicate that the conditioned fear association is not erased(47, 48). Instead, the formation of a new extinction memory that acts to inhibit the initial conditioned fear association is purported to be the underlying mechanism of extinction training(40). The process of forming the extinction memory and the manner by which it inhibits the fear response is mediated by neurocircuitry that is somewhat different from that of fear conditioning. Structures that are relevant to fear extinction include the ventromedial prefrontal cortex, BLA, intercalated cell clusters of the amygdala, and the hippocampus. In the vmPFC, the infralimbic cortex plays a role in inhibiting conditioned fear following extinction(49, 50), whereas the prelimbic cortex is primarily involved in the behavioral expression of fear (i.e. freezing)(50, 51). In addition, the IL also seems to modulate fear-response inhibition by activating GABAergic ITC clusters(52). These receive excitatory input from BLA, and send inhibitory projections to central nucleus of the amygdala (53). During extinction, IL inputs activate the ITC, subsequently inhibiting CE and reducing the fear response (Figure 3)(54).
Figure. 3.
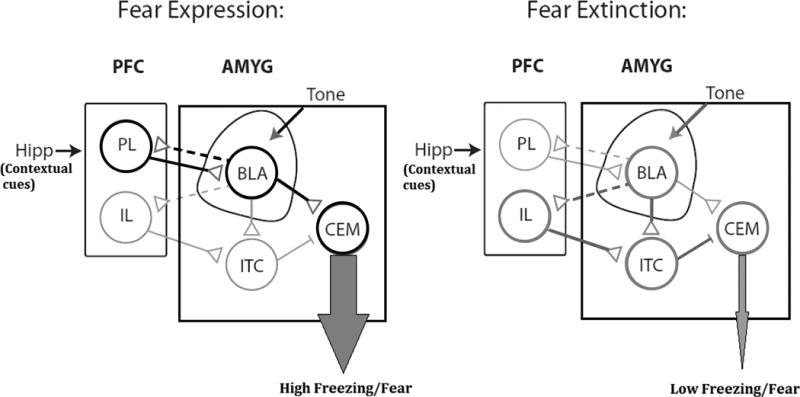
Basic schematic diagram illustrating important interactions between structures involved in fear conditioning and extinction. Black and dark gray thick lines indicate circuitry active in fear expression and extinction, respectively. Interrupted lines indicate competing memory traces that result in opposing behavioral responses. PFC; Prefrontal Cortex. PL; Prelimbic Cortex. IL; Infralimbic cortex. AMYG; Amygdala. BLA; Basolateral amygdala. ITC; Intercalated cell clusters. CEM; Central nucleus of Amygdala. Hipp; Hippocampus. Reprinted from reference (108) with permission from Elsevier.
As for the hippocampus, while both ventral and dorsal regions are involved in mechanisms of contextual-based conditioning and extinction, only the vHPC modulate defensive behaviors associated with contextual fear(55).
Extinction Learning: Relevance to PTSD
The literature spanning preclinical and clinical studies has demonstrated that brain areas implicated in rodent models are also robustly involved in human fear learning and extinction(39, 56, 57). Neuroimaging studies of PTSD patients have been crucial in providing insight into the structural and functional abnormalities associated with this disorder(58). In agreement with animal studies, the areas most commonly associated with PTSD include the amygdala, hippocampus, and prefrontal cortex.
Studies using both functional Magnetic Resonance Imaging (fMRI) and Positron Emission Tomography (PET) have found an increased activity in the amygdala of patients with PTSD in response to trauma-related cues(59), during emotional processing tasks(60), and at rest(61). Similar amygdala responses have also been documented when patients were presented both consciously(62) or unconsciously(63) to trauma or fear-related stimuli and during emotionally neutral tasks(64).
The ventromedial prefrontal cortex, the rough anatomical equivalent of the rodent IL/ventral PL cortex (Figure 2), is implicated in the processing and modulation of fear and negative emotional affect in humans(65). In contrast to the amygdala, this region seems to be hyporesponsive in PTSD(66). PET studies using trauma-related imagery, sounds, or narratives showed decreased blood flow in the vmPFC of combat exposed veterans(67) and sexually abused woman with PTSD(68). A reduced activity in the vmPFC was also reported in PTSD patients during fear processing(62), in response to trauma-reminding cues(69), and during resting state(70). However, a meta-analysis of 15 fMRI studies underscored the finding that PTSD is associated with significant vmPFC hypoactivation but suggested an increased activation of the amygdala(71).
While functional imaging studies of the amygdala and mPFC have been somewhat consistent, investigations of hippocampus activity in PTSD have yielded mixed results. Reduced hippocampal activity was reported in patients during a Verbal Declarative Memory Task(72) and also correlated with more severe PTSD symptoms during a virtual Morris water maze task(73). Furthermore, reduced activity in the hippocampus was reported in PTSD patients during successful encoding of trauma-related stimuli and was also associated with high arousal symptoms on the Clinician-Administered PTSD Scale (CAPS)(74). Conversely, a separate study found enhanced activation of the left hippocampus in PTSD in comparison to controls during encoding and recognition of emotional words in declarative memory task(75).
TREATMENT RESISTANCE IN PTSD
Treatments for PTSD largely fall into two main categories: psychotherapy and medications. In addition, non-invasive neuromodulation treatments, such as transcranial magnetic stimulation (TMS), are currently being investigated with promising results(76). Widely employed psychotherapeutic interventions include cognitive behavioral therapy, exposure therapy, group therapy and eye movement desensitization and reprocessing. With regards to medication, the most commonly prescribed agents are selective serotonin reuptake inhibitors (SSRI; e.g. paroxetine, sertraline, and fluoxetine). Other classes of drugs such as atypical antipsychotics, benzodiazepines, and α-adrenergic drugs have also been used(77).
Both psychotherapy and pharmacological interventions are effective for the treatment of PTSD. A large meta-analysis of over 112 PTSD treatment studies (both psychotherapeutic and pharmacological) conducted over the last 35 years found that cognitive behavioral therapy and exposure therapy account for the majority of all psychotherapy-based studies and also show the largest treatment effect sizes(77).
Among pharmacological interventions, antidepressants are the most commonly studied class of drugs. Watts and colleagues(77) reported that the only effective antidepressants superior to placebo were the selective serotonin reuptake inhibitors (SSRIs) paroxetine, fluoxetine, and sertraline, as well as the selective norepinephrine reuptake inhibitor venlafaxine, all of which showed moderate treatment effects. Other major categories including anticonvulsants, benzodiazepines, and α-adrenergic drugs did not differ statistically from placebo.
Despite the evidence for clinical efficacy described-above, a substantial number of individuals continue to experience PTSD symptoms despite appropriate medical treatment. In psychotherapy-based studies, over 30% of patients that completed a full course of treatment continue to meet criteria for PTSD(78). Response rates to treatment with SSRIs are usually no higher than 60%(79).
While there is no commonly accepted definition of treatment-resistant PTSD (TR-PTSD), an individual who, despite adequate treatment with antidepressants and cognitive behavioral therapy, continues to meet the criteria for PTSD is considered treatment-resistant(80). Resistance to treatment has been thought to be associated with more severe cases of PTSD, usually occurring in patients with multiple traumas or chronic trauma exposure, as well as comorbidity with other psychiatric illnesses, such as substance abuse and mood disorders(80).
DEEP BRAIN STIMULATION
Over the last decades, knowledge gained with the use of DBS for the treatment of movement disorders has been leveraged to other fields, including psychiatric disorders. Several clinical trials have been conducted to investigate the use of DBS for treating conditions such as obsessive-compulsive disorder (OCD)(81–83), addiction(84), anorexia(85) and depression(86–88).
Preclinical Studies using DBS in models of Conditioned Fear and Anxiety
In preclinical models, stimulation has been delivered to multiple structures either to study the behavioral consequences of such treatment or to understand the role of specific regions in mechanisms of conditioned fear, extinction and anxiety. Targets explored in such studies include the basolateral amygdala(89–91), ventral striatum(92), hippocampus(93), and prefrontal cortex(50, 94).
The effects of BLA DBS have been mainly studied in animals during defensive burying, an innate rodent behavior in which animals tend to bury objects they consider to be dangerous, threatening or associated with an unpleasant experience. Overall, treatments that reduce defensive burying are considered to be anxiolytic. Stimulation at high frequencies (e.g. 150–200Hz) delivered prior to testing has been shown to reduce burying latency(89, 90). However, a potential side effect in animals stimulated with high current intensities in BLA is the development of epileptiform after-discharges(91).
The effects of DBS in the ventral striatum have been largely tested in fear conditioning/extinction-based paradigms. Once conditioned with tones/footshocks, animals received DBS at 130Hz for 1h before, 1h during, and 1h after extinction training(92). DBS delivered bilaterally through electrodes that were dorsal to the anterior commissure led to a significant decrease in freezing when animals were re-exposed to conditioned cues.
High frequency stimulation (e.g. 100Hz) delivered in trains or bursts to the hippocampus after conditioning and/or extinction improved extinction learning and/or reduced freezing during recall(95, 96). In contrast, low frequency hippocampal stimulation at 2Hz delivered after extinction, but not following conditioning, impaired extinction learning and the development of hippocampal-PFC plasticity(93, 97).
Similar to what has been observed in studies of the hippocampus, fear conditioning/extinction findings in animals given PFC stimulation varied according to the timing of current delivery and target(49, 94). When administered within 100–400ms of CS, PL stimulation impaired, while IL stimulation facilitated extinction learning(50). An improvement in extinction learning has also been demonstrated when either pulses or trains of high frequency stimulation (100Hz) were applied to the IL following conditioning/extinction and/or reconditioning of the animals to the conditioning context(98, 99).
Clinical use of DBS in PTSD
Though a clinical trial to treat PTSD with DBS has been proposed(100), data is still lacking. In the only case report published to date, Langevin and colleagues have treated a combat veteran with BLA DBS (Figure 4)(101). After enrollment, 18F-fluorodeoxyglucose PET was obtained at rest and during an activated task, which consisted in asking the patient to recall the traumatic event. When compared to the rest scan, images obtained while he recalled the traumatic event showed higher metabolism in the amygdala. Attesting to the safety of the procedure, video-electroencephalogram carried out after the implantation of BLA electrodes and stimulation ruled out the development of seizures or afterdischarges. At eight months postoperatively, clinical improvement was noticeable with a 37.8% reduction in CAPS scores as compared to baseline. Stimulation settings were 60μsec, 160Hz, 1.4V on the right and 0.7V on the left.
Figure. 4.
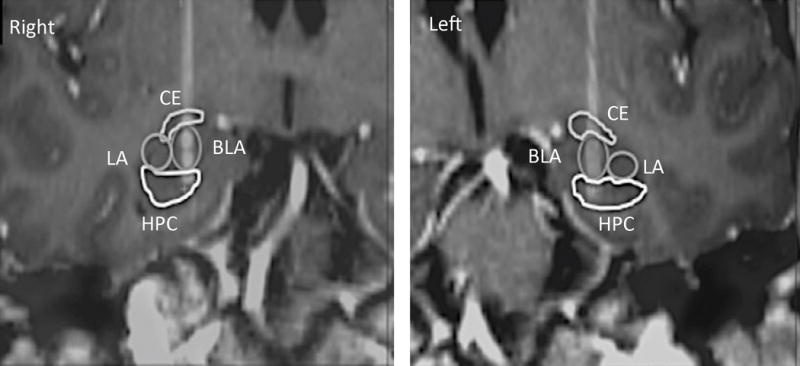
Postoperative CT fused to the preoperative MRI in a coronal plane across the electrode trajectories. Individual contact can be seen at the distal tip of the electrodes. Central nucleus (CE); Basolateral nucleus (BLA); Hippocampus (HPC); Lateral nucleus (LA). Reprinted from reference (101) with permission from Elsevier.
CONCLUSIONS
To date, the circuitry and mechanisms of fear, anxiety and to some extent PTSD are relatively well understood(102). Preclinical work suggests that stimulation at high frequency delivered at particular timeframes to different targets, including the amygdala, ventral striatum, hippocampus and prefrontal cortex may facilitate fear extinction and improve anxiety-like behavior in rodents. As these structures are all part of the neurocircuitry associated with PTSD they may be considered as suitable DBS targets to be investigated in the future. That said, several caveats need to be considered when data from animal models are to be translated. As noted in this review, preclinical paradigms used to date are more suited for the study of fear and anxiety in the relative short-term, and may therefore be somewhat limited for modeling PTSD. Furthermore, in most studies, naïve rodents exposed to trauma or stress are given DBS as a first-line treatment (i.e. without failing medical therapy). Lastly, in contrast to clinical applications of DBS, stimulation is usually given for short time intervals rather than continuously for prolonged periods. Despite these limitations, studies in animal models may help us understand mechanisms of DBS and improve the efficacy of this treatment (19). Future studies should aim to characterize the chronic effects of DBS in animal preparations that include paradigms to assess multiple behavioral domains.
To date, there is a paucity of viable options for patients with refractory PTSD. As such, efforts have been made to investigate novel approaches to treatment development, leading to promising results from preliminary clinical studies(103). Neuromodulation therapies and their associated side effects are generally well tolerated. Nevertheless, the invasive nature of DBS brings with it a certain level of risk which should always be fully disclosed by the investigators and appreciated by the patient. As PTSD is a multi-symptomatic disorder, patients included in investigational studies should ideally be treated and managed by multidisciplinary teams, including psychiatrists, psychologists and neurosurgeons. Informed consent has to be carefully obtained, taking into account the competency of the patient.
In the only clinical report published so far, a patient implanted with BLA electrodes has shown striking improvements in PTSD symptoms(101). Though encouraging, these results certainly require further corroboration. Within the realm of psychiatric disorders, DBS is only offered as an investigational treatment to refractory patients. In contrast to OCD or major depression, criteria to define this population in PTSD are not well established. To date, clinical trials using pharmacological interventions have been largely conducted in previously untreated patients or subjects receiving isolated therapies. Patients who do not respond to psychotherapy or first line drug regimens are often those with co-morbid psychiatric conditions and personality disorders (80). The presence of the latter often comprises an exclusion criterion in most DBS trials. Also unknown in patients with treatment-refractory PTSD is the magnitude of a placebo response.
The above-mentioned considerations are just a portion of those needed to be addressed in order to design appropriate clinical studies of DBS in PTSD. Optimal stimulation parameters, targets, mechanisms of action and the kinetics of stimulation will also need to be characterized prior to the launch of larger scale studies.
Acknowledgments
Funding Statement: This work was supported in part with funds from the National Institutes of Health (R21 MH110846).
Footnotes
Authorship Statement: Roman Reznikov and Clement Hamani wrote and proofread the manuscript. Both authors approved the final version.
Conflict of Interest Statement: The authors have no conflicts of interest to declare.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM V) 4th. Washington, DC: 2013. Revised. [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 4.Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351:13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- 5.Kang HK, Natelson BH, Mahan CM, Lee KY, Murphy FM. Post-traumatic stress disorder and chronic fatigue syndrome-like illness among Gulf War veterans: a population-based survey of 30,000 veterans. Am J Epidemiol. 2003;157:141–148. doi: 10.1093/aje/kwf187. [DOI] [PubMed] [Google Scholar]
- 6.Sareen J, Cox BJ, Afifi TO, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry. 2005;62:1249–1257. doi: 10.1001/archpsyc.62.11.1249. [DOI] [PubMed] [Google Scholar]
- 7.Breslau N. The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma Violence Abuse. 2009;10:198–210. doi: 10.1177/1524838009334448. [DOI] [PubMed] [Google Scholar]
- 8.Breslau N, Davis GC, Andreski P, Peterson E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry. 1991;48:216–222. doi: 10.1001/archpsyc.1991.01810270028003. [DOI] [PubMed] [Google Scholar]
- 9.Stein MB, Jang KL, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am J Psychiatry. 2002;159:1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- 10.True WR, Rice J, Eisen SA, et al. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50:257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- 11.Trickey D, Siddaway AP, Meiser-Stedman R, Serpell L, Field AP. A meta-analysis of risk factors for post-traumatic stress disorder in children and adolescents. Clin Psychol Rev. 2012;32:122–138. doi: 10.1016/j.cpr.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:748–766. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- 13.Broekman BF, Olff M, Boer F. The genetic background to PTSD. Neurosci Biobehav Rev. 2007;31:348–362. doi: 10.1016/j.neubiorev.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Yehuda R. Advances in understanding neuroendocrine alterations in PTSD and their therapeutic implications. Ann N Y Acad Sci. 2006;1071:137–166. doi: 10.1196/annals.1364.012. [DOI] [PubMed] [Google Scholar]
- 15.Angelucci F, Ricci V, Gelfo F, et al. BDNF serum levels in subjects developing or not post-traumatic stress disorder after trauma exposure. Brain Cogn. 2014;84:118–122. doi: 10.1016/j.bandc.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Southwick SM, Paige S, Morgan CA, 3rd, Bremner JD, Krystal JH, Charney DS. Neurotransmitter alterations in PTSD: catecholamines and serotonin. Semin Clin Neuropsychiatry. 1999;4:242–248. doi: 10.153/SCNP00400242. [DOI] [PubMed] [Google Scholar]
- 17.Yehuda R, Brand S, Yang RK. Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry. 2006;59:660–663. doi: 10.1016/j.biopsych.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 18.Hamani C, Nobrega JN. Preclinical studies modeling deep brain stimulation for depression. Biol Psychiatry. 2012;72:916–923. doi: 10.1016/j.biopsych.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamani C, Temel Y. Deep brain stimulation for psychiatric disease: contributions and validity of animal models. Sci Transl Med. 2012;4:142rv148. doi: 10.1126/scitranslmed.3003722. [DOI] [PubMed] [Google Scholar]
- 20.Reznikov R, Binko M, Nobrega JN, Hamani C. Deep Brain Stimulation in Animal Models of Fear, Anxiety, and Posttraumatic Stress Disorder. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lissek S, Powers AS, McClure EB, et al. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behav Res Ther. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Eysenck HJ. The conditioning model of neurosis. Behavioural and Brain Sciences. 1979;2:155–199. [Google Scholar]
- 23.Pitman RK, Orr SP, Shalev AY, Metzger LJ, Mellman TA. Psychophysiological alterations in post-traumatic stress disorder. Semin Clin Neuropsychiatry. 1999;4:234–241. doi: 10.153/SCNP00400234. [DOI] [PubMed] [Google Scholar]
- 24.Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav Res Ther. 2007;45:2019–2033. doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109:290–298. [PubMed] [Google Scholar]
- 26.Romanski LM, Clugnet MC, Bordi F, LeDoux JE. Somatosensory and auditory convergence in the lateral nucleus of the amygdala. Behav Neurosci. 1993;107:444–450. doi: 10.1037//0735-7044.107.3.444. [DOI] [PubMed] [Google Scholar]
- 27.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 28.Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19:613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- 29.Orsini CA, Kim JH, Knapska E, Maren S. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J Neurosci. 2011;31:17269–17277. doi: 10.1523/JNEUROSCI.4095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson LR, McGuire J, Lazarus R, Palmer AA. Pavlovian fear memory circuits and phenotype models of PTSD. Neuropharmacology. 2012;62:638–646. doi: 10.1016/j.neuropharm.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 31.LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwata J, Chida K, LeDoux JE. Cardiovascular responses elicited by stimulation of neurons in the central amygdaloid nucleus in awake but not anesthetized rats resemble conditioned emotional responses. Brain Res. 1987;418:183–188. doi: 10.1016/0006-8993(87)90978-4. [DOI] [PubMed] [Google Scholar]
- 33.Hsu DT, Chen FL, Takahashi LK, Kalin NH. Rapid stress-induced elevations in corticotropin-releasing hormone mRNA in rat central amygdala nucleus and hypothalamic paraventricular nucleus: an in situ hybridization analysis. Brain Res. 1998;788:305–310. doi: 10.1016/s0006-8993(98)00032-8. [DOI] [PubMed] [Google Scholar]
- 34.Rosen JB, Donley MP. Animal studies of amygdala function in fear and uncertainty: relevance to human research. Biol Psychol. 2006;73:49–60. doi: 10.1016/j.biopsycho.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 36.Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 39.VanElzakker MB, Dahlgren MK, Davis FC, Dubois S, Shin LM. From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol Learn Mem. 2014;113:3–18. doi: 10.1016/j.nlm.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 41.Bonanno GA. Loss, trauma, and human resilience: have we underestimated the human capacity to thrive after extremely aversive events? Am Psychol. 2004;59:20–28. doi: 10.1037/0003-066X.59.1.20. [DOI] [PubMed] [Google Scholar]
- 42.Rothbaum BO, Schwartz AC. Exposure therapy for posttraumatic stress disorder. Am J Psychother. 2002;56:59–75. doi: 10.1176/appi.psychotherapy.2002.56.1.59. [DOI] [PubMed] [Google Scholar]
- 43.Taylor S, Thordarson DS, Maxfield L, Fedoroff IC, Lovell K, Ogrodniczuk J. Comparative efficacy, speed, and adverse effects of three PTSD treatments: exposure therapy, EMDR, and relaxation training. J Consult Clin Psychol. 2003;71:330–338. doi: 10.1037/0022-006x.71.2.330. [DOI] [PubMed] [Google Scholar]
- 44.Foa EB. Psychosocial treatment of posttraumatic stress disorder. J Clin Psychiatry. 2000;61(Suppl 5):43–48. discussion 49–51. [PubMed] [Google Scholar]
- 45.van Minnen A, Wessel I, Dijkstra T, Roelofs K. Changes in PTSD patients’ narratives during prolonged exposure therapy: a replication and extension. J Trauma Stress. 2002;15:255–258. doi: 10.1023/A:1015263513654. [DOI] [PubMed] [Google Scholar]
- 46.Tarrier N, Sommerfield C, Pilgrim H, Humphreys L. Cognitive therapy or imaginal exposure in the treatment of post-traumatic stress disorder. Twelve-month follow-up. Br J Psychiatry. 1999;175:571–575. doi: 10.1192/bjp.175.6.571. [DOI] [PubMed] [Google Scholar]
- 47.Quirk GJ. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn Mem. 2002;9:402–407. doi: 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rescorla RA. Retraining of extinguished Pavlovian stimuli. J Exp Psychol Anim Behav Process. 2001;27:115–124. [PubMed] [Google Scholar]
- 49.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 50.Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corcoran KA, Quirk GJ. Recalling safety: cooperative functions of the ventromedial prefrontal cortex and the hippocampus in extinction. CNS Spectr. 2007;12:200–206. doi: 10.1017/s1092852900020915. [DOI] [PubMed] [Google Scholar]
- 52.Amano T, Unal CT, Pare D. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci. 2010;13:489–494. doi: 10.1038/nn.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Royer S, Martina M, Pare D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci. 1999;19:10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ballesteros CI, de Oliveira Galvao B, Maisonette S, Landeira-Fernandez J. Effect of dorsal and ventral hippocampal lesions on contextual fear conditioning and unconditioned defensive behavior induced by electrical stimulation of the dorsal periaqueductal gray. PLoS One. 2014;9:e83342. doi: 10.1371/journal.pone.0083342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 58.Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- 59.Shin LM, McNally RJ, Kosslyn SM, et al. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. Am J Psychiatry. 1999;156:575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- 60.Rauch SL, Whalen PJ, Shin LM, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 61.Chung YA, Kim SH, Chung SK, et al. Alterations in cerebral perfusion in posttraumatic stress disorder patients without re-exposure to accident-related stimuli. Clin Neurophysiol. 2006;117:637–642. doi: 10.1016/j.clinph.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 62.Shin LM, Wright CI, Cannistraro PA, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 63.Bryant RA, Kemp AH, Felmingham KL, et al. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Hum Brain Mapp. 2008;29:517–523. doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bryant RA, Felmingham KL, Kemp AH, et al. Neural networks of information processing in posttraumatic stress disorder: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:111–118. doi: 10.1016/j.biopsych.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 65.Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage. 2011;58:275–285. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- 66.Hughes KC, Shin LM. Functional neuroimaging studies of post-traumatic stress disorder. Expert Rev Neurother. 2011;11:275–285. doi: 10.1586/ern.10.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry. 2005;57:832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 68.Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shin LM, Orr SP, Carson MA, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 70.Sripada RK, King AP, Garfinkel SN, et al. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci. 2012;37:241–249. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carrion VG, Haas BW, Garrett A, Song S, Reiss AL. Reduced hippocampal activity in youth with posttraumatic stress symptoms: an FMRI study. J Pediatr Psychol. 2010;35:559–569. doi: 10.1093/jpepsy/jsp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Astur RS, St Germain SA, Tolin D, Ford J, Russell D, Stevens M. Hippocampus function predicts severity of post-traumatic stress disorder. Cyberpsychol Behav. 2006;9:234–240. doi: 10.1089/cpb.2006.9.234. [DOI] [PubMed] [Google Scholar]
- 74.Hayes JP, LaBar KS, McCarthy G, et al. Reduced hippocampal and amygdala activity predicts memory distortions for trauma reminders in combat-related PTSD. J Psychiatr Res. 2011;45:660–669. doi: 10.1016/j.jpsychires.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomaes K, Dorrepaal E, Draijer NP, et al. Increased activation of the left hippocampus region in Complex PTSD during encoding and recognition of emotional words: a pilot study. Psychiatry Res. 2009;171:44–53. doi: 10.1016/j.pscychresns.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 76.Cohen H, Kaplan Z, Kotler M, Kouperman I, Moisa R, Grisaru N. Repetitive transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in posttraumatic stress disorder: a double-blind, placebo-controlled study. Am J Psychiatry. 2004;161:515–524. doi: 10.1176/appi.ajp.161.3.515. [DOI] [PubMed] [Google Scholar]
- 77.Watts BV, Schnurr PP, Mayo L, Young-Xu Y, Weeks WB, Friedman MJ. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. J Clin Psychiatry. 2013;74:e541–550. doi: 10.4088/JCP.12r08225. [DOI] [PubMed] [Google Scholar]
- 78.Bradley R, Greene J, Russ E, Dutra L, Westen D. A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry. 2005;162:214–227. doi: 10.1176/appi.ajp.162.2.214. [DOI] [PubMed] [Google Scholar]
- 79.Berger W, Mendlowicz MV, Marques-Portella C, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:169–180. doi: 10.1016/j.pnpbp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hamner MB, Robert S, Frueh BC. Treatment-resistant posttraumatic stress disorder: strategies for intervention. CNS Spectr. 2004;9:740–752. doi: 10.1017/s1092852900022380. [DOI] [PubMed] [Google Scholar]
- 81.Hamani C, Pilitsis J, Rughani AI, et al. Deep brain stimulation for obsessive-compulsive disorder: systematic review and evidence-based guideline sponsored by the American Society for Stereotactic and Functional Neurosurgery and the Congress of Neurological Surgeons (CNS) and endorsed by the CNS and American Association of Neurological Surgeons. Neurosurgery. 2014;75:327–333. doi: 10.1227/NEU.0000000000000499. quiz 333. [DOI] [PubMed] [Google Scholar]
- 82.Mallet L, Polosan M, Jaafari N, et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359:2121–2134. doi: 10.1056/NEJMoa0708514. [DOI] [PubMed] [Google Scholar]
- 83.Denys D, Mantione M, Figee M, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67:1061–1068. doi: 10.1001/archgenpsychiatry.2010.122. [DOI] [PubMed] [Google Scholar]
- 84.Stelten BM, Noblesse LH, Ackermans L, Temel Y, Visser-Vandewalle V. The neurosurgical treatment of addiction. Neurosurg Focus. 2008;25:E5. doi: 10.3171/FOC/2008/25/7/E5. [DOI] [PubMed] [Google Scholar]
- 85.Lipsman N, Woodside DB, Giacobbe P, et al. Subcallosal cingulate deep brain stimulation for treatment-refractory anorexia nervosa: a phase 1 pilot trial. Lancet. 2013;381:1361–1370. doi: 10.1016/S0140-6736(12)62188-6. [DOI] [PubMed] [Google Scholar]
- 86.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 87.Dougherty DD, Rezai AR, Carpenter LL, et al. A Randomized Sham-Controlled Trial of Deep Brain Stimulation of the Ventral Capsule/Ventral Striatum for Chronic Treatment-Resistant Depression. Biol Psychiatry. 2015;78:240–248. doi: 10.1016/j.biopsych.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 88.Schlaepfer TE, Bewernick BH, Kayser S, Madler B, Coenen VA. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 2013;73:1204–1212. doi: 10.1016/j.biopsych.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 89.Langevin JP, De Salles AA, Kosoyan HP, Krahl SE. Deep brain stimulation of the amygdala alleviates post-traumatic stress disorder symptoms in a rat model. J Psychiatr Res. 2010;44:1241–1245. doi: 10.1016/j.jpsychires.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 90.Stidd DA, Vogelsang K, Krahl SE, Langevin JP, Fellous JM. Amygdala deep brain stimulation is superior to paroxetine treatment in a rat model of posttraumatic stress disorder. Brain Stimul. 2013;6:837–844. doi: 10.1016/j.brs.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 91.Saldivar-Gonzalez JA, Posadas-Andrews A, Rodriguez R, et al. Effect of electrical stimulation of the baso-lateral amygdala nucleus on defensive burying shock probe test and elevated plus maze in rats. Life Sci. 2003;72:819–829. doi: 10.1016/s0024-3205(02)02335-4. [DOI] [PubMed] [Google Scholar]
- 92.Rodriguez-Romaguera J, Do Monte FH, Quirk GJ. Deep brain stimulation of the ventral striatum enhances extinction of conditioned fear. Proc Natl Acad Sci U S A. 2012;109:8764–8769. doi: 10.1073/pnas.1200782109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Garcia R, Spennato G, Nilsson-Todd L, Moreau JL, Deschaux O. Hippocampal low-frequency stimulation and chronic mild stress similarly disrupt fear extinction memory in rats. Neurobiol Learn Mem. 2008;89:560–566. doi: 10.1016/j.nlm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 94.Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- 95.Deschaux O, Motanis H, Spennato G, Moreau JL, Garcia R. Re-emergence of extinguished auditory-cued conditioned fear following a sub-conditioning procedure: effects of hippocampal and prefrontal tetanic stimulations. Neurobiol Learn Mem. 2011;95:510–518. doi: 10.1016/j.nlm.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 96.Farinelli M, Deschaux O, Hugues S, Thevenet A, Garcia R. Hippocampal train stimulation modulates recall of fear extinction independently of prefrontal cortex synaptic plasticity and lesions. Learn Mem. 2006;13:329–334. doi: 10.1101/lm.204806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Deschaux O, Thevenet A, Spennato G, Arnaud C, Moreau JL, Garcia R. Low-frequency stimulation of the hippocampus following fear extinction impairs both restoration of rapid eye movement sleep and retrieval of extinction memory. Neuroscience. 2010;170:92–98. doi: 10.1016/j.neuroscience.2010.06.067. [DOI] [PubMed] [Google Scholar]
- 98.Nachon O, Cleren C, Husson S, et al. Prefrontal tetanic stimulation, following fear reconditioning, facilitates expression of previously acquired extinction. Neurobiol Learn Mem. 2014;113:62–68. doi: 10.1016/j.nlm.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 99.Maroun M, Kavushansky A, Holmes A, Wellman C, Motanis H. Enhanced extinction of aversive memories by high-frequency stimulation of the rat infralimbic cortex. PLoS One. 2012;7:e35853. doi: 10.1371/journal.pone.0035853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Koek RJ, Langevin JP, Krahl SE, et al. Deep brain stimulation of the basolateral amygdala for treatment-refractory combat post-traumatic stress disorder (PTSD): study protocol for a pilot randomized controlled trial with blinded, staggered onset of stimulation. Trials. 2014;15:356. doi: 10.1186/1745-6215-15-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Langevin JP, Koek RJ, Schwartz HN, et al. Deep Brain Stimulation of the Basolateral Amygdala for Treatment-Refractory Posttraumatic Stress Disorder. Biol Psychiatry. 2016;79:e82–84. doi: 10.1016/j.biopsych.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 102.Taghva A, Oluigbo C, Corrigan J, Rezai AR. Posttraumatic stress disorder: neurocircuitry and implications for potential deep brain stimulation. Stereotact Funct Neurosurg. 2013;91:207–219. doi: 10.1159/000343148. [DOI] [PubMed] [Google Scholar]
- 103.Bowers ME, Ressler KJ. An Overview of Translationally Informed Treatments for Posttraumatic Stress Disorder: Animal Models of Pavlovian Fear Conditioning to Human Clinical Trials. Biol Psychiatry. 2015;78:E15–27. doi: 10.1016/j.biopsych.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fox AS, Oler JA, Tromp do PM, Fudge JL, Kalin NH. Extending the amygdala in theories of threat processing. Trends Neurosci. 2015;38:319–329. doi: 10.1016/j.tins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lein ES, Hawrylycz MJ, Ao N, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 106.Mai JK, Majtanik M, Paxinos G. Atlas of the Human Brain. 4th. Academic Press; 2015. [Google Scholar]
- 107.Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM. The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry. 2011;69:301–308. doi: 10.1016/j.biopsych.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 108.Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


