Abstract
Aims: A kidney-brain interaction has been described in acute kidney injury, but the mechanisms are uncertain. Since we recently described a reno-cerebral reflex, we tested the hypothesis that renal ischemia-reperfusion injury (IRI) activates a sympathetic reflex that interlinks the renal and cerebral renin-angiotensin axis to promote oxidative stress and progression of the injury.
Results: Bilateral ischemia-reperfusion activated the intrarenal and cerebral, but not the circulating, renin-angiotensin system (RAS), increased sympathetic activity in the kidney and the cerebral sympathetic regulatory regions, and induced brain inflammation and kidney injury. Selective renal afferent denervation with capsaicin or renal denervation significantly attenuated IRI-induced activation of central RAS and brain inflammation. Central blockade of RAS or oxidative stress by intracerebroventricular (ICV) losartan or tempol reduced the renal ischemic injury score by 65% or 58%, respectively, and selective renal afferent denervation or reduction of sympathetic tone by ICV clonidine decreased the score by 42% or 52%, respectively (all p < 0.05). Ischemia-reperfusion-induced renal damage and dysfunction persisted after controlling blood pressure with hydralazine.
Innovation: This study uncovered a novel reflex pathway between ischemic kidney and the brain that sustains renal oxidative stress and local RAS activation to promote ongoing renal damage.
Conclusions: These data suggest that the renal and cerebral renin-angiotensin axes are interlinked by a reno-cerebral sympathetic reflex that is activated by ischemia-reperfusion, which contributes to ischemia-reperfusion-induced brain inflammation and worsening of the acute renal injury. Antioxid. Redox Signal. 27, 415–432.
Keywords: : acute kidney injury, renin-angiotensin system, sympathetic nervous system, brain, kidney
Introduction
Acute kidney injury (AKI) is common and carries high in-hospital mortality (19, 24). Dysfunction of distant organs, including brain, worsens the progression and prognosis of AKI (19, 38). AKI increases cerebral microglial cells (brain macrophages) and pyknotic neurons in the hippocampus (Hippo), activates neurons in brain regions associated with the stress response or autonomic activation, and impairs motor activity (35, 40). This has been related to systemic inflammation that can increase the permeability of the blood–brain barrier (9, 35). Such crosstalk between the kidney and brain could be mediated by the release of humoral factors from damaged kidneys. However, it remains unknown whether the brain is merely a passive victim of the consequences of the renal injury or it actually contributes to the progression of AKI. Our recent description that rats fed a high-salt diet after renal mass reduction develop a reno-cerebral reflex involving renal afferent nerves that activates the brain sympathetic outflow, which, in turn, activates renal efferent sympathetic nerves and promotes renal damage, provides an alternative pathway (6). Several mechanisms may account for the worsening of AKI and its progression to chronic kidney disease (CKD), including angiotensin II (Ang II) and inflammation (16), salt intake (3), renal nerves (26), and reactive oxygen species (ROS) (34), but how these are integrated is not yet clear.
Innovation.
A kidney-brain interaction has been described in acute kidney injury, but the mechanisms are uncertain. This study demonstrates that renal ischemia-reperfusion injury activates a sympathetic reflex that interlinks the renal and cerebral renin-angiotensin system (RAS) axis to promote oxidative stress and progression of the kidney injury. This identifies a novel mechanism underlying reno-cerebral interaction in response to renal ischemia-reperfusion, and therefore could lead to new interventional approaches, such as the use of RAS inhibitors, sympatholytic agents, or renal nerve ablation.
Renal angiotensinogen (AGT) level is rate limiting for Ang II generation (10, 30). Urinary AGT has been proposed as a marker of the activity of the intrarenal renin-angiotensin system (RAS) (27, 29, 51) and a biomarker for AKI (1, 53), which suggests that the intrarenal RAS may modulate the severity of AKI. Indeed, pretreatment with RAS inhibitors mitigates renal ischemia-reperfusion injury (IRI) in several experimental models (13, 37, 49). Importantly, most organs possess a local RAS that is regulated independently and is somewhat compartmentalized from the circulation (10, 46). Our recent study reported activation of the local RAS not only in the damaged kidney but also in the brain in a high-salt model of CKD (6). The link between the renal and cerebral RAS axes was provided by a reflex activation of renal afferent sympathetic nerves by salt that increased cerebral and renal ROS (15) and promoted progression of CKD via activation of efferent sympathetic nerves (6). Thus, there can be a self-sustaining enhancement of the brain and the intrarenal RAS through neuronal pathways. Oxidative stress mediated much of the effect of the activated RAS to enhance damage in the kidney and to activate the brain sympathetic outflow (41). Interestingly, overactivation of the renal sympathetic nervous system (17, 33, 45) and severe intrarenal oxidative stress (23, 25) have been reported in IRI-induced AKI. Renal Nox4 expression is increased by hypoxia (12), suggesting the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in ischemic AKI.
In the present study, we used a mouse bilateral renal IRI model to test the hypothesis that the renal and cerebral RAS, which we have termed the “reno-cerebral RAS axis,” interact via changes in renal afferent and efferent sympathetic nerve activity to contribute to the renal and cerebral oxidative stress and to the progression of ischemic AKI.
Results
Time course of blood pressure, heart rate, and humoral parameters after IRI and effect of interventions
The mean arterial pressure (MAP) and heart rate increased over 1 to 3 days after IRI, accompanied by evidence of sympathetic activation (increase in serum norepinephrine), oxidative stress (elevation of serum 8-iso-prostaglandin F2α), and inflammation (increase in serum keratinocyte-derived cytokine [KC]) (Table 1). These parameters returned to baseline by 1 week, except for serum 8-iso-prostaglandin F2α that remained elevated, although it markedly reduced from the peak at 1 day. Plasma renin activity and Ang II level did not change significantly during the course of the study.
Table 1.
Changes in Blood Pressure, Heart Rate, and Biochemical and Metabolic Parameters in Sham and IRI Mice
| Time postischemia | ||||||
|---|---|---|---|---|---|---|
| Parameters | 1 h | 3 h | 6 h | 1 day | 3 day | 1 w |
| MAP (mmHg) | ||||||
| Sham | / | / | / | 116 ± 3 | 115 ± 3 | 115 ± 4 |
| IRI | / | / | / | 131 ± 5* | 125 ± 4* | 118 ± 3 |
| Heart rate (/min) | ||||||
| Sham | / | / | / | 585 ± 26 | 565 ± 28 | 557 ± 42 |
| IRI | / | / | / | 674 ± 23* | 591 ± 21* | 552 ± 31 |
| Serum K+ (mM) | ||||||
| Sham | 5.1 ± 0.5 | 5.4 ± 1.1 | 5.1 ± 0.7 | 4.8 ± 0.8 | 5.4 ± 1.2 | 5.2 ± 0.9 |
| IRI | 5.8 ± 1.9 | 6.6 ± 1.2 | 10.1 ± 0.9* | 9.4 ± 2.3* | 6.2 ± 0.6 | 5.7 ± 1.0 |
| Serum Na+ (mM) | ||||||
| Sham | 150 ± 3 | 148 ± 3 | 150 ± 2 | 152 ± 2 | 150 ± 1 | 151 ± 2 |
| IRI | 152 ± 1 | 151 ± 2 | 155 ± 2 | 154 ± 3 | 153 ± 2 | 152 ± 1 |
| Plasma Ang II (pg/ml) | ||||||
| Sham | 121 ± 30 | 123 ± 27 | 119 ± 32 | 117 ± 20 | 120 ± 24 | 125 ± 31 |
| IRI | 117 ± 21 | 110 ± 29 | 104 ± 31 | 106 ± 21 | 112 ± 26 | 119 ± 32 |
| Plasma renin activity (ng Ang I/ml/h) | ||||||
| Sham | 6.0 ± 1.8 | 5.8 ± 1.4 | 6.5 ± 1.2 | 5.7 ± 0.9 | 6.2 ± 0.7 | 6.0 ± 0.8 |
| IRI | 5.5 ± 1.7 | 5.1 ± 1.8 | 5.3 ± 1.5 | 6.2 ± 2.2 | 6.0 ± 2.0 | 5.8 ± 1.3 |
| Serum norepinephrine (ng/ml) | ||||||
| Sham | 11 ± 4 | 10 ± 3 | 10 ± 2 | 8 ± 3 | 9 ± 3 | 8 ± 2 |
| IRI | 13 ± 3 | 15 ± 3* | 17 ± 3* | 19 ± 5* | 12 ± 4* | 8 ± 3 |
| Serum 8-iso-PGF2α (ng/ml) | ||||||
| Sham | 0.9 ± 0.3 | 1.5 ± 0.5 | 1.1 ± 0.3 | 1.0 ± 0.4 | 0.9 ± 0.2 | 0.9 ± 0.3 |
| IRI | 41 ± 6* | 50 ± 5* | 62 ± 4* | 96 ± 6* | 11 ± 3* | 8 ± 2* |
| Serum KC (pg/ml) | ||||||
| Sham | 1.4 ± 0.3 | 1.6 ± 0.4 | 1.3 ± 0.5 | 1.5 ± 0.5 | 1.4 ± 0.3 | 1.3 ± 0.6 |
| IRI | 3.1 ± 1.0 | 75.9 ± 12.8* | 41.6 ± 15.2* | 25.1 ± 11.9* | 4.2 ± 1.9* | 1.7 ± 0.8 |
Data are expressed as mean ± SD (Each experiment has been replicated three times. For each time, each study group consists of six mice.).
p < 0.05 versus sham mice at the same time point.
8-iso-PGF2α, 8-iso-Prostaglandin2α; Ang II, angiotensin II; IRI, ischemia-reperfusion injury; KC, keratinocyte-derived cytokine; MAP, mean arterial pressure; SD, standard deviation.
The MAP and heart rate decreased after blockade of RAS (intragastric [IG] or intracerebroventricular [ICV] losartan [Los]), blockade of sympathetic outflow (ICV clonidine [Clo]) or afferent signal (renal arterial capsaicin), and treatment with anti-oxidant (ICV tempol [Tem]), accompanied by a parallel reduction in serum 8-iso-prostaglandin F2α and KC (Table 2). Importantly, IG hydralazine (Hyd) reduced mean blood pressure comparably but it did not change 8-iso-prostaglandin F2α and KC. No interventions significantly changed plasma Ang II.
Table 2.
Changes in Blood Pressure, Heart Rate, and Biochemical and Metabolic Parameters Among Treatment Groups 24 H After IRI
| Circulating parameters | |||||||
|---|---|---|---|---|---|---|---|
| Intervention | MAP (mmHg) | Heart rate (/min) | Ang II (pg/ml) | 8-iso-PGF2α (ng/ml) | KC (pg/ml) | K+ (mM) | Na+ (mM) |
| IG Los (mg/kg) | |||||||
| 0 | 129 ± 4 | 696 ± 32 | 101 ± 31 | 108 ± 19 | 26.5 ± 9.4 | 9.3 ± 2.4 | 152 ± 3 |
| 1 | 128 ± 3 | 688 ± 14 | 105 ± 26 | 99 ± 15 | 22.8 ± 6.3 | 9.6 ± 1.1 | 155 ± 2 |
| 75 | 112 ± 5* | 567 ± 13* | 119 ± 20 | 21 ± 9* | 4.2 ± 2.5* | 9.8 ± 1.2 | 153 ± 2 |
| ICV aCSF | 130 ± 4 | 686 ± 15 | 103 ± 29 | 98 ± 19 | 25.2 ± 7.1 | 9.2 ± 1.5 | 154 ± 3 |
| ICV Los (1 mg/kg) | 107 ± 2* | 563 ± 52* | 106 ± 28 | 23 ± 8* | 3.4 ± 1.2* | 9.5 ± 1.7 | 151 ± 2 |
| ICV Clo (10 μg/kg) | 111 ± 6* | 554 ± 34* | 103 ± 29 | 38 ± 8* | 7.8 ± 3.2* | 8.9 ± 2.0 | 152 ± 2 |
| Capsaicin treatment | 118 ± 3* | 583 ± 31* | 105 ± 34 | 56 ± 6* | 8.6 ± 3.7* | 9.3 ± 2.6 | 155 ± 3 |
| ICV Tem (400 μmol/kg) | 108 ± 5* | 551 ± 22* | 100 ± 24 | 24 ± 7* | 6.3 ± 1.6* | 9.1 ± 1.8 | 152 ± 2 |
| IG Hyd (15 mg/kg) | 104 ± 5* | 705 ± 25 | 95 ± 33 | 105 ± 10 | 23.7 ± 9.9 | 10.5 ± 1.9 | 156 ± 4 |
Data are expressed as mean ± SD (Each experiment has been replicated three times. For each time, each study group consists of six mice.).
p < 0.05 versus ischemic mice given vehicle.
aCSF, artificial cerebrospinal fluid; Clo, clonidine; Hyd, hydralazine; ICV, intracerebroventricular; IG, intragastric; Los, losartan; Tem, tempol.
Renal IRI activated renal RAS, sympathetic activity, and oxidative stress
Bilateral renal clamping or bilateral nephrectomy in mice increased serum creatinine (24 h postoperation: sham versus 45 min of IRI versus bilateral nephrectomy: 0.58 ± 0.12 versus 2.0 ± 0.61 versus 2.6 ± 0.43 mg/dl; p < 0.05) and decreased glomerular filtration rate, which was accompanied by marked renal histological injury in the corticomedullary junction and outer medulla (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/ars). IRI also increased urinary albumin excretion and renal KC level (Supplementary Fig. S1), and it elevated serum potassium concentration as well (Table 1).
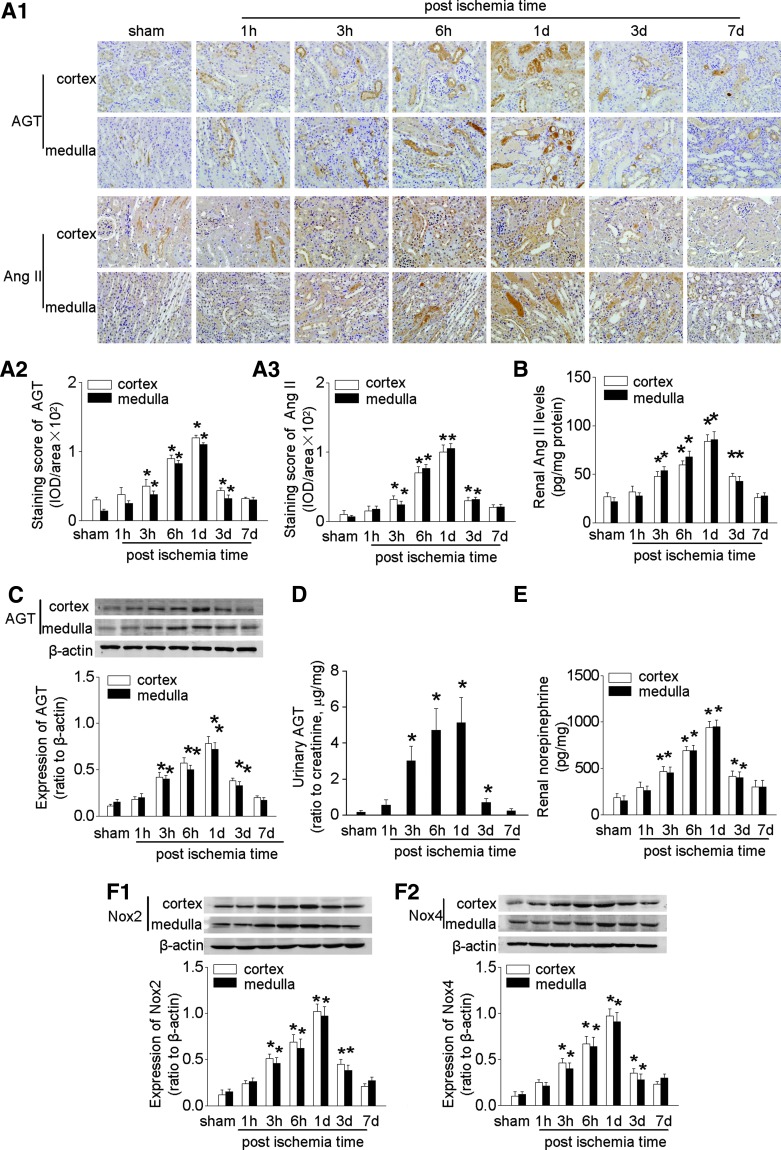
IRI led to a transient increase in renal cortical and medullar expression of AGT protein that peaked at 1 day (Fig. 1A, C). However, there were opposite changes in AGT mRNA at this time after IRI (Supplementary Fig. S2B). This suggests that the increased renal Ang II was not generated from local synthesized AGT but rather from AGT that derived from the systemic circulation and was filtered through a permeable glomerular membrane. The upregulation of the intrarenal RAS after IRI was accompanied by increased renal tissue Ang II levels (Fig. 1A, B) and AGT excretion (Fig. 1D). There were strong correlations between albumin excretion and AGT excretion (r = 0.86, p < 0.05), and between AGT excretion and renal tissue AGT expression (r = 0.88, p < 0.05, Supplementary Fig. S1G, H). This contrasts with an unchanged renal renin expression that was confined to the juxtaglomerular apparatus and an unchanged plasma renin activity and plasma Ang II after IRI (Supplementary Fig. S2A, Table 1). This indicated that the regulation of intrarenal RAS was distinct from the systemic RAS.
FIG. 1.
Overactivation of intrarenal RAS, sympathetic nervous system, and oxidative stress in mice after IRI. (A) Representative photos of intrarenal RAS expression showed by immunohistochemical staining of AGT and Ang II (A1), semi-quantitative data of AGT (A2) and Ang II (A3). (B) The concentration of Ang II in renal homogenates. (C) Expression of intrarenal AGT measured by Western blot. (D) The level of urinary AGT excretion. (E) Concentration of renal norepinephrine. (F) Expression of intrarenal Nox2 (F1) and Nox4 (F2). Data are expressed as mean ± SD (Each experiment has been replicated three times. For each time, each study group consists of six mice.). *p < 0.05 versus sham mice at the same time point. The unedited blots have been provided in Supplementary Figure S7. AGT, angiotensinogen; Ang II, angiotensin II; IRI, ischemia-reperfusion injury; RAS, renin-angiotensin system.
Renal IRI was associated with increased renal norepinephrine level and upregulated expression of the NADPH oxidase subunits Nox2 and Nox4 (Fig. 1E, F).
There was no difference in renal RAS, sympathetic activity, oxidative stress, and inflammation among sham groups at different time points postoperation (data not shown); thus, only the data at 1 day were shown.
Renal tubular RAS was upregulated in mice with renal IRI
Overexpression of AGT in ischemic kidneys was localized mainly in the cortex in proximal tubular cells and in the medulla in the descending thin limb of Henle's loop (Fig. 2A). The expression of Ang II (Fig. 2B) was apparent mainly in the renal medulla in the descending thin limb and ascending thick limbs of Henle's loop, distal convoluted tubules, and collecting ducts.
FIG. 2.
Localization of activated RAS in renal tubules in mice after IRI. (A) Representative photos of intrarenal AGT expression determined by double staining with antibodies against AGT and the markers of renal tubular segments. (B) Location of Ang II determined by double staining with antibodies against Ang II and the markers of renal tubular segments. AQP-1, aquaporin 1 (proximal tubule, and descending thin limb of Henle's loop); AQP-2, aquaporin 2 (collecting duct); NCCT, thiazide-sensitive NaCl cotransporter (distal convoluted tubule); THP, Tamm-Horsfall protein (ascending thick limbs of Henle's loop).
Brain RAS, tyrosine hydroxylase, Noxs, and inflammation were increased in mice with renal IRI
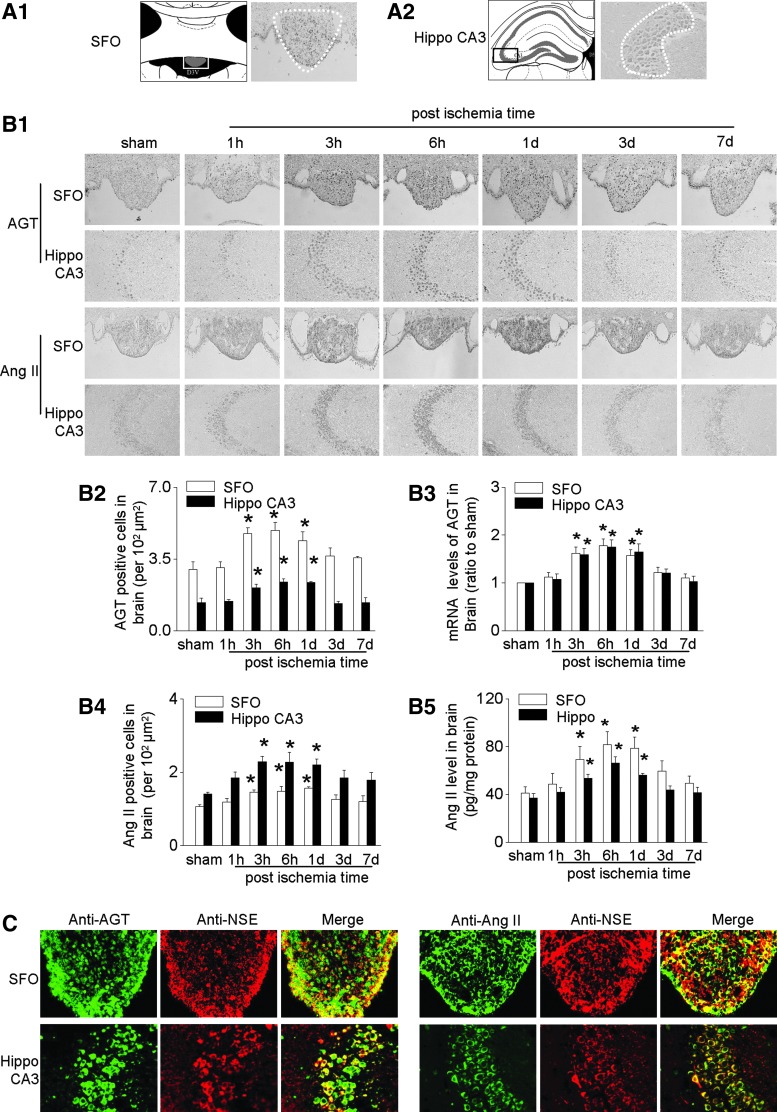
Activation of neuron has been reported in AKI in the stress-sensitive regions (paraventricular nucleus, cingulate cortex, piriform cortex, Hippo), the regions involving regulation of salt and water balance (subfornical organ [SFO]), and area involving autonomic regulations (lateral hypothalamic area) (40).
The expression of AGT and Ang II was upregulated by IRI in all these regions (Fig. 3, Supplementary Fig. S2E–H). We, therefore, chose SFO and Hippo CA3 as the representative study regions. IRI significantly increased the numbers of AGT- and Ang II-expressing cells in SFO and Hippo CA3 and upregulated AGT mRNA and Ang II levels after IRI (Fig. 3B). The mRNA level of brain renin was detectable in SFO and Hippo, but it did not change after IRI (Supplementary Fig. S2C). Bilateral nephrectomy also increased AGT modestly in the brain, but to a much less extent (Supplementary Fig. S3A).
FIG. 3.
Activation of brain RAS in mice after IRI. (A) Schematic drawings of study area (in gray) and light-field photomicrograph of coronal sections of SFO (A1) and hippocampus (Hippo) CA3 (A2). (B) Representative photos of immunohistochemical staining of AGT and angiotensin (Ang) II in SFO and Hippo CA3 (B1), semi-quantitative data of AGT (B2), mRNA level of AGT (B3), semi-quantitative data of Ang II (B4), and the concentration of Ang II in homogenates of SFO and Hippo (B5). (C) Localization of central AGT or Ang II determined by double staining with antibodies against AGT or Ang II (green) and an antibody recognizing NSE (red). Data are expressed as mean ± SD (Each experiment has been replicated three times. For each time, each study group consists of six mice.). *p < 0.05 versus sham mice at the same time point. Hippo, hippocampus; NSE, neuron-specific enolase; SFO, subfornical organ.
Using double immunofluorescence staining with antibodies recognizing the neuron-specific enolase (NSE) or glial fibrillary acidic protein (GFAP), we found that IRI-induced expression of AGT and Ang II was located mainly in neurons in SFO and Hippo CA3 (Fig. 3C), with a less degree in glial cells in the edge area of SFO and the surrounding area of Hippo CA3 (Supplementary Fig. S4). IRI-induced expression of AGT and Ang II was also observed in glial cells in the corpus callosum and cerebral cortex (Supplementary Fig. S4).
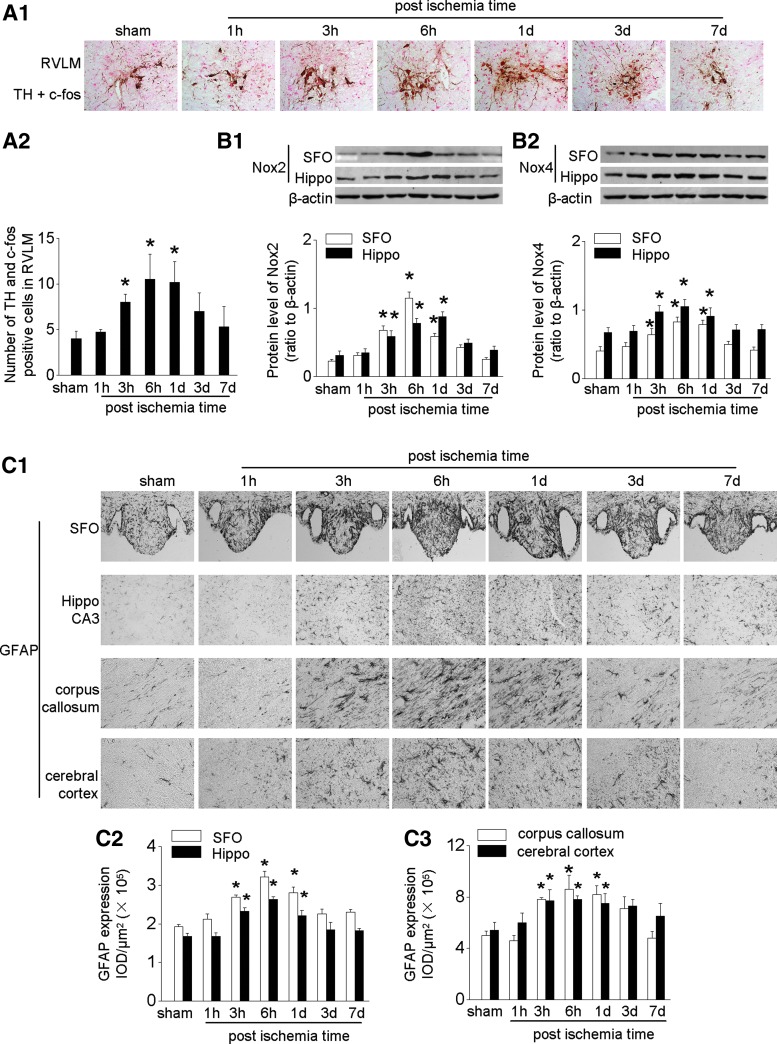
Tyrosine hydroxylase (TH) is the rate-limiting enzyme for cerebral norepinephrine synthesis. Its expression was upregulated after IRI in activated neurons (c-fos-positive) in the rostral ventrolateral medulla (RVLM), which is the gateway for activation of the central sympathetic nervous system (Fig. 4A). Upregulated Nox2 and Nox4 followed a similar time course (Fig. 4B).
FIG. 4.
Overexpression of brain TH, Noxs, and inflammation in mice after IRI. (A) Enhanced expression of TH (brown) in activated neurons (c-fos positive, red) in the RVLM: representative photographs (A1) and quantitative data (A2). (B) Expression of Nox2 (B1) and Nox4 (B2) in SFO and Hippo CA3 assessed by Western blot. (C) Expression of GFAP: representative photos of GFAP expression in SFO or Hippo CA3 and their surrounding area, corpus callosum, and cerebral cortex (C1) and semi-quantitative data (C2 and C3). Data are expressed as mean ± SD (Each experiment has been replicated three times. For each time, each study group consists of six mice.). *p < 0.05 versus sham mice at the same time point. The unedited blots have been provided in Supplementary Figure S7. GFAP, glial fibrillary acidic protein; RVLM, rostral ventrolateral medulla; TH, tyrosine hydroxylase.
IRI induced cerebral inflammation, as evidenced by an increase in GFAP-positive cells (astrocytes) in the SFO, Hippo CA3, corpus callosum, and cerebral cortex. The increase in GFAP-positive cells was mainly located in the edge area of SFO and the surrounding area of Hippo CA3 (Fig. 4C). IRI-induced cerebral inflammation was further demonstrated by increased levels of cytokines such as KC and granulocyte-colony stimulating factor in these regions (Supplementary Fig. S5A). There was only modest cerebral inflammation after bilateral nephrectomy (Supplementary Fig. S3B).
IRI-induced cerebral inflammation and TH expression were prevented by blockade of central AT1 receptors or prevention of oxidative stress
ICV administration of Los, at ∼1.3% of the dose of IG Los, inhibited the expression of TH and Noxs (Fig. 5) in the brain, whereas ICV Clo was ineffective (Fig. 5).
FIG. 5.
Blockade of central RAS or oxidative stress or renal deafferentation inhibits central TH and Noxs expression in mice after IRI. (A) Central administration of Tem or renal deafferentation with capsaicin downregulates expression of central RAS in SFO and Hippo: representative photos of immunohistochemistry staining (A1), semi-quantitative data of AGT (A2) and angiotensin (Ang) II (A3). (B) The concentration of Ang II in homogenates of SFO and Hippo. (C) Central administration of Los or Tem or renal deafferentation with capsaicin downregulates central Nox2 (C1) and Nox4 (C2). (D) Central administration of Los or Tem or renal deafferentation with capsaicin decreases the expression of TH in c-fos-positive neurons in RVLM. Data are expressed as mean ± SD (Each experiment has been replicated three times. For each time, each study group consists of six mice.). *p < 0.05 versus mice with renal IRI. The unedited blots have been provided in Supplementary Figure S7. aCSF, artificial cerebrospinal fluid; Clo, clonidine; Hyd, hydralazine; ICV, intracerebroventricular; IG, intragastric; Los, losartan; Tem, tempol.
Remarkably, selective blockade of renal afferent signals by capsaicin or renal denervation prevented the IRI-induced upregulation of the brain RAS, TH, and Noxs (Fig. 5 and Supplementary Fig. S6). Brain inflammation, as indicated by GFAP, KC, and granulocyte-colony stimulating factor, was decreased by blockade of cerebral RAS, or blockade of renal afferent signals, or treatment with anti-oxidant Tem (Fig. 6 and Supplementary Fig. S5B).
FIG. 6.
Blockade of central RAS, sympathetic outflow, oxidative stress, or renal deafferentation inhibits central inflammation in mice after IRI. (A) Central administration of Los or Tem or renal deafferentation with capsaicin downregulates expression of GFAP: representative photos of GFAP expression in SFO or Hippo CA3 and their surrounding area, corpus callosum, and cerebral cortex (A1). Semi-quantitative data of GFAP staining (A2 and A3). Data are expressed as mean ± SD (Each experiment has been replicated three times. For each time, each study group consists of six mice.). *p < 0.05 versus mice with renal IRI.
The activation of the central RAS, sympathetic nervous system, oxidative stress, or inflammation persisted after controlling blood pressure with Hyd (Figs. 5 and 6).
IRI-induced renal RAS activation was prevented by blockade of RAS, reno-cerebral reflex, or oxidative stress
ICV administration of Los reduced IRI-induced overexpression of the intrarenal RAS (Fig. 7A, B), decreased concentration of Ang II in renal homogenates (Fig. 7C), and reduced urinary AGT excretion (Fig. 7D). Remarkably, blockade of renal afferent sympathetic activity by capsaicin or renal denervation, or efferent signals by ICV Clo, or anti-central oxidative stress by ICV Tem all alleviated the IRI-induced activation of the intrarenal RAS (Fig. 7 and Supplementary Fig. S6). Thus, renal nerves and central oxidative stress activated the intrarenal RAS after IRI, and this persisted after lowering the blood pressure with Hyd.
FIG. 7.
Blockade of central RAS, sympathetic outflow, oxidative stress, or renal deafferentation inhibits activation of intrarenal RAS in mice after IRI. (A) Central administration of Los or Tem or renal deafferentation with capsaicin downregulates overexpression of renal RAS: representative photos (A1) and semi-quantitative analysis (A2 and A3). (B) Expression of intrarenal AGT analyzed by Western blot. (C) The concentration of Ang II in renal homogenates. (D) The level of urinary AGT excretion. Data are expressed as mean ± SD (Each experiment has been replicated three times. For each time, each study group consists of six mice.). *p < 0.05 versus mice with ischemic AKI given vehicle. The unedited blots have been provided in Supplementary Figure S7. AKI, acute kidney injury; PBS, phosphate-buffered saline.
Renal injury and dysfunction after IRI were attenuated by blockade of RAS, sympathetic activity, or oxidative stress
ICV administration of Los decreased the renal tissue injury score (Fig. 8A) and renal KC level (Fig. 8B), reduced serum creatinine (Fig. 8C), decreased the renal norepinephrine concentration (Fig. 8D), and downregulated the renal Nox2 and Nox4 expression (Fig. 8E). Blockade of sympathetic traffic by ICV Clo or renal denervation, or renal afferent nerve output with capsaicin, or anti-oxidative stress with ICV Tem all reduced renal injury and dysfunction in mice with ischemic AKI (Fig. 8 and Supplementary Fig. S6) whereas the reduction of blood pressure with Hyd was ineffective (Fig. 8). Overall, renal injury score in this model was increased 2.2-fold compared with sham mice, but ICV Tem, Los, or Clo reduced this by 58%, 65%, or 52%, respectively, and renal denervation or renal deafferentaion by 49% or 42%, suggesting robust, but not exclusive, roles for the brain and the renal nerves in the renal dysfunction in this ischemic AKI model.
FIG. 8.
Blockade of central RAS, sympathetic outflow, oxidative stress, or renal deafferentation attenuates renal injury in mice after IRI. (A) Central administration of Los or Tem or renal deafferentation with capsaicin inhibits acute tubular injury in mice after ischemic-reperfusion: representative photos of ischemic renal injury showed by HE staining (A1) and semi-quantitative data (A2). (B) Changes in mRNA level of KC. (C) Changes in serum creatinine. (D) Concentration of renal norepinephrine. (E) Expression of intrarenal Nox2 (E1) and Nox4 (E2). Data are expressed as mean ± SD (Each experiment has been replicated three times. For each time, each study group consists of six mice.). *p < 0.01 versus mice with ischemic AKI given vehicle. The unedited blots have been provided in Supplementary Figure S7. HE, hematoxylin-eosin; KC, keratinocyte-derived cytokine.
Discussion
The present study has uncovered a new reflex pathway between damaged kidneys and the brain that sustains renal oxidative stress and renal RAS activation to promote ongoing renal damage. Renal IRI co-activated the intrarenal and cerebral RAS and oxidative stress, independent of systemic Ang II or blood pressure, via a robust intercommunication provided by renal afferent and efferent sympathetic nerves. Reflex activation of this reno-cerebral RAS and oxidative stress axis contributed to the brain inflammation and to worsening of the ischemic renal damage in ischemic AKI. To our knowledge, this is the first study demonstrating that interorgan crosstalk between kidney and brain may occur directly through a neuronal pathway in ischemic AKI (illustrated in Fig. 9).
FIG. 9.
Schematic diagram summarizing co-activation of oxidative stress and RAS in the ischemic kidney and brain linked via the renal afferent and efferent sympathetic nerves in a positive feedback mode.
Activation of intrarenal RAS is a well-established pathway for progression of CKD (4, 10), but its role in the pathophysiology of AKI remains poorly understood. Renal AGT and Ang II were upregulated in the IRI model, suggesting an over-activation of intrarenal RAS. Overexpression of AGT was most prominent in the renal cortex in proximal tubules, whereas Ang II was most prominent in the renal medulla in distal tubules. Enhancement of the intrarenal RAS components was regulated independently from circulating RAS, since plasma renin activity and circulating Ang II remained unchanged during IRI, and although leading to systemic hypertension, was independent of blood pressure since it persisted after prevention of hypertension with Hyd. This extends previous reports of increased Ang II in renal homogenates after IRI (2, 11, 31).
Whether the increases in urinary AGT in AKI reflect increased glomerular passage (36, 43) or intrarenal AGT formation (28, 30) is currently unknown. In a transgenic mouse model of podocyte-selective injury, increased renal Ang II content and markedly increased tubular and urinary AGT were attributed to increased glomerular passage of circulating AGT (36). Urinary albumin is generally believed to be plasma derived. We confirm that the levels of urinary albumin and AGT are strongly correlated, suggesting that much of the urinary AGT can be derived from the plasma. Indeed, the finding that renal tissue AGT protein expression increased approximately fivefold 1 day after IRI, yet renal tissue AGT mRNA expression fell by approximately eightfold implies that much of the increased renal AGT was not locally synthesized but rather derived by filtration from the systemic circulation.
Since activation of the intrarenal RAS was upregulated strongly in ischemic AKI, it became important to understand its cause. Previous studies have shown that central Ang II activates sympathetic tone (5, 55). We found likewise that activation of intrarenal RAS after IRI was mediated by overexpression of the brain RAS that, in turn, activated the sympathetic nervous system. Mice with ischemic AKI exhibited an increased generation of cerebral TH, a rate-limiting enzyme for norepinephrine synthesis, thereby indicating an increased central sympathetic drive. These mice also had increased renal levels of norepinephrine, indicating an enhanced renal efferent sympathetic nerve activity. This central sympathoexcitation is secondary to oxidative stress, since centrally administration of Ang II potently activates ROS in cardiovascular neurons (60). Indeed, brain sympathoexcitation during ischemic AKI was clearly dependent on the central RAS and ROS, because their blockade with ICV Los or Tem prevented the activation of intrarenal RAS and the increase in renal norepinephrine, and attenuated renal injury and dysfunction induced by IRI. In turn, the changes in renal structure and function were dependent on increased central sympathetic outflow, since its blockade with ICV Clo suppressed renal RAS activation and injury after IRI. Because ICV Clo did not affect the brain RAS activity, we conclude that brain RAS activation is upstream from central sympathetic outflow, thereby linking activation of the brain RAS and ROS with exacerbation of renal injury. Similarly, blockade of brain RAS or sympathetic outflow in salt-loaded CKD rats attenuates the renal damage (6).
After IRI, the mRNA expression of renin in the SFO increased ∼30% (not a significant change), whereas AGT expression in the SFO increased by ∼80% and Ang II expression increased by ∼90%. These figures suggest that the increased levels of Ang II in the SFO related primarily to an increase in AGT. The protein expression for AGT in the SFO increased by ∼60%, which was quite comparable to the increase of ∼80% in the local levels of AGT mRNA. This suggests that the brain AGT was synthesized locally. There were rather similar changes in the Hippo. Moreover, IRI reduced circulating Ang II by about 15%. Therefore, we consider our data compatible with the increased levels of Ang II in the brain after IRI originating from increased local expression of AGT but cannot rule out a contribution from circulating Ang II.
Interestingly, we found that capsaicin treatment (14) to interrupt renal afferent nerves or renal denervation inhibited the activation of the brain-kidney RAS axis and alleviated the renal injury, implying an essential role for renal afferent sympathetic nerves in the central sympathoexcitation and consequent renal injury. This extends previous reports in rats of activation of renal afferent nerves by ROS during neurogenic hypertension (39, 56), Moreover interruption of afferent renal nerves inhibits the activation of the brain-kidney RAS axis in salt-loaded CKD rats (6).
Blockade of the cerebral RAS/ROS attenuated IRI-induced renal damage by ∼60%, suggesting a robust but not exclusive effect of RAS/ROS activation. Other pathways might be also involved in kidney damage after IRI. A pathway for distant brain inflammation in the presence of AKI might be uremic solute retention, which, in turn, triggers a whole cascade of central proinflammatory reactions (35). Consistent with this view, our study also confirmed the presence of systemic inflammation after IRI. Furthermore, bilateral nephrectomy also induced cerebral RAS activation and inflammation, although to a much less degree. However, uremia was not a dominant cause, since brain RAS activation and inflammation occurred at 3 h of IRI when azotemia was not evident.
An unexpected finding was that renal deafferentation with capsaicin, or central blockade of Ang II type 1 receptor with ICV Los or prevention of central oxidative stress with ICV Tem, or prevention of sympathetic activation with ICV Clo all prevented the increase in mean blood pressure and systemic parameters of oxidative stress and inflammation after IRI. This suggests that a more widespread hypertensive, oxidative, and inflammatory stimulus originated from the postischemia kidney that was mediated via the renal afferent nerves, the central RAS, and the efferent sympathetic nerves. Although the focus of this study was on renal damage, the profound effects on the blood pressure, heart rate and these systemic parameters suggest that this pathway likely extends to other organs in the body. This is important, since AKI increases morbidity and mortality beyond what can be ascribed to the effect of angiotensin or uremia. Indeed, the ∼250-fold increase in serum 8-iso-prostaglandin F2α may be sufficient to activate systemic thromboxane-prostanoid receptors that are themselves implicated in hypertension, renal vasoconstriction, oxidative stress, thrombosis (47, 48), and increased mortality from shock (44).
In conclusion, this study demonstrates that ischemia-reperfusion induces AKI in a mouse model, at least in part, by activation of a reno-cerebral RAS axis that is interlinked by renal afferent and efferent sympathetic nerves. This identifies a novel mechanism underlying reno-cerebral interaction in response to renal ischemia-reperfusion, and, therefore, could lead to new interventional approaches, such as the use of RAS inhibitors, sympatholytic agents, or even renal nerve ablation.
Materials and Methods
Animals
Six-week-old male C57BL/6J mice (Nanfang Hospital Animal Experiment Center, Guangzhou, China) were maintained in a pathogen-free facility under controlled temperature (24°C ± 2°C) and humidity (55% ± 5%), with a 12-h light/dark cycle. All animal experiments were approved by the Animal Ethics Committee of Nanfang Hospital.
Study design
Protocol 1
Renal IRI, bilateral nephrectomy, or sham operation (sham) was performed at 7 weeks of age as previously described (35). Animals were hydrated with warm saline on a heating pad (40°C), keeping body temperatures constantly at 37°C until full recovery from anesthesia. An atraumatic vascular clamp was placed on both renal pedicles for 45 min to induce IRI. Sham-operated animals underwent the identical procedure but without placement of the vascular clamps. Bilateral nephrectomy animals underwent similar procedures, except that both renal pedicles were ligated and the kidneys were removed. The success of the AKI model was confirmed by increased serum creatinine concentration and renal histological damage after 24 h.
Protocol 2
Mice were assigned randomly to 10 groups and matched for body weight (n = 6 in each group). All except group 7 received the following treatments at 48, 24, and 1.5 h before IRI: (a) IG vehicle (phosphate-buffered saline, pH 7.4) (group 1) or Los (Sigma Chemical, Saint Louis, MO) at 1 mg/kg (group 2), or 75 mg/kg (group 3); (b) ICV injection of vehicle (artificial cerebrospinal fluid [aCSF]) (group 4) or Los at 1 mg/kg in 3 μl of aCSF (20) (group 5); (c) ICV Clo (Sigma Chemical) at 10 μg/kg in 3 μl of aCSF as previously described (32) (group 6); (d) Renal deafferentation with capsaicin (Sigma Chemical) treatment was performed 10 min before ischemia-reperfusion as previously described (14) (group 7); (e) Renal denervation was performed 5 min before IRI as previously described (17) (group 8); (f) ICV Tem (Sigma Chemical) at 400 μmol/kg in 3 μl of aCSF (20) (group 9); and (g) IG Hyd (Sigma Chemical) at 15 mg/kg (group 10). Animals were subjected to renal IRI and were sacrificed 24 h after ischemia for sample harvesting.
Treatment procedure
ICV administration of drug
The doses of ICV Los, Clo, or Tem were chosen from preliminary studies in which an intravenous injection of these doses had no detectable effect on Ang II-induced increase in blood pressure or renal norepinephrine levels. The accuracy of the ICV injection was confirmed by using the tracer Evans blue. The dose of IG Los was chosen to achieve similar improvement of renal injury to those given ICV Los. The dose of IG Hyd was chosen to reduce the blood pressure similarly to mice given ICV Los.
Selective renal deafferentation
Capsaicin-mediated selective renal afferent denervation was performed according to a previous report (14). Briefly, the renal vessels were exposed by gently dissecting the surrounding fat and wrapped with a small piece of gauze that was soaked in a capsaicin solution (33 mM in 5% ethanol, 5% tween 80, and 90% normal saline) for 15 min. The efficacy of capsaicin treatment was confirmed by reduced expression of calcitonin gene-related peptide, which is an afferent nerve-specific marker (Supplementary Fig. S5C) (14).
Renal denervation
Renal denervation was performed as previously described (17). Briefly, the renal vessels were exposed by gently dissecting the surrounding fat, and renal denervation was performed by surgically stripping the renal arteries and veins of adventitia, cutting all visible renal nerve bundles under a dissection microscope. Renal vessels were painted with 10% phenol to ensure the destruction of any remaining nerves. Effectiveness of renal denervation was demonstrated by a reduction of renal norepinephrine content to less than 5% of the control.
Measurement of renal function and mean arterial pressure
Serum creatinine concentration was measured as a marker of renal function by using the Quantichrom Creatinine assay kit (BioAssay Systems, Hayward, CA). Serum potassium and sodium concentrations were measured with an automated chemistry analyzer (AU480; Beckman Coulter). Urinary albumin excretion was measured with an ELISA kit (IMTEC Diagnostics). Glomerular filtration rate was determined by inulin clearance as previously described (22).
Mean blood pressure was measured in conscious rats via a catheter in the carotid artery by telemetry using TA11PA-C10 probes (Data Sciences International, St. Paul, MN), which were implanted 1 week before the renal IRI or sham operation as previously described (18).
Tissue injury analysis
Evaluation of renal injury
The kidneys were dissected and processed for hematoxylin-eosin staining as previously described (52). Morphologic damage at the corticomedullary junction and outer medullary area after IRI was assessed by two experienced blinded renal pathologists using a grading scale of 0 to 5, as described (52).
Evaluation of tissue inflammation
Whole brain was dissected and cut into thick coronal slices (2 mm) with a mouse brain slicer matrix (Zivic Instruments, Pittsburgh, PA). Slices were postfixed, embedded in paraffin, and cut into 5 μm serial thick sections. The stress-related regions, including SFO, Hippo CA3, corpus callosum, and cerebral cortex, were identified under a microscope and immunohistochemical staining was performed by using an anti-GFAP (Millipore, Temecula, CA) antibody to determine the expression of GFAP. The integrated optical density/area of the positive staining for GFAP was assessed in three sections from each part of the brain by two blinded pathologists using an Image-Pro Plus software (version 6.0, Media Cybernetics, Rockville, MD) (21).
The expression of KC and granulocyte-colony stimulating factor in homogenates of brain or kidney was determined by a real-time polymerase chain reaction (PCR) as previously described (57, 58).
RAS expression and activity
Immunohistochemistry analysis
To evaluate the expression of intrarenal RAS, 5-μm-thick sections were processed (7) by using rabbit anti-mouse AGT (1:200; IBL, Japan), rabbit anti-Ang II (1:800; Peninsula Laboratories, San Carlos, CA), and goat anti-mouse renin (1:50; Santa Cruz, Dallas, TA) antibodies. RAS expression in the renal cortex and medulla was quantitated as described (50), and intrarenal renin expression was assessed as previously described (42).
The cerebral expression of AGT and Ang II was quantitated with immunohistochemical staining as described in our previous study (6) by using the antibodies mentioned earlier.
Localization of tissue RAS
For localization of intrarenal RAS, 5-μm paraffin-embedded kidney sections were double stained by using first primary antibodies against AGT (1:200; IBL), or Ang II (1:800; Peninsula Laboratories), in separate sections, and second primary antibodies against the markers of tubular epithelial cells: anti-aquaporin 1 (AQP-1, a marker of proximal tubular epithelial cells and the descending thin limb of Henle's loop; 1:100; Abcam, UK); anti-Tamm-Horsfall protein (a marker of ascending thick limbs of Henle's loop; 1:100, Santa Cruz); anti-thiazide-sensitive NaCl cotransporter (NCCT, a marker of distal convoluted tubular epithelial cells; 1:500; Millipore); and anti-aquaporin 2 (AQP-2, a marker of collecting duct epithelial cells; 1:100; Novus Biologicals, Littleton, CO) antibodies in separate sections, separately.
Cerebral localization of AGT and Ang II was determined by double staining using the anti-AGT or anti-Ang II antibody mentioned earlier as the first primary antibody, and anti-NSE (Millipore) or anti-GFAP (Millipore) antibodies as the second primary antibody (50).
Western blot and real-time PCR
The levels of AGT in renal homogenates were determined as previously described (8) by using rabbit anti-mouse AGT (IBL) and rabbit anti-β-actin (Cell Signaling Technology, Danvers, MA) antibodies. AGT and renin mRNA expression were determined by real-time PCR as previously described (59).
ELISA
AGT levels in urine collected via cystocentesis were assessed by an ELISA kit (IBL) according to the manufacturer's instructions.
Radioimmunoassay
Ang II concentrations in plasma and renal homogenates and plasma renin activity were determined by the radioimmunoassay according to the manufacturer's instructions (Beijing North Institute of Biological Technology, China). To prevent angiotensin from degradation, blood samples were collected in ice-cooled tubes containing a cocktail of protease inhibitors (0.01 mM p-hydroxylmercuribenzoate, 1.5 mM 1,10-phenanthroline, 0.01 mM PMSF, 0.05 mM pepstatin A, and 10 mM EDTA). Renal and brain tissue was homogenized on ice with 0.045 M HCl in ethanol containing 0.9 mM p-hydroxylmercuribenzoate, 131.5 mM 1,10-phenanthroline, 0.9 mM PMSF, 1.75 mM pepstatin A, 1.1 mM EDTA, and 0.0043% protease-free BSA (11).
Evaluation of sympathetic activity
Norepinephrine concentrations
Concentrations of norepinephrine in serum and homogenates of renal tissue were assessed with an ELISA kit (Demeditec Diagnostics, Germany) according to the manufacturer's protocol.
Expression of TH in brain
Five-micrometer-thick brain stem sections were double stained with rabbit anti-mouse TH (Millipore) and rat anti-mouse c-fos (Santa Cruz) antibodies as previously described (54). The number of neurons labeled by TH and c-fos in the RVLM was quantified as previously described (54).
Evaluation of oxidative stress and inflammation
Systemic oxidative stress and inflammation
Serum concentrations of 8-iso-prostaglandin F2α and KC, markers of systemic oxidative stress and inflammation, were quantified by the ELISA kits (Enzo Life Sciences, NY; Qiagen, Valencia, CA).
Expression of Noxs in kidney and brain
The expression of NADPH oxidase subunits Nox2 and Nox4 in renal and brain homogenates was determined by Western blot using anti-Nox2 (Abcam) or Nox4 antibody (Santa Cruz) (8).
Statistic analyses
All data are expressed as mean ± SD of at least three independent experiments. Continuous variables among groups were compared by using one-way analysis of variance, followed by LSD test. An unpaired t-test was used to compare the parameters between IRI and sham mice or between animals treated with IRI and bilateral nephrectomy at the same time point. Histology scores were analyzed by nonparametric testing. Statistical analyses were conducted with SPSS 17.0 for Windows (SPSS, Inc., Chicago, IL). A value of p < 0.05 was considered statistically significant.
Supplementary Material
Abbreviations Used
- 8-iso-PGF2α
8-iso-Prostaglandin2α
- aCSF
artificial cerebrospinal fluid
- AGT
angiotensinogen
- AKI
acute kidney injury
- Ang II
angiotensin II
- CGRP
calcitonin gene-related peptide
- CKD
chronic kidney disease
- Clo
clonidine
- GFAP
glial fibrillary acidic protein
- HE
hematoxylin-eosin
- Hippo
hippocampus
- Hyd
hydralazine
- ICV
intracerebroventricular
- IG
intragastric
- IRI
ischemia-reperfusion injury
- KC
keratinocyte-derived cytokine
- Los
losartan
- MAP
mean arterial pressure
- NADPH
nicotinamide adenine dinucleotide phosphate
- NSE
neuron-specific enolase
- PBS
phosphate-buffered saline
- RAS
renin-angiotensin system
- ROS
reactive oxygen species
- RVLM
rostral ventrolateral medulla
- SFO
subfornical organ
- Tem
tempol
- TH
tyrosine hydroxylase
Acknowledgments
This study was supported by the National Key Technology Support Program of China (2013BAI09B06 and 2015BAI2B07 to F.F.H.), the State Key Program of National Natural Science Foundation of China (81430016 to F.F.H.), the Major International (Regional) Joint Research Project of National Natural Science Foundation of China (81620108003 to F.F.H.), the Foundation for Innovation Research Groups of the National Natural Science Foundation of China (81521003 to Y.L.), the National Natural Science Foundation of China (81570619 to W.C., 81270825 and 31201751 to A.L.), the Major Scientific and Technological Planning Project of Guangzhou (15020010 to F.F.H.), NIH (HL-68686; DK-049870; DK-036079 to C.S.W.), and funds from the George E. Schreiner Chair of Nephrology and the Georgetown University Hypertension, Kidney and Vascular Research Center (to C.S.W.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Alge JL, Karakala N, Neely BA, Janech MG, Tumlin JA, Chawla LS, Shaw AD, and Arthur JM. Urinary angiotensinogen and risk of severe AKI. Clin J Am Soc Nephrol 8: 184–193, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allred AJ, Chappell MC, Ferrario CM, and Diz DI. Differential actions of renal ischemic injury on the intrarenal angiotensin system. Am J Physiol Renal Physiol 279: F636–F645, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Averina VA, Othmer HG, Fink GD, and Osborn JW. A mathematical model of salt-sensitive hypertension: the neurogenic hypothesis. J Physiol 593: 3065–3075, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becher UM, Endtmann C, Tiyerili V, Nickenig G, and Werner N. Endothelial damage and regeneration: the role of the renin-angiotensin-aldosterone system. Curr Hypertens Rep 13: 86–92, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Campese VM, Ye S, and Zhong H. Downregulation of neuronal nitric oxide synthase and interleukin-1beta mediates angiotensin II-dependent stimulation of sympathetic nerve activity. Hypertension 39: 519–524, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Cao W, Li A, Wang L, Zhou Z, Su Z, Bin W, Wilcox CS, and Hou FF. A salt-induced reno-cerebral reflex activates renin-angiotensin systems and promotes CKD progression. J Am Soc Nephrol 26: 1619–1633, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao W, Xu J, Zhou ZM, Wang GB, Hou FF, and Nie J. Advanced oxidation protein products activate intrarenal renin-angiotensin system via a CD36-mediated, redox-dependent pathway. Antioxid Redox Signal 18: 19–35, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao W, Zhou QG, Nie J, Wang GB, Liu Y, Zhou ZM, and Hou FF. Albumin overload activates intrarenal renin-angiotensin system through protein kinase C and NADPH oxidase-dependent pathway. J Hypertens 29: 1411–1421, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Chou AH, Lee CM, Chen CY, Liou JT, Liu FC, Chen YL, and Day YJ. Hippocampal transcriptional dysregulation after renal ischemia and reperfusion. Brain Res 1582: 197–210, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Crowley SD. and Coffman TM. Recent advances involving the renin-angiotensin system. Exp Cell Res 318: 1049–1056, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.da Silveira KD, Pompermayer Bosco KS, Diniz LR, Carmona AK, Cassali GD, Bruna-Romero O, de Sousa LP, Teixeira MM, Santos RA, Simoes e Silva AC, and Ribeiro Vieira MA. ACE2-angiotensin-(1-7)-Mas axis in renal ischaemia/reperfusion injury in rats. Clin Sci (Lond) 119: 385–394, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Diebold I, Petry A, Hess J, and Gorlach A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol Biol Cell 21: 2087–2096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Efrati S, Berman S, Hamad RA, Siman-Tov Y, Ilgiyaev E, Maslyakov I, and Weissgarten J. Effect of captopril treatment on recuperation from ischemia/reperfusion-induced acute renal injury. Nephrol Dial Transplant 27: 136–145, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Foss JD, Wainford RD, Engeland WC, Fink GD, and Osborn JW. A novel method of selective ablation of afferent renal nerves by periaxonal application of capsaicin. Am J Physiol Regul Integr Comp Physiol 308: R112–R122, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francis J. and Davisson RL. Emerging concepts in hypertension. Antioxid Redox Signal 20: 69–73, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franco M, Martinez F, Rodriguez-Iturbe B, Johnson RJ, Santamaria J, Montoya A, Nepomuceno T, Bautista R, Tapia E, and Herrera-Acosta J. Angiotensin II, interstitial inflammation, and the pathogenesis of salt-sensitive hypertension. Am J Physiol Renal Physiol 291: F1281–F1287, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Fujii T, Kurata H, Takaoka M, Muraoka T, Fujisawa Y, Shokoji T, Nishiyama A, Abe Y, and Matsumura Y. The role of renal sympathetic nervous system in the pathogenesis of ischemic acute renal failure. Eur J Pharmacol 481: 241–248, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Genest DS, Falcao S, Michel C, Kajla S, Germano MF, Lacasse AA, Vaillancourt C, Gutkowska J, and Lavoie JL. Novel role of the renin-angiotensin system in preeclampsia superimposed on chronic hypertension and the effects of exercise in a mouse model. Hypertension 62: 1055–1061, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Grams ME. and Rabb H. The distant organ effects of acute kidney injury. Kidney Int 81: 942–948, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Grande MT, Pascual G, Riolobos AS, Clemente-Lorenzo M, Bardaji B, Barreiro L, Tornavaca O, Meseguer A, and Lopez-Novoa JM. Increased oxidative stress, the renin-angiotensin system, and sympathetic overactivation induce hypertension in kidney androgen-regulated protein transgenic mice. Free Radic Biol Med 51: 1831–1841, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Hao LY, Hao XQ, Li SH, and Li XH. Prenatal exposure to lipopolysaccharide results in cognitive deficits in age-increasing offspring rats. Neuroscience 166: 763–770, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Hueper K, Gutberlet M, Rong S, Hartung D, Mengel M, Lu X, Haller H, Wacker F, Meier M, and Gueler F. Acute kidney injury: arterial spin labeling to monitor renal perfusion impairment in mice-comparison with histopathologic results and renal function. Radiology 270: 117–124, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Johnson KJ. and Weinberg JM. Postischemic renal injury due to oxygen radicals. Curr Opin Nephrol Hypertens 2: 625–635, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Kelly KJ. Acute renal failure: much more than a kidney disease. Semin Nephrol 26: 105–113, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Kim KY, Jang HS, Yoshida T, Tsuchiya K, Nitta K, Park JW, Bonventre JV, and Park KM. Role of cytosolic NADP+-dependent isocitrate dehydrogenase in ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol 296: F622–F633, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J. and Padanilam BJ. Renal denervation prevents long-term sequelae of ischemic renal injury. Kidney Int 87: 350–358, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobori H, Harrison-Bernard LM, and Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int 61: 579–585, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobori H NM, Navar LG, and Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Kobori H, Ohashi N, Katsurada A, Miyata K, Satou R, Saito T, and Yamamoto T. Urinary angiotensinogen as a potential biomarker of severity of chronic kidney diseases. J Am Soc Hypertens 2: 349–354, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobori H, Ozawa Y, Suzaki Y, Prieto-Carrasquero MC, Nishiyama A, Shoji T, Cohen EP, and Navar LG. Young scholars award lecture: intratubular angiotensinogen in hypertension and kidney diseases. Am J Hypertens 19: 541–550, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kontogiannis J. and Burns KD. Role of AT1 angiotensin II receptors in renal ischemic injury. Am J Physiol 274: F79–F90, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Kotagale NR, Taksande BG, Gahane AY, Ugale RR, and Chopde CT. Repeated agmatine treatment attenuates nicotine sensitization in mice: modulation by alpha2-adrenoceptors. Behav Brain Res 213: 161–174, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Kurata H, Fujii T, Tsutsui H, Katayama T, Ohkita M, Takaoka M, Tsuruoka N, Kiso Y, Ohno Y, Fujisawa Y, Shokoji T, Nishiyama A, Abe Y, and Matsumura Y. Renoprotective effects of l-carnosine on ischemia/reperfusion-induced renal injury in rats. J Pharmacol Exp Ther 319: 640–647, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Lai EY, Luo Z, Onozato ML, Rudolph EH, Solis G, Jose PA, Wellstein A, Aslam S, Quinn MT, Griendling K, Le T, Li P, Palm F, Welch WJ, and Wilcox CS. Effects of the antioxidant drug tempol on renal oxygenation in mice with reduced renal mass. Am J Physiol Renal Physiol 303: F64–F74, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu M, Liang Y, Chigurupati S, Lathia JD, Pletnikov M, Sun Z, Crow M, Ross CA, Mattson MP, and Rabb H. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol 19: 1360–1370, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, Nishiyama A, and Ichikawa I. Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol 23: 1181–1189, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molinas SM, Cortes-Gonzalez C, Gonzalez-Bobadilla Y, Monasterolo LA, Cruz C, Elias MM, Bobadilla NA, and Trumper L. Effects of losartan pretreatment in an experimental model of ischemic acute kidney injury. Nephron Exp Nephrol 112: e10–e19, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Nongnuch A, Panorchan K, and Davenport A. Brain-kidney crosstalk. Crit Care 18: 225, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliveira-Sales EB, Colombari DS, Davisson RL, Kasparov S, Hirata AE, Campos RR, and Paton JF. Kidney-induced hypertension depends on superoxide signaling in the rostral ventrolateral medulla. Hypertension 56: 290–296, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Palkovits M, Sebekova K, Gallatz K, Boor P, Sebekova K, Jr., Klassen A, Bahner U, and Heidland A. Neuronal activation in the CNS during different forms of acute renal failure in rats. Neuroscience 159: 862–882, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Peterson JR, Sharma RV, and Davisson RL. Reactive oxygen species in the neuropathogenesis of hypertension. Curr Hypertens Rep 8: 232–241, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Pupilli C, Chevalier RL, Carey RM, and Gomez RA. Distribution and content of renin and renin mRNA in remnant kidney of adult rat. Am J Physiol 263: F731–F738, 1992 [DOI] [PubMed] [Google Scholar]
- 43.Roksnoer LC, Verdonk K, van den Meiracker AH, Hoorn EJ, Zietse R, and Danser AH. Urinary markers of intrarenal renin-angiotensin system activity in vivo. Curr Hypertens Rep 15: 81–88, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Sparks MA, Makhanova NA, Griffiths RC, Snouwaert JN, Koller BH, and Coffman TM. Thromboxane receptors in smooth muscle promote hypertension, vascular remodeling, and sudden death. Hypertension 61: 166–173, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Tsutsui H, Sugiura T, Hayashi K, Yukimura T, Ohkita M, Takaoka M, and Matsumura Y. Protective effect of moxonidine on ischemia/reperfusion-induced acute kidney injury through alpha2/imidazoline I1 receptor. Eur J Pharmacol 718: 173–180, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Velez JC. The importance of the intrarenal renin-angiotensin system. Nat Clin Pract Nephrol 5: 89–100, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Wang D, Chabrashvili T, and Wilcox CS. Enhanced contractility of renal afferent arterioles from angiotensin-infused rabbits: roles of oxidative stress, thromboxane prostanoid receptors, and endothelium. Circ Res 94: 1436–1442, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Wang D, Luo Z, Wang X, Jose PA, Falck JR, Welch WJ, Aslam S, Teerlink T, and Wilcox CS. Impaired endothelial function and microvascular asymmetrical dimethylarginine in angiotensin II-infused rats: effects of tempol. Hypertension 56: 950–955, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z, Liu Y, Han Y, Guan W, Kou X, Fu J, Yang D, Ren H, He D, Zhou L, and Zeng C. Protective effects of aliskiren on ischemia-reperfusion-induced renal injury in rats. Eur J Pharmacol 718: 160–166, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Xavier LL, Viola GG, Ferraz AC, Da Cunha C, Deonizio JM, Netto CA, and Achaval M. A simple and fast densitometric method for the analysis of tyrosine hydroxylase immunoreactivity in the substantia nigra pars compacta and in the ventral tegmental area. Brain Res Brain Res Protoc 16: 58–64, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto T, Nakagawa T, Suzuki H, Ohashi N, Fukasawa H, Fujigaki Y, Kato A, Nakamura Y, Suzuki F, and Hishida A. Urinary angiotensinogen as a marker of intrarenal angiotensin II activity associated with deterioration of renal function in patients with chronic kidney disease. J Am Soc Nephrol 18: 1558–1565, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Yang L, Brooks CR, Xiao S, Sabbisetti V, Yeung MY, Hsiao LL, Ichimura T, Kuchroo V, and Bonventre JV. KIM-1-mediated phagocytosis reduces acute injury to the kidney. J Clin Invest 125: 1620–1636, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang X, Chen C, Tian J, Zha Y, Xiong Y, Sun Z, Chen P, Li J, Yang T, Ma C, Liu H, Wang X, and Hou FF. Urinary angiotensinogen level predicts AKI in acute decompensated heart failure: a prospective, two-stage study. J Am Soc Nephrol 26: 2032–2041, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yao ST. and May CN. Intra-carotid angiotensin II activates tyrosine hydroxylase-expressing rostral ventrolateral medulla neurons following blood-brain barrier disruption in rats. Neuroscience 245: 148–156, 2013 [DOI] [PubMed] [Google Scholar]
- 55.Ye S, Zhong H, Duong VN, and Campese VM. Losartan reduces central and peripheral sympathetic nerve activity in a rat model of neurogenic hypertension. Hypertension 39: 1101–1106, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Ye S, Zhong H, Yanamadala S, and Campese VM. Oxidative stress mediates the stimulation of sympathetic nerve activity in the phenol renal injury model of hypertension. Hypertension 48: 309–315, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y, Viennois E, Xiao B, Baker MT, Yang S, Okoro I, and Yan Y. Knockout of Ste20-like proline/alanine-rich kinase (SPAK) attenuates intestinal inflammation in mice. Am J Pathol 182: 1617–1628, 2013 [DOI] [PubMed] [Google Scholar]
- 58.Zhou J, Wu R, High AA, Slaughter CA, Finkelstein D, Rehg JE, Redecke V, and Hacker H. A20-binding inhibitor of NF-kappaB (ABIN1) controls Toll-like receptor-mediated CCAAT/enhancer-binding protein beta activation and protects from inflammatory disease. Proc Natl Acad Sci U S A 108: E998–E1006, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou L, Li Y, Hao S, Zhou D, Tan RJ, Nie J, Hou FF, Kahn M, and Liu Y. Multiple genes of the renin-angiotensin system are novel targets of Wnt/beta-catenin signaling. J Am Soc Nephrol 26: 107–120, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zimmerman MC, Lazartigues E, Sharma RV, and Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res 95: 210–216, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.