Abstract
The abundance and accessibility of a primary virus receptor are critical factors that impact the susceptibility of a host cell to virus infection. The Coxsackievirus and adenovirus receptor (CAR) has two transmembrane isoforms that occur due to alternative splicing and differ in localization and function in polarized epithelia. To determine the relevance of isoform-specific expression across cell types, the abundance and localization of both isoforms were determined in ten common cell lines, and correlated with susceptibility to adenovirus transduction relative to polarized primary human airway epithelia. Data show that the gene and protein expression for each isoform of CAR varies significantly between cell lines and polarization, as indicated by high transepithelial resistance, is inversely related to adenovirus transduction. In summary, the variability of polarity and isoform-specific expression among model cells are critical parameters that must be considered when evaluating the clinical relevance of potential adenovirus-mediated gene therapy and anti-adenovirus strategies.
Keywords: Adenovirus, coxsackievirus and adenovirus receptor (CAR), non-polarized, polarized, tight junction, epithelia, apical and basolateral
Graphical abstract
Differential accessibility of CAR for adenovirus infection of non-polarized or polarized cells. A. CAREx7 and CAREx8 are exposed and accessible to mediate the infection of incoming adenovirus in non-polarized epithelia. B. CAREx7 localizes beneath the tight junctions at the basolateral surface of polarized epithelia and is not accessible to incoming adenovirus. C. CAREx8 localizes at the apical surface and in the recycling endosomes of polarized epithelia. Apical CAREx8 allows adenovirus to bind, internalize, and infect cells from the apical surface of polarized epithelia.
1. Introduction
Understanding the development, maintenance, and composition of the lung epithelial barrier is of great importance to human respiratory health and disease. This barrier segregates the microbial infested external environment from the body’s sterile internal environment. Each polarized epithelial cell has two distinct compartments: an apical or mucosal surface exposed to the air, and a basolateral surface in close communication with the internal environment. Investigations into the mechanisms of pathogenic microbial penetration of the lung epithelial barrier provide insights into its structure and regulation (Zihni et al., 2014). Interestingly, many viruses use basolateral adhesion proteins as their primary receptors. Three groups of viruses, coxsackie B viruses (CVB), all but group B human adenoviruses, and swine vesicular disease virus, utilize the coxsackievirus and adenovirus receptor (CAR) as their primary receptor (Bergelson et al., 1997; Carson et al., 1997; Tomko et al., 1997).
Several protein isoforms have been described for CAR (Excoffon et al., 2014; Excoffon et al., 2010; Raschperger et al., 2006). Alternative splicing not only regulates protein expression, but also allows multiple proteins to be expressed from the same gene resulting in significant proteomic diversity. Human CAR was initially isolated as a seven exon encoded protein (Bergelson et al., 1997). In contrast to other species, mouse CAR (mCAR) was initially cloned as a protein composed of eight exons (Tomko et al., 1997). Transcripts for the seven-exon mouse and eight-exon human forms were subsequently identified (Bergelson et al., 1998). A detailed analysis of protein expression and localization in mice has revealed differential tissue dependent expression and localization for the mCAREx7 (mCAR2) and mCAREx8 (mCAR1) isoforms (Excoffon et al., 2010; Mirza et al., 2006; Nalbantoglu et al., 2001; Nalbantoglu et al., 1999; Raschperger et al., 2006). This suggests that protein-protein interactions and potentially the functional importance of these two isoforms may be distinct. We are the first group to investigate the importance of human CAREx8 in polarized epithelia (Excoffon et al., 2010; Kolawole et al., 2012; Kotha et al., 2015; Sharma et al., 2012a; Sharma et al., 2012b; Yan et al., 2015). The alternative splicing event that creates CAREx8 occurs at a cryptic splice site within the seventh exon. Thus, these two isoforms contain identical extracellular and transmembrane domains. The majority of the cytoplasmic C-terminal domain is also identical except for the last 26 amino acids encoded by exon 7 (CAREx7) that are replaced by 13 distinct amino acids encoded by exon 8 (CAREx8).
Along with others, we have previously shown that CAREx7 resides on the basolateral surface of polarized epithelia where it behaves as a homophilic epithelial junction adhesion protein and a heterophilic leukocyte adhesion protein that plays a role in neutrophil transepithelial migration and gammadelta T cell activation (Cohen et al., 2001; Excoffon et al., 2010; Kotha et al., 2015; Verdino et al., 2010; Walters et al., 2002; Witherden et al., 2010; Zen et al., 2005). CAREx7 also plays a role in trafficking proteins to cell-cell junctions (Coyne et al., 2004; Excoffon et al., 2004; Excoffon et al., 2012; Lim et al., 2008; Mirza et al., 2005; Sollerbrant et al., 2003; Weber et al., 2014; Yan et al., 2015).
CAREx8 can also behave as a homo- and heterophilic adhesion protein; however, in contrast to CAREx7, CAREx8 is found at the apical surface of polarized epithelia where it is unlikely to interact with CAR molecules on adjacent cells (Excoffon et al., 2010). Instead, we have shown that CAREx8 mediates heterophilic adhesion with neutrophils that have transmigrated from the basal to apical surface (Kotha et al., 2015). The apical CAREx8 isoform also mediates adenovirus entry from the apical surface and, surprisingly, neutrophils further enhance adenovirus entry. The protein levels of apical CAREx8 are tightly regulated by intracellular proteins, such as the cytoplasmic scaffolding protein MAGI-1, and are increased by extracellular signals such as interleukin-8 (IL-8) and side-stream cigarette smoke (Excoffon et al., 2010; Kolawole et al., 2012; Kotha et al., 2015; Sharma et al., 2012a).
The abundance and accessibility of a primary viral receptor is a critical factor that defines the susceptibility of a host cell to viral infection. Many distinct model cells have been investigated for CAR-binding virus infection. In this manuscript we characterize cellular polarity and isoform-specific expression of both transmembrane isoforms of CAR in eleven commonly studied cell types. The cell lines tested were divided in two groups based on their polarity; 1) non-polarized cells (CHO-K1, COS-7, HeLa, HEK-293, 293T and A549) that are unable to form tight junctions and hence unable to polarize at the air-liquid interface; 2) cells able to form tight junctions (MDCK, Caco-2, Calu-3, NuLi-1 and HAE) and therefore polarize on a semipermeable membrane resulting in two distinct compartments (apical and basolateral). Together, these data will allow those investigating CAR-binding virus infections a clearer understanding of cellular polarity and receptor availability that will enhance data interpretation and advance the understanding of the factors that regulate susceptibility to infection.
2. Materials and methods
2.1 Cell Lines
Ten immortal cell lines and primary human airway epithelia were investigated: Chinese hamster ovary (CHO-K1, ATCC, CCL-61), transformed monkey kidney (COS-7, ATCC, CRL-1651), human cervical adenocarcinoma (HeLa, ATCC- CCL-2), human embryonic kidney (HEK-293, ATCC, CRL-1573), subclone of human embryonic kidney (293T, Clontech, Mountain View, CA), human lung carcinoma (A549, ATCC, CCL-185), Madin Darby canine kidney (MDCK, ATCC, CCL-34), human colonic adenocarcinoma (Caco-2, ATCC, HTB-37), human lung adenocarcinoma (Calu-3, ATCC, HTB-55), normal lung transformed epithelial cells (NuLi-1, a kind gift from Dr. Joseph Zabner, University of Iowa) and human airway epithelial (HAE, Human Donors)
HAE were originally isolated under approval by the Institutional Review Board of the University of Iowa (IRB ID No. 9507432) from discarded and deidentified trachea and bronchi of donor lungs. This study used discarded lung tissue, thus IRB deemed consent was not needed. HAE were cultured and differentiated as previously described (Karp et al., 2002; Kotha et al., 2015; Liu et al., 2012).
2.2 Culture Media
The cells were cultured in appropriate sterile culture medium (Gibco™, Invitrogen Corporation, Grand Island, NY) supplemented with 10% fetal bovine serum (except MDCK, supplemented with 5% FBS). All the culture media were further supplemented with 0.5% penicillin and streptomycin antibiotics and with 3.024 g/L sodium bicarbonate, 0.5% by appropriate volume percentage.
2.3 Total Cellular RNA Extraction
Total cellular RNA was extracted from cells using TRIzol Reagent (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer’s protocol. The RNA pellet was dissolved in diethyl pyrocarbonate (DEPC)-treated ddH2O and the purity and concentration of the extracted RNA were determined with a nanodrop spectrophotometer (Thermo Scientific, Waltham, MA).
2.4 Quantitative reverse transcription and PCR (qPCR)
Complementary DNA (cDNA) was synthesized using Quanta First Strand Kit (Quanta BioSciences, Gaithersburg, MD). qPCR was performed using SYBR Green with low ROX (Quanta BioSciences) in Stratagene's Real Time PCR System (Agilent Technologies, Santa Clara, CA) using 3000p software v2.0.1 for data analysis as previously described (Kotha et al., 2015; Sharma et al., 2012a; Sharma et al., 2012b). Primers used were: CAR-qPCR-F: 5’ TCGGCAGTAATCATTCATCCCTGG; CAREx7-qPCR-R: 5′ ATAGACCCATCCTTGCTCTGTGCT; CAREX8-qPCR-R: 5’ ACTGTAATTCCATCAGTCTTGTAAGGG. Abundance relative to GAPDH gene expression was calculated for each gene of interest in human cells. GAPDH-F: 5’ CACCCTGTTGCTGTAGCCAAA; GAPDH-R: 5’ CAACAGCGACACCCACTCCT. In dog cells, CAR was normalized to MDCK-Actin-F: 5’ AAGATCTGGCACCACACCTTCTAC; MDCK-Actin-R: 5’ ATCTGGGTCATCTTCTCACGGTTG (Kotha et al., 2015). In CHO cells, CAR was normalized to CHO-Actin-F-5’ TGGCATCCACGAAACTACAT; CHO-Actin-R-5’ TGGTACCACCAGACAGCACT.
2.5 Immunocytochemistry
Cells seeded and polarized on millicells (0.4 µm pore; Millipore, St. Louis, MO) were kept on ice for 5 min, and washed 3 times with ice-cold PBS supplemented with Mg2+ and Ca2+ (PBS +/+). The cells were then fixed with ice-cold methanol containing 1% paraformaldehyde for 20 min at −20°C. Cells were rinsed with ice-cold PBS +/+, allowed to come to room temperature, and blocked with 2% bovine serum albumin (BSA) in SuperBlock (Pierce, Rockford, IL) for 45 min. Primary antibodies, anti- rabbit 1605 (rα1605) for total CAR, anti-rabbit 5678 (rα5678) and anti-mouse actin as previously published (Excoffon et al., 2014; Excoffon et al., 2010; Excoffon et al., 2005) were added to cells for 2.5 h at 37°C or overnight at 4°C. Cells were rinsed, reblocked, and then incubated with secondary antibodies (Invitrogen, Carlsbad, CA). The cells were rinsed and mounted onto glass slides using Vectashield mounting media with DAPI (Vector Laboratories Inc., Burlingame, CA). Staining was evaluated by laser scanning confocal microscopy (Olympus FV1000) with a 60× oil immersion lens; images are shown as single X-Y sections.
2.6 Cell Polarization
For polarization studies, 2 × 104 – 2 × 106 cells per well were seeded on 12 mm diameter polyester Millicell filters consisting of a semi-permeable membrane with a pore size of 0.4 µm (Millipore, St. Louis, MO). Media on the apical surface of cells was removed every alternate day in order to establish and maintain an air-liquid interface. Polarized cells actively transport fluid from the apical to the basolateral surface and thus maintain a defined apical surface fluid composition.
2.7 Transepithelial electrical resistance (TER) measurement
Transepithelial electrical resistance was measured with a chopstick ohmmeter (World Precision Instruments, Sarasota, FL) every other day. Media was aspirated from the wells and replaced with 400 µL fresh media at the basolateral surface. The same amount of PBS +/+ was applied to the apical surface. The background electrical resistance was determined by adding media to the basolateral chamber and PBS +/+ to the apical chamber of a blank Millicell filter. The TER measurements were recorded as Ω•cm2.
2.8 Western blotting
Cell culture plates were placed on ice for 5 min, washed with ice-cold PBS +/+, and lysed in buffer (50 mM Tris pH 7.4, 150 mM NaCl, 1% Triton X-100, protease inhibitors (20 µg/ml, leupeptin, aprotinin, 10 µg/ml pepstatin, and 17.4 µg/ml phenylmethylsulfonyl fluoride)) by rocking at 4°C for 10 min. Cells were scraped into a tube, sonicated five times and centrifuged at 17000 × g for 10 minutes at 4°C. The supernatant was transferred to fresh tubes and subjected to protein estimation by Bio-Rad protein assay according to manufacturer’s instructions. Aliquots of each lysate were used for SDS polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA), blocked with 5% BSA in TBST, washed, and probed with primary antibody, as indicated in the text, followed by HRP-conjugated secondary antibodies (Jackson Immuno Research, West Grove, PA). Protein bands were detected with ECL reagents (Pierce, Rockford, IL) and imaged on a Fuji LAS 4000 and/or developed in an X-ray Medical film processor (Konica SRX 101). Blots shown where chosen to show bands in all wells. Quantitation was performed at multiple exposures for blots from three separate experiments (see also Table 1).
Table 1.
Calu-3 and NuLi-1 cells have similar CAREx7 and CAREx8 gene and protein expression relative to HAE. Ratio of CAREx7:CAREx8 gene and protein expression in cell lines as determined by qRT-PCR and Western blot.
| Cell lines CAR Isoform Gene Expression (Relative to HAE) |
CAR Isoform Protein Expression (Relative to actin) |
|||||
|---|---|---|---|---|---|---|
| Ex7 | Ex8 | Ex7:Ex8 | Ex7 | Ex8 | Ex7:Ex8 | |
| CHO-K1 | N.D. | N.D. | N.D. | N.D. | N.D | N.D. |
| COS-7 | 1.5 | 0.6 | 2.6 | 2.83 | 0.52 | 5.49 |
| HeLa | 0.4 | 0.8 | 0.4 | 3.70 | 1.67 | 2.21 |
| HEK-293 | 0.4 | 0.7 | 0.6 | 6.38 | 3.27 | 1.95 |
| 293T | 0.4 | 0.7 | 0.6 | 5.24 | 6.30 | 0.83 |
| A549 | 0.1 | 0.6 | 0.2 | 0.98 | 1.37 | 0.72 |
| MDCK | 4.8 | 0.0 | 641.4 | 3.69 | 0.39 | 9.48 |
| Caco-2 | 0.5 | 1.7 | 0.3 | 3.43 | 0.73 | 4.66 |
| Calu-3 | 0.7 | 0.6 | 1.1 | 3.67 | 1.47 | 2.50 |
| NuLi-1 | 0.5 | 0.4 | 1.4 | 0.83 | 0.63 | 1.33 |
| HAE | 1.0 | 1.0 | 1.0 | 0.86 | 1.08 | 0.80 |
2.9 Adenovirus transduction
The apical surfaces of cells, seeded on millicells, were rinsed with PBS+/+. Recombinant human adenovirus type 5 containing the β-galactosidase gene (AdV5-β-Gal) driven by the CMV-IE promoter (University of Iowa Vector Core, Iowa City, IA), diluted in Opti-Mem serum-free culture media (Gibco, Invitrogen), was added to the apical surface at a multiplicity of infection (MOI) of 100 pfu/cell and returned to the incubator. After 1 h, the inoculum was removed and cells were rinsed once with Opti-MEM. Cells were lysed 24 h later and analyzed by β-galactosidase assay (Galactolight Plus β-Galalactosidase Reporter Gene Assay, Fisher Scientific). Luminescence values were normalized to the amount of protein (Bio-Rad protein assay). Error bars represent standard error over three experiments with 3–5 replicates per cell line per experiment.
3. Results
3.1 MDCK, Caco-2, Calu-3, NuLi-1 and HAE cells polarize into epithelia
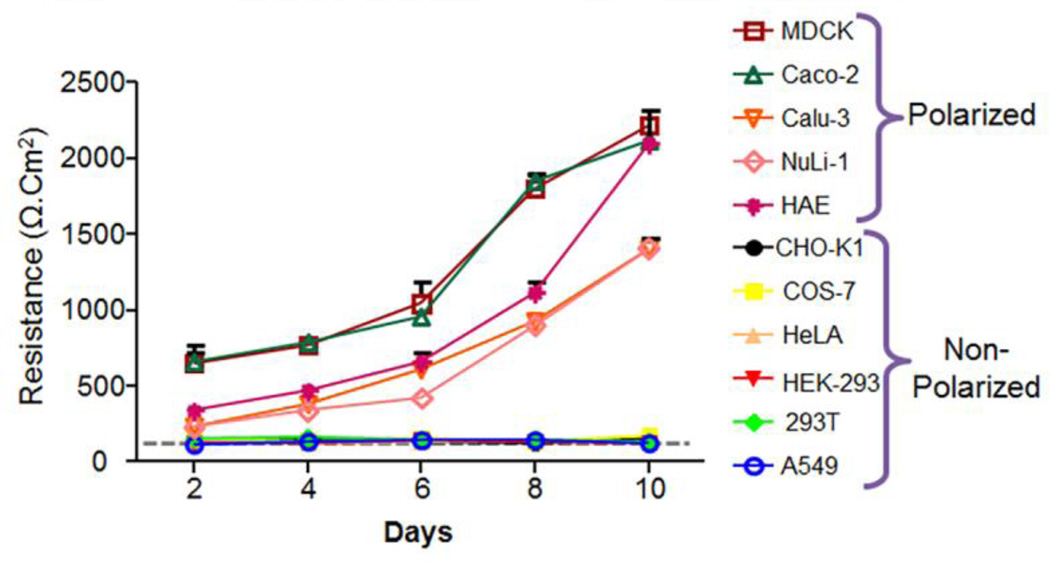
Many cells transform from non-polarized to polarized states during development. Epithelial polarization occurs by the formation of tight junctions and membrane segregation into apical and basolateral surfaces, each with a distinct distribution of proteins and lipids. Furthermore, these unique surfaces are known to mediate differential susceptibility to pathogens (Vogelmann et al., 2004). The formation, polarization, and integrity of epithelial tight junctions of various commonly used immortal cell lines were determined and compared to primary human airway epithelial cells. The cells were seeded onto semi-permeable membranes and transepithelial electrical resistance (TER) was measured and recorded over a 10 day period. Of the epithelial cells known to polarize (MDCK, Caco-2, Calu-3, NuLi-1 and HAE), MDCK and Caco-2 cells displayed the most rapid increase in TER after seeding with a marked TER within 2 days after seeding (Fig. 1). Calu-3, NuLi-1, and HAE cells were also able to polarize although the polarization was not as rapid as MDCK and Caco-2 cells. It took about 5 days for these other epithelial cell types to attain a resistance above background (> 300 Ω·cm2), indicative of a functional tight junction. Furthermore, upon polarization, primary human airway epithelial cells (HAE) were able to differentiate and be maintained at an air-liquid interface for more than 2 months with TER between 1000–2500 Ω·cm2. By contrast, CHO-K1, COS-7, HeLa, HEK-293, 293T, and A549 never increased above the background levels (dotted line) when grown on a semipermeable membrane, indicating that these cells fail to fully polarize (Fig. 1).
Figure 1.
Transepithelial electrical resistance (TER) increases over time in cell lines able to polarize into epithelia. No increase in the TER above background level (grey dotted line) was observed in cell lines unable to polarize on semipermeable membranes (CHO-K1, COS-7, HeLa, HEK-293, 293T and A549). By contrast, a rapid increase in TER over time was observed for most epithelial cell lines (MDCK, Caco-2, Calu-3, NuLi-1, and primary human airway epithelia (HAE)).
3.2 CAREx7 isoform-specific RNA is more abundant than CAREx8 RNA in cells expressing both isoforms
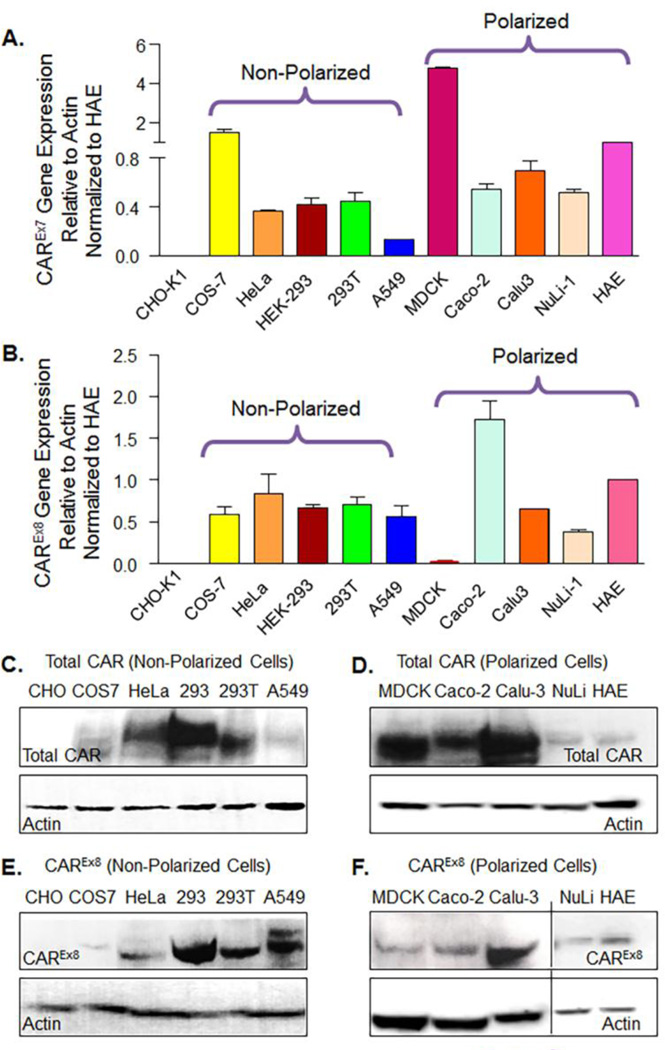
Previously we have shown that mRNA for CAREx7 is more abundant than mRNA for CAREx8 in primary human airway epithelia (HAE) (Excoffon et al., 2010). To determine the relative abundance of these two isoforms in other cell lines, RNA was isolated from each cell line and gene expression was quantified by quantitative RT-PCR (qPCR). RNA levels were measured with respect to β-actin and normalized to the CAR negative CHO-K1 cell line (Table 1) or HAE (Fig. 2 A and B and Table 1). With the exception of CHO-K1 cells, all the cell lines tested expressed RNA specific for each isoform. We first compared relative gene expression to CHO-K1 in order to illustrate the difference from baseline. Among the cell lines expressing both CAR isoforms, similar to HAE, CAREx7 transcript levels were more abundant than CAREx8 transcript levels (Table 1). However, the relative abundance of CAR isoforms varied significantly between cell lines, with the greatest isoform difference found in MDCK cells. We next compared expression relative to HAE and found that MDCK and COS-7 cells have much more CAREx7 relative to CAREx8 than HAE, Calu-3 and NuLi-1 were similar to HAE, and the other cell lines had a lower ratio.
Figure 2.
CAREx7 transcript and protein levels are more abundant than CAREx8 transcript and protein levels. A) CAREx7 or B) CAREx8 gene expression was quantified by qPCR with exon specific primers after cDNA synthesis from total RNA obtained from non-polarized and polarized cell types grown on semi-permeable membranes. Gene expression shown is relative to HAE (see also Table 1). A comparison to CHO-K1 cells is shown in Supplementary Table 1. C–F) Lysate from each cell type was subjected to SDS–PAGE and analyzed by Western blotting. Total CAR expression was detected in C) the cell lines that do not polarize or D) polarized cells with a CAR-specific polyclonal antibody (rabbit anti-CAR-1605p) that recognizes the C-terminus of both CAR isoforms. CAREx8 specific protein expression was detected in E) the cell lines that do not polarize or F) polarized cells by a CAREx8-specific antibody (rabbit anti-CAREx8-5678p). As expected, no CAREx7 or CAREx8 protein was detectable in CHO-K1 cells.
3.3 CAR protein expression varies between cell lines
CAREx7 and CAREx8 protein expression was determined in ten different cell lines and HAE. Western blot with a rabbit polyclonal Ab that detects both isoforms (rα1605) (Excoffon et al., 2005; Sharma et al., 2012a) demonstrated a CAR-specific band at approximately 46 kDa in all cell lines except CHO-K1 (Fig. 2). We found that CAR protein expression levels vary greatly between the investigated cell lines. In non-polarized cells, HEK-293 cells expressed the highest level of total CAR (Fig. 2C) and CAREx8 protein (Fig. 2D). In the case of polarized cells, MDCK cells had the highest protein expression of the CAREx7 isoform (Fig. 2D) and, consistent with the mRNA expression results, MDCK cells also had very low endogenous protein levels for the apical CAREx8 isoform (Fig. 2E and F). These data support the use of the MDCK cell line as a suitable cell line for studying CAREx7 with little CAREx8 to confound results. The protein ratio of the two transmembrane isoforms studied in all the cell lines is shown in Table 1 and suggests that the CAR gene transcript levels in MDCK, NuLi-1 and HAE correlate relatively well to the amount of protein expression, but do not correlate in the other cell lines. These data suggest that while all tested cell lines are candidates for CAR expression studies, the variability of CAR isoform gene and protein expression should be considered when choosing a cell line for the desired experiment.
3.4 CAR is localized at cell junctions
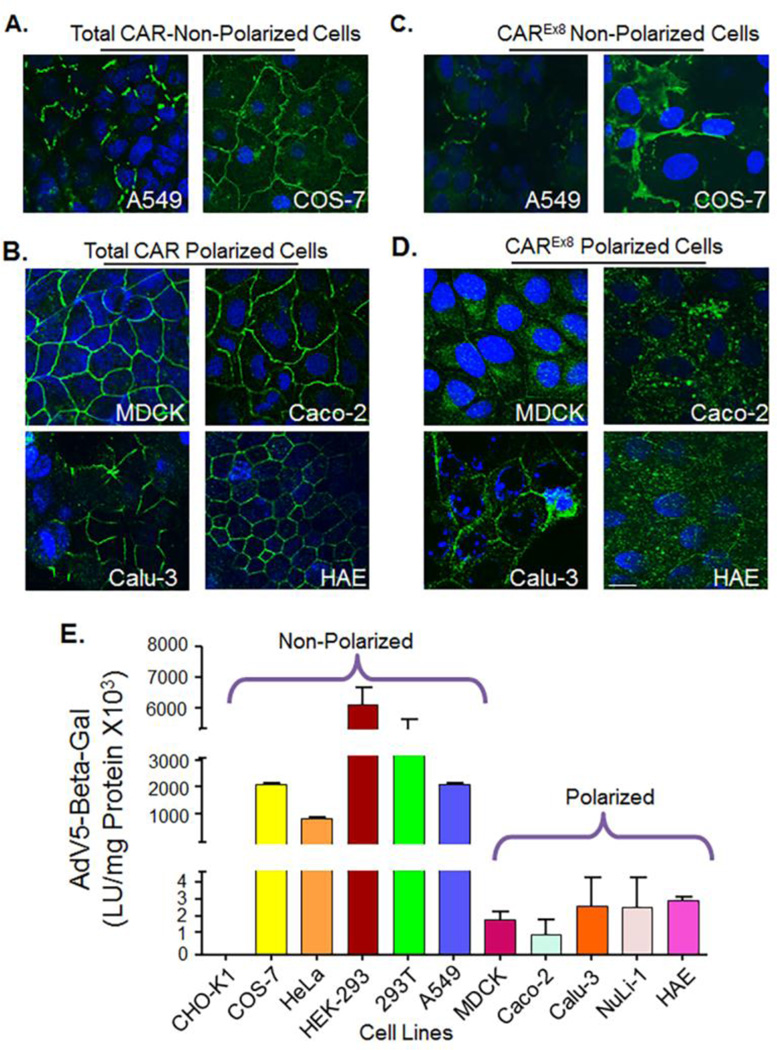
To determine the localization of CAR, cells were seeded and polarized on semi-permeable membranes, after which the cells were fixed and subjected to immunocytochemistry for total CAR or CAREx8 (Fig. 3 A, B, C and D). Both CAR isoforms (green) were localized at the cell junctions of non-polarized A549 and COS-7 cells (total CAR, Fig. 3A; CAREx8, Fig. 3B). Polarized cell lines showed robust basolateral junctional localization of CAREx7 (Fig. 3A and C). The smooth “chickenwire” outline surrounding the cells suggests that tight junctions are well formed in these cells. This is in contrast to A549 and COS-7 cells where CAR is much less organized and does not form a smooth chickenwire pattern around each cell suggesting a lack of polarization (Sharma et al., 2012b). CAREx8 was mainly found in within the cytoplasm region consistent with the sub-apical compartment (Fig. 3D). In summary, in non-polarized immortal cell lines, both CAREx7 and CAREx8 isoforms are localized at cell junctions and no differential localization is observed. By contrast, immortal polarizable cell lines express both CAR isoforms in a similar manner to primary human airway epithelia. The characteristics shown by these cell lines confirm that they are suitable epithelial cells lines for investigating endogenous CAR isoform-specific expression in polarized epithelia.
Figure 3.
CAREx7 localizes at basolateral junctions while CAREx8 localizes within the subapical and apical compartments and epithelial polarization decreases susceptibility to adenovirus transduction. A) and B) Immunocytochemistry for total CAR staining (rabbit anti-CAR-1605p) in green and nuclei in blue (DAPI). A) In non-polarized cells (A549 and COS-7), CAREx7 staining and localization is discontinuous due to lack of formation of tight junction, B) whereas CAREx7 localization in polarized cells (MDCK, Caco-2, Calu-3, and HAE) presents as a “chicken wire” pattern due to the formation of tight junctions. C) and D) CAREx8 localization (rabbit anti-CAREx8-5678p) is shown in green and nuclei in blue (DAPI). C) CAREx8 localization is similar to CAREx7 in non-polarized cells. D) However, CAREx8 localization in well differentiated and polarized epithelial cells is distinct and is found in the cytoplasm consistent with the subapical compartment. (60× oil immersion confocal microscopy, white bar equals 10 µm. E) All cell types were seeded onto semi-permeable membranes and maintained at the air-liquid interface prior to infection from the “apical” surface with recombinant adenovirus carrying the gene for beta-galactosidase (AdV5-Beta-Gal) and Beta-galactosidase activity evaluated 24 h post-transduction.
3.5 Adenovirus transduction depends on primary receptor expression and cell polarity
We next correlated the expression of the CAR isoforms to adenovirus transduction. All of the cell lines were seeded on semipermeable membranes, allowed to polarize, infected with recombinant adenovirus containing beta-galactosidase gene (AdV5-β-Gal), and assayed using a luminescence assay relative to the amount of protein (Fig. 3E). With the exception of CAR-negative CHO-K1 cells, all the non-polarized cells were much more susceptible to adenovirus infection when compared to polarized cells. Among the non-polarized cells, HEK-293 and 293T demonstrated the highest expression of β-Galactosidase, which directly correlates with the primary receptor expression. Also, HEK-293 and 293T cells have an insertion of adenovirus E1A and E1B genes that transcomplement the production of first generation E1/E3 deleted recombinant vectors, such as the AdV5-β-Gal vector used here (Graham et al., 1977), and therefore also reflect viral replication. In the case of polarized cells, epithelia were approximately 3 log less susceptible to adenovirus infection and demonstrated little variability in apical adenovirus infection.
4. Discussion
In contrast to previous beliefs that adenoviral infection occurs only from the basolateral surface of polarized cells, recent evidence shows that viral infection can occur at the apical surface of polarized epithelia (Excoffon et al., 2014; Excoffon et al., 2010; Kolawole et al., 2012; Kotha et al., 2015; Lutschg et al., 2011; Sharma et al., 2012a; Sharma et al., 2012b). CAR has two transmembrane isoforms, CAREx7 and CAREx8, each having a unique ability to initiate viral entry from distinct epithelial surfaces (Excoffon et al., 2014). This raises the question of the abundance and cellular localization of the two CAR isoforms, and their relative contribution to viral infection of commonly used cells lines. Ten immortal cell lines that have previously been used to study the function of viral receptors were investigated including: CHO-K1, COS-7, HeLa, HEK-293, 293T, A549, MDCK, Caco-2, Calu-3, NuLi-1, and compared to primary HAE. As expected, the two airway epithelial model cell lines, Calu-3 and NuLi-1, were most similar to HAE.
Cell lines were first tested for their ability to polarize and form tight junctions. Cells grown on semipermeable membranes were effaced every other day in order to determine the ability for each line to form electrically tight junctions and a unique “dry” apical surface at the air-liquid interface. As expected, media from the basolateral surface leaked into the apical compartment of millicells containing CHO-K1, COS-7, HeLa, HEK-293, 293T, or A549 cells. By contrast MDCK, Caco-2, Calu-3, NuLi-1, and HAE developed a “dry” apical surface and high transepithelial resistance. All the immortal polarized cell lines were found to polarize similarly to airway epithelia but the number of days they took to achieve polarity is different (Fig. 1). Moreover, of these 3 cell lines, MDCK cells polarize most rapidly, confirming that MDCK cells are an ideal line for investigating rapid epithelial polarization.
We next examined the expression and localization of both CAR isoforms (Fig. 2, 3, Table 1, S1). With the exception of CHO-K1 cells, all cell lines were found to contain a sufficient amount of RNA for CAREx8 protein production. CAREx7 transcript and protein levels were consistently more abundant than CAREx8 (Fig. 2, Table S1), both isoforms are expressed in multiple cell lines. Although most cell types had similar CAREx7 and CAREx8 transcript levels, higher CAREx7 transcript levels were observed in COS-7 and MDCK, lower CAREx7 transcript levels were found in A549 cells, and very low CAREx8 transcript levels were found in MDCK. A few studies have shown that the STAT1 pathway and transcription factors, SP1, SNAIL1, and SMAD3/4, transcriptionally regulate CAR (Chung et al., 2011; Ruppert et al., 2008; Vincent et al., 2009). The only cell line overlapping with our study is HeLa, and it was shown that deletion of the SP1 promoter motif impaired CXADR (CAR gene) promoter activity (Chung et al., 2011). How each of these pathways impact transcriptional regulation of CXADR in the other cell lines in our study is currently unclear. Moreover, to our knowledge, no studies have investigated the regulation of alternative mRNA splicing for CAR. Given the variability in CAR isoform transcript levels in COS-7, A549, and MDCK cells, these likely represent candidate cell lines to elucidate novel CAR-isoform specific transcriptional regulation pathways.
With the exception of MDCK, NuLi-1, and HAE, transcript levels did not directly correlate with protein levels (Fig. 2). Multiple factors could play a role in translational regulation. For example, we have previously shown that the activation of the Akt pathway via IL-8 stimulation increases CAREx8 protein synthesis (Kotha et al., 2015), whereas inhibition of GSK-3β increased both transcript and proteins levels for CAREx8 (Sharma et al., 2012a). Both pathways lead to increased apical localization of CAREx8 and increased adenovirus transduction.
Both isoforms localize to cell-cell junctions in non-polarized cells (Fig. 3.). In polarized MDCK, Caco-2, Calu-3 and HAE epithelia, CAREx7 shows robust junctional localization, whereas little CAREx8 is present at basolateral junctions. Differential isoform expression and localization has previously been investigated in mice. Raschperger et al compared CAR transmembrane isoform expression in tissues from adult mice using isoform specific antibodies (Raschperger et al., 2006). The murine CAREx8 equivalent, mCAR-1 (TVV), and CAREx7, mCAR-2 (SIV), were predominately expressed in epithelial cells lining organs, although immunoreactivity was also found in endothelial cells, at the neuromuscular junction, and at cardiac intercalated discs. Although differences in localization were primarily observed in liver and kidney tissues, large differences in the relative amount of mCAR-1 protein expression was found between liver, kidney, pancreas and colon, and relative to mCAR-2. Differential isoform localization has also been shown in skeletal muscle, where mCAR-2 is the primary isoform at the neuromuscular junction in mice and both CAREx7 and CAREx8 are present in human (Shaw et al., 2004). Perhaps the most striking difference in isoform localization has been observed in mature spermatozoa where each isoform was present in a distinct region of the acrosome (Mirza et al., 2006), however, subsequent studies that conditionally knocked out both isoforms in mice did not show any defect in spermatogenesis or fertilization (Sultana et al., 2014). These findings are consistent with differential localization of the two CAR transmembrane isoforms in multiple cell types in mice and in humans.
Finally we investigated the susceptibility of each cell line to adenovirus transduction after growth on a semipermeable membrane (Fig. 3). Adenovirus transduction was approximately 3 logs lower in polarized epithelia in comparison to non-polarized cells. The variability in adenovirus-mediated gene expression was primarily observed in non-polarized cells and ranged from no transduction of CHO-K1 cells (CAR negative) to the highest expression in HEK-293 and 293T cells. HEK-293 and 293T contain adenovirus 5 DNA and transcomplement the production of adenovectors that have genomic deletions that render them replication defective in the absence of transcomplementation. Since a high level of AdV5-β-Gal replication will have occurred in these cells by 24 h, β-galactosidase activity does not solely reflect viral transduction as it does in the other cell types. Moreover, the CMV promoter is known to vary from cell type to cell type by up to a log difference in gene expression (Qin et al., 2010; Xia et al., 2006). Given the large 3 log difference between polarized and non-polarized cells, it is unlikely that a difference in promoter or enzymatic activity would explain our findings. Transduction was evaluated rather than viral binding since binding may not lead to viral entry. Moreover, not all adenovirus genomes that enter cells end up in the nucleus where genome transcription occurs. Transduction is a readout for the genomes that reach the nucleus and can successfully express a transgene – a critical parameter for gene therapy and wild type infection alike. Future studies will focus on the correlations between binding, entry, transduction, and wild type adenovirus infection in polarized cell types.
The cell lines in this study were chosen based on their instrumental roles in the initial discovery and advancement of our understanding of CAR and adenovirus infection. For example, CHO-K1 and HeLa cells were critical in the discovery of CAR and its alternative splice products, adenovirus co-receptors, and the step-wise dismantling of adenovirus during infection (Bergelson et al., 1997; Carson et al., 1997; Greber et al., 1993; Thoelen et al., 2001; Wickham et al., 1993). A549 cells have been used extensively in the production, characterization, and titering of wild type adenovirus. Interpretation of studies in this cell line should take into account that A549 cells demonstrate only partial polarization and therefore do not reflect infection from distinct apical or basolateral surfaces. MDCK cells have been used to elucidate the clathrin-mediated trafficking of CAREx7 in polarized cells (Carvajal-Gonzalez et al., 2012; Diaz et al., 2009). We consider MDCK to be a particularly strong experimental system given the high amount of endogenous CAREx7, but low levels of CAREx8. We have taken advantage of this imbalance and created doxycycline-inducible MDCK cell lines that contain the cDNAs for human CAREx8, CAREx7, or mCherry, to investigate the important physiological effects of CAREx8 expression on viral infection and innate immunity (Kotha et al., 2015). CAREx8 overexpression leads to increased apical trafficking of CAREx8, which, in turn, increases adenoviral infection from the apical surface. This evidence suggests that, contrary to a decrease in TER or a break in the junctions, apical adenovirus infection can occur by apically localized CAREx8.
Applications of these data include the ability to test innovative medical treatments for respiratory infection. The effect that CAREx8 exerts on viral entry in polarized cells could potentially be targeted to develop novel therapies that regulate receptor expression and/or trafficking. Down-regulation of CAREx8 could prevent adenovirus-mediated exacerbation of diseases, such as asthma, cystic fibrosis (CF), and chronic obstructive pulmonary disease (COPD), and protect immunosuppressed populations. Moreover, the up-regulation of CAREx8 could be used to facilitate viral entry during gene therapy using adenovirus as vectors. Understanding CAR expression and function in epithelial cells may facilitate the development and evaluation of novel treatment options for both new and persistent respiratory and gastrointestinal infections in humans.
Supplementary Material
Highlights.
-
-
Variability in CAR expression in model cell lines is compared to primary epithelia
-
-
Polarization reduces adenovirus transduction efficiency
-
-
Localization of CAR transmembrane isoforms impacts adenovirus transduction
Acknowledgments
We thank Dr. Joseph Zabner and the University of Iowa Cell Culture Model Core for providing primary human airway epithelia, and Mahmoud Alghamri and James Readler for technical assistance and manuscript review. Research reported in this publication was supported by the National Institute of Allergy And Infectious Diseases of the National Institutes of Health under Award Number R15AI090625. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- Bergelson JM, Krithivas A, Celi L, Droguett G, Horwitz MS, Wickham T, Crowell RL, Finberg RW. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J Virol. 1998;72:415–419. doi: 10.1128/jvi.72.1.415-419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson SD, Chapman NN, Tracy SM. Purification of the putative coxsackievirus B receptor from HeLa cells. Biochem Biophys Res Commun. 1997;233:325–328. doi: 10.1006/bbrc.1997.6449. [DOI] [PubMed] [Google Scholar]
- Carvajal-Gonzalez JM, Gravotta D, Mattera R, Diaz F, Perez Bay A, Roman AC, Schreiner RP, Thuenauer R, Bonifacino JS, Rodriguez-Boulan E. Basolateral sorting of the coxsackie and adenovirus receptor through interaction of a canonical YXXPhi motif with the clathrin adaptors AP-1A and AP-1B. Proc Natl Acad Sci U S A. 2012;109:3820–3825. doi: 10.1073/pnas.1117949109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SK, Kim JY, Lim JY, Park YM, Hwang HY, Nam JH, Park SI. Transcription factor Sp1 is involved in expressional regulation of coxsackie and adenovirus receptor in cancer cells. J Biomed Biotechnol. 2011;2011:636497. doi: 10.1155/2011/636497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, Bergelson JM. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci USA. 2001;98:15191–15196. doi: 10.1073/pnas.261452898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne CB, Voelker T, Pichla SL, Bergelson JM. The coxsackievirus and adenovirus receptor interacts with the multi-PDZ domain protein-1 (MUPP-1) within the tight junction. J Biol Chem. 2004;279:48079–48084. doi: 10.1074/jbc.M409061200. [DOI] [PubMed] [Google Scholar]
- Diaz F, Gravotta D, Deora A, Schreiner R, Schoggins J, Falck-Pedersen E, Rodriguez-Boulan E. Clathrin adaptor AP1B controls adenovirus infectivity of epithelial cells. Proc Natl Acad Sci U S A. 2009;106:11143–11148. doi: 10.1073/pnas.0811227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffon KJ, Bowers JR, Sharma P. 1. Alternative splicing of viral receptors: A review of the diverse morphologies and physiologies of adenoviral receptors. Recent Res Dev Virol. 2014;9:1–24. [PMC free article] [PubMed] [Google Scholar]
- Excoffon KJ, Gansemer ND, Mobily ME, Karp PH, Parekh KR, Zabner J. Isoform-specific regulation and localization of the coxsackie and adenovirus receptor in human airway epithelia. PLoS One. 2010;5:e9909. doi: 10.1371/journal.pone.0009909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffon KJ, Hruska-Hageman A, Klotz M, Traver GL, Zabner J. A role for the PDZ-binding domain of the coxsackie B virus and adenovirus receptor (CAR) in cell adhesion and growth. J Cell Sci. 2004;117:4401–4409. doi: 10.1242/jcs.01300. [DOI] [PubMed] [Google Scholar]
- Excoffon KJ, Kolawole AO, Kusama N, Gansemer ND, Sharma P, Hruska-Hageman AM, Petroff E, Benson CJ. Coxsackievirus and adenovirus receptor (CAR) mediates trafficking of acid sensing ion channel 3 (ASIC3) via PSD-95. Biochem Biophys Res Commun. 2012;425:13–18. doi: 10.1016/j.bbrc.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffon KJ, Traver GL, Zabner J. The role of the extracellular domain in the biology of the coxsackievirus and adenovirus receptor. Am J Respir Cell Mol Biol. 2005;32:498–503. doi: 10.1165/rcmb.2005-0031OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Greber UF, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- Karp PH, Moninger TO, Weber SP, Nesselhauf TS, Launspach JL, Zabner J, Welsh MJ. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol Biol. 2002;188:115–137. doi: 10.1385/1-59259-185-X:115. [DOI] [PubMed] [Google Scholar]
- Kolawole AO, Sharma P, Yan R, Lewis KJ, Xu Z, Hostetler HA, Ashbourne Excoffon KJ. The PDZ1 and PDZ3 domains of MAGI-1 regulate the eight-exon isoform of the coxsackievirus and adenovirus receptor. J Virol. 2012;86:9244–9254. doi: 10.1128/JVI.01138-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotha PL, Sharma P, Kolawole AO, Yan R, Alghamri MS, Brockman TL, Gomez-Cambronero J, Excoffon KJ. Adenovirus entry from the apical surface of polarized epithelia is facilitated by the host innate immune response. PLoS Pathog. 2015;11:e1004696. doi: 10.1371/journal.ppat.1004696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim BK, Xiong D, Dorner A, Youn TJ, Yung A, Liu TI, Gu Y, Dalton ND, Wright AT, Evans SM, Chen J, Peterson KL, McCulloch AD, Yajima T, Knowlton KU. Coxsackievirus and adenovirus receptor (CAR) mediates atrioventricular-node function and connexin 45 localization in the murine heart. J Clin Invest. 2008;118:2758–2770. doi: 10.1172/JCI34777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ory V, Chapman S, Yuan H, Albanese C, Kallakury B, Timofeeva OA, Nealon C, Dakic A, Simic V, Haddad BR, Rhim JS, Dritschilo A, Riegel A, McBride A, Schlegel R. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012;180:599–607. doi: 10.1016/j.ajpath.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutschg V, Boucke K, Hemmi S, Greber UF. Chemotactic antiviral cytokines promote infectious apical entry of human adenovirus into polarized epithelial cells. Nat Commun. 2011;2:391. doi: 10.1038/ncomms1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza M, Hreinsson J, Strand ML, Hovatta O, Soder O, Philipson L, Pettersson RF, Sollerbrant K. Coxsackievirus and adenovirus receptor (CAR) is expressed in male germ cells and forms a complex with the differentiation factor JAM-C in mouse testis. Exp Cell Res. 2006;312:817–830. doi: 10.1016/j.yexcr.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Mirza M, Raschperger E, Philipson L, Pettersson RF, Sollerbrant K. The cell surface protein coxsackie- and adenovirus receptor (CAR) directly associates with the Ligand-of-Numb Protein-X2 (LNX2) Exp Cell Res. 2005;309:110–120. doi: 10.1016/j.yexcr.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Nalbantoglu J, Larochelle N, Wolf E, Karpati G, Lochmuller H, Holland PC. Muscle-specific overexpression of the adenovirus primary receptor CAR overcomes low efficiency of gene transfer to mature skeletal muscle. J Virol. 2001;75:4276–4282. doi: 10.1128/JVI.75.9.4276-4282.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbantoglu J, Pari G, Karpati G, Holland PC. Expression of the primary coxsackie and adenovirus receptor is downregulated during skeletal muscle maturation and limits the efficacy of adenovirus-mediated gene delivery to muscle cells. Hum Gene Ther. 1999;10:1009–1019. doi: 10.1089/10430349950018409. [DOI] [PubMed] [Google Scholar]
- Raschperger E, Thyberg J, Pettersson S, Philipson L, Fuxe J, Pettersson RF. The coxsackie- and adenovirus receptor (CAR) is an in vivo marker for epithelial tight junctions, with a potential role in regulating permeability and tissue homeostasis. Exp Cell Res. 2006;312:1566–1580. doi: 10.1016/j.yexcr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Ruppert V, Meyer T, Pankuweit S, Jonsdottir T, Maisch B. Activation of STAT1 transcription factor precedes up-regulation of coxsackievirus-adenovirus receptor during viral myocarditis. Cardiovasc Pathol. 2008;17:81–92. doi: 10.1016/j.carpath.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Sharma P, Kolawole AO, Core SB, Kajon AE, Excoffon KJ. Sidestream smoke exposure increases the susceptibility of airway epithelia to adenoviral infection. PLoS One. 2012a;7:e49930. doi: 10.1371/journal.pone.0049930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Kolawole AO, Wiltshire SM, Frondorf K, Excoffon KJ. Accessibility of the coxsackievirus and adenovirus receptor and its importance in adenovirus gene transduction efficiency. J Gen Virol. 2012b;93:155–158. doi: 10.1099/vir.0.036269-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw CA, Holland PC, Sinnreich M, Allen C, Sollerbrant K, Karpati G, Nalbantoglu J. Isoform-specific expression of the Coxsackie and adenovirus receptor (CAR) in neuromuscular junction and cardiac intercalated discs. BMC Cell Biol. 2004;5:42. doi: 10.1186/1471-2121-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollerbrant K, Raschperger E, Mirza M, Engstrom U, Philipson L, Ljungdahl PO, Pettersson RF. The Coxsackievirus and adenovirus receptor (CAR) forms a complex with the PDZ domain-containing protein ligand-of-numb protein-X (LNX) J Biol Chem. 2003;278:7439–7444. doi: 10.1074/jbc.M205927200. [DOI] [PubMed] [Google Scholar]
- Sultana T, Hou M, Stukenborg JB, Tohonen V, Inzunza J, Chagin AS, Sollerbrant K. Mice depleted of the coxsackievirus and adenovirus receptor display normal spermatogenesis and an intact blood-testis barrier. Reproduction. 2014;147:875–883. doi: 10.1530/REP-13-0653. [DOI] [PubMed] [Google Scholar]
- Thoelen I, Magnusson C, Tagerud S, Polacek C, Lindberg M, Van Ranst M. Identification of alternative splice products encoded by the human coxsackie-adenovirus receptor gene. Biochem Biophys Res Commun. 2001;287:216–222. doi: 10.1006/bbrc.2001.5535. [DOI] [PubMed] [Google Scholar]
- Tomko RP, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci U S A. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdino P, Witherden DA, Havran WL, Wilson IA. The molecular interaction of CAR and JAML recruits the central cell signal transducer PI3K. Science. 2010;329:1210–1214. doi: 10.1126/science.1187996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent T, Neve EP, Johnson JR, Kukalev A, Rojo F, Albanell J, Pietras K, Virtanen I, Philipson L, Leopold PL, Crystal RG, de Herreros AG, Moustakas A, Pettersson RF, Fuxe J. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-beta mediated epithelial-mesenchymal transition. Nat Cell Biol. 2009;11:943–950. doi: 10.1038/ncb1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelmann R, Amieva MR, Falkow S, Nelson WJ. Breaking into the epithelial apical-junctional complex--news from pathogen hackers. Curr Opin Cell Biol. 2004;16:86–93. doi: 10.1016/j.ceb.2003.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters RW, Freimuth P, Moninger TO, Ganske I, Zabner J, Welsh MJ. Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell. 2002;110:789–799. doi: 10.1016/s0092-8674(02)00912-1. [DOI] [PubMed] [Google Scholar]
- Weber DA, Sumagin R, McCall IC, Leoni G, Neumann PA, Andargachew R, Brazil JC, Medina-Contreras O, Denning TL, Nusrat A, Parkos CA. Neutrophil-derived JAML inhibits repair of intestinal epithelial injury during acute inflammation. Mucosal Immunol. 2014;7:1221–1232. doi: 10.1038/mi.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- Witherden DA, Verdino P, Rieder SE, Garijo O, Mills RE, Teyton L, Fischer WH, Wilson IA, Havran WL. The junctional adhesion molecule JAML is a costimulatory receptor for epithelial gammadelta T cell activation. Science. 2010;329:1205–1210. doi: 10.1126/science.1192698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Sharma P, Kolawole AO, Martin SC, Readler JM, Kotha PL, Hostetler HA, Excoffon KJ. The PDZ3 domain of the cellular scaffolding protein MAGI-1 interacts with the Coxsackievirus and adenovirus receptor (CAR) Int J Biochem Cell Biol. 2015 doi: 10.1016/j.biocel.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zen K, Liu Y, McCall IC, Wu T, Lee W, Babbin BA, Nusrat A, Parkos CA. Neutrophil migration across tight junctions is mediated by adhesive interactions between epithelial coxsackie and adenovirus receptor and a junctional adhesion molecule-like protein on neutrophils. Mol Biol Cell. 2005;16:2694–2703. doi: 10.1091/mbc.E05-01-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zihni C, Balda MS, Matter K. Signalling at tight junctions during epithelial differentiation and microbial pathogenesis. J Cell Sci. 2014;127:3401–3413. doi: 10.1242/jcs.145029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.