Abstract
The canonical NF-κB signaling pathway is a mediator of the cellular inflammatory response and a target for developing therapeutics for multiple human diseases. The furthest downstream proteins in the pathway, the p50/p65 transcription factor heterodimer, have been recalcitrant towards small molecule inhibition despite the substantial number of compounds known to inhibit upstream proteins in the activation pathway. Given the roles of many of these upstream proteins in multiple biochemical pathways, targeting the p50/p65 heterodimer offers an opportunity for enhanced on-target specificity. Towards this end, the p65 protein presents two non-disulfide cysteines, Cys38 and Cys120, at its DNA-binding interface that are amenable to targeting by covalent molecules. The natural product helenalin, a sesquiterpene lactone, has been previously shown to target Cys38 on p65 and ablate its DNA-binding ability. Using helenalin as inspiration, simplified helenalin analogues were designed, synthesized, and shown to inhibit induced canonical NF-κB signaling in cell culture. Moreover, two simplified helenalin probes were proficient at forming covalent protein adducts, binding to Cys38 on recombinant p65, and targeting p65 in HeLa cells without engaging canonical NF-κB signaling proteins IκBα, p50, and IKKα/β. These studies further support that targeting the p65 transcription factor-DNA interface with covalent small molecule inhibitors is a viable approach towards regulating canonical NF-κB signaling.
Graphical Abstract
INTRODUCTION
Aberrant activation of canonical p50/p65 NF-κB transcription factors and concomitant expression of their target genes has been implicated in a spectrum of human diseases, including chronic inflammatory disease, atherosclerosis, arthritis, and cancer.1–4 Consequently, interventional strategies to regulate canonical NF-κB signaling may be broadly useful for multiple therapeutic indications.5–8 Accordingly, significant research efforts have been devoted towards the discovery of inhibitors of canonical p50/p65 NF-κB signaling with the targeting of upstream kinases that facilitate p50/p65 activation (via release from its repressor protein, IκBα) being the prominent strategy.9, 10 However, most of these enzymes have degenerate activities with other cellular processes, and therefore, their inhibition as a strategy to regulate canonical NF-κB signaling results in off-target effects.9 Targeting the most downstream proteins in the canonical NF-κB signaling pathway, the p50/p65 transcription factor heterodimer itself, would ablate such specificity issues.
Chemical modulation of transcription factor-DNA interfaces has shown promise as a strategy to regulate aberrant transcription factor signaling. Pyrrole-imidazole polyamides that target the DNA minor groove with sequence specificity and disrupt transcription factor-DNA binding have demonstrated promising utilities in both cell culture and animal models, including modulation of canonical NF-κB signaling.11–15 Conversely, targeting transcription factors with protein-binding small molecules has proven more problematic, which has been attributed to the typical shallow protein binding pockets and non-discrete protein tertiary structure, which result in weak binding by putative inhibitors.16, 17 Irreversible covalent binding by inhibitors to nucleophilic amino acids on transcription factors may provide another strategy for transcriptional regulation via direct protein binding provided the amino acids that are covalently modified are intolerant of chemical modification (e.g., chemical modification prevents DNA binding and/or transcriptional activation).6
Many sesquiterpene lactone (SL) natural products are known modulators of NF-κB signaling.18, 19 In particular, helenalin (Figure 1A), a SL isolable from some Arnica and Helenium species,20–22 has provided inspiration for the rational design of transcription factor-targeting NF-κB inhibitors that disrupt DNA binding. The medicinal properties of helenalin have been known for over a century,23 which are structurally endowed, in part, by its α-methylene-γ-butyrolactone; a moiety found in scores of bioactive natural products.24 Exocyclic methylene butyrolactones undergo hetero-Michael addition with biological thiols to form covalent adducts. Helenalin also contains an endocyclic α,β-unsaturated ketone (cyclopentenone) that can also undergo hetero-Michael addition with thiols. Removal of one of the two Michael acceptors of helenalin, such as reduction of the cyclopenteone (yielding 2,3-dihydrohelenalin) or α-methylene-γ-butyrolactone (yielding 11,13-dihydrohelenalin; plenolin), significantly diminishes cytotoxicity in comparison to the parent natural product.25–28 Reduction of both Michael acceptors on helenalin ablates all activity.26, 27 The cyclopentenone and α-methylene-γ-butyrolactone of helenalin can engage biological thiols, such as glutathione and cysteine, yielding covalent adducts.29, 30 Previous studies by Merfort and colleagues have shown that helenalin covalently targets Cys38 of NF-κB p65,31, 32 which is positioned at the DNA-binding interface upon heterodimerization with p50 and DNA engagement (Figure 1B).33 Alkylation of p65 by helenalin sterically prevents DNA binding of the p50/p65 heterodimer and inhibits its transcriptional activation.34 Interestingly, molecular modeling of helenalin enabled the hypothesis that it may engage in tandem hetero-Michael additions with Cys38 and Cys120, which are 7.7 Å apart when bound to DNA;35,36 however, the lack of sensitivity of helenalin to a Cys120→Ser mutation has drawn this crosslinking model into question.18, 34 Nonetheless, the non-disulfide, nucleophilic cysteines, Cys38 and Cys120, located at the p65 DNA-binding interface constitute unique structural features of p65 that lends itself towards the development of covalent chemical modulators. Furthermore, previous studies from other groups have demonstrated covalent engagement of p65 Cys38 with diverse small molecules,6, 37–41 as well as covalent targeting of p65 Cys120.42
Figure 1.
(A) Structure of the sesquiterpene lactone helenalin and the design of helenalin mimics that contain both electrophiles found in the parent natural product. The secondary alcohol is amenable to chemical modifications to install reporter tags, such as alkynes. Based on two reported crystal structures of helenalin, the distance between the two electrophilic carbons (red asterisks) is 6.2–6.4 Å.48, 49 (B) X-ray crystal structure of the NF-κB p50-p65 heterodimer bound to DNA (pdb 1VKX).33 Notably, p65 cysteine residues 38 and 120 are adjacent in the DNA-binding interface (7.7 Å S-S distance, depicted with dashed line).
We hypothesized that chemical probes containing two structurally similar, electrophilic heterocycles configured in comparable chemical space as that of helenalin may recapitulate the interesting biological activity of the parent natural product. Consequently, we devised a strategy to develop structurally simplified helenalin analogues that could be amenable to structure-activity relationship studies, serve as suitable chemical mimics of the parent natural product, and yield powerful chemical biology tools for specificity studies and target annotation by protein pulldown-mass spectrometry analysis. Recently, bis-Michael acceptors have been developed to regulate the Keap1/Nrf2/ARE pathway, which supports our approach.43 Our design would also enable the rapid synthesis of analogues in comparison to helenalin, which required lengthy total syntheses.44–47 Herein, we report the design, synthesis, and characterization of simplified helenalin analogues and their utilization in cell culture to target p65 of the canonical NF-κB signaling pathway.
RESULTS AND DISCUSSION
Design and Synthesis of Simplified Helenalin Analogues
The probe design was based on retaining both the cyclopentenone and α-methylene-γ-butyrolactone of helenalin, which are required for covalent reactivity at Cys38 and Cys120 of p65, but structurally simplifying the central 7-membered ring whereas the two above-mentioned ring systems would be tethered in only one position (Figure 1A). In support of this design, computational modeling of 6a and 6b predicted distances between the electrophilic carbons (analogous to the electrophilic carbons of helenalin denoted in Figure 1A) on both simplified analogues to be 6.2 Å, which is comparable to those distances measured from published x-ray crystal structures of helenalin (6.2–6.4 Å; see Figure S1 for modeling data).48, 49 Additionally, the calculated distances between the analogous electrophilic carbons on alkynylated probes 1a and 1b was similar (1a: 6.1 Å; 1b: 5.4 Å). An additional consideration in our design was the utility of the diastereoselective, Barbier-type, aldehyde allylation chemistry developed by Hodgson and coworkers for synthesizing β-substituted α-methylene-γ-butyrolactones.50 Accordingly, we devised the 5-step racemic synthesis of structurally simplified helenalin probe 6a* shown in Scheme 1. The known, racemic cyclopentanone rac-251–53 was oxidized to the α,β-unsaturated ketone 3 using IBX in moderate yield.54 β-keto ester 3 was reduced to the diol with LiAlH4, and then subsequently oxidized to the desired aldehyde 4 with PCC in a 40% yield over the two steps. Notably, aldehyde 4 is volatile and unstable and must be used immediately following careful purification and isolation. The α-(bromo)methyl unsaturated furanone 5 was synthesized in one step from α-methylene-γ-butyrolactone by a known procedure.50, 55 With building blocks 4 and 5 in-hand, the diastereoselective Barbier coupling (vide supra) was performed, which resulted in inseparable diastereomers 6a* and 6b*. Only one diastereomer was observed in the Barbier coupling with respect to β-substitution of α-methylene-γ-butyrolactone ring in accord with literature precedence of a 6-membered transition state. However, metal-catalyzed allylation of aldehyde 4, bearing a stereogenic center, occurs without facial selectivity, yielding diastereomeric products 6a* and 6b* that are racemic. Coupling the inseparable mixture of 6a* and 6b* with 4-pentynoic acid and DCC yielded racemic 1a and 1b that were separable on silica gel. The overall yield for the synthesis of 1a and 1b was 13% (5 steps).
Scheme 1. Racemic synthesis of bifunctional helenalin mimics and their alkyne analogues.a.
aReagents and Conditions: (a) IBX, DMSO, 75 °C, 66%; (b) (i) LiAlH4, Et2O 0 °C; (ii) PCC, CH2Cl2, RT, 40% (2 steps); (c) Zn0, NH4Cl (aq.), DMF, RT, 60%; (d) 4-pentynoic acid, DCC, DMAP, CH2Cl2, 40 °C, 80% (separable diastereomers). *Denotes 6a and 6b prepared from racemic starting materials.
A stereoselective synthesis of 6a was also developed to deliver a simplified helenalin probe with the appropriate overall stereochemistry as that found in helenalin and to overcome our inability to separate diastereomers 6a* and 6b* from the Barbier coupling in the racemic synthesis. Since our stereoselective synthesis would employ a different aldehyde coupling partner, an opportunity to separate the diastereomers resulting from the Barbier coupling would exist. Our synthesis began with the stereoselective introduction of the quaternary methyl group, yielding (S)-2, using an established protocol for the stereoselective methylation of 1,3-ketoesters (Scheme 2).56–59 Accordingly, 1,3-ketoester 7 was condensed with L-tert-butylvaline to obtain enamine 8 in 75% yield. Enamine 8 was deprotonated with LDA in toluene at –78 °C, and then two equivalents of THF were added, followed by the addition of excess CH3I at the same temperature. After formation of the quaternary center, the crude product was isolated and the resulting Schiff base was hydrolyzed with aqueous HCl to obtain (S)-2 in 53% yield and 93:7 er (85% ee) by chiral-GC analysis. Cyclopentanone (S)-2 was then converted to the TBS-protected enol ether, followed by 2-electron reduction of the ester to aldehyde 9 using DIBAL-H in 27% yield (two steps). Notably, addition of the TBS protecting group resulted in a non-volatile and stable aldehyde 9 in comparison to 4. Aldehyde 9 was then subjected to the aforementioned Barbier conditions with 5 to obtain diastereomers 10a and 10b in 1:1 dr (64% yield) that were separable by silica gel chromatography. Diastereomers 10a and 10b were oxidized separately to their corresponding cyclopentenones under catalytic Saegusa-Ito oxidation conditions60, 61 in DMSO under 1 atm of O2 in 46% yield (for 6a, 93:7 er) and 56% yield (for 6b, 91:9 er). It is noteworthy that 10a, 10b, 6a, and 6b should not be dried under high vacuum because of their propensity to decompose (drying under low vacuum for a short period of time is recommended). Enantiomerically pure 6a and 6b were synthesized in six steps in 3% and 4% overall yields, respectively.
Scheme 2. Enantioselective synthesis of 6a and 6b.a.
aReagents and Conditions: (a) L-Valine tert-butyl ester, BF3·OEt, PhH, reflux (Dean-Stark), 75%; (b) (i) LDA, toluene, THF (2 eq.), −78 °C; MeI; (ii) 3M HCl (aq.), THF, RT, 53% (2 steps), 93:7 er; (c) (i) LiHMDS, THF; TBSCl, −78 °C to RT; (ii) DIBAL-H, CH2Cl2, −78 °C, 27% (2 steps); (d) 5, Zn0, NH4Cl (aq.), DMF, RT, 64% (separable diastereomers); (e) Pd(OAc)2, O2 (1 atm), DMSO, 46% (6a, 93:7 er), 56% (6b, 91:9 er).
Stereochemical Determination of Simplified Helenalin Probes
The Barbier coupling utilized for the synthesis of our molecular probes resulted in a highly stereocontrolled β-substitution of the butyrolactone ring. However, the lack of facial selectivity for aldehyde allylation resulted in diastereomers with respect to the quaternary center on the cyclopentanone ring and secondary alcohol. To unambiguously assign the stereochemistry of our probes by x-ray crystallography, we esterified diastereomer 10b from the enantioselective synthesis with 4-bromobenzoic acid, a moiety that bears a heavy atom for determination of absolute configuration (Scheme 3). Deprotection of the silyl enol ether to the ketone using TFA in DCM afforded 11, which was crystallized and the x-ray structure solved (Scheme 3). Based on this information, diagnostic 1H NMR coupling constants, and the known, absolute configuration of 9, we unambiguously assigned the structures of our chemical probes.
Scheme 3. Synthesis and X-ray Crystal Structure of 11a.
aReagents and Conditions: (a) 4-bromobenzoic acid, 4-DMAP, DCC, DCM, 40 °C (99%) (b) TFA, DCM, RT (53%). Bottom: ORTEPII plot of the x-ray diffraction data of 11.
Simplified Helenalin Probes Inhibit NF-κB Signaling in Cell Culture
Simplified helenalin analogues 6a and 6b and alkynylated probes 1a and 1b were screened for inhibitory activity towards canonical p50/p65 NF-κB signaling with a cellular luciferase assay (Figure 2).62 Helenalin was utilized as a benchmark and exhibited low micromolar inhibition of induced NF-κB signaling (53.7 ± 14.1% NF-κB activity at 2.5 µM). In comparison, alkyne-functionalized probes 1a and 1b were comparably active at 5 µM (54.4 ± 16.7% and 52.9 ± 7.1% NF-κB activity, respectively). Notably, 1a elicits more cellular cytotoxicity (64.5 ± 3.0% cell viability) during this 8-hour assay compared to 1b (97.8 ± 15.1 % cell viability) at 10 µM treatment. Cellular cytotoxicity for 1a at 20 µM treatment was comparable to that observed at 10 µM, whereas cellular viability was >80% for all other doses of all compounds shown in Figure 2 (refer to Figure S2 for cellular viability data). Intriguingly, enantiomerically pure 6b, the diastereomer with the ‘incorrect’ stereochemistry with respect to helenalin, has approximately 6-fold more NF-κB inhibitory activity at 25 µM compared to 6a, the enantiomerically pure ‘correct’ diastereomer from the Barbier coupling (6b: 14.5 ± 7.4% NF-κB activity; 6a: 88.8 ± 4.9% NF-κB activity). Furthermore, 6b achieves the same NF-κB inhibitory activity as helenalin at only a 4-fold higher dose (6b: 51.6 ± 16.6% NF-κB activity at 10 µM; helenalin: 53.7 ± 14.1% NF-κB activity at 2.5 µM). These data suggests the stereochemistries of our helenalin analogues are important with respect to cellular potency. The observation that 1a and 1b are equivalently potent in this assay hints at the possibility that 6a may have poor uptake properties or may be poorly retained in cells; however, no data currently exists to support this proposal.
Figure 2.
NF-κB-luciferase inhibition assay in A549 cells. Compounds were dosed to A549 cells containing a stably transfected NF-κB luciferase reporter construct and stimulated with TNF-α for eight hours (except for non-induced control, Non-Ind). Luminescence was normalized to the no compound induced (Ind) control and plotted as % NF-κB luciferase activity. Mean ± standard deviation values are shown (n ≥ 3 biological replicates). Compound-mediated toxicity to A549 cells was measured concurrently (Figure S4) and no significant toxicity was observed under these assay conditions.
Simplified Helenalin Probes Covalently Bind Proteins in Cell Culture
To qualitatively assess the protein-binding properties of our probes in cell culture, we performed protein labeling studies in HeLa cells. Alkyne-functionalized 1a and 1b were dosed separately to HeLa cells for one hour, cells were then lysed and the lysate cleared, and then protein-probe adducts were labeled with tetramethylrhodamine-azide (TAMRA-N3) via a copper mediated [3+2] Huisgen reaction (click chemistry).63 Protein-1a/1b-TAMRA adducts were separated by denaturing PAGE and fluorescence visualization of TAMRA (Figure 3). To demonstrate uniform protein labeling, total proteins were also imaged. Both 1a and 1b labeled multiple proteins in a concentration dependent manner (Figure 3). Gratifyingly, protein bands at approximately 65 kDa were observed for both 1a and 1b, which would be expected for targeting NF-κB p65. Off-target binding by 1a and 1b, which is evident by the multiple protein bands observed in our qualitative analysis, was observed and was certainly anticipated given the designed probes are bis-electrophiles. However, the distinct labeling patterns of 1a and 1b further reinforce the relevance of the stereochemistry of our probes with respect to protein targeting. Probe 1a (which contains the correct stereochemistry with respect to helenalin) qualitatively labels more proteins compared to 1b by visualization of the intense bands seen at 50 and 10 µM. Additionally, pre-treatment of cells with helenalin prior to labeling studies with 1a competes away protein binding properties by 1a, suggesting that our designed probe mimics the proteome reactivity properties of the parent natural product (Figure S3).
Figure 3.
Proteome labeling of 1a and 1b in HeLa cells. Compounds were dosed to HeLa cells at the concentrations shown, the cells were lysed, and then TAMRA-N3 was conjugated to 1a and 1b via azide-alkyne click chemistry. Proteins were separated by denaturing PAGE and gels were imaged for TAMRA fluorescence. Total proteins were then visualized by staining with Oriole® fluorescent protein stain followed by gel imaging.
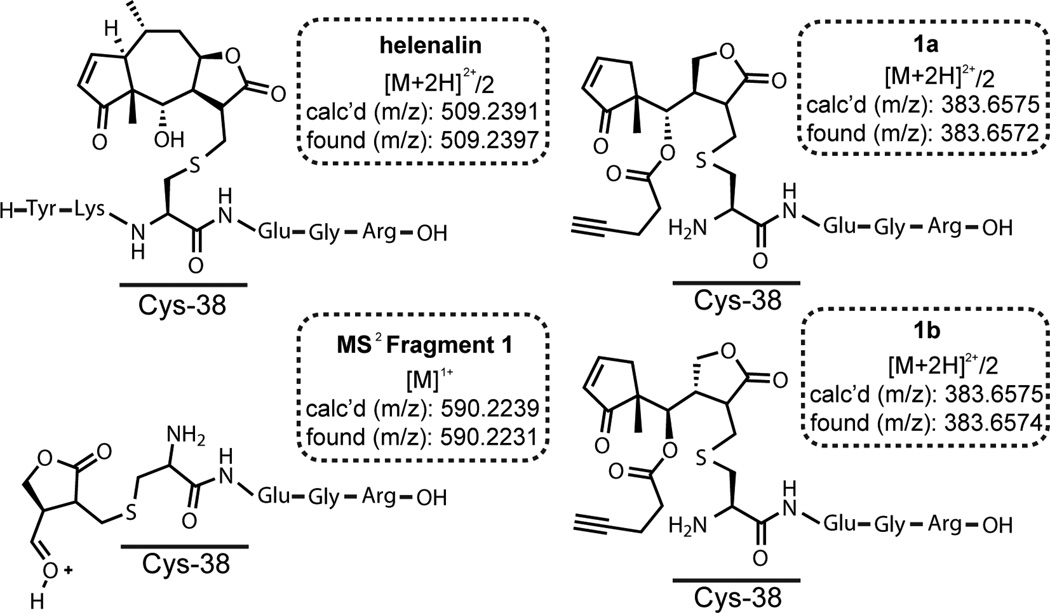
Helenalin and Simplified Helenalin Probes Covalently Label Cys38 of Recombinant p65
Previous studies with wild-type p65 and Cys→Ser mutant p65 proteins have demonstrated that helenalin covalently targets Cys38.34 To evaluate if helenalin mimics 1a and 1b also target Cys38, recombinant human p65 was incubated with 1a and 1b, proteins were digested, and LC-MS/MS was performed to identify adducted thiols (Figure 4). The predicted trypsin digestion of p65 proximal to Cys38 yields C38EGR41 or Y36KCEGR41 if the cleavage site closest to Cys38 is missed. Searching for the exact masses of the probes as a cysteine modification found the expected probe-peptide adducts for helenalin (Y36KCEGR41 adduct), 1a (C38EGR41 adduct) and 1b (C38EGR41 adduct). Analysis of the MS2 data for studies with 1a and 1b and recombinant p65 consistently revealed a fragment that was cleaved at the secondary hydroxyl group, which demonstrates the α-methylene-γ-butyrolactone was reacting with Cys38 (Figure 4 and Figures S4–S6). On the other hand, no fragments were found to support reactivity at the endocyclic enones of 1a and 1b, although such adducts might be reversible and unstable to digestion and MS analysis. Our reactivity data is consistent with previous reports demonstrating that α-methylene-γ-butyrolactones form irreversible hetero-Michael addition adducts with cysteines.30, 31 Interestingly, attempts to identify Cys38 adducts for 6a and 6b were unsuccessful, although adducts to a surface cysteine on p65, Cys105, was observed (Figure S7). Cys105 adducts were not found in experiments with 1a, 1b, or helenalin. The biological significance of labeling Cys105 on p65 remains unclear.
Figure 4.
Annotation of compound binding sites on p65. Helenalin, 1a and 1b were incubated separately with recombinant human p65 in 1X PBS buffer for 1 hr. Proteins were digested with trypsin and peptides analyzed by LC-MS/MS. Peptide-probe adducts were identified by extracting the theoretical mass from the total ion chromatogram and then analysis of the MS2 fragmentation pattern. Helenalin, 1a and 1b all bound Cys38 as expected. The MS2 Fragment 1 (shown on the bottom left) was detected after dosing 1a and 1b indicating that covalent adducts are forming at the exocyclic methylene butyrolactone.
Simplified Helenalin Probes Covalently Target p65 in Cell Culture
After determining that simplified helenalin probes 1a and 1b can inhibit canonical NF-κB signaling in cell culture, covalently label cellular proteins, and target Cys38 of recombinant p65, we turned our attention to evaluating if 1a and 1b can directly bind p65 in cell culture and avoid other well-established canonical NF-κB signaling enzymes. Accordingly, 1a and 1b were dosed to HeLa cells at 50 µM and incubated for 30 minutes. Cells were then harvested, washed extensively to remove unincorporated probe, and then lysed by sonication. Protein-1a/1b adducts were then labeled with biotin-azide (biotin-N3) via a copper mediated [3+2] Huisgen reaction (click chemistry).63 Protein-1a/1b-biotin adducts were enriched with a monomeric avidin column and separated by denaturing PAGE. Protein-1a/1b-biotin adducts were transferred to a membrane and then incubated with primary antibodies for p65 (primary target), IκBα (p50/p65 repressor protein; for specificity assessment), p50 (forms transcription factor heterodimer with p65; for specificity assessment), and IKKα/β (upstream kinases that contribute to activation of the NF-κB signaling pathway; for specificity assessment; antibody recognizes both proteins). To our delight, simplified helenalin probes 1a and 1b successfully pulled down p65 from live HeLa cells, but did not target IκBα, p50, or IKKα/β (Figure 5). The negative control demonstrates no proteins are pulled down in the absence of probes and the input lysates show that all proteins being evaluated are present in the input lysate (prior to monomeric avidin isolation of protein-1a/1b-biotin adducts). Furthermore, a head-to-head comparison of the ability of 1a and 1b to target p65 reveals comparable efficiencies (Figure 5C).
Figure 5.
Immunodetection of NF-κB signaling pathway proteins for covalent binding by 1a and 1b. Compounds were dosed to HeLa cells at the concentrations shown, the cells were lysed, and then Biotin-N3 was conjugated to 1a and 1b via azide-alkyne click chemistry. Protein-1a/1b adducts were isolated on monomeric avidin resin and identified by Western blotting with the antibodies shown. Notably, IKKα/β is recognized with the same antibody. (A & B) 1a (for A) and 1b (for B) label p65, but not other NF-κB pathway proteins, as shown in pulldown experiments. Competition experiments were performed by pre-treating cells with 6a and 6b 20 min before addition of 1a (for A) or 1b (for B). (C) Head-to-head comparison of labeling efficiency of 1a and 1b. Input lysate is the crude protein products before monomeric avidin enrichment.
We also wanted to determine if 6a and 6b could compete away binding of alkyne probes 1a and 1b in live cells; especially since p65 adducts were not observed with 6a and 6b by MS analysis. To this end, 6a and 6b were dosed to HeLa cells at 50 µM for 20 minutes prior to dosing 1a or 1b. Interestingly, 6b was able to completely block labeling and pulldown by both 1a and 1b, whereas 6a exhibited only partial activity at the same concentration (Figure 5). These data suggest that 6a and 6b target Cys38 on p65. Additionally, the difference in efficiencies between the two compounds is consistent with the cellular reporter data (Figure 2), in which 6a was dramatically less potent than 6b for inhibition of canonical NF-κB signaling.
CONCLUSIONS
We have developed simplified helenalin probes that target Cys38 of NF-κB p65 by a covalent mechanism of action. Our chemical probes are easily accessed in 6 synthetic steps (to probes 1a and 1b) and contain an alkyne handle for imaging and protein pulldown applications. Simplified helenalin probes 1a and 1b inhibit induced, canonical NF-κB signaling in a cellular reporter assay with low micromolar efficiencies and selectively engage p65 over other well-established proteins whose targeting by small molecules affects canonical NF-κB signaling; namely, IκBα, p50, and IKKα/β. The strategy of covalent targeting of solvent accessible, nucleophilic amino acids at key transcription factor interfaces, such as the DNA-binding cleft for p65 in this work, represents a viable strategy to rationally design small molecule transcription factor binders with biochemical utilities for transcriptional control of aberrant gene expression in human diseases. Current efforts are focused on improving the specificities and potencies of the first-generation helenalin-based probes reported in this work, with a particular focus on the size and rigidity of the moieties that mimic the central 7-membered ring of helenalin.
MATERIALS AND METHODS
Materials and Synthetic Methods
Unless otherwise noted, reactions were performed in flame-dried glassware under a nitrogen or argon atmosphere and stirred with a Teflon-coated magnetic stir bar. Liquid reagents and solvents were transferred via syringe and cannula using standard techniques. Reaction solvents dichloromethane (DCM), N,N-dimethylformamide (DMF), tetrahydrofuran (THF) and diethyl ether (Et2O) were dried by passage over a column of activated alumina using a solvent purification system (MBraun). All other chemicals were used as received unless otherwise noted. Helenalin was purchased from Enzo Life Sciences and purified by SiO2 flash column chromatography before use in biological assays. The molarities of n-butyllithium solutions were determined by titration against diphenylacetic acid as an indicator (average of three determinations). Reaction temperatures above 23 °C refer to oil bath temperature, which was controlled by a temperature modulator. Reaction progress was monitored by thin layer chromatography using EMD Chemicals Silica Gel 60 F254 glass plates (250 µm thickness) and visualized by UV irradiation (at 254 nm) and/or KMnO4 stain. Silica gel chromatography was performed on a Teledyne-Isco Combiflash Rf-200 instrument utilizing Redisep Rf High Performance silica gel columns (Teledyne-Isco) or flash column chromatography was performed using SiliCycle silica gel (32–63 µm particle size, 60 Å pore size). 1H NMR (500 MHz), 13C NMR (125 MHz), and 19F NMR (470 MHz) spectra were recorded on a Bruker Avance NMR spectrometer. 1H and 13C chemical shifts (δ) are reported relative to the solvent signal, CHCl3 (δ = 7.26 for 1H NMR and δ = 77.00 for 13C NMR). Some spectra contain TMS (0.05% v/v). All NMR spectra were obtained at room temperature unless otherwise specified. High resolution mass spectral data were obtained at the Analytical Biochemistry Core Facility of the University of Minnesota Masonic Cancer Center on an LTQ Orbitrap Velos Mass Spectrometer (Thermo Fisher).
The purity of all compounds tested in biological assays were checked via analytical HPLC analysis on an Agilent 1200 series instrument equipped with a diode array detector (wavelength monitored = 215 nm) and a Zorbax SBC18 column (4.6×150 mm, 3.5 µm, Agilent Technologies). All compounds tested in biological assays were >95% pure by HPLC. Information regarding the HPLC method and purity traces can be found in the Supporting Information.
Enantiopurity of (S)-2 was determined by chiral-GC/MS using an Agilent 7200B GC/Q-TOF with an Agilent J&W CycloSil-B GC column (30 m x 0.25 mm, 0.25 µm film). The injector port was set at 250 °C and the column flow (helium gas) at 1.0 mL/min. The temperature method began at 35 °C and increased to 180 °C (8 °C/min) over 18.1 min. The mass spectrometer electron ionization source temperature was set to 250 °C for detection. Area under the peak for each enantiomer was used to determine the enantiopurity; reported as enantiomeric ratio (er). The enantiopurity of other compounds was determined using normal phase chiral-HPLC or 19F analysis after derivatization with (S)-(-)-α-methoxy-α-(trifluoromethyl)phenylacetic acid ((S)-MTPA). Information regarding these two methods can be found in the Supporting information.
Ethyl 1-methyl-2-oxocyclopent-3-enecarboxylate (3).51–53
To a stirred solution of rac-2 (1.00 g, 5.88 mmol) in DMSO (20 mL) was added 4.11 g (14.7 mmol) of IBX64 and the solution was heated to 85 °C for 18 h. The reaction was cooled to 0 °C and aqueous NaHCO3 (sat’d, 40 mL) was added slowly. The reaction was filtered to remove IBX decomposition products. The solution was extracted with Et2O (30 mL, 3X), and the organic layer was dried over Na2SO4, and concentrated in vacuo. The crude mixture was SiO2 purified with EtOAc (10–30%) in hexanes to afford 3 (0.52 g, 53% yield) as a slightly tinted yellow oil. 1H NMR (CDCl3): 7.76-7.71 (m, 1H), 6.19-6.15 (m, 1 H), 4.14 (q, J = 7.2 Hz, 2 H), 3.25 (d, J = 19.1 Hz, 1 H), 2.53 (d, J = 19.2 Hz, 1 H), 1.39 (s, 3H), 1.21 (t, J = 7.2 Hz, 3H) ppm. 13C NMR (CDCl3): 206.9, 171.7, 163.2, 131.8, 61.7, 53.5, 42.9, 20.8, 14.2 ppm HRMS-ESI+ (m/z): calc’d [M+H]+ for C9H12O3: 169.0859, found 169.0855.
5-(Hydroxymethyl)-5-methylcyclopent-2-enol
To a stirred suspension of LiAlH4 (0.66 g, 17 mmol) in EtO2 (25 mL) at 0 °C was added 3 (0.75 g, 4.4 mmol) in EtO2 (6 mL) dropwise. The reaction was stirred at 0 °C for 2 h. The reaction was carefully quenched with H2O (1 mL), then aqueous NaOH (10%, 0.5 mL), and finally H2O (2 mL) and stirred overnight. The reaction was then filtered through celite, and the filtrate was concentrated in vacuo. The crude mixture was SiO2 purified with EtOAc (30–100%) in hexanes to afford a clear oil (0.46 g, 80% yield). 1H NMR analysis was consistent with that reported previously.65–67
1-Methyl-2-oxocyclopent-3-enecarbaldehyde (4)
The diol product (0.31 g, 2.3 mmol) from above was dissolved in DCM (20 mL) at room temperature and stirred. PCC (1.51 g, 7.03 mmol) was added in 3 equivalent portions over 4 h while stirring. This reaction was not kept under an inert atmosphere. The reaction was vacuum filtered through celite. The resulting solution was carefully concentrated on a rotary evaporator (no water bath; 150 torr vacuum) until only a small amount of solution remained. This solution was introduced onto a silica column (1 in. diameter, 4 in. length) and quickly purified (10% Et2O in pentanes). The fractions containing the desired compound were collected and concentrated carefully (as described above) to afford a volatile, clear oil 4 (0.16 g, 55% yield). Note: Concentration until no residual solvent is left will result in loss of product and decreased yields; do not use high vacuum to dry product. After isolation, the product was used immediately in the next reaction. If stored at −20° C in DCM the compound degrades in the span of 24 h. 1H NMR (CDCl3): 9.43 (s, 1H), 7.82-7.77 (m, 1H), 6.16-6.12 (m, 1H), 3.37 (dt, J = 19.4, 2.5 Hz, 1H), 2.41 (dt, J = 19.4, 2.3 Hz, 1H), 1.45 (s, 3H) ppm. 13C NMR (CDCl3): 206.0, 198.1, 164.4, 132.0, 60.6, 37.4, 18.2 ppm. HRMS-ESI+ (m/z): calc’d [M+H]+ for C7H8O2: 125.0597, found 125.0594.
Rac-(R)-4-((S)-Hydroxy((R)-1-methyl-2-oxocyclopent-3-en-1-yl)methyl)-3-methylenedihydrofuran-2(3H)-one (6a*) and rac-(S)-4-((R)-hydroxy((R)-1-methyl-2-oxocyclopent-3-en-1-yl)methyl)-3-methylenedihydrofuran-2(3H)-one (6b*)
Compounds 4 (69 mg, 0.56 mmol) and 550, 55 (55 mg, 0.84 mmol) were combined in a round bottom flask containing DMF (3 mL) and a stir bar. Activated Zn0 (109 mg, 1.68 mmol) was added to the stirring solution. Zn0 was freshly activated before each reaction by stirring in aqueous HCl (4M) for 15 min, and then filtered, washed with H2O (200 mL, 3X), MeOH (200 mL), EtOAc (200 mL), Et2O (100 mL), and dried under high vacuum for at least 1 h. Aqueous NH4Cl (sat’d, 1 drop) was added to the solution. The reaction was degassed and backfilled with Ar (g) 3X and then allowed to stir at RT for 16 h. The reaction was quenched with H2O (15 mL) and extracted with Et2O (20 mL, 3X) then dried over Na2SO4, and concentrated in vacuo to a crude oil. The crude oil was SiO2 purified with EtOAc (0 to 70%) in hexanes to give 74 mg (60% yield) of a clear oil containing 6a* and 6b* that was an inseparable mixture of diastereomers. The NMR characterization data for the separated, enantiomerically enriched compounds are below. See the Supporting Information for methodology used for enantiopurity analysis of synthesized compounds. For 6a*, a modest chiral induction was observed in the Barbier coupling reaction in a 32:68 dr for the resulting esterified, (S)-MTPA analogues by 19F NMR. For 6b*, no chiral induction was observed, as evidenced by the 1:1 er by chiral-HPLC analysis.
Rac-(S)-((R)-1-Methyl-2-oxocyclopent-3-en-1-yl)((R)-4-methylene-5-oxotetrahydrofuran-3-yl)methyl pent-4-ynoate (1a) and rac-(R)-((R)-1-methyl-2-oxocyclopent-3-en-1-yl)((S)-4-methylene-5-oxotetrahydrofuran-3-yl)methyl pent-4-ynoate (1b)
The previously isolated mixture of 6a* and 6b* (14 mg, 0.06 mmol) were dissolved in DCM (5 mL), then 4-pentynoic acid (12 mg, 0.13 mmol) was added to this solution. 4-DMAP (31 mg, 0.25 mmol) was then added followed by DCC (39 mg, 0.19 mmol). The reaction was heated to 40 °C for 4 h. The reaction was quenched with H2O (5 mL) and extracted with DCM (10 mL, 3X). The organic layer was dried over Na2SO4, filtered, and concentrated in vacuo to afford a crude oil. The crude product was SiO2 purified with EtOAc (0–70%) in hexanes to afford two white solids with an overall yield of 15 mg (80% yield, 1:1 ratio of 1a:1b). 1a:1H NMR (CDCl3): 7.73-7.68 (m, 1H), 6.38 (s, 1H), 6.19-6.14 (m, 1H), 5.72 (s, 1H), 5.17 (d, J = 4.9 Hz, 1H), 4.36 (dd, J = 9.5, 7.6 1H), 4.27 (dd, J = 9.6, 2.8 Hz, 1H), 3.60-3.52 (m, 1H), 2.97 (dt, J = 19.0, 2.4 Hz, 1H), 2.49-2.33 (m, 5H), 1.95 (t, J = 2.3 Hz, 1H), 1.20 (s, 3H) ppm. 13C NMR (CDCl3): 210.1, 170.4, 169.8, 163.2, 134.5, 132.0, 125.4, 82.0, 77.0, 69.9, 69.3, 49.8, 40.8, 40.2, 33.2, 20.3, 14.1 ppm. HRMS-ESI+ (m/z): calc’d [M+H]+ for C18H20O3: 303.1227, found 303.1222.
1b: 1H NMR (CDCl3): 7.76-7.72 (m, 1H), 6.28 (s, 1H), 6.26-6.20 (m, 1H), 5.56 (s, 1H), 5.10 (d, J = 6.3 Hz, 1H), 4.30-4.27 (m, 2H), 3.56-3.50 (m, 1H), 2.92 (dt, J = 19.8, 2.4 Hz, 1H), 2.54-2.42 (m, 5H), 1.98 (t, J = 2.4 Hz, 1H), 1.19 (s, 3H) ppm. 13C NMR (CDCl3): 209.8, 169.9, 168.8, 162.4, 133.5, 132.4, 124.3, 81.0, 76.8, 69.0, 68.5, 48.4, 40.2, 38.5, 32.4, 21.6, 13.2 ppm. HRMS-ESI+ (m/z): calc’d [M+H]+ for C18H20O3: 303.1227, found 303.1222.
(S)-2-((1-(tert-Butoxy)-3-methyl-1-oxobutan-2-yl)amino)cyclopent-1-ene-1-carboxylate (8)
The synthetic procedure was adapted from that previously reported.56–59 To remove the salt of L-valine tert-butyl ester hydrochloride, the material was dissolved in EtOAc (100 mL), and aqueous NaOH (0.5 M, 100 mL) was added and stirred for 10 minutes. The material was extracted with EtOAc (100 mL, 3X), washed with brine (15 mL, 1X), dried over Na2SO4, concentrated in vacuo to a clear oil, and then immediately used. To a stirred solution of 7 (2.99 g, 19.1 mmol) and L-valine tert-butyl ester (4.31 g, 24.9 mmol) in benzene (50 mL) was added BF3·OEt2 (1.18 mL, 9.55 mmol). The reaction mixture was refluxed with a Dean-Stark trap for 24 h. The reaction was allowed to cool to RT and quenched with aqueous NaHCO3 (sat’d, 50 mL) and then extracted with Et2O (50 mL, 3X), washed with brine (10 mL, 1X), and dried over Na2SO4. The organic layer was concentrated in vacuo. The crude product was SiO2 purified with EtOAc (0–5%) in hexanes to afford 8 (4.46 g, 75%) as a white solid. 1H NMR (500 MHz): 7.63 (bs, 1H), 4.22-2.11 (m, 2H), 3.64 (dd, J = 10.0, 5.5 Hz, 1H), 2.52 (t, J = 7.1 Hz, 2H), 2.47 (t, J = 7.6 Hz, 2H), 2.17-2.06 (m, 1H), 1.81 (p, J = 7.4 Hz, 2H), 1.46 (s, 9H), 1.27 (t, J = 7.1 Hz, 3H), 0.98 (app t, 6H) ppm. 13C NMR (125 MHz): 171.3, 168.2, 163.1, 94.7, 81.6, 63.9, 58.6, 32.3, 31.8, 29.3, 28.0, 20.9, 19.2, 17.8, 14.8. HRMS-ESI+ (m/z): calc’d [M+H]+ for C17H29NO4: 312.2169, found 312.2163.
Ethyl (S)-1-methyl-2-oxocyclopentane-1-carboxylate ((S)-2)
Stereoselective methylation was adapted from that previously reported.56–59 n-BuLi in hexanes (2.0 M solution, 3.56 mL, 7.13 mmol) was added to a solution of DIPA (1.00 mL, 7.13 mmol) at –78 °C in anhydrous toluene (30 mL) and the reaction was warmed to 0 °C and stirred for 30 min. The reaction mixture was cooled to –78 °C and 8 (1.85 g, 5.94 mmol) in anhydrous toluene (10 mL) was added dropwise and stirred for 1 h. THF (0.96 mL, 11 mmol) was added to the reaction and stirred for 2 h. MeI (1.85 mL, 29.7 mmol) was added and the reaction was stirred at –78 °C for 16 hours. The reaction was then quenched with aqueous NH4Cl (sat’d, 50 mL) and extracted with Et2O (30 mL, 3X), washed with brine (15 mL, 1X), dried over Na2SO4, and concentrated in vacuo. The crude products were then dissolved in THF (50 mL) and aqueous HCl (3M, 50 mL) was added and stirred at RT for 6 hours. The reaction was extracted with Et2O (50 mL, 3X), washed with water (10 mL, 1X), then brine (15 mL, 1X), and dried over Na2SO4. The solvent was concentrated in vacuo. The crude mixture was SiO2 purified with EtOAc (0%-20%) in hexanes to afford (S)-2 as a colorless oil (0.54 g, 53%, 93:7 er by chiral-GCMS). NMR characterization was consistent with previously reported data for this compound.51
Ethyl (S)-2-((tert-butyldimethylsilyl)oxy)-1-methylcyclopent-2-ene-1-carboxylate
LiHMDS solution in THF (1 M solution, 4.20 mL, 4.19 mmol) was added to anhydrous THF (30 mL) at – 78 °C. Next, a solution of 9 (0.59 g, 3.5 mmol) in THF (5 mL) was added dropwise and stirred for 1 hr at –78 °C. A solution of TBSCl (1.05 g, 6.98 mmol) in THF (10 mL) was then added dropwise at –78 °C and then the reaction was allowed to slowly come to RT and stirred for 16 h. The reaction mixture was quenched with aqueous NH4Cl (sat’d, 30 mL) and extracted with Et2O (30 mL, 3X). The organic layer was washed with brine (15 mL, 1X), and dried over Na2SO4, and concentrated in vacuo. The crude material was SiO2 purified with EtOAc (0 to 10%) in hexanes, resulting in a clear oil (0.78 g, 79% yield). 1H NMR (500 MHz): 4.61 (t, J = 2.2 Hz, 1H), 4.12 (q, J = 7.2 Hz, 2H), 2.40-2.29 (m, 2H) 2.29-2.19 (m, 1H), 1.77-1.67 (m, 1H), 1.72 (m, 1H), 1.30 (s, 3H), 1.24 (t, J = 7.1 Hz, 3H), 0.90 (s, 9H), 0.16 (s, 3H), 0.15 (s, 3H). 13C NMR (125 MHz): 176.1, 156.2, 101.2, 60.5, 54.3, 35.3, 26.2, 25.5, 21.3, 18.0, 14.2, −5.0, −5.2 ppm. HRMS-ESI+ (m/z): calc’d [M+H]+ for C15H28O3Si: 285.1881, found 285.1883.
(S)-2-((tert-Butyldimethylsilyl)oxy)-1-methylcyclopent-2-enecarbaldehyde (9)
To a solution of anhydrous DCM (20 mL) was added the silyl enol ether from the above reaction (0.91 g, 3.19 mmol) and the resulting solution was cooled to –78 °C. A solution of DIBAL-H in DCM (1M, 3.19 mL, 3.19 mmol) was added dropwise to the reaction mixture and stirred for 4 h at –78 °C. The reaction was then carefully quenched with H2O (1 mL), aqueous NaOH (0.5M, ~0.1 mL) was added, and then an additional aliquot of H2O (1 mL). The reaction was gravity filtered through qualitative filter paper, washed with brine (10 mL, 1X), dried over Na2SO4, and concentrated in vacuo. The crude product was SiO2 purified with EtOAc (0–5%) in hexanes to afford a clear oil (0.29 g, 38% yield). 1H NMR (500 MHz): 9.53 (s, 1H), 4.75 (t, J = 2.3 Hz, 1H), 2.34-2.25 (m, 3H), 1.72 – 1.60 (m, 1H), 1.20 (s, 3H), 0.90 (s, 9H), 0.17 (s, 3H), 0.15 (s, 3H) ppm. 13C NMR (125 MHz): 202.5, 154.2, 103.2, 59.3, 30.7, 26.0, , 25.5, 18.0, 17.6, −4.9, −5.0; impurities at 25.7, −3.6 ppm. HRMS-ESI+ (m/z): calc’d [M+H]+ for C13H24O2Si: 241.1618, found 241.1616.
(R)-4-((S)-((R)-2-((tert-Butyldimethylsilyl)oxy)-1-methylcyclopent-2-en-1-yl)(hydroxy)methyl)-3-methylenedihydrofuran-2(3H)-one (10a) and (S)-4-((R)-((R)-2-((tert-Butyldimethylsilyl)oxy)-1-methylcyclopent-2-en-1-yl)(hydroxy)methyl)-3-methylenedihydrofuran-2(3H)-one (10b)
To a solution of DMF (3 mL), 9 (0.29 g, 1.2 mmol) and 550, 55 (0.32 g, 1.8 mmol) was added powdered Zn0 (0.32 g, 4.8 mmol, activated as described for 6a*/6b*). Aqueous NH4Cl (sat’d, one drop) was added and the reaction was degassed and backfilled with Ar (g) (3X). The reaction was then stirred at RT for 16 h. The reaction mixture was quenched with H2O (15 mL) and extracted with Et2O (15 mL, 3X). The organic layer was washed with H2O (10 mL, 1X) and then brine (10mL, 1X). The subsequent organic layer was dried over Na2SO4 and concentrated in vacuo. The crude mixture was SiO2 purified with an isocratic eluent system EtOAc (20%) in hexanes to afford the separated diastereomers 10a and 10b as white solids (0.41 g, 64% yield, 1:1 dr) Note: 10a/10b will polymerize when concentrated and/or the silyl enol ether will be deprotected if allowed to sit at RT as a solid for an extended period of time. To avoid this, do not dry under high vacuum, and after concentration with a low vacuum pump immediately take 10a and 10b on to the next reaction. 10a: 1H NMR (500 MHz): 6.42 (d, J = 1.6 Hz, 1H), 5.91 (d, J = 1.0 Hz, 1H), 4.66 (t, J = 2.4 Hz, 1H), 4.22 (dd, J = 9.2, 4.1 Hz, 1H), 4.22 (dd, J = 9.2, 4.1 Hz, 1H), 3.62 (dd, J = 6.6, 4.1 Hz, 1H), 3.29-3.22 (m, 1H), 2.27-2.19 (m, 2H), 2.17-2.08 (m, 1H), 2.0 (d, J = 4.2 Hz, 1H), 1.62-1.51 (m, 1H), 1.10 (s, 3H), 0.93 (s, 9H), 0.20 (s, 3H), 0.17 (s, 3H) ppm. 13C NMR (125 MHz): 170.8, 156.9, 135.0, 126.2, 102.0, 76.1, 70.3, 51.9, 41.3, 31.1, 25.7, 25.4, 21.8, 18.0, −4.6, −5.3 ppm. HRMS-ESI+ (m/z): calc’d [M+H]+ for C18H30O4Si: 339.1986, found 339.1982. 10b: 1H NMR (500 MHz): 6.40 (d, J = 1.1 Hz, 1H), 6.01 (s, 1H), 4.62 (t, J = 2.3 Hz, 1H), 4.24 (dd, J = 9.4, 3.1 Hz, 1H), 4.24 (dd, J = 9.4, 5.3 Hz, 1H), 3.64 (dd, J = 8.6, 3.7 Hz, 1H), 3.23 – 3.16 (m, 1H), 2.51 (d, J = 3.7 Hz, 1H), 2.30– 2.12 (m, 2H), 2.07–1.99 (m, 1H), 1.63–1.55 (m, 1H), 1.20 (s, 3H), 0.94 (s, 9H), 0.22 (s, 3H), 0.18 (s, 3H) ppm. 13C NMR 170.9, 157.4, 135.7, 126.0, 101.9, 77.4, 68.9, 51.3, 42.4, 30.7, 25.8, 25.6, 22.7, 18.0, −4.4, −5.5 (125 MHz). HRMS-ESI+ (m/z): calc’d [M+H]+ for C18H30O4Si: 339.1986, found 339.1978.
(R)-4-((S)-Hydroxy((R)-1-methyl-2-oxocyclopent-3-en-1-yl)methyl)-3-methylenedihydrofuran-2(3H)-one (6a)
10a (22 mg, 0.074 mmol) was dissolved in DMSO (2 mL) and Pd(OAc)2 (7.0 mg, 0.013 mmol) was added. The reaction was placed under 1 atm of O2 (g) and stirred at RT for 24 h. The reaction mixture was quenched with H2O (10 mL) and extracted with Et2O (15 mL, 3X). The organic layer was washed with H2O (10 mL, 1X), then brine (10mL, 1X), dried over Na2SO4, and concentrated in vacuo. The crude mixture was SiO2 purified with EtOAc (0–80%) in hexanes to afford 6a (10 mg, 46% yield, 93:7 er), as a clear oil. 1H NMR (500 MHz): 7.78–7.72 (m, 1H), 6.46 (d, J = 2.1 Hz, 1H), 6.20–6.16 (m, 1H), 5.81 (d, J = 1.9 Hz, 1H), 4.43 (dd, J = 9.4, 7.7 Hz, 1H), 4.24 (dd, J = 9.4, 3.1 Hz, 1H), 3.97 (d, J = 5.7 Hz, 1H), 3.35–3.29 (m, 1H), 3.10 (app dt, J = 18.9, 2.5 Hz, 1H), 2.35 (app dt, J = 18.9, 2.8 Hz, 1H), 1.14 (s, 3H) ppm. 13C NMR (125 MHz): 213.0, 170.3, 164.3, 134.6, 132.0, 126.3, 75.9, 69.9, 51.4, 41.4, 39.5, 21.1 ppm. HRMS-ESI+ (m/z): calc’d [M+H]+ for C12H14O4: 223.0965, found 223.0963.
(S)-4-((R)-Hydroxy((R)-1-methyl-2-oxocyclopent-3-en-1-yl)methyl)-3-methylenedihydrofuran-2(3H)-one (6b)
10b (12 mg, 0.04 mmol) was dissolved in DMSO (2 mL) and Pd(OAc)2 (1.00 mg, 0.004 mmol) was added. The reaction was placed under 1 atm of O2 (g) and stirred at RT for 24 h. The reaction mixture was quenched with H2O (10 mL) and extracted with Et2O (15 mL, 3X). The organic layer was washed with H2O (10 mL, 1X) and then brine (10 mL, 1X). The subsequent organic layer was dried over Na2SO4 and concentrated in vacuo. The crude mixture was SiO2 purified with EtOAc (0–80%) in hexanes to afford 6b (4.0 mg, 56% yield, 91:9 er) as a clear oil. 1H NMR (500 MHz): 7.77–7.73 (m, 1H), 6.39 (d, J = 2.7 Hz, 1H), 6.24–6.16 (m, 1H), 5.98 (d, J = 2.3 Hz, 1H), 4.32–4.23 (m, 1H), 4.15 (dd, J = 9.4, 5.2 Hz, 1H), 3.77 (dd, J = 7.9, 2.6 Hz, 1H), 3.45 (d, J = 2.8 Hz, 1H), 3.41-3.32 (m, 1H), 2.87 (dt, J = 19.5, 2.5 Hz, 1H), 2.44 (dt, J = 19.4, 2.4 Hz, 1H), 1.26 (s, 3H). 13C NMR (125 MHz): 213.8, 170.4, 163.8, 134.9, 132.7, 126.4, 75.4, 68.5, 49.9, 42.0, 41.1, 20.8 ppm. HRMS-ESI+ (m/z): calc’d [M+H]+ for C12H14O4: 223.0965, found 223.0963.
Rac-(R)-((R)-2-((tert-Butyldimethylsilyl)oxy)-1-methylcyclopent-2-en-1-yl)((S)-4-methylene-5-oxotetrahydrofuran-3-yl)methyl 4-bromobenzoate
10b (0.150 g, 0.443 mmol) was dissolved in DCM (4 mL). Next 4-DMAP (0.325 g, 2.66 mmol) and 4-bromobenzoic acid (0.445 g, 2.22 mmol) was added, followed by DCC (0.457 g, 2.22 mmol). The reaction was was heated to 40 °C for 16 h. The reaction was allowed to cool to RT and quenched with H2O (5 mL). The mixture was extracted with DCM (20 mL, 3X). The organic layer was dried over Na2SO4 and concentrated in vacuo. The crude reaction product was SiO2 purified with EtOAc (0–20%) in hexanes. The reaction afforded 0.180 g (99% yield) of a white solid. 1H NMR (500 MHz): 7.89-7.82 (m, 2H), 7.63-7.56 (m, 2H), 6.12 (d, J = 2.5 Hz, 1H), 5.51 (d, J = 2.1 Hz, 1H), 5.11 (d, J = 7.5 Hz, 1H), 4.71 (t, J = 2.3 Hz, 1H), 4.46 (dd, J = 9.5, 3.8 Hz, 1H), 4.34 (dd, J = 9.5, 7.9 Hz, 1H), 3.50–3.41 (m, 1H), 2.38-2.21 (m, 3H), 1.84-1.74 (m, 1H), 1.14 (s, 3H), 0.96 (s, 9H), 0.23 (s, 3H), 0.19 (s, 3H) ppm. 13C NMR (125 MHz): 170.1, 165.1, 155.8, 134.9, 132.0, 131.0, 128.7, 128.5, 125.2, 102.8, 79.5, 69.7, 51.5, 40.3, 30.5, 26.1, 25.7, 24.3, 18.1, 4.4, 5.4, impurity at 53.4 ppm. HRMS-ESI+ (m/z): calc’d [M+H]+ for C25H33BrO5Si: 521.1353, found 521.1331.
Rac-(R)-((R)-1-Methyl-2-oxocyclopentyl)((S)-4-methylene-5-oxotetrahydrofuran-3-yl)methyl 4-bromobenzoate (11)
The 4-bromo benzoate silyl enol ether (0.180 g, 0.035 mmol) from the previous reaction was dissolved in DCM (2 mL) and TFA (200 µL) was added to the reaction at RT. The reaction was stirred for 1 h, then quenched with aqueous NaHCO3 (sat’d, 10 mL) and extracted with DCM (10 mL, 3X). The organic layer was dried over Na2SO4 and concentrated in vacuo. The crude product was SiO2 purified with EtOAc (0–40%) in hexanes to afford a white solid (0.100 g, 53% yield). 1H NMR (500 MHz): 7.83-74 (m, 2H), 7.62-7.54 (m, 2H), 6.14 (d, J = 2.4 Hz, 1H), 5.53 (d, J = 1.7 Hz, 1H), 5.19 (d, J = 6.7 Hz, 1H), 4.47-4.35 (m, 2H), 3.87-3.81 (m, 1H), 2.52-2.42 (m, 1H), 2.33-2.21 (m, 1H), 2.15-2.07 (m, 1H), 2.05-1.88 (m, 3H), 1.17 (s, 3H) ppm. 13C NMR (125 MHz): 220.0, 169.7, 164.8, 134.2, 132.1, 131.0, 128.9, 128.1, 126.0, 78.5, 69.8, 51.4, 39.9, 38.8, 34.2, 20.2, 18.6 ppm. HRMS-ESI+ (m/z): calc’d [M+H]+ for C19H19BrO5: 407.0489, found 407.0471.
Cell Culture
All cell lines were maintained in a humidified 5% CO2 environment at 37 °C in tissue culture flasks (Corning) under normoxic conditions. Adherent cells were dissociated using Trypsin-EDTA solution (0.25%, Gibco). A549-NF-κB luciferase cells were cultured as described previously.62 HeLa were cultured in MEM media (Cellgro) supplemented with 10% FBS (Gibco), penicillin (100 I.U./mL, ATCC), and streptomycin (100 µg/mL, ATCC).
A549-Luciferase NF-κB reporter assay
The NF-κB-luciferase assay in stably transfected A549 cells was conducted according to a previously described protocol.62
Labeling in HeLa Cell Culture
HeLa cells were grown to 90% confluency in a 75 cm2 culture flask. The culture flasks were dosed with the respective probe concentrations or a DMSO control and incubated for 1 h at 37 °C under normoxic conditions. The cells were detached with non-enzymatic cell dissociation solution (Life Technologies) and washed with cold 1X PBS buffer (10 mL, 3X). The cells were pelleted after each suspension for five minutes at 1000 rpm. After the last wash the cells were suspended in cold 1X PBS buffer (1 mL) containing Complete EDTA-Free Protease Cocktail (Promega). The cells were lysed via sonication with a Vibra Cell VCX 750 (750 W, 20 kHz, 120 V) at 40% power for 30 seconds, while on ice. The lysates were stored at –80 °C until further use.
Lysate was allowed to thaw and kept on ice. The protein concentration was measured via BCA analysis (Pierce BCA Protein Assay Kit, Thermo Scientific) and all lysates were normalized to the sample with the lowest concentration. Click reagents were added to each sample (1 µL CuSO4, 100 mM stock in ddH2O; 1 µL TBTA, 20 mM stock in DMSO; 0.5 µL TAMRA-N368, 40 mM stock in DMSO; 2 µL TCEP, 100 mM in ddH2O) and allowed to react for 3 h at room temperature. LDS 4X Sample Buffer (8 µL, NuPAGE) and of 10X Sample Reducing Agent (2 µL, NuPAGE) was then added to each sample and heated to 90 °C for five minutes before being pipetted into a 15-well NuPAGE Novex 4–12% polyacrylamide bis-tris gel and separated with electrophoresis (180V, 54 min) in NuPAGE MES SDS running buffer (1X). Gels were imaged using a TyphoonFLA7000 gel imager (General Electric). Images were analyzed using ImageQuant TL v7.0 software.
Pulldown Experiments
HeLa cells were allowed to grow to 90% confluency in a 150 cm2 flask under normoxic conditions at 37 °C in a humidified CO2 incubator. The media was replaced with 20 mL of fresh media and the competition compounds 6a, 6b, or a DMSO control was dosed to achieve the final concentration (50 µM, DMSO concentration <0.05%) in each flask and incubated for 20 min. Alkyne probes 1a or 1b were dosed at 50 µM and incubated for an additional 30 min. After incubation, the media was removed and the cells were washed with cold 1X PBS (10 mL). The cells were then dissociated from the flask using non-enzymatic dissociation media (4 mL, Life Technologies). The cells were collected in 1X PBS (8 mL) and centrifuged (1000 rpm, 5 min, RT) in a conical tube. The cells were washed with cold 1X PBS (10 mL) and centrifuged again. The cells were then taken up in 1X PBS (2.5 mL) containing protease inhibitor (Complete EDTA-free protease inhibitor cocktail, Life Technologies). The cells were lysed via sonication with a Vibra Cell VCX 750 (750 W, 20 kHz, 120 V) at 40% power for 30 seconds, while on ice. The lysates were stored at –80 °C until further use.
After thawing, the samples were centrifuged at 4000 RPM for 20 min at 0 °C to clear the lysate. The samples were transferred to clean conical tubes and 200 µL of 10 w/v% SDS in ddH2O were added and heated to 65 °C for 10 minutes. The protein concentration of each sample was measured via BCA analysis (Pierce BCA Protein Assay Kit, Thermo Scientific) and all lysates were normalized to the sample with the lowest concentration (between 1.0 to 1.6 mg/mL). Click reagents were added (10 µL CuSO4, 100 mM stock in ddH2O; 20 µL TBTA, 20 mM stock in DMSO; 20 µL Biotin-N3 [Sigma-Aldrich 762024; CAS:875770-34-6], 20 mM stock in DMSO; 10 µL TCEP, 100 mM in ddH2O) and allowed to react for 3 h at room temperature. After incubation 15 µL of each sample was collected and saved for the input lysate control.
The samples were then separated on a monomeric avidin column according to the manufacturer’s instructions (Pierce) at 4 °C. The biotinylated samples were eluted using the regeneration buffer (0.1 M HCl glycine buffer, pH 2.8) Note: biotinylated samples did not elute using the elution biotin buffer. After the samples are collected they were concentrated using a 10 kDa molecular weight cut-off filter (Amicon) and diluted with ddH2O (20 mL, 2X) and then finally concentrated to ~500 µL. The samples were collected and concentrated to dryness in a SpeedVac for 10 h at RT. These samples were then dissolved in ddH2O (20 µL), 10X reducing agent (2 µL, NuPAGE), and 4X sample buffer (10 µL, NuPAGE) and vortexed. Sample buffer and reducing agent was added to the input lysates in the same fashion and all samples were heated to 90 °C for five minutes before pipetting 15 µL of each sample into a 15-well NuPAGE Novex 4–12% polyacrylamide bis-tris gel and separated with electrophoresis (180V, 54 min) in NuPAGE MES SDS running buffer (1X). The samples were separated and transferred (30 V, 1 h, RT) in 1X TBE buffer to a PVDF membrane (Immobilon-FL) for Western blot analysis. Membranes were incubated with the respective primary antibodies (p65, Santa Cruz, sc-372; p50, Santa Cruz sc-8414; IκBα, Santa Cruz sc-371; IKKα/β, Santa Cruz sc-7607) with a 1:1,000 dilution in 0.5% non-fat milk (BioRad) in 1X PBS (10 mL) overnight at 4 °C. Secondary HRP-conjugated antibodies (anti-rabbit poly-HRP, Pierce cat # 32260; secondary anti-mouse, Novex HRP cat # A16072) were added to 0.5% non-fat milk (BioRad) in 1X PBS (10 mL) at a 1:5,000 dilution for 1 h at RT. Membranes were washed in ddH2O between each incubation (30 mL for 1 min, 5X). Super Signal West Dura Extended Duration Luminol/Enhancer Solution (1 mL) and Stable Peroxide Buffer (1 mL) were added to the top of the membrane and imaged with a Li-COR Odyssey Fc imaging system. After each antibody was detected the membrane was stripped with Restore PLUS Western Blot Stripping Buffer (Thermo), washed with ddH2O for 30 min, and blocked overnight at 4 °C in 0.5% w/v non-fat dry milk (BioRad) in 1X PBS before incubating with the next antibody.
Labeling Recombinant Human p65
Recombinant human p65 in buffer (this clone has five point mutations compared to the p65 sequence listed under accession no. AAA36408: L159V, P180S, F309S, A439V and V462M; Active Motif) was placed in an Eppendorf tube (3 µL, 100 ng/µL) with ddH2O (6 µL) and incubated with 100 µM of helenalin, 6a, 6b, 1a, or 1b for 1 h at RT. LDS 4X Sample Buffer (4 µL, NuPAGE) and 10X Sample Reducing Agent (1 µL, NuPAGE) was then added to each sample and heated to 90 °C for five minutes before being pipetted into a 15-well NuPAGE Novex 4–12% polyacrylamide bis-tris gel and run into the top of the gel with electrophoresis (180V, 5 min) in NuPAGE MES SDS running buffer (1X). The top of each well where the protein was located was excised and placed into an Eppendorf tube.
The gel pieces were then processed by in-gel trypsin digestion according to a previously reported protocol.69, 70 The peptides were desalted using a P10 C18 Zip-Tip (Millipore). Samples were dried with a SpeedVac for 3 h at RT and the dried peptides were dissolved in 95:5 H2O:MeCN 0.1% formic acid solution (12 µL) for HPLC- ESI+-MS/MS analysis.
HPLC-ESI+-MS/MS analyses of tryptic peptides were conducted using an Orbitrap Fusion mass spectrometer (Thermo Scientific) equipped with a Dionex Ultimate UHPLC pump (Thermo Scientific), a nanospray source, and Xcalibur 3.0.63 software for instrument control. Peptide mixtures were directly injected onto a nanoHPLC column (75 µm i.d., 10 cm packed bed, 15 µm orifice) created by hand packing a commercially purchased fused-silica emitter (New Objective) with Zorbax SB-C18 5 µm separation media (Agilent). The gradient program started from 0–17 min at 2% MeCN:H2O (1% formic acid) with a flow rate of 3 µL/min, followed by a linear increase to 30% MeCN:H2O (1% formic acid) from 17–80 min, followed by a linear increase to 80% MeCN:H2O (1% formic acid) from 80–91 min. Finally, the column was equilibrated with 2% MeCN:H2O (1% formic acid) from 91–99 min with a flow rate of 9 µL/min. Liquid chromatography was carried out at an ambient temperature. The mass spectrometer was calibrated prior to each analysis, and the spray voltage was adjusted to ensure a stable spray. The MS tune parameters were as follows: spray voltage of 2.460 kV, capillary temperature of 300 °C, and an S-lens RF level of 60%. MS/MS spectra were collected using simultaneous data-dependent scanning and target mass analysis, in which one full scan mass spectrum is acquired in the Orbitrap detector (R = 120,000, scan range 320–2000 m/z), followed by a target list analyzing masses corresponding to expected theoretical probe-peptide adducts (343.6443, 489.2235, 640.8083, 770.8567, 229.4320, 326.4848, 427.5413, 514.2402, 923.7783, 693.0855, 569.7730, 383.6574, 529.2365, 353.1601, 680.8214, 454.2167 m/z), followed by 12 data-dependent MS/MS spectra acquired with the Orbitrap detector (R = 15,000) with charge states 2–7, dynamic exclusion after one detection for 20 s, an intensity threshold of 5.0 × 104, and a mass tolerance of 10.00 ppm. The method uses an isolation width of 1.6 m/z, maximum injection time of 150 ms, 40% HCD collision energy, and 1 AGC microscan. Spectral data were analyzed using Proteomic Discoverer software package (v1.4.0.288, ThermoFisher). Data was processed using the SEQUEST v.27 algorithm.71 Peptide spectra were searched against the UniProt Human Protein Database. Helenalin (+262.1205 Da), 6a and 6b (+222.0892), 1a and 1b (+302.3260 Da), and/or cysteine carboxamidomethylation (+57.0215 Da) was set as a dynamic modification. Precursor mass tolerance was set to 10 ppm within the calculated mass, and fragment ion mass tolerance was set to 10 mmu of their monoisotopic mass. Probe-peptide adducts found using the Proteome Discoverer software were further scrutinized by manually extracting the mass using the Xcalibur software from the total ion chromatogram. The MS2 fragmentation data were analyzed manually to confirm the identity of probe-peptide adducts.
Supplementary Material
Acknowledgments
The work was generously supported by the University of Minnesota (Academic Health Center Seed Grant #2010.01), The V Foundation for Cancer Research (V Scholar Award to DAH), Hyundai Hope on Wheels (Hope Grant), and the NIH (R21-CA194661). J.C.W. acknowledges the University of Minnesota, College of Pharmacy for a Bighley Graduate Fellowship. Mass spectrometry was performed at the Analytical Biochemistry Core Facility of the Masonic Cancer Center, which is supported by the NIH (P30-CA77598 and S10 RR-024618). We gratefully acknowledge Victor G. Young, Jr. (University of Minnesota, Department of Chemistry, X-Ray Crystallographic Laboratory) for solving the structure of 11 and funding from the NSF/MRI (#1229400) and the University of Minnesota for the purchase of the Bruker-AXS D8 Venture diffractometer. The Minnesota Supercomputing Institute (MSI) at the University of Minnesota is acknowledged for molecular modeling resources.
ABREVIATIONS
- Cys
cysteine
- IBX
2-Iodoxybenzoic acid
- DMSO
dimethyl sulfoxide
- PCC
pyridinium chlorochromate
- DCC
N,N’-dicyclohexylcarbodiimide
- RT
room temperature
- PhH
benzene
- LDA
lithium diisopropylamine
- LiHMDS
Lithium bis(trimethylsilyl)amide
- 4-DMAP
4-dimethylaminopyridine
- TBSCl
tert-butyldimethylsilyl chloride
- atm
atmosphere
- sec
seconds
- min
minutes
- dr
diastereomeric ratio
- er
enantiomeric ratio
- sat.
saturated
- aq.
aqueous
- GC
gas chromatography
- TFA
trifluoroacetic acid
- LC-MS
liquid chromatography-mass spectrometry
- SDS-PAGE
sodium dodecylsulfate-polyacrylamide gel electrophoresis
- (S)-MTPA
(S)-α-Methoxy-α-trifluoromethylphenylacetic acid
- TMS
tetramethylsilane
- EtOAc
ethyl acetate
- EDTA
Ethylenediaminetetraacetic acid
- ESI
electrospray ionization
- HPLC
high-performance liquid chromatography
- UHPLC
ultra high-performance liquid chromatography
- TBE
tris boric acid EDTA buffer
- ddH2O
distilled and deionized water
- HRMS-ESI
high resolution mass spectrometry-electrospray ionization
- PVDF
polyvinylidene difluoride
Footnotes
ASSOCIATED CONTENT
Supporting information is available free of charge at the ACS publications website at DOI:
NMR and analytical HPLC characterization of synthesized compounds, determination of enantiomeric ratio of 6a and 6b, peptide-probe MS2 fragmentation data, and crystallographic information.
CIF file for 11
The authors declare no competing financial interest.
REFERENCES
- 1.Xiao G, Fu J. NF-kappaB and Cancer: a Paradigm of Yin-Yang. Am. J. Cancer Res. 2011;1:192–221. [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal BB. Nuclear factor-kappaB the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Huxford T, Ghosh G. A structural guide to proteins of the NF-kappaB signaling module. Cold Spring Harb. Perspect. Biol. 2009;1:a000075. doi: 10.1101/cshperspect.a000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Greten FR, Karin M. The IKK/NF-kappaB activation pathway-a target for prevention and treatment of cancer. Cancer Lett. 2004;206:193–199. doi: 10.1016/j.canlet.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 6.Pande V, Sousa SF, Ramos MJ. Direct covalent modification as a strategy to inhibit nuclear factor-kappa B. Curr. Med. Chem. 2009;16:4261–4273. doi: 10.2174/092986709789578222. [DOI] [PubMed] [Google Scholar]
- 7.Wang W, Nag SA, Zhang R. Targeting the NFkappaB signaling pathways for breast cancer prevention and therapy. Curr. Med. Chem. 2015;22:264–289. doi: 10.2174/0929867321666141106124315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karin M, Yamamoto Y, Wang QM. The IKKNF-kappa B system: A treasure trove for drug development. Nat. Rev. Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- 9.Gilmore TD, Herscovitch M. Inhibitors of NF-kappaB signaling: 785 and counting. Oncogene. 2006;25:6887–6899. doi: 10.1038/sj.onc.1209982. [DOI] [PubMed] [Google Scholar]
- 10.Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting NF-kappaB activation by small molecules as a therapeutic strategy. Biochim. Biophys. Acta. 2010;1799:775–787. doi: 10.1016/j.bbagrm.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raskatov JA, Meier JL, Puckett JW, Yang F, Ramakrishnan P, Dervan PB. Modulation of NF-kappaB-dependent gene transcription using programmable DNA minor groove binders. Proc. Natl. Acad. Sci. USA. 2012;109:1023–1028. doi: 10.1073/pnas.1118506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kojima T, Wang X, Fujiwara K, Osaka S, Yoshida Y, Osaka E, Taniguchi M, Ueno T, Fukuda N, Soma M, Tokuhashi Y, Nagase H. Inhibition of human osteosarcoma cell migration and invasion by a gene silencer, pyrrole-imidazole polyamide, targeted at the human MMP9 NF-kappaB binding site. Biol. Pharm. Bull. 2014;37:1460–1465. doi: 10.1248/bpb.b14-00147. [DOI] [PubMed] [Google Scholar]
- 13.Wurtz NR, Pomerantz JL, Baltimore D, Dervan PB. Inhibition of DNA binding by NF-kappa B with pyrrole-imidazole polyamides. Biochemistry. 2002;41:7604–7609. doi: 10.1021/bi020114i. [DOI] [PubMed] [Google Scholar]
- 14.Chenoweth DM, Poposki JA, Marques MA, Dervan PB. Programmable oligomers targeting 5’-GGGG-3’ in the minor groove of DNA and NF-kappaB binding inhibition. Bioorg. Med. Chem. 2007;15:759–770. doi: 10.1016/j.bmc.2006.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang F, Nickols NG, Li BC, Szablowski JO, Hamilton SR, Meier JL, Wang CM, Dervan PB. Animal toxicity of hairpin pyrrole-imidazole polyamides varies with the turn unit. J. Med. Chem. 2013;56:7449–7457. doi: 10.1021/jm401100s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koehler AN. A complex task? Direct modulation of transcription factors with small molecules. Curr. Opin. Chem. Biol. 2010;14:331–340. doi: 10.1016/j.cbpa.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berg T. Inhibition of transcription factors with small organic molecules. Curr. Opin. Chem. Biol. 2008;12:464–471. doi: 10.1016/j.cbpa.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 18.Merfort I. Perspectives on sesquiterpene lactones in inflammation and cancer. Curr. Drug Targets. 2011;12:1560–1573. doi: 10.2174/138945011798109437. [DOI] [PubMed] [Google Scholar]
- 19.Siedle B, Garcia-Pineres AJ, Murillo R, Schulte-Monting J, Castro V, Rungeler P, Klaas CA, Da Costa FB, Kisiel W, Merfort I. Quantitative structure - Activity relationship of sesquiterpene lactones as inhibitors of the transcription factor NF-kappa B. J. Med. Chem. 2004;47:6042–6054. doi: 10.1021/jm049937r. [DOI] [PubMed] [Google Scholar]
- 20.Adams RHW. Helenalin. I. Isolation and Properties. J. Am. Chem. Soc. 1949;71:2546–2551. [Google Scholar]
- 21.Kos O, Lindenmeyer MT, Tubaro A, Sosa S, Merfort I. New sesquiterpene lactones from Arnica tincture prepared from fresh flowerheads of Arnica montana. Planta Med. 2005;71:1044–1052. doi: 10.1055/s-2005-871284. [DOI] [PubMed] [Google Scholar]
- 22.Pettit GR, Budzinsk JC, Cragg GM, Brown P, Johnston LD. Antineoplastic Agents. 34. Helenium-Autumnale-L. J. Med. Chem. 1974;17:1013–1016. doi: 10.1021/jm00255a024. [DOI] [PubMed] [Google Scholar]
- 23.Lamson PD. On the Pharmacological Action of Helenin, the Active Principle of Helenium Autumnale. J. Pharmacol. Exp. Ther. 1913;4:471–489. [Google Scholar]
- 24.Kitson RR, Millemaggi A, Taylor RJ. The Renaissance of alpha-methylene-gamma-butyrolactones: New Synthetic Approaches. Angew. Chem. Int. Ed. Engl. 2009;48:9426–9451. doi: 10.1002/anie.200903108. [DOI] [PubMed] [Google Scholar]
- 25.Lee KH, Ibuka T, Mar EC, Hall IH. Anti-tumor agents. 31. Helenalin sym-dimethylethylenediamine reaction products and related derivatives. J. Med. Chem. 1978;21:698–701. doi: 10.1021/jm00205a022. [DOI] [PubMed] [Google Scholar]
- 26.Lee KH, Huang ES, Furukawa H. Antitumor Agents. 3. Synthesis and Cytotox Activity of Helenalin Amine Adducts and Related Derivatives. J. Med. Chem. 1972;15:609–611. doi: 10.1021/jm00276a010. [DOI] [PubMed] [Google Scholar]
- 27.Lee KH, Kim SH, Furukawa H, Piantadosi C, Huang ES. Antitumor Agents. 11. Synthesis and Cytotox Activity of Epoxides of Helenalin Related Derivatives. J. Med. Chem. 1975;18:59–63. doi: 10.1021/jm00235a013. [DOI] [PubMed] [Google Scholar]
- 28.Grippo AA, Hall IH, Kiyokawa H, Muraoka O, Shen YC, Lee KH. The cytotoxicity of helenalin, its mono and difunctional esters, and related sesquiterpene lactones in murine and human tumor cells. Drug Des. Discov. 1992;8:191–206. [PubMed] [Google Scholar]
- 29.Hall IH, Lee KH, Mar EC, Starnes CO, Waddell TG. Antitumor Agents. 21. A Proposed Mechanism for Inhibition of Cancer Growth by Tenulin and Helenalin and Related Cyclopentenones. J. Med. Chem. 1977;20:333–337. doi: 10.1021/jm00213a003. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt TJ. Helenanolide-type sesquiterpene lactones--III. Rates and stereochemistry in the reaction of helenalin and related helenanolides with sulfhydryl containing biomolecules. Bioorg. Med. Chem. 1997;5:645–653. doi: 10.1016/s0968-0896(97)00003-5. [DOI] [PubMed] [Google Scholar]
- 31.Lyss G, Knorre A, Schmidt TJ, Pahl HL, Merfort I. The Anti-inflammatory Sesquiterpene Lactone Helenalin Inhibits the Transcription Factor NF-κB by Directly Targeting p65. J. Biol. Chem. 1998;273:33508–33516. doi: 10.1074/jbc.273.50.33508. [DOI] [PubMed] [Google Scholar]
- 32.Buchele B, Zugmaier W, Lunov O, Syrovets T, Merfort I, Simmet T. Surface Plasmon Resonance Analysis of Nuclear Factor-κB Protein Interactions with the Sesquiterpene Lactone Helenalin. Anal. Biochem. 2010;401:30–37. doi: 10.1016/j.ab.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 33.Chen FE, Huang DB, Chen YQ, Ghosh G. Crystal Structure of p50/p65 Heterodimer of Transcription Factor NF-κB Bound to DNA. Nature. 1998;391:410–413. doi: 10.1038/34956. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Pineres AJ, Castro V, Mora G, Schmidt TJ, Strunck E, Pahl HL, Merfort I. Cysteine 38 in p65/NF-κB Plays a Crucial Role in DNA Binding Inhibition by Sesquiterpene Lactones. J. Biol. Chem. 2001;276:39713–39720. doi: 10.1074/jbc.M101985200. [DOI] [PubMed] [Google Scholar]
- 35.Cys38 and Cys120 of p65 are spatially separated by 7.7 Å in the PDB 1VKX. This distance is similar to spatial separation of the two electrophilic carbons in helenalin (~ 7 Å) from two published crystal structures and a computationally minimized structure.
- 36.Rungeler P, Castro V, Mora G, Goren N, Vichnewski W, Pahl HL, Merfort I, Schmidt TJ. Inhibition of transcription factor NF-kappaB by sesquiterpene lactones: a proposed molecular mechanism of action. Bioorg. Med. Chem. 1999;7:2343–2352. doi: 10.1016/s0968-0896(99)00195-9. [DOI] [PubMed] [Google Scholar]
- 37.Kastrati I, Siklos MI, Calderon-Gierszal EL, El-Shennawy L, Georgieva G, Thayer EN, Thatcher GR, Frasor J. Dimethyl Fumarate Inhibits the Nuclear Factor κB Pathway in Breast Cancer Cells by Covalent Modification of p65 Protein. J. Biol. Chem. 2016;291:3639–3647. doi: 10.1074/jbc.M115.679704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han Y, Englert JA, Yang R, Delude RL, Fink MP. Ethyl Pyruvate Inhibits Nuclear Factor-κB-dependent Signaling by Directly Targeting p65. J. Pharmacol. Exp. Ther. 2005;312:1097–1105. doi: 10.1124/jpet.104.079707. [DOI] [PubMed] [Google Scholar]
- 39.Sethi G, Ahn KS, Aggarwal BB. Targeting Nuclear factor-κB Activation Pathway by Thymoquinone: Role in Suppression of Antiapoptotic Gene Products and Enhancement of Apoptosis. Mol. Cancer Res. 2008;6:1059–1070. doi: 10.1158/1541-7786.MCR-07-2088. [DOI] [PubMed] [Google Scholar]
- 40.Liang MC, Bardhan S, Pace EA, Rosman D, Beutler JA, Porco JA, Jr, Gilmore TD. Inhibition of Transcription Factor NF-κB Signaling Proteins IKKβ and p65 Through Specific Cysteine Residues by Epoxyquinone A Monomer: Correlation with its Anti-cancer Cell Growth Activity. Biochem. Pharmacol. 2006;71:634–645. doi: 10.1016/j.bcp.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Rana S, Blowers EC, Tebbe C, Contreras JI, Radhakrishnan P, Kizhake S, Zhou T, Rajule RN, Arnst JL, Munkarah AR, Rattan R, Natarajan A. Isatin Derived Spirocyclic Analogues with α-methylene-γ-butyrolactone as Anticancer Agents: A Structure-Activity Relationship Study. J. Med. Chem. 2016;59:5121–5127. doi: 10.1021/acs.jmedchem.6b00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto M, Horie R, Takeiri M, Kozawa I, Umezawa K. Inactivation of NF-κB Components by Covalent Binding of (-)-dehydroxymethylepoxyquinomicin to Specific Cysteine Residues. J. Med. Chem. 2008;51:5780–5788. doi: 10.1021/jm8006245. [DOI] [PubMed] [Google Scholar]
- 43.Deny LJ, Traboulsi H, Cantin AM, Marsault E, Richter MV, Belanger G. Bis-Michael Acceptors as Novel Probes to Study the Keap1/Nrf2/ARE Pathway. J. Med. Chem. 2016;59:9431. doi: 10.1021/acs.jmedchem.6b01132. [DOI] [PubMed] [Google Scholar]
- 44.Grieco PA, Majetich GF, Ohfune Y. Pseudoguaianolides. 1. Stereospecific Total Synthesis of the Ambrosanolides dl-Ambrosin and dl-Damsin. J. Am. Chem. Soc. 1982;104:4226–4233. [Google Scholar]
- 45.Grieco PA, Ohfune Y, Majetich GF, Wang CLJ. Pseudoguaianolides .2. Stereocontrolled Total Synthesis of the Helenanolide dl-Helenalin. J. Am. Chem. Soc. 1982;104:4233–4240. [Google Scholar]
- 46.Ohfune Y, Grieco PA, Wang CLJ, Majetich G. Stereospecific Total Synthesis of dl-Helenalin - General Route to Helenanolides and Ambrosanolides. J. Am. Chem. Soc. 1978;100:5946–5948. [Google Scholar]
- 47.Roberts MR, Schlessinger RH. Total Synthesis of dl-Helenalin. J. Am. Chem. Soc. 1979;101:7626–7627. [Google Scholar]
- 48.Watson WH, Kashyap RP. The Structures of 2 Sesquiterpene Lactones. Acta Cryst. C. 1990;46:1524–1528. [Google Scholar]
- 49.Fronczek FR, Ober AG, Fischer NH. Helenalin, a Pseudoguaianolide from Helenium-Amarum. Acta Cryst. C. 1987;43:358–360. [Google Scholar]
- 50.Hodgson DM, Talbot EPA, Clark BP. Stereoselective Synthesis of β-(hydroxymethylaryl/alkyl)-α-methylene-γ-butyrolactones. Org. Lett. 2011;13:2594–2597. doi: 10.1021/ol200711f. [DOI] [PubMed] [Google Scholar]
- 51.Ramachandran PV, Chen GM, Brown HC. Chiral Synthesis Via Organoboranes. 42. Selective Reductions. 57. Efficient Kinetic Resolution of Representative α-tertiary Ketones with B-chlorodiisopinocampheylborane. J. Org. Chem. 1996;61:88–94. [Google Scholar]
- 52.Sato KSS, Kojima Y. A New Synthesis of 3-Methylcyclopent-2-en-2-ol-1-one. J. Org. Chem. 1967;32:339–341. [Google Scholar]
- 53.Lee KH, Mar EC, Okamoto M, Hall IH. Antitumor Agents 32. Synthesis and Antitumor Activity of Cyclopentenone Derivatives Related to Helenalin. J. Med. Chem. 1978;21:819–822. doi: 10.1021/jm00206a021. 21, 819-822. [DOI] [PubMed] [Google Scholar]
- 54.Nicolaou KC, Montagnon T, Baran PS, Zhong YL. Iodine(V) Reagents in Organic Synthesis. Part 4. O-iodoxybenzoic acid as a Chemospecific Tool for Single Electron Transfer-based Oxidation Processes. J. Am. Chem. Soc. 2002;124:2245–2258. doi: 10.1021/ja012127+. [DOI] [PubMed] [Google Scholar]
- 55.Fuchs M, Schober M, Orthaber A, Faber K. Asymmetric Synthesis of β-Substituted α-methylenebutyrolactones via TRIP-Catalyzed Allylation: Mechanistic Studies and Application to the Synthesis of (S)-(-)-Hydroxymatairesinol. Adv. Synth. Catal. 2013;355:2499–2505. doi: 10.1002/adsc.201300392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ando K, Takemasa Y, Tomioka K, Koga K. Stereoselective Reactions .21. Asymmetric Alkylation of Alpha Alkyl Beta-Keto-Esters to Alpha,Alpha-Dialkyl Beta-Keto-Esters Having Either (R)-Chiral or (S)-Chiral Quaternary Center Depending on the Solvent System. Tetrahedron. 1993;49:1579–1588. [Google Scholar]
- 57.Fukuyama Y, Matsumoto K, Tonoi Y, Yokoyama R, Takahashi H, Minami H, Okazaki H, Mitsumoto Y. Total syntheses of neuroprotective mastigophorenes A and B. Tetrahedron. 2001;57:7127–7135. [Google Scholar]
- 58.Fukuyama Y, Kiriyama Y, Kodama M. Total synthesis of herbertenediol, an isocuparane sesquiterpene isolated from liverworts. Tetrahedron Lett. 1996;37:1261–1264. [Google Scholar]
- 59.Lee JN, Oya S, Snyder JK. Asymmetric Oxidation of Beta-Ketoesters with Benzoyl Peroxide - Enantioselective Formation of Protected Tertiary Alcohols. Tetrahedron Lett. 1991;32:5899–5902. [Google Scholar]
- 60.Ito Y, Hirao T, Saegusa T. Synthesis of Alpha,Beta-Unsaturated Carbonyl-Compounds by Palladium(Ii)-Catalyzed Dehydrosilylation of Silyl Enol Ethers. J. Org. Chem. 1978;43:1011–1013. [Google Scholar]
- 61.Larock RC, Hightower TR, Kraus GA, Hahn P, Zheng D. A Simple, Effective, New, Palladium-Catalyzed Conversion of Enol Silanes to Enones and Enals. Tetrahedron Lett. 1995;36:2423–2426. [Google Scholar]
- 62.Hexum JK, Tello-Aburto R, Struntz NB, Harned AM, Harki DA. Bicyclic Cyclohexenones as Inhibitors of NF-kappa B Signaling. ACS Med. Chem. Lett. 2012;3:459–464. doi: 10.1021/ml300034a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. Engl. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 64.Frigerio M, Santagostino M, Sputore S. A user-friendly entry to 2-iodoxybenzoic acid (IBX) J. Org. Chem. 1999;64:4537–4538. [Google Scholar]
- 65.Kozawa I, Akashi Y, Takiguchi K, Sasaki D, Sawamoto D, Takao K, Tadano K. Stereoselective double alkylation of the acetoacetate ester alpha-carbon on a D-glucose-derived template: Application to the synthesis of enantiopure cycloalkenones bearing an asymmetric quaternary carbon. Synlett. 2007:399–402. [Google Scholar]
- 66.Kato K, Suzuki H, Tanaka H, Miyasaka T, Baba M, Yamaguchi K, Akita H. Stereoselective Synthesis of 4’-α-alkylcarbovir Derivatives Based on an Asymmetric Synthesis or Chemoenzymatic Procedure. Chem. Pharm. Bull. 1999;47:1256–1264. [Google Scholar]
- 67.Kumar R, Rej RK, Halder J, Mandal H, Nanda S. Enantiopure Hydroxymethylated Cycloalkenols as Privileged Small Molecular Multifunctional Scaffolds for the Asymmetric Synthesis of Carbocycles. Tetrahedron-Asymmetr. 2016;27:498–512. [Google Scholar]
- 68.Friscourt F, Fahrni CJ, Boons GJ. A Fluorogenic Probe for the Catalyst-free Detection of Azide-tagged Molecules. J. Am. Chem. Soc. 2012;134:18809–18815. doi: 10.1021/ja309000s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shevchenko A, Wilm M, Vorm O, Mann M. Mass Spectrometric Sequencing of Proteins from Silver Stained Polyacrylamide Gels. Anal. Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 70.Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. n-gel Digestion for Mass Spectrometric Characterization of Proteins and Proteomes. Nature Prot. 2006;1:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- 71.Yates JR, 3rd, Eng JK, McCormack AL. Mining Genomes: Correlating Tandem Mass Spectra of Modified and Unmodified Peptides to Sequences in Nucleotide Databases. Anal. Chem. 1995;67:3202–3210. doi: 10.1021/ac00114a016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.