Abstract
Digital pathology is becoming technically possible to implement for routine pathology work. At our institution, we have been using digital pathology for second opinion intraoperative consultations for over 10 years. Herein, we describe our experience in converting to a digital pathology platform for primary pathology diagnosis. We implemented an incremental rollout for digital pathology on subspecialty benches, beginning with cases that contained small amounts of tissue (biopsy specimens). We successfully scanned over 40,000 slides through our digital pathology system. Several lessons (both challenges and opportunities) were learned through this implementation. A successful conversion to digital pathology requires pre-imaging adjustments, integrated software and post-imaging evaluations.
Keywords: Diagnostic imaging, Digital imaging, Imaging informatics, Experiential, Implementation, Whole slide imaging, Digital pathology
Background
Digital pathology has matured to the point where it is technically possible to produce high-quality digital images that can be used for diagnostic interpretation [1, 2]. Whole slide imaging (WSI) is the digitization of glass slides by whole slide scanners. WSI files are accurate and high-resolution images captured from traditionally prepared specimens on glass slides, where many individual microscopic fields are quilted together to form a nearly seamless digital image. Several studies have compared WSI to glass slides with favorable opinions [3, 4]. In the USA, digital pathology is mainly used for consultations, medical education, and research [5–8].
The organization investigated is a large multi-facility healthcare organization that consists of more than 20 hospitals and 500 outpatient sites located over a wide geographic area in western Pennsylvania, USA. The institution also has international partners, including a hospital located in Italy and reference lab in China, from which it receives consultations via telepathology. This institution has one of the largest academic pathology departments in the USA, with more than 100 diagnostic anatomic pathologists. This organization has been using digitized pathology slides, including whole slide images, for over a decade to support the internal triaging of difficult intraoperative pathology consultations to appropriate experts and to provide remote teleconsultations to external organizations across the world [7].
This case study investigates the journey of our organization’s conversion to a digital pathology platform for primary diagnosis sign out. An integrated digital pathology solution was implemented across an integrated hospital system. The solution was fully interfaced with the laboratory information system (LIS) and includes histology workstations, pathologist workstations, short- and long-term archives, and WSI scanners.
Process/Workflow
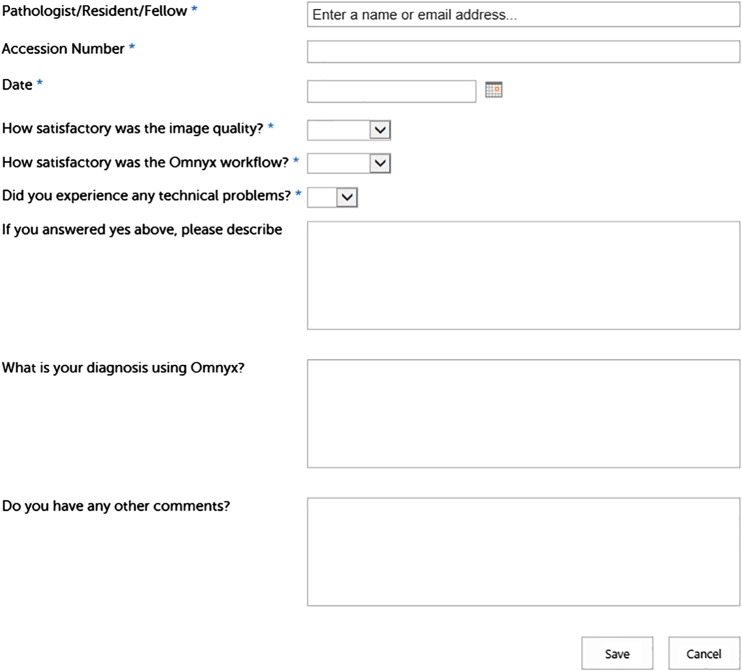
Workflow data was collected from a high-volume histology laboratory to determine which processes would benefit most from going digital. Several use cases were piloted and evaluated in different subspecialties including dermatopathology; autopsy; and gastrointestinal, gynecologic/breast, pediatric, and genitourinary pathology as well as central core services such as performing immunohistochemistry. Of note, the tissue specimen types selected for implementation contained small tissue fragments and few slides to be scanned. Immediately following sign out of WSI, pathologists were asked to provide feedback via an electronic survey link on their desktop (Fig. 1). Additional data (e.g., slide scan time, case turnaround time, interface downtime, diagnostic concordance) were collected to measure the impact on a workflow of hardware and software failures.
Fig. 1.
Sample feedback form for WSI to be filled out by a pathologist before review of glass slides
A digital pathology workflow provides highly reliable images of diagnostic quality that are archived (easy to retrieve) and portable (easy to share). This enabled our organization to route pathology cases comprised of digitized (virtual) whole slides by subspecialty regardless of the physical location of the glass slides and the pathologists. Imaging information was presented in a digital cockpit (composed of two HP Z24X monitors, situated side-by-side with landscape orientation) along with other patient information (e.g., gross pathology findings and prior specimens transmitted via the LIS) in an organized manner (Fig. 2). This provided efficiency in the workflow and improved turnaround time without hampering diagnostic accuracy. Even more powerful was the ability to couple images with computer-aided image analysis tools (specifically for breast cancer biomarkers), which are under continued development.
Fig. 2.
Sign out of the first digital case in August 2015 by Dr. Hartman
However, along with the significant rewards derived from a digital workflow are several problems and important planning decisions. The biggest challenges that an organization has to face when adopting a fully digital workflow are technical readiness, operational readiness, and cost [5].
Technical Readiness
Hardware and software problems can have a significant negative impact on the cost and adoption rate for digital pathology implementation. Hardware issues are related to scanner throughput (overall daily slide capacity and scan time) and rate of scan failures (i.e., inability to digitize a slide). Scanner problems can affect image quality (e.g., poor focus and/or resolution). Software difficulties include interface downtime and delayed network connectivity. Understanding such variables will ensure that there is appropriate redundancy so that a workflow is not interrupted. The most frequent failures experienced were dropped slides on the stage (4.5 times per month). The frequency of errors per month ranged from 0.5 to 4.5 times per month. The downtime ranged from 28 to 85 min. The longest failure was for powering off and clearing all slides. Other errors included camera error, robot error (slide on stage sideways), robot dropped slide at the edge locator, and unexpected slide in a gripper.
A review of existing practices and thorough validation of proposed workflows was a necessary step in the evaluation of a pure digital workflow. For example, marks (e.g., pen markings) on the glass slides could cause problems with the focus of the optics of the scanner, causing aberrations and rendering the whole slide image not suitable for diagnostic interpretation. Label or cover slip overhangs can cause issues with the instrument’s gripper and cause a slide to be dropped and potentially broken by the robotics in the scanner (Fig. 3).
Fig. 3.
Examples of slide label variabilities for both initial labels and additional labels for consultation cases
Even the evaluation of histology materials (e.g., glass slide size and thickness, slide labels, barcodes, mounting medium, chemical like xylene in automated instruments) used is important when considering a pure digital workflow in the histology lab. For example, the type of label used could potentially cause failures related to the slide grip mechanics, in some cases with extreme consequences including the physical destruction of the glass slide (Fig. 4).
Fig. 4.
The right panel demonstrates a gripper with sticky material. The left panel demonstrates mass spectroscopy results of material from the gripper and adhesive from the slide labels
Some failures can cause significant disruption, because they may require multiple actions. For example, the disassembly of the hardware, troubleshooting, installation of any replacement parts, and reassembly of the scanner require highly specialized hardware technicians and are a timely task. Also, in the case of large scanners that cannot be easily transported, technicians need to be able to perform services on these instruments on site. Some repairs to bring a scanner back into operation may even require replacement parts that need to be shipped from a remote location.
In order to reduce the resolution time in such instances, it is possible for an organization to maintain storage of common parts that may be required for repairs. One option to mitigate interruptions of a workflow is to maintain some level of redundancy in the number of scanners being used. Throughput of individual scanners should be measured, including any setup and loading time between runs and any preparation checks performed on the scanner as well as glass slide preparation required prior to scanning. However, the method used to determine the level of redundancy required in a laboratory can have undesired results if one fails to consider the average failure rate for an individual scanner. While the scan failure rates reported by vendors for their devices may appear to be acceptable over an extended period of time, when such downtimes actually occur during a working shift for a system in live production, it can significantly impact clinical operations.
For example, if the average throughput of a scanner is 50 slides/h and a scanner has an uptime warranty of 98%, it would be possible with a simple formula to calculate the expected throughput of slides during a shift of 8 h based on the number of scanners employed (Table 1). However, this method would be short sighted due to the fact that the uptime warranty of a scanner is based on total available time. A manufacturer uptime warranty of 98% means that it is possible for a scanner to be down on average for over 14 h over the course of a month. The downtime events can be relatively short in duration, such that may occur when recalibrating the optics or responding to an error that requires some repositioning of glass slides or robotic arms. However, as alluded before, if there is an event that requires replacement of electronic components or mechanical parts, there could be an extended downtime, which could potentially impact 100% of the duration of one shift. A simple recommendation is to use the nominal throughput value as the base number of scanners required for desired throughput, and then add an extra scanner. However, this approach may be cost prohibitive in addition to creating challenges of physical space in the laboratory.
Table 1.
An example of throughput analysis (slides per shift) compared to 2% downtime calculation
| Number of scanners | Throughput (slides/shift) | |
|---|---|---|
| Nominal | 2% Downtime | |
| 2 | 800 | 784 |
| 3 | 1200 | 1176 |
| 4 | 1600 | 1568 |
| 5 | 2000 | 1960 |
Operational Readiness
The introduction of a digital workflow does not entirely eliminate the legacy tasks of a histology lab (e.g., microtomy) and may even add incremental steps (e.g., slide preparation prior to scanning, WSI instrument quality control checks). If only a portion of glass slides are scanned in a laboratory, dealing with hybrid digital and glass slides can result in disruptive and even competing workflows. Specimens must still be processed and glass slides must still be prepared before they can be digitized. This ultimately results in an incremental workload as no efficiency is gained in the histology lab (Table 2).
Table 2.
Comparison of the legacy workflow to the digital workflow. The final column evaluates a workload between the two workflows
| Legacy workflow | Digital workflow | Workload of digital workflow |
|---|---|---|
| Accessioning | Accessioning | Same |
| Grossing | Grossing | Same |
| Histology slide creation | Histology slide creation | Same |
| Case validation in digital solution | More: potential duplicate step | |
| Issue management/calibration | More: monitor against errors | |
| Scanning/imaging tasks | More: loading/unloading slides | |
| Case assembly (physical) | Case assembly (digital) | Equivalent (but less error prone) |
| Quality check (physical) | Quality check (digital) | Equivalent |
| Delivery (physical) | Delivery (digital) | Less |
While efficiencies are gained in the digital delivery of pathology cases to the appropriate pathologist, the actual impact of imaging tasks, monitoring the scanner against errors and mechanical failures, managing interruptions, and resolving problems can add a significant workload and in most cases may require additional personnel (Fig. 5).
Fig. 5.
A diagram comparing the traditional workflow for slides from the histology laboratory with the workflow for digital slides
Operational readiness also includes hiring and training of the appropriate personnel to manage the required technology both in information technology (IT) and in the histology lab. From an IT perspective, there is a need for dedicated staff to deploy, validate, and maintain the digital pathology system. Such effort is equivalent to that which is required to support a traditional Picture and Archiving Communication System (PACS) in radiology, with the additional challenge of managing higher requirements for network connectivity, storage, and imaging lifecycle. In the histology lab, the personnel dedicated to managing scanners will have to be a specialized technologist with an interest in imaging and expertise in image management, who has the ability to monitor, troubleshoot, and resolve the scanner issues. Additional operational readiness includes all of the challenges associated with budgeting, change management, orientation, and adoption of new workflows, as well as engagement of all the key stakeholders. The key stakeholders involved include pathologists, IT division, lab supervisors/managers, and hospital administration.
Table 3 summarizes the advantages and disadvantages of rapidly implementing a digital workflow as compared to a more staged, incremental adoption. An accelerated rollout requires a higher investment at the beginning of the process and enables an organization to quickly transition from legacy workflows with focused efforts in support and devoted to change management. Conversely, an incremental adoption allows more time for clinical validation and a more mature adoption. However, the latter approach causes the need for extended support of the rollout and the coexistence of both legacy and digital workflows.
Table 3.
Comparison of advantages and disadvantages of accelerated rollout to incremental adoption of digital pathology
| Accelerated rollout | Incremental adoption | ||
|---|---|---|---|
| Advantages | Disadvantages | Advantages | Disadvantage |
| Full span | Higher upfront cost | Lower upfront cost | Limited span |
| Focused rollout support | Immature adoption | Mature adoption | Extended rollout support |
| Fast adoption | Limited use cases | Lower refresh cost | Slow adoption |
| Focused change management | Impact on operations | More time for clinical validation | Long change management |
Conclusion
Digital pathology is a key enabler of improved patient care and provides an organization with the ability to introduce economies of scale, centralization of services, and subspecialty coverage. Our institution is an early adopter of WSI for primary diagnosis. Our implementation was more challenging due to this, as no blueprint for implementation previously existed. We are committed to leading digital transformation within the pathology community. Given the current limitations of WSI scanner hardware, certain centers of excellence (e.g., cytology and hematopathology), educational cases, and frozen sections were excluded from our implementation use cases [9, 10]. For full implementation of digital pathology within a department, these areas would need to be included. Laboratory information system integration is critical for efficiency and functionality with digital pathology as pointed out by Guo et al. and Cheng et al. [11, 12]. Additionally, our health system represents a unique combination of academic and community pathology practices, and our experience may not extrapolate to other institutions.
Certain IT factors are also important for a successful implementation. These include maintaining both a test and live environment, boosting network connectivity (1 Gbps) to the WSI scanners, using a central image data repository, and policy for handling images (purge images or maintain them). Certain operational factors needed to be changed/adjusted for digital pathology implementation. Facility renovations (i.e., sign out rooms to accommodate digital workstations), pre-imaging factors to enhance scanning (e.g., barcodes, rapid tissue processors), and dedicated resources for training pathologists (25 pathologists were trained) were needed. Along with establishing a few pathologists as champions of digital pathology, communication with the pathology staff through frequent memos, giving departmental presentations, and involving community pathologists with the implementation studies were necessary for a successful implementation.
Since our implementation, we have scanned over 40,000 slides. We were able to accomplish this because there was a concomitant top-down (buy-in from leaders) and bottom-up (empowering users) approach. Additionally, we crafted flexible plans for implementation such that we were able to adapt to changes in technology over the time of implementation (such as web-based version of the software). We set out to validate our use cases (primary diagnosis, internal consults, IHC review) according to the College of American Pathologists’ recommendations [4, 13, 14]. However, implementing a fully digital pathology workflow can be costly and requires significant changes to existing workflows, an assessment of the operational readiness and technological infrastructure to ensure appropriate levels of service. Of note, this study does not address return on investment for digital pathology as this has been discussed in other papers [15].
Footnotes
This work was completed while Gonzalo Romero Lauro was employed at the University of Pittsburgh Medical Center.
References
- 1.Montalto MC. An industry perspective: An update on the adoption of whole slide imaging. Journal of pathology informatics. 2016;7:18. doi: 10.4103/2153-3539.180014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thorstenson S, Molin J, Lundstrom C. Implementation of large-scale routine diagnostics using whole slide imaging in Sweden: Digital pathology experiences 2006-2013. Journal of pathology informatics. 2014;5(1):14. doi: 10.4103/2153-3539.129452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goacher E, Randell R, Williams B, Treanor D 2016. The Diagnostic Concordance of Whole Slide Imaging and Light Microscopy: A Systematic Review. Archives of pathology & laboratory medicine. [DOI] [PubMed]
- 4.Snead DR, Tsang YW, Meskiri A, Kimani PK, Crossman R, Rajpoot NM, et al. Validation of digital pathology imaging for primary histopathological diagnosis. Histopathology. 2016;68(7):1063–72. doi: 10.1111/his.12879. [DOI] [PubMed] [Google Scholar]
- 5.Al-Janabi S, Huisman A, Van Diest PJ. Digital pathology: current status and future perspectives. Histopathology. 2012;61(1):1–9. doi: 10.1111/j.1365-2559.2011.03814.x. [DOI] [PubMed] [Google Scholar]
- 6.Pantanowitz L. Digital images and the future of digital pathology. Journal of pathology informatics. 2010;1. [DOI] [PMC free article] [PubMed]
- 7.Romero Lauro G, Cable W, Lesniak A, Tseytlin E, McHugh J, Parwani A, et al. Digital pathology consultations-a new era in digital imaging, challenges and practical applications. Journal of digital imaging. 2013;26(4):668–77. doi: 10.1007/s10278-013-9572-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pantanowitz L, Valenstein PN, Evans AJ, Kaplan KJ, Pfeifer JD, Wilbur DC, et al. Review of the current state of whole slide imaging in pathology. Journal of pathology informatics. 2011;2:36. doi: 10.4103/2153-3539.83746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stathonikos N, Veta M, Huisman A, van Diest PJ. Going fully digital: Perspective of a Dutch academic pathology lab. Journal of pathology informatics. 2013;4:15. doi: 10.4103/2153-3539.114206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins C. Applications and challenges of digital pathology and whole slide imaging. Biotechnic & histochemistry : official publication of the Biological Stain Commission. 2015;90(5):341–7. doi: 10.3109/10520295.2015.1044566. [DOI] [PubMed] [Google Scholar]
- 11.Guo H, Birsa J, Farahani N, Hartman DJ, Piccoli A, O'Leary M, et al. Digital pathology and anatomic pathology laboratory information system integration to support digital pathology sign-out. Journal of pathology informatics. 2016;7:23. doi: 10.4103/2153-3539.181767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng CL, Azhar R, Sng SH, Chua YQ, Hwang JS, Chin JP, et al. 2016 Enabling digital pathology in the diagnostic setting: navigating through the implementation journey in an academic medical centre. Journal of clinical pathology. [DOI] [PubMed]
- 13.Thrall MJ, Wimmer JL, Schwartz MR. Validation of multiple whole slide imaging scanners based on the guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Archives of pathology & laboratory medicine. 2015;139(5):656–64. doi: 10.5858/arpa.2014-0073-OA. [DOI] [PubMed] [Google Scholar]
- 14.Buck TP, Dilorio R, Havrilla L, O'Neill DG. Validation of a whole slide imaging system for primary diagnosis in surgical pathology: A community hospital experience. Journal of pathology informatics. 2014;5(1):43. doi: 10.4103/2153-3539.145731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho J, Ahlers SM, Stratman C, Aridor O, Pantanowitz L, Fine JL, et al. Can digital pathology result in cost savings? A financial projection for digital pathology implementation at a large integrated health care organization. Journal of pathology informatics. 2014;5(1):33. doi: 10.4103/2153-3539.139714. [DOI] [PMC free article] [PubMed] [Google Scholar]