Abstract
Translocator protein (TSPO) is a validated target for molecular imaging of a variety of human diseases and disorders. Given its involvement in cholesterol metabolism, TSPO expression is commonly elevated in solid tumors, including glioma, colorectal cancer, and breast cancer. TSPO ligands capable of detection by optical imaging are useful molecular tracers for a variety of purposes that range from quantitative biology to drug discovery. Leveraging our prior optimization of the pyrazolopyrimidine TSPO ligand scaffold for cancer imaging, we report herein a new generation of TSPO tracers with superior binding affinity and suitability for optical imaging and screening. In total, seven candidate TSPO tracers were synthesized and vetted in this study; the most promising tracer identified (29, Kd = 0.19 nM) was the result of conjugating a high-affinity TSPO ligand to a fluorophore used routinely in biological sciences (FITC) via a functional carbon linker of optimal length. Computational modeling suggested that an n-alkyl linker of eight carbons in length allows for positioning of the bulky fluorophore distal to the ligand binding domain and toward the solvent interface, minimizing potential ligand−protein interference. Probe 29 was found to be highly suitable for in vitro imaging of live TSPO-expressing cells and could be deployed as a ligand screening and discovery tool. Competitive inhibition of probe 29 quantified by fluorescence and 3H-PK11195 quantified by traditional radiometric detection resulted in equivalent affinity data for two previously reported TSPO ligands. This study introduces the utility of TSPO ligand 29 for in vitro imaging and screening and provides a structural basis for the development of future TSPO imaging ligands bearing bulky signaling moieties.
Graphical Abstract

INTRODUCTION
Alterations in cellular metabolism distinguish cancer cells from many surrounding, otherwise normal, tissues. While many tumors exhibit elevated metabolism of glucose and glutamine,1,2 elevated cholesterol metabolism is also a characteristic of many human tumors. Translocator protein (TSPO) is an 18 kDa protein, primarily localized to mitochondria. One important role of TSPO is to facilitate cholesterol transport and metabolism. TSPO is highly expressed in numerous solid tumors, including those of the CNS,3–6 oral cavity,7 breast,8–10 and colon,11–13 where elevated levels tend to be associated with poor outcome. These findings support TSPO as a potentially important biomarker in oncology and suggest that molecular imaging probes can be designed to target TSPO specifically to image cancers and diseases with elevated TSPO expression.
Given the important role of TSPO in cancer imaging, several structurally diverse TSPO ligands have been reported, including PK 11195,14 Ro-54864,15 DPA-713,16 DPA-714,17,18 PBR28,19,20 and ER17621 (Figure 1), which can be utilized to further develop positron emission tomography (PET) tracers by replacing a certain atom with its isotope. Our laboratory’s previous research has focused on the discovery, development, and clinical translation of TSPO ligands for cancer imaging.3,5,11,22–25 Recently, we reported on the discovery of a novel TSPO ligand, 2-(5,7-diethyl-2-(4-(2-fluoroethoxy)phenyl)-pyrazolo[1,5-a]pyrimidin-3-yl)-N,N-diethylacetamide (VUIIS 1008), which exhibited a 36-fold enhancement in affinity compared with DPA-714.3
Figure 1.
Compound structures of TSPO ligands cited in the text.
As a noninvasive molecular imaging modality, optical imaging utilizes inexpensive and simple instrumentation, making it more affordable and accessible for preclinical research and clinical imaging.26 Optical imaging may capitalize upon optical tracers that emit photons following excitation, without the radiation exposure to subject and worker as is typical for nuclear medicine. Furthermore, the lack of radiochemical half-life renders optical tracers easier to store, deliver, and use, with the added benefit that large-scale manufacturing is possible.27 Unlike most nuclear medicine tracers, optical tracers are suitable for lengthy procedures that may include surgical navigation or tracking disease progression longitudinally.28 One can also utilize several probes that emit a diversity of colors and measure the signals from different channels simultaneously, allowing for the investigation of a series of molecular properties in paralleling.27,29,30 Optical tracers are also uniquely suitable for high-resolution molecular imaging such as microscopy, as well as biochemical assays. However, optical imaging may not be applicable for deep tissues because of the strong attenuation of the signal. It is also difficult to generate tomographic images with optical imaging because almost all the detected photons have been scattered multiple times.31
Early in 1997, Kozikowski et al.32 developed TSPO optical probe using 4-chloro-7-nitrobenzofurazan (NBD chloride) and utilized the probe to label MA-10 Leydig cells and C6 rat glioma cells. Further, Laquintana et al.33 reported a series of TSPO fluorescent probes and investigated the impact of linker and ligand units on both fluorescence characteristics and binding affinity. Taliani et al.34,35 described several new TSPO fluorescent probes, and most interestingly, they found that the polarity of the medium can have a huge impact on quantum yield. By conjugating PK 11195 with Lissamine-Rhodamine B sulfonyl chloride, Manning et al.22,30 discovered probes for glioma cell imaging. Bai et al.36 separated two isomers of Lissamine-Rhodamine B sulfonyl chloride conjugated probe, and utilized the one with higher molar extinction coefficient for imaging. By using fluorescein isothiocyanate (FITC) to conjugate with high potent TSPO ligand, Denora et al.27 developed two novel fluorescent probes with high affinity, favorable stability, and good blood-brain barrier penetration.
Herein, we developed a series of new TSPO ligands with appended FITC allowing for straightforward detectability by conventional optical measures and microscopy. A library of candidate ligands generated and subjected to competitive binding assays that explored the effects of linker length selection (n = 2−8). The best candidate compound, 29, which exhibited exceptional TSPO affinity (Ki = 0.31 nM) and negligible central benzodiazepine receptor (CBR) activity, exhibited suitable in vitro imaging characteristics and high target specificity. To the best of our knowledge, this is the highest affinity for any TSPO optical probe that has been reported. The new probe was also found to be very useful for small molecule screening studies in multiwell plate format. Competitive inhibition of compound 29 quantified by fluorescence compared with 3H-PK 11195 quantified by traditional radiometric detection was equivalent for two reported TSPO ligands. This work illustrates that TSPO optical probes afford opportunities to introduce high-resolution in vitro imaging and fast screening assay without using radioactive materials. We anticipate that the structurally based modeling and analysis in this study may expand opportunities to develop novel TSPO imaging ligands bearing bulky moieties.
RESULTS AND DISCUSSION
Synthesis.
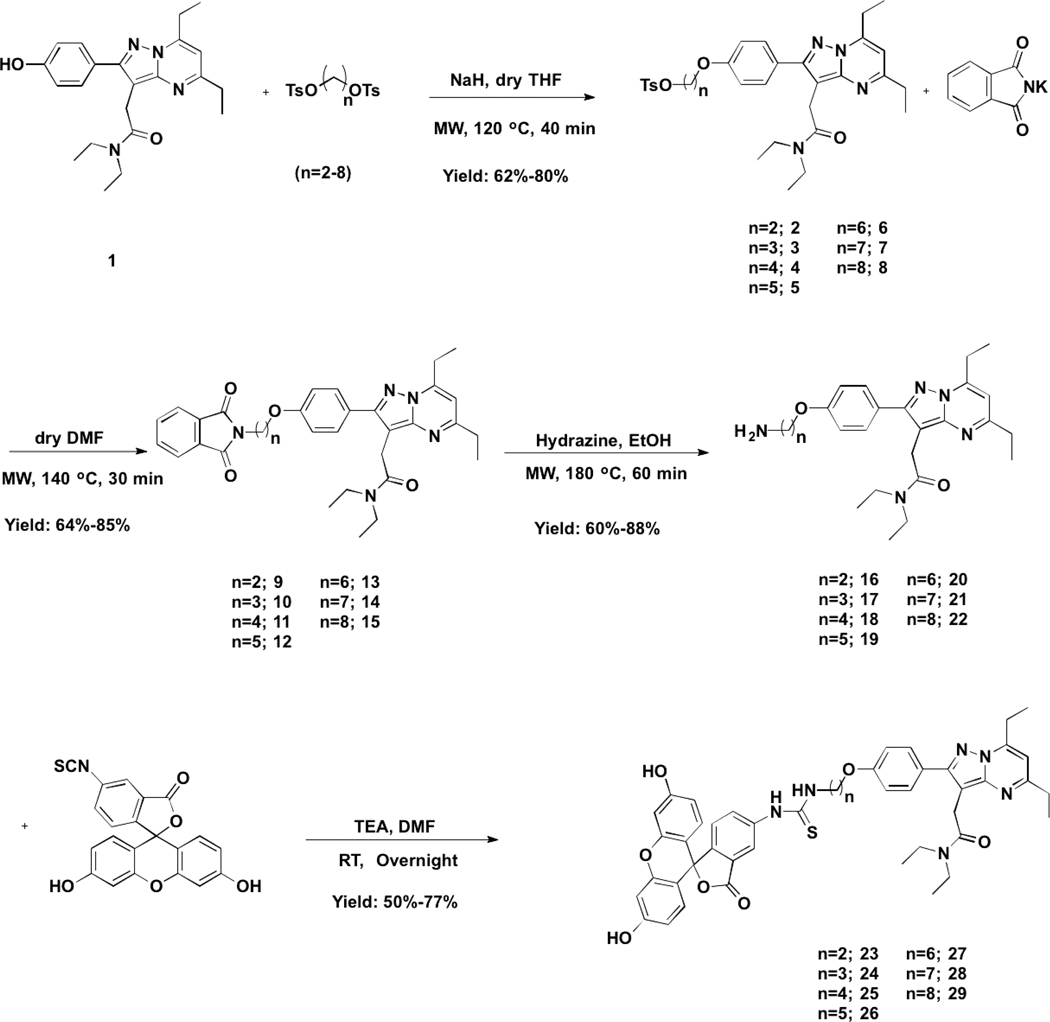
TSPO fluorescent probes 23−29 were synthesized through conjugating the TSPO ligand with the fluorescent compound FITC employing various linker lengths (2−8 carbons) via a four-step synthetic route starting from compound 1 (Scheme 1).3 Briefly, compounds 2−8 were prepared by condensation of the available para-hydroxyl group in 1 with various straight chained ditosylates, which were all formed by the reaction of the corresponding diols with tosylchloride at room temperature. Compounds 9−15 were subsequently formed by reaction of compounds 2−8 with phthalimide potassium salt in anhydrous DMF. Treatment of compounds 9−15 with hydrazine in ethanol reveals the terminal amine present in compounds 16−22. Final products were prepared by the direct condensation of compounds 16−22 with the isothiocyanate of FITC under basic conditions in anhydrous DMF at room temperature.
Scheme 1.
Synthesis of TSPO Fluorescent Ligands 23−29
Receptor Binding Affinity and Spectroscopic Characterization.
The binding affinity of probes 23−29 to TSPO were assayed and recorded in Table 1. Binding of probes to CBR was ruled out by a [3H]-flunitrazepam challenge assay. The Ki values of the synthesized probes for TSPO varied from submicromolar to subnanomolar. The tracer bearing an 8-carbon linker (29) exhibited the highest binding affinity (Ki = 0.31 nM), which was 590-fold higher than the least potent tracer in the series, 26 (Ki = 184.86 nM). Notably, the binding affinity of 29 was essentially equivalent to the parent TSPO ligand VUIIS 1008 (Ki = 0.27 nM).3 This represents the highest binding affinity for a fluorescent TSPO probe reported to date.
Table 1.
TSPO−Ligand Interaction for Probes 23−29
| entry | Ki (nM)a,b |
|---|---|
| 23 (n = 2) | 13.84 ± 4.99 |
| 24 (n = 3) | 110.88 ± 25.63 |
| 25 (n = 4) | 155.47 ± 8.94 |
| 26 (n = 5) | 184.86 ± 15.39 |
| 27 (n = 6) | 11.67 ± 4.86 |
| 28 (n = 7) | 16.29 ± 5.01 |
| 29 (n = 8) | 0.31 ± 0.02 |
| VUIIS 1008c | 0.27 |
All Ki values were measured from the displacement of 3H-PK 11195 and averaged from triple runs with corresponding standard deviation.
Ki versus 3H-flunitrazepam >10 000 nM.
See ref 3.
It was noted that the apparent binding affinity did not change correspondingly with the linker length. Given the size of the fluorescent unit, the longer linker was expected to benefit the binding affinity. However, 23, which contained a 2-carbon linker, displayed an elevated binding affinity over 24−26, which contained 3-, 4-, and 5-carbon linkers, respectively. This trend was similar to the previous research of Bai et al.,37 which showed that the binding affinity decreased first, and then increased with the increment of linker length.
To further explain this phenomenon, probe 29 was evaluated computationally (Figure 2). The best scoring pose positioned the ligand unit within the TSPO hydrophobic pocket formed by five transmembrane α-helices, while the fluorescent unit was completely distal to the binding pocket. It is anticipated that this arrangement decreases influence from the bulky dye unit. Of note, it was found that the computational model also estimated that two side chains near the bottom of the TSPO protein binding pocket, tryptophan TRP51 and phenylalanine PHE90, are potentially interacting with the pyrazole ring of the ligand unit through an apparent π−π stacking interaction.
Figure 2.
Best score modeling structure of probe 29 interaction with human TSPO (see all poses in Supporting Information).
After coupling with the FITC, the probes exhibited similar aqueous spectroscopy (Figure 3), with the maximum absorption wavelength at approximately 500 nm and the maximum emission wavelength at approximately 522 nm. The molar extinction coefficient of the highest binding compound 29 was found to be 13 913.04 L·mol−1·cm−1 (Figure 3) and the quantum efficiency was 0.33 compared with the efficiency of FITC. Thus, the presence of the TSPO ligand had only a minor effect on the aqueous phase spectroscopy of 29 compared with the parent dye FITC.
Figure 3.
Fluorescent probe 29 aqueous spectroscopy (A) and molar extinction coefficient curve (B).
Saturation Curve and Displacement Experiments.
Ligand saturation and displacement of 29 in vitro was assayed using a modified multiwell assay that employed optical imaging. Specific binding of 29 was dominant at concentrations up to 10 nM (Figure 4). Upon further increasing of the probe concentration while maintaining the same amount of TSPO, nonspecific binding was observed. The dissociation constant (Kd) of probe 29 was found to be 0.19 nM (Figure 4) by subtracting the nonspecific binding from the displacement curve.
Figure 4.
Saturation curve of fluorescent probe 29 binding to C6 glioma cell lysate (A black curve, and B) and displacement experiments (A red curve). C6 glioma cell lysates were coincubated with 0.50−100 nM fluorescent probe and 100 nM of its parent ligand.
Cell Imaging.
We evaluated the uptake and intracellular behavior of 29 in live C6 rat glioma cells. MitoTracker Red was used to label the mitochondria. As shown in Figure 5, fluorescent probe 29 labeled the cells very efficiently. Furthermore, the specific localization of 29 and MitoTracker Red were found to be similar.
Figure 5.
Confocal microscopy images of C6 rat glioma cells incubated with optical probe 29. (A,D) fluorescent images of optical probe; (B,E) fluorescent images of MitoTracker Red; (C,F) merged images of optical probe and MitoTracker Red. (D,E,F) fluorescent images of the chosen area in (A); (G,H,I) and (J,K,L) images of displacement experiment. C6 cells were coincubated with 0.1 μM optical probe 29 and 10 μM of its parent ligand (G,H,I) or PK 11195 (J,K,L); (M,N,O) images of C6 glioma cells dosed with MitoTracker Red only.
Displacement experiments were performed to further evaluate the TSPO specificity. C6 glioma cells were treated with fluorescent probe 29 and 100-fold higher concentration of nonfluorescent parent ligand VUIIS 1008 or PK 11195, as well as MitoTracker Red. As shown in Figure 5, both of them completely displaced the fluorescent probe, further confirming that the fluorescent probe 29 bound to the same binding sites as its TSPO parent ligand and PK 11195.
Ligand Screening Assay.
Presently, 3H-PK 11195 is routinely used in the standard radiometric screening assays to determine the binding affinity of TSPO ligands. However, current binding assays have several limitations, including the inherent safety concerns about the use of radioactive material, the monetary cost of purchasing the radioactive compounds, reagents, and sample containers, and the long counting times for proper measurement of the large number of samples used for each assay. Leveraging the existence of a fluorescent unit within the optical ligand to measure the signal, probe 29 can be utilized to develop a robust and efficient screening assay to replace the traditional 3H-PK 11195. The new assay using probe 29 was utilized to screen two reported TSPO ligands, PK 11195 and VUIIS 1006, in a 24-well plate format. As shown in Table 2, the binding affinity measured by the two assays were comparable to each other, which demonstrated that the optical probe can be used to replace 3H-PK 11195 to develop a fast and nonradioactive screening assay.
Table 2.
Ligand Binding Affinity Screened with Optical Probe 29 and 3H-PK11195
CONCLUSIONS
We developed a series of novel TSPO fluorescent probes by conjugating our previously reported TSPO ligand with a fluorophore (FITC). By changing the linker length from two to eight carbons, optical properties of the probes remained similar, while TSPO binding affinities varied greatly. This led to the discovery of a new TSPO optical compound with a Ki value of 0.31 nM, the highest binding affinity among TSPO fluorescent probes reported to date. Subsequent saturation curve determination, displacement experimentation, and cell imaging illustrated the specific binding of the probe to TSPO. The new optical ligand was also found to be very promising for development of a fast tracer screening assay to replace 3H-PK 11195.
This work lays the foundation for the development of a battery of fluorescence-based TSPO ligands that could potentially be translated to in vivo studies, especially with further refinement of the fluorescence properties. In addition, we envision a role for these probes as precision, point-of-care diagnostics, given the important role of TSPO in many human diseases.
EXPERIMENTAL PROCEDURES
Synthesis.
The synthetic route is presented in Scheme 1.
Compounds 2−8.
To a solution of 2-(5,7-diethyl-2-(4-hydroxyphenyl)pyrazolo[1,5-a]pyrimidin-3-yl)-N,N-diethylace-tamide38 (Compound 1, 100 mg, 1 equiv) in dry tetrahydrofuran (THF, 7 mL) was added NaH (18.9 mg, 3 equiv). The reaction mixture was then stirred at 0 °C for 10 min and allowed to warm up to room temperature before adding the di(tosyloxy) compound39 (3 equiv). The reaction mixture was then irradiated in a Biotage Initiator at 120 °C for 40 min (pre-stirring: 1 min, absorption level: normal). After that, the reaction was acidified with 1.0 M aqueous HCl and extracted with dichloromethane (DCM) three times. The organic solutions were then collected, dried over magnesium sulfate, and concentrated to dryness. The residue was purified by flash chromatography using 19:1 DCM/methanol to yield compounds 2−8 as yellow solids.
Compounds 9−15.
To a solution of compounds 2−8 (100 mg, 1 equiv) in 6 mL dry dimethylformamide (DMF) was added potassium 1,3-dioxoisoindolin-2-ide (2 equiv). The reaction mixture was irradiated in a Biotage Initiator at 140 °C for 30 min (pre-stirring: 1 min, absorption level: normal). The mixture was then concentrated and purified by flash chromatography using 19:1 DCM/methanol to yield compounds 9−15 as yellow solids.
Compounds 16−22.
To a solution of compounds 9−15 (50 mg, 1 equiv) in 4 mL of ethanol was added hydrazine (5 equiv). The reaction mixture was irradiated in a Biotage Initiator at 180 °C for 60 min (pre-stirring: 1 min, absorption level: high). The mixture was then concentrated and purified by flash chromatography using 6:4 DCM/methanol to yield compounds 16−22 as yellow solids.
Fluorescent Probe Compounds 23−29.
To a solution of compounds 16−22 (20 mg, 1 equiv) and fluorescent compound FITC (2 equiv) in 3 mL DMF was added triethylamine (TEA, 4 equiv). The reaction mixture was stirred in the dark at room temperature overnight. The mixture was then purified with a Gilson to yield compounds 23−29 as orange solids.
Spectroscopic Characterization.
After preparing 20 μM of 23−29 aqueous solutions in 25 mM phosphate buffer (pH 7.2), absorption and emission spectra were measured with an Agilent 8453 UV−visible spectrophotometer and PTI 814 photomultiplier detection system at room temperature, respectively.
Receptor Binding Assay.
Binding affinity of the fluorescent probes using C6 glioma cell lysates were carried out as previously reported.3,23,32 All experiments were conducted in triplicate. Binding affinities were calculated with GraphPad Prism 6.
Saturation Curves.
The saturation curves for Kd were determined utilizing 0.50−100 nM of optical probe, followed by incubation with C6 glioma cell lysates. All experiments were conducted in triplicate and imaging was carried out in a Maestro imaging system (CRi) with the same exposure time for each sample. The saturation curve (Kd) was normalized and calculated with GraphPad Prism 6.
Displacement Experiments.
Displacement experiments using C6 glioma cell lysate were performed by incubating 0.50−100 nM optical probe for 30 min and subsequently adding 100 nM 2-(5,7-diethyl-2-(4-methoxyphenyl)pyrazolo-[1,5-a]pyrimidin-3-yl)-N,N-diethylacetamide for 2 h. The solutions were then filtered with a Brandel harvester and imaging was carried out in a Maestro imaging system (CRi) with the same exposure time for each sample.
Cell Culture.
C6 glioma cells were cultured in Dulbecco’s modified Eagle medium (DMEM), supplemented with 10% fetal bovine serum and 1% penicillin−streptomycin. Cells were maintained at 37 °C, 5% CO2 in a tissue culture incubator (Forma Scientific).
Cell Imaging.
C6 cells were incubated with 0.1 μM optical probe in DMEM medium for 2 h in a tissue culture incubator at 37 °C, at 5% CO2. At the end of the incubation, the cells were rinsed with PBS three times, before addition of 50 nM MitoTracker Red in serum-free DMEM and incubation for 20 min. Cells were rinsed with PBS three times, fixed, and imaged using a Zeiss LSM 510 inverted confocal microscope. In the displacement experiments, C6 cells were coincubated with 0.1 μM probe and 10 μM 2-(5,7-diethyl-2-(4-methoxyphenyl)-pyrazolo[1,5-a]pyrimidin-3-yl)-N,N-diethylacetamide in DMEM for 2 h.
Ligand Screening Assay.
C6 cell lysate was incubated with probe 29 and ligands (PK 11195 or VUIIS 1006) at 4 °C for 2 h in a 24-well plate. The solution was washed through a Brandel harvester and collected on filter paper. Filters were transferred and imaged with a CRi Maestro Imaging System. Fluorescence remaining on filters was measured and the binding affinity was calculated using Prism GraphPad software.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge funding from the National Institutes of Health (K25 CA127349, P30 CA068485, P30 DK058404, 1R01 CA163806), The Kleberg Foundation, The Lustgarten Foundation, and the Vanderbilt Center for Molecular Probes. Confocal imaging was performed in part through the use of the Vanderbilt Cell Imaging Shared Resource.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.bioconjchem.6b00711.
All poses generated from computational modeling and analytical data (NMR and HRMS) of all the synthesized compounds (PDF)
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Schulte ML, Dawson ES, Saleh SA, Cuthbertson ML, and Manning HC (2015) 2-Substituted Nγ-glutamylanilides as novel probes of ASCT2 with improved potency. Bioorg. Med. Chem. Lett 25, 113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Mankoff DA, and Bellon JR (2001) Positron-emission tomographic imaging of cancer: glucose metabolism and beyond. Seminars in radiation oncology 11, 16–27. [DOI] [PubMed] [Google Scholar]
- (3).Tang D, McKinley ET, Hight MR, Uddin MI, Harp JM, Fu A, Nickels ML, Buck JR, and Manning HC (2013) Synthesis and Structure−Activity Relationships of 5, 6, 7-Substituted Pyrazolopyrimidines: Discovery of a Novel TSPO PET Ligand for Cancer Imaging. J. Med. Chem 56, 3429–3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Tang D, Nickels ML, Tantawy MN, Buck JR, and Manning HC (2014) Preclinical Imaging Evaluation of Novel TSPO-PET Ligand 2-(5, 7-Diethyl-2-(4-(2-[18F] fluoroethoxy) phenyl) pyrazolo [1, 5-a] pyrimidin-3-yl)-N, N-diethylacetamide ([18F] VUIIS1008) in Glioma. Mol. Imaging Biol 16, 813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Tang D, Hight MR, McKinley ET, Fu A, Buck JR, Smith RA, Tantawy MN, Peterson TE, Colvin DC, Ansari MS, Nickels ML, and Manning HC (2012) Quantitative preclinical imaging of TSPO expression in glioma using N, N-diethyl-2-(2-(4-(2−18F-fluoroethoxy) phenyl)-5, 7-dimethylpyrazolo [1, 5-a] pyrimidin-3-yl) acetamide. J. Nucl. Med 53, 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Starosta-Rubinstein S, Ciliax BJ, Penney JB, McKeever P, and Young AB (1987) Imaging of a glioma using peripheral benzodiazepine receptor ligands. Proc. Natl. Acad. Sci. U. S. A 84, 891–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Nagler R, Ben-Izhak O, Savulescu D, Krayzler E, Akrish S, Leschiner S, Otradnov I, Zeno S, Veenman L, and Gavish M (2010) Oral cancer, cigarette smoke and mitochondrial 18 kDa translocator protein (TSPO)—In vitro, in vivo, salivary analysis. Biochim. Biophys. Acta, Mol. Basis Dis 1802, 454–461. [DOI] [PubMed] [Google Scholar]
- (8).Wyatt SK, Manning HC, Bai M, Bailey SN, Gallant P, Ma G, McIntosh L, and Bornhop DJ (2010) Molecular imaging of the translocator protein (TSPO) in a pre-clinical model of breast cancer. Mol. Imaging Biol 12, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Wyatt SK, Manning HC, Bai M, Ehtesham M, Mapara KY, Thompson RC, and Bornhop DJ (2012) Preclinical molecular imaging of the translocator protein (TSPO) in a metastases model based on breast cancer xenografts propagated in the murine brain. Curr. Mol. Med 12, 458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Hardwick M, Rone J, Han Z, Haddad B, and Papadopoulos V (2001) Peripheral-type benzodiazepine receptor levels correlate with the ability of human breast cancer MDA-MB-231 cell line to grow in scid mice. Int. J. Cancer 94, 322–327. [DOI] [PubMed] [Google Scholar]
- (11).Deane NG, Manning HC, Foutch AC, Washington MK, Aronow BA, Bornhop DJ, and Coffey RJ (2007) Targeted imaging of colonic tumors in smad3−/− mice discriminates cancer and inflammation. Mol. Cancer Res 5, 341–349. [DOI] [PubMed] [Google Scholar]
- (12).Maaser K, Hopfner M, Jansen A, Weisinger G, Gavish M, Kozikowski AP, Weizman A, Carayon P, Riecken E, Zeitz M, and Scherubl H (2001) Specific ligands of the peripheral benzodiazepine receptor induce apoptosis and cell cycle arrest in human colorectal cancer cells. Br. J. Cancer 85, 1771–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Han Z, Slack RS, Li W, and Papadopoulos V (2003) Expression of peripheral benzodiazepine receptor (PBR) in human tumors: relationship to breast, colorectal, and prostate tumor progression. J. Recept. Signal Transduction Res 23, 225–238. [DOI] [PubMed] [Google Scholar]
- (14).Le Fur G, Perrier ML, Vaucher N, Imbault F, Flamier A, Benavides J, Uzan A, Renault C, Dubroeucq MC, and Gueremy C (1983) Peripheral benzodiazepine binding sites: effect of PK 11195, 1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecar-boxamide: I. In vitro studies. Life Sci. 32, 1839–1847. [DOI] [PubMed] [Google Scholar]
- (15).Marangos PJ, Patel J, Boulenger JP, and Clark-Rosenberg R (1982) Characterization of peripheral-type benzodiazepine binding sites in brain using [3H] Ro 5−4864. Mol. Pharmacol 22, 26–32. [PubMed] [Google Scholar]
- (16).James ML, Fulton RR, Henderson DJ, Eberl S, Meikle SR, Thomson S, Allan RD, Dolle F, Fulham MJ, and Kassiou M (2005) Synthesis and in vivo evaluation of a novel peripheral benzodiazepine receptor PET radioligand. Bioorg. Med. Chem 13, 6188–6194. [DOI] [PubMed] [Google Scholar]
- (17).Zheng J, Winkeler A, Peyronneau M, Dolle F, and Boisgard R (2016) Evaluation of PET Imaging Performance of the TSPO Radioligand [18F] DPA-714 in Mouse and Rat Models of Cancer and Inflammation. Mol. Imaging Biol 18, 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Gargiulo S, Anzilotti S, Coda AR, Gramanzini M, Greco A, Panico M, Vinciguerra A, Zannetti A, Vicidomini C, Dollé F, Pignataro G, Quarantelli M, Annunziato L, Brunetti A, Salvatore M, and Pappatà S (2016) Imaging of brain TSPO expression in a mouse model of amyotrophic lateral sclerosis with 18F-DPA-714 and micro-PET/CT. Eur. J. Nucl. Med. Mol. Imaging 43, 1348–1359. [DOI] [PubMed] [Google Scholar]
- (19).Briard E, Zoghbi SS, Imaizumi M, Gourley JP, Shetty HU, Hong J, Cropley V, Fujita M, Innis RB, and Pike VW (2008) Synthesis and evaluation in monkey of two sensitive 11C-labeled aryloxyanilide ligands for imaging brain peripheral benzodiazepine receptors in vivo. J. Med. Chem 51, 17–30. [DOI] [PubMed] [Google Scholar]
- (20).Kreisl WC, Lyoo CH, McGwier M, Snow J, Jenko KJ, Kimura N, Corona W, Morse CL, Zoghbi SS, Pike VW, McMahon FJ, Turner RS, Innis RB, and the Biomarkers Consortium PET Radioligand Project Team (2013) In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain 136, 2228–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Ikawa M, Lohith TG, Shrestha S, Telu S, Zoghbi SS, Castellano S, Taliani S, Da Settimo F, Fujita M, Pike VW, and Innis RB (2017) 11C-ER176, a radioligand for 18-kDa translocator protein (TSPO), has adequate sensitivity to robustly image all three affinity genotypes in human brain. J. Nucl. Med 58, 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Manning HC, Goebel T, Thompson RC, Price RR, Lee H, and Bornhop DJ (2004) Targeted molecular imaging agents for cellular-scale bimodal imaging. Bioconjugate Chem. 15, 1488–1495. [DOI] [PubMed] [Google Scholar]
- (23).Buck JR, McKinley ET, Hight MR, Fu A, Tang D, Smith RA, Tantawy MN, Peterson TE, Colvin D, Ansari MS, Baldwin RM, Zhao P, Guleryuz S, and Manning HC (2011) Quantitative, preclinical PET of translocator protein expression in glioma using 18F-N-fluoroacetyl-N-(2, 5-dimethoxybenzyl)-2-phenoxyaniline. J. Nucl. Med 52, 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Li J, Schulte ML, Nickels ML, and Manning HC (2016) New structure−activity relationships of N-acetamide substituted pyrazolopyrimidines as pharmacological ligands of TSPO. Bioorg. Med. Chem. Lett 26, 3472–3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Tang D, Li J, Buck JR, Tantawy MN, Xia Y, Harp JM, Nickels ML, Meiler J, and Manning HC (2016) Evaluation of TSPO PET Ligands [18F] VUIIS1009A and [18F] VUIIS1009B: Tracers for Cancer Imaging. Mol. Imaging. Biol, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Trapani A, Palazzo C, de Candia M, Lasorsa FM, and Trapani G (2013) Targeting of the translocator protein 18 kDa (TSPO): a valuable approach for nuclear and optical imaging of activated microglia. Bioconjugate Chem. 24, 1415–1428. [DOI] [PubMed] [Google Scholar]
- (27).Denora N, Laquintana V, Trapani A, Suzuki H, Sawada M, and Trapani G (2011) New fluorescent probes targeting the mitochondrial-located translocator protein 18 kDa (TSPO) as activated microglia imaging agents. Pharm. Res 28, 2820–2832. [DOI] [PubMed] [Google Scholar]
- (28).Rosenholm JM, Gulin-Sarfraz T, Mamaeva V, Niemi R, Özliseli E, Desai D, Antfolk D, von Haartman E, Lindberg D, Prabhakar N, Näreoja T, and Sahlgren C (2016) Prolonged Dye Release from Mesoporous Silica-Based Imaging Probes Facilitates Long-Term Optical Tracking of Cell Populations In Vivo. Small 12, 1578–1592. [DOI] [PubMed] [Google Scholar]
- (29).Manning HC, Merchant NB, Foutch AC, Virostko JM, Wyatt SK, Shah C, McKinley ET, Xie J, Mutic NJ, Washington MK, LaFleur B, Tantawy MN, Peterson TE, Ansari MS, Baldwin RM, Rothenberg ML, Bornhop DJ, Gore JC, and Coffey RJ (2008) Molecular imaging of therapeutic response to epidermal growth factor receptor blockade in colorectal cancer. Clin. Cancer Res 14, 7413–7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Manning HC, Smith SM, Sexton M, Haviland S, Bai M, Cederquist K, Stella N, and Bornhop DJ (2006) A peripheral benzodiazepine receptor targeted agent for in vitro imaging and screening. Bioconjugate Chem. 17, 735–740. [DOI] [PubMed] [Google Scholar]
- (31).Peterson TE, and Manning HC (2009) Molecular imaging: 18F-FDG PET and a whole lot more. J. Nucl. Med. Technol 37, 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Kozikowski AP, Kotoula M, Ma D, Boujrad N, Tückmantel W, and Papadopoulos V (1997) Synthesis and biology of a 7-nitro-2, 1, 3-benzoxadiazol-4-yl derivative of 2-phenylindole-3-acetamide: a fluorescent probe for the peripheral-type benzodiazepine receptor. J. Med. Chem 40, 2435–2439. [DOI] [PubMed] [Google Scholar]
- (33).Laquintana V, Denora N, Lopedota A, Suzuki H, Sawada M, Serra M, Biggio G, Latrofa A, Trapani G, and Liso G (2007) N-Benzyl-2-(6, 8-dichloro-2-(4-chlorophenyl) imidazo [1, 2-a] pyridin-3-yl)-N-(6-(7-nitrobenzo [c][1, 2, 5] oxadiazol-4-ylamino) hexyl) acetamide as a new fluorescent probe for peripheral benzodiazepine receptor and microglial cell visualization. Bioconjugate Chem. 18, 1397–1407. [DOI] [PubMed] [Google Scholar]
- (34).Taliani S, Simorini F, Sergianni V, La Motta C, Da Settimo F, Cosimelli B, Abignente E, Greco G, Novellino E, Rossi L, Gremigni V, Spinetti F, Chelli B, and Martini C (2007) New fluorescent 2-phenylindolglyoxylamide derivatives as probes targeting the peripheral-type benzodiazepine receptor: design, synthesis, and biological evaluation. J. Med. Chem 50, 404–407. [DOI] [PubMed] [Google Scholar]
- (35).Taliani S, Da Pozzo E, Bellandi M, Bendinelli S, Pugliesi I, Simorini F, La Motta C, Salerno S, Marini AM, Da Settimo F, Cosimelli B, Greco G, Novellino E, and Martini C (2010) Novel irreversible fluorescent probes targeting the 18 kDa translocator protein: synthesis and biological characterization. J. Med. Chem 53, 4085–4093. [DOI] [PubMed] [Google Scholar]
- (36).Bai M, Wyatt SK, Han Z, Papadopoulos V, and Bornhop DJ (2007) A novel conjugable translocator protein ligand labeled with a fluorescence dye for in vitro imaging. Bioconjugate Chem. 18, 1118–1122. [DOI] [PubMed] [Google Scholar]
- (37).Bai M, Rone MB, Papadopoulos V, and Bornhop DJ (2007) A Novel Functional Translocator Protein Ligand for Cancer Imaging. Bioconjugate Chem. 18, 2018–2023. [DOI] [PubMed] [Google Scholar]
- (38).Tang D, Buck JR, Hight MR, and Manning HC (2010) Microwave-assisted organic synthesis of a high-affinity pyrazolopyrimidinyl TSPO ligand. Tetrahedron Lett. 51, 4595–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Abreu AR, Pereira MM, and Bayón JC (2010) Synthesis of new bis-BINOL-2,2′-ethers and bis-H8BINOL-2,2′-ethers evaluation of their Titanium complexes in the asymmetric ethylation of benzaldehyde. Tetrahedron 66, 743–749. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.