Abstract
The constitutive heparin+ (HP) mast cells (MCs) in mice express mouse MC protease (mMCP)-5 and carboxypeptidase A (mMC-CPA). The amino acid sequence of mMCP-5 is most similar to that of human chymase-1, as are the nucleotide sequences of their genes and transcripts. Using a homologous recombination approach, a C57BL/6 mouse line was created that possessed a disrupted mMCP-5 gene. The resulting mice were fertile and had no obvious developmental abnormality. Lack of mMCP-5 protein did not alter the granulation of the IL-3/IL-9-dependent mMCP-2+ MCs in the jejunal mucosa of Trichinella spiralis-infected mice. In contrast, the constitutive HP+ MCs in the tongues of mMCP-5-null mice were poorly granulated and lacked mMC-CPA protein. Bone marrow-derived MCs were readily developed from the transgenic mice using IL-3. Although these MCs contained high levels of mMC-CPA mRNA, they also lacked the latter exopeptidase. mMCP-5 protein is therefore needed to target translated mMC-CPA to the secretory granule along with HP-containing serglycin proteoglycans. Alternately, mMCP-5 is needed to protect mMC-CPA from autolysis in the cell's granules. Fibronectin was identified as a target of mMCP-5, and the exocytosis of mMCP-5 from the MCs in the mouse's peritoneal cavity resulted in the expression of metalloproteinase protease-9, which has been implicated in arthritis. In support of the latter finding, experimental arthritis was markedly reduced in mMCP-5-null mice relative to wild-type mice in two disease models.
Keywords: arthritis, carboxypeptidase, heparin, mast cell, serine protease
Introduction
Human chymase-1 (hCMA1)3 (1, 2) (GenBankTM Gene ID 1215) is an enzymatically active ∼30-kDa serine protease stored in the secretory granules of the constitutive mast cells (MCs) in most connective tissues at a concentration of ∼4.5 pg/cell ionically bound to heparin (HP)-containing serglycin proteoglycans (SGPGs). Although the functions of hCMA1 in MC-dependent inflammation and connective tissue remodeling have not been determined, some of its identified targets based on in vitro studies are angiotensin-I (3), endothelin-1 (4), IL-1β (5), TGF-β1 (6), and fibronectin (7).
hCMA1 usually is coordinately expressed at an 1:1 molar ratio with human MC carboxypeptidase A (hMC-CPA; also known as carboxypeptidase A3) (8). These hCMA1+/hMC-CPA+/HP+ MCs also contain large amounts of hTryptase-β in their granules. Despite its abundance in many populations of MCs, the hCMA1 transcript and protein are highly restricted. In that regard, only four ESTs of the 8.7 million in GenBankTM originated from its gene. Surprisingly, the hCMA1 gene on human chromosome 14q11.2 gave rise to the seven genes on mouse chromosome 14C3 that encode mouse MC protease-1 (mMCP-1), mMCP-2, mMCP-4, mMCP-5/mCma1 (9), mMCP-8, mMCP-9, and mMCP-10. The reason why mouse MCs express so many serine proteases in this family remains to be determined, but their restricted substrate specificities probably necessitated gene duplication to generate more proteases to give the mouse greater versatility and/or redundancy to carry out varied MC-dependent biologic responses. Whatever the explanation, the amino acid sequence of hCMA1 is much more similar to that of mMCP-5 than any other chromosome 14C3 family member, as is the nucleotide sequences of its gene and transcript. The safranin+ mouse MCs in the tongue, peritoneal cavity, and numerous other connective tissues contain substantial amounts of mMCP-5 protein (10–12), and the storage of this serine protease in the MC's secretory granules is exquisitely dependent on HP (13). The hMCs that increase in the synovium of those with rheumatoid arthritis express hCMA1 (14). Likewise, the corresponding MCs in arthritic WT C57BL/6 (B6) mice express mMCP-5 (15), thereby raising the possibility of adverse roles in this inflammatory disease.
mMCP-5 and mMC-CPA are coordinately expressed in numerous populations of in vivo and in vitro differentiated MCs as occurs in hMCs for their homologs, whereas mMCP-4 and the other chromosome 14C3 family members are not. Based on these and other data, the Human and Mouse Genome Consortiums concluded that mMCP-5 is the ortholog of hCMA1 (see GenBankTM Gene ID 17228). In support of this conclusion, the expression of its family members mMCP-1, mMCP-2, and mMCP-4 in mouse MCs is dominantly regulated by a novel post-transcriptional pathway (16) that is highly dependent on a repetitive UGXCCCC nucleotide sequence (where X is any nucleotide) in its 3′-untranslated region.4 This cis-acting element is not present in the 3′-untranslated regions of the mMCP-5 and hCMA1 transcripts. Not only is mMCP-5 also expressed early in the differentiation of MCs from their committed progenitors, every examined mouse MC line expressed this serine protease in contrast to its other family members.
Like hCMA1, mMCP-5 is translated as a 247-mer zymogen that contains a 19-mer signal peptide, a 2-mer Gly-Glu propeptide, and a C-terminal 226-mer mature catalytic domain. Crystallographic analysis of hCMA1 protein (17) and comparative 3D protein modeling studies of mMCP-5 (18) revealed these MC-restricted serine proteases have substrate specificities more restricted than that of pancreatic chymotrypsin because of substantial conformational changes caused by the insertion of 3 amino acids in the protease's N-terminal domain, the deletion of 7 amino acids in the C-terminal domain, and the loss of a disulfide bond. hCMA1 and mMCP-5 also have conserved faces on their surfaces that are positively charged at the granule pH of ∼5.5. mMCP-5 and hCMA1 use these conformation-dependent, positively charged domains to recognize the negatively charged HP-SGPGs in the MC's Golgi complex, thereby ensuring the targeting and retention of only properly folded protease in the cell's secretory granules. Once in that acidic intracellular compartment, pro-mMCP-5 and pro-hCMA1 are proteolytically activated by dipeptidyl peptidase I/cathepsin C (19). Because the HP-binding domains on the surfaces of mMCP-5 and hCMA1 are Lys- and Arg-rich, both serine proteases remain tightly bound to their HP-SGPGs when exocytosed from activated MCs into the extracellular matrix.
When evaluated using low molecular weight substrates in the absence of HP, the enzymatic specificity of naturally occurring hCMA1 is more similar to that of insect cell-generated mMCP-4 than insect cell-generated mMCP-5 (20). Problematic in this and all other functional studies carried out so far on recombinant mMCP-5, naturally occurring mMCP-5 is exocytosed from activated MCs tightly bound to HP-SGPGs (10) like the tryptase mMCP-6 (21). Thus, no free mMCP-5 exists in vivo. Because HP dominantly regulates the substrate specificity of mMCP-6 (22) and the rat homolog of mMCP-5 (23), the substrate preference of naturally occurring mMCP-5 in its physiologic state ionically bound to HP-SGPG has not been deduced. Likewise, the importance of mMCP-5 in varied MC-dependent diseases remains to be determined.
We now describe the generation of a mMCP-5-null B6 mouse line that has been backcrossed extensively against WT B6 mice to ensure that there is no other disrupted gene in the transgenic mouse line, as well as to ensure the resulting mice are histocompatible with WT B6 mice. We show that these mice can be used to better understand the function of mMCP-5. In that regard, we show mMCP-5 is essential for packaging of mMC-CPA protein in the MC's granule. We then show that fibronectin is a target of naturally occurring mMCP-5 when bound to HP-SGPG, that this serine protease regulates matrix metalloproteinase-9 (MMP-9)/gelatinase B expression in vivo, and that mMCP-5 has prominent adverse roles in two widely used arthritis models.
Results
Generation of a mMCP-5-null B6 Mouse Strain and Characterization of the MCs in These Transgenic Animals
Using a homologous recombination approach and the methodology described under “Experimental Procedures,” a mMCP-5-null B6 mouse strain was created (Fig. 1, A and B). Because most of the mMCP-5 ESTs in the GenBankTM database originated from the mouse's fetus (see UniGene Mm.1252), there was some concern that it might not be possible to obtain an adult mMCP-5-null mouse using a direct knock-out approach. Fortunately, mMCP-5-null mice were born at the expected Mendelian ratio, and these animals exhibited no obvious abnormality as they aged. mMCP-5 is therefore not essential for the fertility or development of the mouse when housed in a pathogen-free animal facility.
FIGURE 1.
Targeted disruption of the mMCP-5 gene in B6 mice. A, in the targeting vector, a portion of the mMCP-5 gene (including part of intron 2 and exon 3) was deleted. The Neor gene was then placed at the disrupted locus. Although the HSV-TK gene also was placed 3′ of the targeting construct in case negative selection had to be used to obtain ES cell clones with a disrupted allele of the mMCP-5 gene, only positive selection was used to obtain ES cell clones that underwent correct homologous recombination. B, a PCR approach was devised to expedite the genotyping of mMCP-5-null mice. Depicted are representative PCR analyses carried out on genomic DNA isolated from the tail of mMCP-5 knock-out (lane 2), heterozygous (lane 3), and WT mice (lane 4) using primers that reside 5′ and 3′of exon 3 of the mMCP-5 gene. The presence of the Neor gene in the mutated mMCP-5 gene causes the generation of a larger DNA fragment. Molecular weight markers are indicated in lane 1.
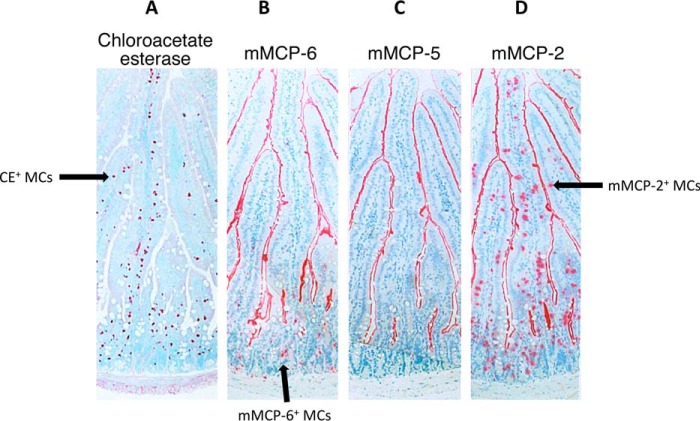
The MCs that increase in number in the jejunal mucosa during a Trichinella spiralis infection of WT mice preferentially express mMCP-2, whereas the constitutive MCs that reside in the jejunal muscle and submucosa preferentially express mMCP-5 and mMCP-6. We therefore investigated the consequences of disruption of the mMCP-5 gene on the different populations of MCs in the jejunum following helminth infection. As occurs in WT mice (24), the number of CE+/mMCP-2+ MCs markedly increased in the jejunal mucosa of helminth-infected mMCP-5-null mice (Fig. 2, A and D). Because the expression of mMCP-2 in this population of MCs is highly dependent on the T-cell cytokines IL-3 (25) and IL-9 (26), the mucosal MCs in our mMCP-5-null mice do not have defects in their IL-3 and IL-9 signaling pathways.
FIGURE 2.
Analysis of the MCs in the jejunum of helminth-infected mice. A–D, sections of jejunum from T. spiralis-infected mMCP-5-null mice at day 14 were evaluated cytochemically for the presence of CE+ MCs (A) or immunohistochemically for the presence of mMCP-6+ (B), mMCP-5+ (C), and mMCP-2+ (D) MCs. Arrows point to muscle/submucosa mMCP-6+ MCs (B) and mucosal CE+ (A)/mMCP-2+ (D) MCs present in mMCP-5-null mice (C).
In agreement with the genotyping data (Fig. 1), the constitutive MCs in the muscle and submucosa of mMCP-5-null mice lacked this chromosome 14C3 family member (Fig. 2C). Despite the absence of mMCP-5 protein, these muscle/submucosal MCs contained abundant amounts of mMCP-6 protein as assessed immunohistochemically (Fig. 2B). Thus, disruption of the mMCP-5 gene did not diminish the expression and granule accumulation of mMCP-2 or mMCP-6 in the different populations of MCs that reside in the mouse's jejunum. Because the constitutive MCs that reside in muscle, submucosa, and other connective tissues require KL and its receptor Kit/CD117 (for review, see Ref. 27), Kit/KL signaling pathways also were intact in mMCP-5-null MCs.
Although MC-committed progenitors could home to all analyzed tissues of the mMCP-5-null mouse, the constitutive MCs in the tongue (Fig. 3) and peritoneal cavity (data not shown) of the transgenic mice were poorly granulated when compared with WT mice. The MCs in the tongues of WT BALB/c and B6 mice constitutively contained high levels of mMCP-5 (Fig. 3h), mMCP-6 (Fig. 3i), and mMC-CPA (Fig. 3j) protein. In contrast, the corresponding MCs in the transgenic BALB/c and B6 mouse lines contained no mMCP-5 protein (Fig. 3b) and almost no mMC-CPA protein (Fig. 3e).
FIGURE 3.
Histochemical and immunohistochemical evaluation of tongue MCs in WT and mMCP-5-null mice. a–j, serial sections (a–c, d–f, and g–j) of tongue from mMCP-5-null (a–f) or WT (g–j) mice were stained with methylene blue (a, d, and g), anti-mMCP-5 antibody (b and h), anti-mMCP-6 antibody (c, f, and i), or anti-MC-CPA antibody (e and j). The MCs in the tongue of WT mice have granules filled with HP (g), mMCP-5 (h), mMCP-6 (i), and mMC-CPA (j). As expected, disruption of the mMCP-5 gene resulted in MCs unable to express mMCP-5. Although these MCs contained variable amounts of mMCP-6 (c and f), they were unable to store HP (a and d) and mMC-CPA (e) in their granules.
The passive cutaneous anaphylaxis (PCA) reaction in WT mice is dependent on the release of histamine from the IgE/antigen-activated mMCP-5+ MCs in the skin (28). Despite poor granulation, no significant differences were observed in the MC-mediated PCA reaction between mMCP-5-null and WT mice (data not shown). Disruption of the mMCP-5 gene therefore did not significantly affect histamine expression and its IgE/antigen-dependent release in response to MC activation in the skin. Thus, FcϵRI signaling pathways in mMCP-5-null MCs also was intact.
Because MCs could be generated in vitro from mMCP-5-null mice using IL-3 (Fig. 4A), disruption of the mMCP-5 gene did not affect the MC-committed progenitors in the bone marrow or their ability to respond to IL-3. These data were in agreement with the helminth infection data (Fig. 2). Although WT and mMCP-5-null mouse bone marrow-derived MCs (mBMMCs) contained high levels of the mMCP-6 and mMC-CPA transcripts (Fig. 4B), the latter mBMMCs lacked mMC-CPA protein (Fig. 4C). Disruption of the mMCP-5 gene did not affect the levels of mMCP-6 protein in mBMMCs (data not shown), as occurred in vivo in helminth-infected mice (Fig. 2B). The absence of mMCP-5 protein is therefore selective in terms of its effect on the accumulation of mMC-CPA in mBMMCs. WT mMCP-5+ mBMMCs do not express mMCP-1, mMCP-2, or mMCP-4 because of a post-transcriptional pathway that catabolizes these transcripts as fast as they are produced (16). Targeted inactivation of mMCP-5 gene did not result in a compensatory expression of its other chromosome 14C3 family members in these mBMMCs (Fig. 4B).
FIGURE 4.

Analysis of WT and mMCP-5-null mBMMCs. A, cytospins of mMCP-5-null (−/−) and WT mBMMCs (+/+) were stained with toluidine blue. B, RNA blot analyses were carried out to evaluate the levels of the transcripts that encode mMCP-1, mMCP-2, mMCP-4, mMCP-5, mMCP-6, mMC-CPA, and β-actin in mMCP-5+/+ (data not shown), mMCP-5−/+, and mMCP-5−/− mBMMCs. C, lysates of these three populations of mBMMCs also were evaluated for their mMCP-5 and mMC-CPA protein levels by SDS-PAGE/immunoblot analysis.
The characteristic staining properties of WT MCs when incubated with cationic dyes are due to the presence of negatively charged SGPGs localized within the secretory granules of MCs ionically bound to varied proteases (for review, see Ref. 29). mMCP-5-null mBMMCs failed to stain appreciably when incubated with toluidine or methylene blue (Fig. 4A). To evaluate the effects of mMCP-5 gene disruption on the biosynthesis of SGPGs, WT and mMCP-5-null mBMMCs were metabolically labeled with [35S]sulfate. The [35S]SGPGs were purified and subjected to CL-4B-Sepharose gel filtration chromatography. No significant differences were observed in the amount and size of the synthesized SGPGs by WT and mMCP-5-null mBMMCs (data now shown). These results indicated that mMCP-5 protein affects MC granulation by affecting the packaging and/or targeting of SGPGs to the MC's secretory granules rather than their biosynthesis.
When co-cultured with 3T3 fibroblasts, WT mBMMCs markedly increase their accumulation of mMCP-5 and mMC-CPA protein (30). In this in vitro system, mMC-CPA protein also failed to accumulate in the co-cultured mMCP-5-null mBMMCs (Fig. 5). These results indicated that mMCP-5 protein is needed to target translated mMC-CPA protein to the secretory granule or to protect the later neutral protease from autolysis. Because mMCP-5 is a major granule constituent of IL-3-developed mBMMCs, its absence in mMCP-5-null mBMMCs significantly affected the serine protease activity present in the lysates of mBMMCs before and after fibroblast co-culture, as measured by a [3H]diisopropyl fluorophosphate (DFP) binding assay (data not shown).
FIGURE 5.
mMC-CPA protein levels in mBMMCs before and after co-culture with fibroblasts. mMCP-5+/− (lanes 1 and 2) and mMCP-5−/− (lanes 3 and 4) were cultured in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of 3T3 fibroblasts for 2 weeks. Lysates of the resulting cell suspensions were then evaluated for their levels of mMC-CPA protein by SDS-PAGE/immunoblot analysis.
We previously showed that WT mMCP-4−/mMCP-5+/mMCP-6+ mBMMCs release an undefined serine protease that rapidly degrades fibronectin in vitro (31). As noted in Fig. 6, the relevant protease is mMCP-5 rather than mMCP-6 or another chromosome 14C3 family member. Because mMC-CPA is an exopeptidase with a limited substrate preference for C-terminal aromatic and hydrophobic amino acids (30), the Fig. 6 data were not a consequence of a lack of mMC-CPA protein in mMCP-5-null mBMMCs.
FIGURE 6.
Fibronectin is highly susceptible to naturally occurring mMCP-5 but not mMCP-6. Lysates of mMCP-5-null (lanes 1–3), mMCP-6-null (lanes 4–6), and WT (data not shown) B6 mBMMCs were incubated with fibronectin (Fib.) for 30 (lanes 1 and 4), 60 (lanes 2 and 5), and 120 (lanes 3 and 6) min. The resulting digests were then subjected to SDS-PAGE. Undigested fibronectin (lane 7) was incubated 120 min in the absence of mBMMC lysate as a negative control.
In support of the Fig. 6 data, there was a noticeable difference in the expression and/or post-translational processing of numerous proteins in the fibroblast·mBMMC co-cultures depending on whether the latter immune cells were generated from WT or mMCP-5-null mice (Fig. 7 and supplemental Table S1). For example, protein 38 was selectively expressed in the co-culture that contained WT mBMMCs. The MS fingerprint data of six peptides in its trypsin digest revealed this protein was mMC-CPA, thereby confirming the SDS-PAGE immunoblot data in Fig. 5. This finding also validated the two-dimensional difference gel electrophoresis (2D-DIGE) approach that documented the importance of mMCP-5 in the co-culture system.
FIGURE 7.
2D-DIGE analysis of protein expression in mBMMC·fibroblast co-cultures. Mouse 3T3 fibroblasts were co-cultured with WT or mMCP-5-null mBMMCs. The proteins in the resulting lysates of the two co-cultures were differentially labeled by Cy2 and Cy3, pooled, and subjected to 2D-DIGE. The green-labeled proteins were only found in the co-cultures that contained WT mBMMCs, whereas the red-labeled proteins were only found in the co-cultures that contained mMCP-5-null mBMMCs. Yellow-labeled proteins were found in both. Those differentially expressed proteins selected for MALDI-ToF MS proteomic analysis (also see supplemental Table S1) are indicated.
The observation that three of the differentially expressed proteins in the mBMMC·fibroblast co-cultures were isoforms of serpinB1 (supplemental Table S1) suggests that this protease inhibitor is a naturally occurring inactivator of mMCP-5. Mouse 3T3 fibroblasts lose their contact inhibition and increase their cellular proliferation when co-cultured with WT mBMMCs (32). Interestingly, some of the other differentially expressed and/or post-translationally modified proteins in the two co-cultures corresponded to those intracellular proteins that participate in cellular adhesion and/or proliferation (e.g. vimentin, scinderin, and vinculin). The latter data raise the possibility that mMCP-5 is one of the factors constitutively released from the co-cultured WT mBMMCs that regulates varied intracellular processes in the co-cultured fibroblasts.
mMCP-5-dependent Expression of Biologically Active Proteins in the Peritoneal Cavity
The constitutive MCs in the peritoneal cavity of WT mice degranulate when exposed in vivo or in vitro to compound 48/80 (C48/80). This reagent was therefore injected into the peritoneal cavities of WT and mMCP-5-null B6 mice. 30 and 120 min post-challenge, the peritoneal cavity fluids were harvested, and the levels of 96 biologically active proteins in the samples were determined at each time point (Fig. 8 and supplemental Table S2). Differences were noted in the levels of eotaxin-2, MIP-1, p-selectin, Vcam-1, and insulin-like growth factor-1. However, the most impressive data were markedly reduced levels of MMP-9/gelatinase B in the peritoneal cavity fluid of mMCP-5-null mice 2 h after exposure to C48/80 relative to similarly treated WT mice.
FIGURE 8.
Expression of biologically active proteins in the peritoneal cavities of WT and mMCP-5-null mice after MC activation. A, RayBiotech mouse G series 1000 antibody arrays III and IV were used to evaluate the presence of 96 biologically active proteins in the peritoneal cavity exudates of WT (samples 1 and 2) and mMCP-5-null (samples 3 and 4) B6 mice 30 min (samples 1 and 3) and 120 min (samples 2 and 4) after these animals were given C48/80. B, MMP-9 levels were selectively increased in the peritoneal cavity of WT mice 2 h after given C48/80 (also see supplemental Table S2).
Inflammatory Arthritis
Whether mice were subjected to the K/BxN mouse serum transfer (Fig. 9) or the methylated albumin (meBSA)/IL-1β disease models (Fig. 10), arthritis was significantly reduced in mMCP-5-null B6 mice relative to WT B6 mice. The clinical index (Fig. 9A), ankle thickness (Fig. 9B), inflammation (Fig. 9C), bone erosion (Fig. 9C), cartilage destruction (Fig. 9C), and aggrecan proteoglycan loss from the diseased cartilage (Fig. 9D) were all significantly reduced when mMCP-5-null B6 mice were subjected to the K/BxN arthritis model relative to similarly treated WT B6 mice. Similar data were obtained when the two mouse strains were subjected to the meBSA/IL-1β arthritis model (Fig. 10, A–C).
FIGURE 9.
Inflammatory K/BxN arthritis in WT and mMCP-5-null mice. A–D, WT (●) (n = 15) and mMCP-5-null (▵) (n = 15) B6 mice were subjected to K/BxN arthritis. Arthritis was significantly reduced in mMCP-5-null B6 mice relative to WT B6 as assessed by clinical index (A), ankle thickness (B), inflammation (C), bone erosion (C), cartilage destruction (C), and aggrecan proteoglycan loss from the diseased cartilage (D).
FIGURE 10.
Inflammatory meBSA/IL1β arthritis in WT and mMCP-5-null mice. A–C, meBSA/IL-1β arthritis were induced in 10 knees of 5 WT B6 mice and 10 knees of 5 mMCP-5-null B6 mice. A, representative histochemistry data are shown. B, cellular inflammation of the joint space, pannus size, and the extent of cartilage and bone erosion were all significantly reduced in the diseased mMCP-5-null mice relative to the diseased WT mice. C, the mean total scores for the respective groups were 17.8 versus 12.0 (WT versus 5-KO). *, p < 0.05; **, p < 0.01.
Discussion
Of the seven mouse MC chromosome 14C3 family members, mMCP-5 (9) is the closest homolog of hCMA1 (1) at the gene, transcript, and protein levels. A homologous recombination approach therefore was used to disrupt the mMCP-5 gene in B6 mice (Fig. 1) to evaluate the function of this mouse chromosome 14C3 family member in disease models. WT mice experience a pronounced MC hyperplasia in their intestinal mucosa during a helminth infection that peaks at day 14 (12). These chloroacetate esterase (CE)+ MCs express mMCP-2 (33), in contrast to the constitutive mMCP-5+/mMCP-6+ MCs that reside in the muscle/submucosa layer of the jejunum. Large numbers of IL-3/IL-9-dependent CE+/mMCP-2+ MCs accumulated in the intestinal mucosa of the helminth-infected mMCP-5-null animals (Fig. 2). Likewise, mMCP-6+ MCs were found in the submucosa/muscle region of the jejunum of these transgenic mice. In contrast, the constitutive HP+ MCs in the tongue (Fig. 3), skin, and other connective tissues of mMCP-5-null mice were poorly granulated when compared with their WT littermates. Despite these data, mMCP-5-null B6 mice could mount a MC-dependent PCA reaction, thereby revealing no significant defect in histamine expression and its FcϵRI-dependent release.
As assessed by immunohistochemistry, tongue MCs in mMCP-5-null mice contained mMCP-6 protein (Figs. 2 and 3) but lacked mMCP-5 and mMC-CPA protein (Fig. 3). Disruption of the mMCP-5 gene therefore led to a selective loss of mMC-CPA protein in this population of MCs. In support of these data, Feyerabend and co-workers (34) discovered that targeted inactivation of the mMC-CPA gene adversely affected the accumulation of mMCP-5 protein in the granules of connective tissue-type MCs.
The N-deactylase-N-sulfotransferase-2 (NDST-2) gene encodes an enzyme required for the biosynthesis of fully sulfated HP in MCs. When the NDST-2 gene was disrupted in mice, the constitutive MCs in the resulting transgenic animals could not store appreciable amounts of mMCP-5 and mMC-CPA protein in their secretory granules (13). Although NDST-2-null mBMMCs also could not store mMCP-5 and mMC-CPA in their granules, the levels of mMCP-6 protein in these in vitro differentiated MCs were comparable with that in WT mBMMCs.
The histochemical, biochemical, and morphological similarities of the MCs in mMCP-5- and NDST-2-null B6 mice suggested a critical role for mMCP-5 in regulating the biosynthesis and/or the packaging and targeting of SGPGs and/or mMC-CPA in the secretory granules of constitutive MCs. Consistent with this conclusion, in vivo differentiated MCs (Fig. 3) and in vitro differentiated mBMMCs from mMCP-5-null mice (Fig. 4A) showed poor granulation when compared with the mBMMCs from their +/+ and +/− littermates.
The ability of WT MCs to be stained by varied cationic dyes is due to the presence of negatively charged SGPGs. No significant differences were observed in the SGPGs synthesized by WT and mMCP-5-null mBMMCs employing a biochemical approach. As assessed by RNA blot analysis, mBMMCs from mMCP-5-null and WT mice contained high levels of the mMC-CPA transcript (Fig. 4B). Despite these mRNA data, mMCP-5-null mBMMCs lacked mMC-CPA protein (Fig. 4C). Co-culture of these immature MCs on 3T3 fibroblasts also fail to induce mMC-CPA protein accumulation in mMCP-5-null mBMMCs in contrast to mMCP-5+/− and mMCP-5+/+ mBMMCs (Fig. 5).
mMCP-5 and mMC-CPA are coordinately expressed in mouse MCs, as is hCMA1 and hMC-CPA in hMCs. Like pancreatic CPA, mMC-CPA has a 94-residue propeptide that is predicted to cover its active site. Pancreatic CPA forms a complex with chymotrypsinogen C that is dependent on its propeptide (35). Based on these data, it is likely that the pro-mMCP-5 and pro-mMC-CPA form a similar binary complex in the MC's endoplasmic reticulum. Because HP biosynthesis is completed in the Golgi, it is presumed that the pro-mMCP-5·pro-mMC-CPA complex comes in contact with HP-SGPGs just before the macromolecular complex is targeted to the cell's secretory granule. If either mMCP-5, mMC-CPA, or HP-SGPG is absent, the 3-mer complex cannot accumulate in the cell's granules. This novel packaging mechanism presumably evolved to ensure the close juxtaposition of positively charged mMCP-5 and mMC-CPA in a 1:1 molar ratio along the SGPG's negatively charged HP glycosaminoglycan chains as likely occurs in hCMA1+/hMC-CPA+/HP-SGPG+ hMCs. The ability of both proteases to remain tightly bound to HP after the macromolecular complex is exocytosed from activated MCs ensures mMCP-5 and mMC-CPA stay close to one another in the extracellular matrix. The large size of the macromolecular complex also sterically prevents the inactivation of mMCP-5 by α2-macrogloblin and possibly other protease inhibitors.
mMCP-5 and mMC-CPA are major granule constituents of in vitro IL-3-dependent mBMMCs. The absence of mMCP-5 significantly affected the endopeptidase activity in lysates of mMCP-5-null mBMMCs, as assessed by the [3H]DFP assay. This dramatic decrease in serine protease activity also abolished fibronectin degradation by mMCP-5-null mBMMCs lysates (Fig. 6). It was previously shown that an undefined mMCP can degrade fibronectin in a HP-dependent manner based on the observation that the digestion of fibronectin was reduced in NDST-2-null MCs (36). Although it was concluded that the relevant protease probably was mMCP-4, WT mBMMCs lack mMCP-4 mRNA and protein (37) (Fig. 4B) even though these MCs contain an undefined serine protease that rapidly degrades fibronectin (31) (Fig. 6). It is now apparent that the relevant protease in IL-3-developed mBMMCs is mMCP-5 rather than mMCP-4. Nevertheless, we cannot rule out the possibility that mMCP-4 and mMCP-5 have overlapping bioactivities in other populations of MCs as occurs for the MC's tetramer-forming tryptases, namely mMCP-6 and mMCP-7. In that regard, the α chain of fibrinogen is susceptible to both mMCP-6 (38) and mMCP-7 (39) in vivo, even though these tryptases have somewhat different substrate preferences (22).
Histamine+/hTryptase-β+/hCMA1+ MCs are present in increased numbers at the sites of cartilage erosion in the joints of patients with rheumatoid arthritis (14), and it has been concluded they comprise up to 5% of the immune cells in the diseased synovial tissue (40). Histamine and hTryptase-β have been detected in the synovial fluids of patients with arthritis (41). Despite the considerable amount of circumstantial data implicating MCs in human arthritis, it was not possible to conclusively determine the adverse roles of these immune cells in experimental arthritis when MC-deficient W/Wv, Wsh/Wsh, Sl/Sld, mMCP-5-Cre, or CPA3Cre/+ mice were used. One of the problems using Kit/KL-defective W/Wv, Wsh/Wsh, Sl/Sld mice in MC in vivo studies is that these animals have abnormalities in neutrophils and other cell types. In regard to CPA3Cre/+ (42) and mMCP-5-Cre (43) mice, data interpretation is highly dependent on the efficiency of Cre-mediated MC ablation in each experimental animal.
An even greater problem using MC-deficient mice in a biologic system is that these immune cells release >50 biologically active factors, many of which have contrasting activities in vivo. For example, activated MCs produce cytokines that promote (e.g. IL-1β and TNF-α) and inhibit (e.g. IL-4 and IL-13) arthritis. Further complicating data interpretation from MC-deficient mice, these immune cells express activating (e.g. FcϵRI, FcγRI, protease-activated receptor 2, toll-like receptor 4, and CD88) and inhibitory (e.g. gp49B1/Lilrb4, CD200R, CD300a, CD300f, and FcγRIIB) receptors. The latter inhibitory receptors would not have been identified had investigators relied solely on MC-deficient mice in their studies. We therefore concluded the only way to definitively determine whether mMCP-5 participates in a disease model is to selectively knock out its gene using a homologous recombination approach. Although arthritis was reduced in mMCP-6-null B6 mice, inflammation and joint destruction were less pronounced in HP-deficient mice (44–47). The latter data suggested that an unidentified HP-binding endopeptidase released from the activated MCs in the diseased joint contributes significantly to experimental arthritis.
MMP-9 has been implicated in arthritis (48). Because the level of MMP-9 was reduced in the peritoneal cavities of C48/80-treated mMCP-5-null mice (Fig. 7) and because the MCs in the synovium express mMCP-5 (15), mMCP-5-null mice were subjected to two widely used inflammatory arthritis models. As noted in Figs. 9 and 10, mMCP-5 had prominent adverse roles in both disease models.
It is likely that mMCP-5 participates in arthritis by multiple mechanisms that are not mutually exclusive. Insulin and many other peptide hormones are generated by a two-step proteolytic processing event involving serine protease-dependent cleavage of the pro-hormone followed by carboxypeptidase-dependent maturation of the biologically active precursor hormone. The observation that mMCP-5 and mMC-CPA are co-expressed in mice at a 1:1 molar ratio and are exocytosed tightly bound to HP-SGPG (as occurs for their human homologs) raises the possibility that the tertiary complex is used to create biologically active factors that participate in arthritis and other MC-dependent diseases. This could be explored in the future by evaluating other MC-associated diseases.5
The finding that the levels of MMP-9 are increased in the peritoneal cavities of WT mice but not mMCP-5-null mice when these animals are given C48/80 (Fig. 8) raises the alternate possibility that mMCP-5 and hCMA1 regulate the expression and/or activation of certain MMPs that participate in connective tissue remodeling. In regard to this hypothesis, an undefined chymase in dog mastocytoma activates pro-MMP-1 and pro-MMP-3 (49). Moreover, mMCP-6 activates pro-MMP-3 and pro-MMP-13, which are additional MMPs implicated in arthritis (50).
Fragments of fibronectin are often present in abundance in the synovial fluid of those with arthritis (51), and the fibronectin·arthritis link has been well established. The finding that fibronectin is a target of mMCP-5 (Fig. 6) raises the additional possibility that mMCP-5 disrupts fibronectin·integrin signaling events in the body. In this regard, it is noteworthy that the tetramer-forming tryptases mMCP-6, mMCP-7, and hTryptase-β alter fibrinogen·integrin signaling events because the α chain of fibrinogen is a target of these tryptases (38, 39).
There is no evidence that mouse or human MC-CPA participates in arthritis, and the fibronectin data (Fig. 6) are not a consequence of mMCP-5-dependent expression of mMC-CPA in mBMMCs. Nevertheless, we cannot rule out the more remote possibility that mMCP-5 participates in arthritis in vivo in a more indirect manner by impacting the expression of mMC-CPA or another factor in MCs.
Whatever the mechanism by which mMCP-5 participates in arthritis, transgenic mice that lack this homolog of hCMA1 have no developmental abnormality. Moreover, loss of mMCP-5 does not negatively impact the expansion and phenotype of the MCs in the animal's jejunum. Importantly, the MCs in our mMCP-5-null mice continue to express mMCP-6 (the mouse homolog of hTryptase-β), which is a tetramer-forming tryptase that has beneficial roles in innate immunity, acquired immunity, and the prevention of life-threatening fibrin-platelet clots internally. In terms of the human relevance of our mouse data, anti-hCMA1 therapy might be effective in the treatment of those with arthritis without causing serious adverse side effects as probably would occur in humans given a hTryptase-β inhibitor.
Experimental Procedures
Generation of a mMCP-5-null B6 Mouse Strain Using a Homologous Recombination Approach
A 129/Sv mouse genomic library was screened with a mMCP-5-specific cDNA (9) to isolate the 3.2-kb 5-exon mMCP-5 gene and its 5′ and 3′ flanking sequences. A targeting construct (Fig. 1A) was created in which the gene's second intron and most of its third exon were replaced by the Neomycin resistance (Neo) gene. If homologous recombination takes place in the transfected ES cells, the resulting mutated mMCP-5 gene will lack codons that encode critical amino acids in this serine protease, including the catalytic triad residue Asp110. Thus, no functional mMCP-5 protein can be expressed even if truncated transcripts and/or proteins are generated in the created transgenic animals.
In the targeting construct, 5.8 and 1.7 kb of genomic sequence at the mMCP-5 locus was inserted 5′ and 3′ of the Neo gene, respectively. The disrupted mMCP-5 gene was inserted into the vector pBS-SK/HSVtk immediately upstream of the HSV-TK (herpes simplex virus thymidine kinase) gene. J1 mouse ES cells were transfected with the targeting construct, and the resulting cells were cultured in the presence of geneticin to obtain cloned cells. Genomic DNA (∼20 μg) was prepared from the clones and incubated with the restriction enzyme StuI (New England Biolabs, Beverly, MA). The digests were fractionated on 1% agarose gels, the separated DNA fragments were blotted onto nylon membranes, and the resulting blots were exposed to a radiolabeled probe corresponding to the 3′ end of the mMCP-5 gene outside of the targeting construct to identify those clones that possessed the disrupted allele.
One of the obtained ES cell clones was microinjected into mouse blastocysts, which were then implanted into pseudopregnant foster mothers to generate the initial chimera. Using a standard mating approach, mMCP-5+/− mice were backcrossed 10 times with WT B6 mice and 3 times with WT BALB/c mice. A PCR approach was used to genotype the backcrossed animals to create the final B6 mouse strain used in most experiments. In this screening assay, samples of tail genomic DNA were subjected to 30 cycles of PCR using primers (5′-TCACTTGTCGGGATCTAC-3′ and 5′-GGTTAGCTTGGCTTTCTC-3′) that flank exon 3 of the endogenous mMCP-5 gene. Each cycle of the PCR consisted of a denaturation step at 94 °C for 1 min, an annealing step at 51 °C for 1 min, and an extension step of 72 °C for 2 min. Heterozygotes containing the WT and disrupted alleles of the mMCP-5 gene yielded DNA fragments of ∼1 and 1.5 kb, respectively, in this PCR-based assay (Fig. 1B). All animal studies were approved by our Institutions' Animal Care and Use Committees.
Histochemistry, Enzyme Cytochemistry, and Immunohistochemistry
mBMMCs were obtained by culturing isolated bone marrow cells from mMCP-5-null B6 mice and their WT littermates for at least 3 weeks in IL-3 enriched medium (52). Some of the resulting mBMMCs were then co-cultured for an additional 2 weeks with Swiss albino mouse 3T3 fibroblasts (line CCL-92; American Type Culture Collection), as previously described (53). This was done to induce granule maturation and the accumulation of mMC-CPA protein (30).
For histological examination, cytocentrifuge preparations of mBMMCs and 1.5-μm-thick glycolmethacrylate sections of tissue from different organs of WT and mMCP-5-null mice were stained with 0.5% solutions of methylene or toluidine blue in 0.6 m HCl. mMCP-5 and its other chromosome 14C3 family members cleave the substrate naphthol AS-D chloroacetate (Sigma-Aldrich) (12). Those MCs that contained substantial amounts of CE activity were identified cytochemically (12). Tissue sections and cultured mBMMCs also were incubated with rabbit anti-peptide antibodies specific for mMCP-5 (10) and other MC-restricted granule proteases, as described in our earlier publications. To confirm the immunohistochemical and enzyme cytochemical data, lysates of mBMMCs developed from WT and mMCP-5-null B6 mice were electrophoresed in 12% SDS-PAGE gels. The resulting protein blots were probed with anti-mMCP-5 antibody.
DFP is an active site inhibitor of the serine proteases in the MC's secretory granules (31). Lysates of WT and mMCP-5-null mBMMCs were therefore suspended in 33 μl of 0.15 m NaCl, 20 mm Tris-HCl pH 7.4, buffer supplemented with 8 μCi of [3H]DFP (4 Ci/nmol; Ci = 37 Gbq; Amersham Biosciences). The treated samples were incubated for 60 min at 37 °C. SDS-PAGE loading buffer was added, and each sample was boiled for 5 min before the [3H]DFP-labeled proteins were subjected to SDS-PAGE. The resulting gels were treated with EN3HANCE, dried, and exposed to X-ray film.
RNA Analyses
Total RNA was isolated from IL-3-dependent mBMMCs developed from WT and mMCP-5-null B6 mice. The RNA samples were applied to 1.2% agarose-formaldehyde gels. The gels were subjected to electrophoresis for 17 h, the separated RNA were transferred to nylon membranes, and the resulting blots were analyzed with gene-specific probes for the transcripts that encode mMCP-1, mMCP-2, mMCP-4, mMCP-5, mMCP-6, mMC-CPA, and β-actin. The cDNA probes used in these analyses were random primed with [32P]dCTP using the Rediprime kit (GE Healthcare-Amersham Biosciences). They were hybridized to the RNA blots at 65 °C for 2 h in QuikHyb hybridization solution (Stratagene, San Diego, CA). The blots were washed at room temperature in 2× SSC containing 0.1% SDS and then at 60 °C in 0.2× SSC containing 0.1% SDS before exposure to BIOMAX film. RT-PCR assays using primer sets from Qiagen were employed to confirm the RNA blot data.
Evaluation of the Ability of the Serine Proteases in WT and mMCP-5-null mBMMCs to Cleave Fibronectin and Other Fibroblast Proteins
We previously showed (31) that lysates of mBMMCs developed from WT mice contain an undefined serine protease that rapidly degrades fibronectin in vitro, thereby impacting fibronectin·intregin signaling pathways. mMCP-5 (9) and mMCP-6 (54) are the most abundant serine proteases in B6 mBMMCs, and fibronectin is highly susceptible to hCMA1 in vitro (55). IL-3-developed mBMMCs were chosen for investigation because they do not express mMCP-1, mMCP-2, mMCP-4, mMCP-7, mMCP-8, mMCP-9, or mMCP-10. Because of these data, 106 mBMMCs from mMCP-5-null, mMCP-6-null (56), and WT B6 mice were sonicated separately on ice in 1 ml of serum-free Hanks' balanced salt solution to liberate their enzymatically active proteases. Samples of the lysates were subjected to a fibronectin susceptibility assay (31) in which 20 μl of a 1 μg/μl solution of fibronectin (Sigma-Aldrich) was incubated 30 min at 37 °C with 2, 4, and 10 μl of each mBMMC lysate. Fifteen μl of each digest was then added to an equal volume of 2× SDS-PAGE buffer supplemented with DTT. After the samples were boiled to denature their proteins, they were subjected to SDS-PAGE. The substrate:enzyme ratio was >100:1 in these in vitro experiments.
We previously showed that complex bilateral interactions occur when WT mBMMCs are co-cultured for 2 weeks with mouse fibroblasts (53). Relevant to the present study, the amount of mMC-CPA protein markedly increased in the co-cultured WT mBMMCs (30). Because these MC·fibroblast interactions are highly dependent on Kit/KL (57) and IL-33/IL1RL1 (58) signaling pathways on the surfaces of the two cell types, they are adherent to one another and to the extracellular matrix-coated plastic-culture dishes. To begin to determine the roles of mMCP-5 in these co-cultures, WT and mMCP-5-null mBMMCs were co-cultured with mouse 3T3 fibroblasts for 2 weeks. Although the MCs were not induced to undergo exocytosis in these experiments via the cell's high affinity IgE receptors, we previously showed biologically active factors were constitutively transferred between the two cell types (53, 59).
After 2 weeks, the resulting co-cultures were washed extensively with PBS to remove contaminating fetal calf serum proteins from the culture medium. Denaturing buffer (7 m urea, 2 m thiourea, 4% CHAPS, and 30 mm Tris-HCl, pH 8.8) containing a protease inhibitor mixture (Sigma-Aldrich) was added. The liberated cell- and extracellular matrix-associated proteins were enriched by TCA precipitation. They were then subjected to Applied Biomics's 2D-DIGE/MALDI-ToF MS proteomic analysis, using the company's methodology. Briefly, the proteins in the mBMMC·fibroblast lysate samples were labeled with either Cy3 or Cy2. Approximately equal amounts of the tagged proteins in the two samples were pooled and subjected to 2D-DIGE. After electrophoresis, the resulting gel of the separated proteins was scanned using a Typhoon image scanner at different excitation wavelengths. The fluorescent green spots in the scanned gel corresponded to proteins only present in the WT mBMMC·fibroblast co-culture. In contrast, the fluorescent red spots corresponded to proteins only present in the mMCP-5-null mBMMC·fibroblast co-culture. Fluorescent yellow spots correspond to proteins present in both co-cultures. Differentially expressed proteins were picked from the 2D gel using an Ettan Spot Picker, exhaustively digested by trypsin, and then identified whenever possible by MALDI-ToF MS using a peptide-mass fingerprinting approach that is based on the predicted molecular weights of the trypsin-generated peptides of every known mouse protein.
SGPG Expression in mBMMCs
WT mBMMCs increase their biosynthesis and granule accumulation of HP-SGPGs when co-cultured with fibroblasts (53). To evaluate the consequences of disruption of the mMCP-5 gene on the expression of SGPGs in MCs, mBMMCs from mMCP-5-null and WT B6 mice were evaluated before and after co-culture with 3T3 fibroblasts. The SGPGs produced by both populations of mMCP-5-null mBMMCs were characterized, as previously described for WT mBMMCs (53). Briefly, the starting mBMMCs and the adherent cells in the mBMMC·fibroblast co-cultures were radiolabeled in culture medium supplemented with 50 μCi of [35S]sodium sulfate/ml. After this step, each culture was washed and then cultured for an additional 2 h in normal medium. The mBMMC·fibroblast co-cultures were then exposed to a solution containing 0.25% trypsin and 1 mm EDTA for 5 min to remove residual trypsin-susceptible, fibroblast-derived [35S]proteoglycans (namely radiolabeled syndecans and glypicans) in the co-culture.
After the detached fibroblasts and MCs were centrifuged, the cell pellets were disrupted by the addition of 150 μl of 1% Zwittergent 3-12 (Calbiochem), followed by 1350 μl of 4 m guanidine hydrochloride. The liberated intracellular [35S]SGPGs were subjected to CsCl density gradient centrifugation (60). Samples of the purified mBMMC-derived [35S]SGPGs before and after fibroblast co-culture were incubated with chondroitinase ABC or nitrous acid to evaluate their relative chondroitin sulfate and HP contents, respectively, as previously described. Other samples of the intracellular [35S]SGPGs produced by the mMCP-5-null and WT mBMMCs were subjected to Sepharose CL-4B column chromatography to evaluate their overall sizes.
mMCP-5-dependent Expression of Biologically Active Proteins in the Peritoneal Cavity
C48/80 induces the release of the MC's preformed mediators (61), including mMCP-5. To evaluate the consequences of the exocytosis of mMCP-5 in vivo, C48/80 was injected into the peritoneal cavities of WT and mMCP-5-null mice, as described by Fawcett (62). 30 or 120 min latter, the treated mice were sacrificed, and PBS (10 ml) was injected into the peritoneal cavity of each mouse. The resulting peritoneal cavity exudates were subjected to RayBio® mouse G series 1000 antibody arrays to measure the levels of 96 biologically active proteins. To minimize false positives and negatives, the level of each protein in the four samples was evaluated twice.
Helminth Infection, PCA, and Arthritis
To evaluate the consequence of disrupted mMCP-5 expression on the IL-3/IL-9-dependent mMCP-2+ population of MCs that increase in number in the jejunal mucosa of helminth-infected mice, mMCP-5-null and WT B6 mice were each infected orally with 400 freshly isolated, stage 3 T. spiralis larvae as previously described (12). The MCs residing in the mucosa and submucosa/muscle areas of the jejunum were phenotyped at the height of the mastocytosis at day 14.
For IgE-dependent PCA reactions, 20 μl of PBS with or without 20 ng of anti-dinitrophenyl IgE (Sigma-Aldrich) was injected into the separate ears of each mMCP-5-null and WT mouse, as previously described for similar studies carried out on HP-deficient transgenic B6 mice created by targeted inactivation of the NDST-2 gene (13). Twenty-four h later, a 100-μl solution of 0.5% Evans blue containing 100 μg of dinitrophenyl-labeled albumin was injected into the tail vein of each animal. The visual uptake of Evans blue into the ears was compared ∼30 min later.
The HP+ MCs in the arthritic synovium of WT mice express mMCP-5 (46, 47). Thus, mMCP-5-null B6 mice also were subjected to the K/BxN mouse serum transfer (44, 45) and meBSA/IL-1β (47) arthritis models using the methodology described in our earlier publications.
Author Contributions
R. L. S., H. P. M., L. A. W., K. S., and G. W. W. performed the experiments. R. L. S., P. M. H., and S. A. K. planned the experiments, analyzed the data, wrote the manuscript, and funded the research.
Supplementary Material
Acknowledgments
We thank Drs. Michael Gurish and Daniel Friend (Brigham and Women's Hospital, Boston, MA) for their assistance in the helminth infection and immunohistochemistry studies of WT and mMCP-5-null mice.
This work was supported in part by funds from the St. George and Sutherland Medical Research Foundation (to R. L. S. and S. K.), the Harvard Club of Australia Foundation (to R. L. S., S. K., and P. M. H.), the Australian Research Council (to P. M. H. and R. L. S.), and the University of Newcastle (to R. L. S.); National Institutes of Health Grants DK084171 (to G. W. W.) and AI059746 (to R. L. S.); a fellowship from the National Health and Medical Research Council of Australia (to P. M. H.); the Brawn Fellowship from the University of Newcastle (to P. M. H.); and a fellowship from Fundação de Amparo a Pesquisa do Estado de São Paulo-FAPESP (to L. A. W.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.

This article contains supplemental Tables S1 and S2.
S. M. Lyons, R. L. Stevens, P. M. Hansbro, and P. Ivanov, unpublished observation.
In preliminary studies carried out on our mMCP-5-null mice that had been backcrossed only four times, mMCP-5 also had adverse roles in experimental ischemia-reperfusion injury (63) and epidermal burn injury (64), thereby confirming the biologic importance of this homolog of hCMA1. The generation of the mMCP-5-null mouse line was not described in either of those earlier studies.
- hCMA1
- human chymase-1
- 2D-DIGE
- two-dimensional difference gel electrophoresis
- B6
- C57BL/6
- CE
- chloroacetate esterase
- DFP
- diisopropyl fluorophosphate
- MC-CPA
- MC carboxypeptidase A
- h
- human
- HP
- heparin
- KL
- Kit ligand
- MC
- mast cell
- mBMMC
- mouse bone marrow-derived MC
- mMCP
- mouse MC protease
- meBSA
- methylated bovine serum albumin
- NDST-2
- N-deactylase-N-sulfotransferase-2
- SGPG
- serglycin proteoglycan
- EST
- expressed sequence tag
- MMP
- matrix metalloproteinase
- PCA
- passive cutaneous anaphylaxis.
References
- 1. Caughey G. H., Zerweck E. H., and Vanderslice P. (1991) Structure, chromosomal assignment, and deduced amino acid sequence of a human gene for mast cell chymase. J. Biol. Chem. 266, 12956–12963 [PubMed] [Google Scholar]
- 2. Urata H., Kinoshita A., Perez D. M., Misono K. S., Bumpus F. M., Graham R. M., and Husain A. (1991) Cloning of the gene and cDNA for human heart chymase. J. Biol. Chem. 266, 17173–17179 [PubMed] [Google Scholar]
- 3. Urata H., Kinoshita A., Misono K. S., Bumpus F. M., and Husain A. (1990) Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J. Biol. Chem. 265, 22348–22357 [PubMed] [Google Scholar]
- 4. Semaan W., Desbiens L., Houde M., Labonté J., Gagnon H., Yamamoto D., Takai S., Laidlaw T., Bkaily G., Schwertani A., Pejler G., Levesque C., Desjardins R., Day R., and D'Orléans-Juste P. (2015) Chymase inhibitor-sensitive synthesis of endothelin-1 (1–31) by recombinant mouse mast cell protease 4 and human chymase. Biochem. Pharmacol. 94, 91–100 [DOI] [PubMed] [Google Scholar]
- 5. Mizutani H., Schechter N., Lazarus G., Black R. A., and Kupper T. S. (1991) Rapid and specific conversion of precursor interleukin 1β to an active IL-1 species by human mast cell chymase. J. Exp. Med. 174, 821–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Y., Shiota N., Leskinen M. J., Lindstedt K. A., and Kovanen P. T. (2001) Mast cell chymase inhibits smooth muscle cell growth and collagen expression in vitro: transforming growth factor-β1-dependent and -independent effects. Arterioscler. Thromb. Vasc. Biol. 21, 1928–1933 [DOI] [PubMed] [Google Scholar]
- 7. Okumura K., Takai S., Muramatsu M., Katayama S., Sakaguchi M., Kishi K., Jin D., and Miyazaki M. (2004) Human chymase degrades human fibronectin. Clin. Chim. Acta 347, 223–225 [DOI] [PubMed] [Google Scholar]
- 8. Irani A. M., Goldstein S. M., Wintroub B. U., Bradford T., and Schwartz L. B. (1991) Human mast cell carboxypeptidase: selective localization to MCTC cells. J. Immunol. 147, 247–253 [PubMed] [Google Scholar]
- 9. McNeil H. P., Austen K. F., Somerville L. L., Gurish M. F., and Stevens R. L. (1991) Molecular cloning of the mouse mast cell protease 5 gene: a novel secretory granule protease expressed early in the differentiation of serosal mast cells. J. Biol. Chem. 266, 20316–20322 [PubMed] [Google Scholar]
- 10. McNeil H. P., Frenkel D. P., Austen K. F., Friend D. S., and Stevens R. L. (1992) Translation and granule localization of mouse mast cell protease 5: immunodetection with specific antipeptide Ig. J. Immunol. 149, 2466–2472 [PubMed] [Google Scholar]
- 11. Stevens R. L., Friend D. S., McNeil H. P., Schiller V., Ghildyal N., and Austen K. F. (1994) Strain-specific and tissue-specific expression of mouse mast cell secretory granule proteases. Proc. Natl. Acad. Sci. U.S.A. 91, 128–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Friend D. S., Ghildyal N., Austen K. F., Gurish M. F., Matsumoto R., and Stevens R. L. (1996) Mast cells that reside at different locations in the jejunum of mice infected with Trichinella spiralis exhibit sequential changes in their granule ultrastructure and chymase phenotype. J. Cell Biol. 135, 279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Humphries D. E., Wong G. W., Friend D. S., Gurish M. F., Qiu W. T., Huang C., Sharpe A. H., and Stevens R. L. (1999) Heparin is essential for the storage of specific granule proteases in mast cells. Nature 400, 769–772 [DOI] [PubMed] [Google Scholar]
- 14. Gotis-Graham I., Smith M. D., Parker A., and McNeil H. P. (1998) Synovial mast cell responses during clinical improvement in early rheumatoid arthritis. Ann. Rheum. Dis. 57, 664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shin K., Gurish M. F., Friend D. S., Pemberton A. D., Thornton E. M., Miller H. R., and Lee D. M. (2006) Lymphocyte-independent connective tissue mast cells populate murine synovium. Arthritis Rheum. 54, 2863–2871 [DOI] [PubMed] [Google Scholar]
- 16. Xia Z., Ghildyal N., Austen K. F., and Stevens R. L. (1996) Post-transcriptional regulation of chymase expression in mast cells: a cytokine-dependent mechanism for controlling the expression of granule neutral proteases of hematopoietic cells. J. Biol. Chem. 271, 8747–8753 [DOI] [PubMed] [Google Scholar]
- 17. McGrath M. E., Mirzadegan T., and Schmidt B. F. (1997) Crystal structure of phenylmethanesulfonyl fluoride-treated human chymase at 1.9 Å. Biochemistry 36, 14318–14324 [DOI] [PubMed] [Google Scholar]
- 18. Sali A., Matsumoto R., McNeil H. P., Karplus M., and Stevens R. L. (1993) Three-dimensional models of four mouse mast cell chymases: identification of proteoglycan binding regions and protease-specific antigenic epitopes. J. Biol. Chem. 268, 9023–9034 [PubMed] [Google Scholar]
- 19. McGuire M. J., Lipsky P. E., and Thiele D. L. (1993) Generation of active myeloid and lymphoid granule serine proteases requires processing by the granule thiol protease dipeptidyl peptidase I. J. Biol. Chem. 268, 2458–2467 [PubMed] [Google Scholar]
- 20. Kunori Y., Koizumi M., Masegi T., Kasai H., Kawabata H., Yamazaki Y., and Fukamizu A. (2002) Rodent α-chymases are elastase-like proteases. Eur. J. Biochem. 269, 5921–5930 [DOI] [PubMed] [Google Scholar]
- 21. Ghildyal N., Friend D. S., Stevens R. L., Austen K. F., Huang C., Penrose J. F., Sali A., Gurish M. F. (1996) Fate of two mast cell tryptases in V3 mastocytosis and normal BALB/c mice undergoing passive systemic anaphylaxis: prolonged retention of exocytosed mMCP-6 in connective tissues, and rapid accumulation of enzymatically active mMCP-7 in the blood. J. Exp. Med. 184, 1061–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang C., Friend D. S., Qiu W. T., Wong G. W., Morales G., Hunt J., and Stevens R. L. (1998) Induction of a selective and persistent extravasation of neutrophils into the peritoneal cavity by tryptase mouse mast cell protease 6. J. Immunol. 160, 1910–1919 [PubMed] [Google Scholar]
- 23. Le Trong H., Neurath H., and Woodbury R. G. (1987) Substrate specificity of the chymotrypsin-like protease in secretory granules isolated from rat mast cells. Proc. Natl. Acad. Sci. U.S.A. 84, 364–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Friend D. S., Ghildyal N., Gurish M. F., Hunt J., Hu X., Austen K. F., and Stevens R. L. (1998) Reversible expression of tryptases and chymases in the jejunal mast cells of mice infected with Trichinella spiralis. J. Immunol. 160, 5537–5545 [PubMed] [Google Scholar]
- 25. Madden K. B., Urban J. F. Jr., Ziltener H. J., Schrader J. W., Finkelman F. D., and Katona I. M. (1991) Antibodies to IL-3 and IL-4 suppress helminth-induced intestinal mastocytosis. J. Immunol. 147, 1387–1391 [PubMed] [Google Scholar]
- 26. Eklund K. K., Ghildyal N., Austen K. F., and Stevens R. L. (1993) Induction by IL-9 and suppression by IL-3 and IL-4 of the levels of chromosome 14-derived transcripts that encode late-expressed mouse mast cell proteases. J. Immunol. 151, 4266–4273 [PubMed] [Google Scholar]
- 27. Galli S. J., Zsebo K. M., and Geissler E. N. (1994) The kit ligand, stem cell factor. Adv. Immunol. 55, 1–96 [DOI] [PubMed] [Google Scholar]
- 28. Halpern B. N., Neveu T., and Spector S. (1963) On the nature of the chemical mediators involved in anaphylactic reactions in mice. Br. J. Pharmacol. Chemother. 20, 389–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stevens R. L., Wong G. W., and Humphries D. E. (2000) Serglycin proteoglycans: the family of proteoglycans stored in the secretory granules of certain effector cells of the immune system. In Proteoglycans: Structure, Biology, and Molecular Interactions (Iozzo R. V., ed) pp. 177–199, Marcel Dekker, Inc., New York [Google Scholar]
- 30. Serafin W. E., Dayton E. T., Gravallese P. M., Austen K. F., and Stevens R. L. (1987) Carboxypeptidase A in mouse mast cells: identification, characterization, and use as a differentiation marker. J. Immunol. 139, 3771–3776 [PubMed] [Google Scholar]
- 31. DuBuske L., Austen K. F., Czop J., and Stevens R. L. (1984) Granule-associated serine neutral proteases of the mouse bone marrow-derived mast cell that degrade fibronectin: their increase after sodium butyrate treatment of the cells. J. Immunol. 133, 1535–1541 [PubMed] [Google Scholar]
- 32. Dayton E. T., Caulfield J. P., Hein A., Austen K. F., and Stevens R. L. (1989) Regulation of the growth rate of mouse fibroblasts by IL-3-activated mouse bone marrow-derived mast cells. J. Immunol. 142, 4307–4313 [PubMed] [Google Scholar]
- 33. Serafin W. E., Reynolds D. S., Rogelj S., Lane W. S., Conder G. A., Johnson S. S., Austen K. F., and Stevens R. L. (1990) Identification and molecular cloning of a novel mouse mucosal mast cell serine protease. J. Biol. Chem. 265, 423–429 [PubMed] [Google Scholar]
- 34. Feyerabend T. B., Hausser H., Tietz A., Blum C., Hellman L., Straus A. H., Takahashi H. K., Morgan E. S., Dvorak A. M., Fehling H. J., and Rodewald H. R. (2005) Loss of histochemical identity in mast cells lacking carboxypeptidase A. Mol. Cell Biol. 25, 6199–6210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gomis-Rüth F. X., Gómez M., Bode W., Huber R., and Avilés F. X. (1995) The three-dimensional structure of the native ternary complex of bovine pancreatic procarboxypeptidase A with proproteinase E and chymotrypsinogen C. EMBO J. 14, 4387–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tchougounova E., Forsberg E., Angelborg G., Kjéllen L., and Pejler G. (2001) Altered processing of fibronectin in mice lacking heparin. a role for heparin-dependent mast cell chymase in fibronectin degradation. J. Biol. Chem. 276, 3772–3777 [DOI] [PubMed] [Google Scholar]
- 37. Serafin W. E., Sullivan T. P., Conder G. A., Ebrahimi A., Marcham P., Johnson S. S., Austen K. F., and Reynolds D. S. (1991) Cloning of the cDNA and gene for mouse mast cell protease 4: demonstration of its late transcription in mast cell subclasses and analysis of its homology to subclass-specific neutral proteases of the mouse and rat. J. Biol. Chem. 266, 1934–1941 [PubMed] [Google Scholar]
- 38. Prieto-García A., Zheng D., Adachi R., Xing W., Lane W. S., Chung K., Anderson P., Hansbro P. M., Castells M., and Stevens R. L. (2012) Mast cell restricted mouse and human tryptase-heparin complexes hinder thrombin-induced coagulation of plasma and the generation of fibrin by proteolytically destroying fibrinogen. J. Biol. Chem. 287, 7834–7844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang C., Wong G. W., Ghildyal N., Gurish M. F., Sali A., Matsumoto R., Qiu W. T., and Stevens R. L. (1997) The tryptase, mouse mast cell protease 7, exhibits anticoagulant activity in vivo and in vitro due to its ability to degrade fibrinogen in the presence of the diverse array of protease inhibitors in plasma. J. Biol. Chem. 272, 31885–31893 [DOI] [PubMed] [Google Scholar]
- 40. Godfrey H. P., Ilardi C., Engber W., and Graziano F. M. (1984) Quantitation of human synovial mast cells in rheumatoid arthritis and other rheumatic diseases. Arthritis Rheum. 27, 852–856 [DOI] [PubMed] [Google Scholar]
- 41. Buckley M. G., Walters C., Wong W. M., Cawley M. I., Ren S., Schwartz L. B., and Walls A. F. (1997) Mast cell activation in arthritis: detection of α- and β-tryptase, histamine and eosinophil cationic protein in synovial fluid. Clin. Sci. 93, 363–370 [DOI] [PubMed] [Google Scholar]
- 42. Feyerabend T. B., Weiser A., Tietz A., Stassen M., Harris N., Kopf M., Radermacher P., Möller P., Benoist C., Mathis D., Fehling H. J., and Rodewald H. R. (2011) Cre-mediated cell ablation contests mast cell contribution in models of antibody- and T cell-mediated autoimmunity. Immunity 35, 832–844 [DOI] [PubMed] [Google Scholar]
- 43. Peschke K., Dudeck A., Rabenhorst A., Hartmann K., and Roers A. (2015) Cre/loxP-based mouse models of mast cell deficiency and mast cell-specific gene inactivation. Methods Mol. Biol. 1220, 403–421 [DOI] [PubMed] [Google Scholar]
- 44. Lee D. M., Friend D. S., Gurish M. F., Benoist C., Mathis D., and Brenner M. B. (2002) Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science 297, 1689–1692 [DOI] [PubMed] [Google Scholar]
- 45. Shin K., Nigrovic P. A., Crish J., Boilard E., McNeil H. P., Larabee K. S., Adachi R., Gurish M. F., Gobezie R., Stevens R. L., and Lee D. M. (2009) Mast cells contribute to autoimmune inflammatory arthritis via their tryptase/heparin complexes. J. Immunol. 182, 647–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shin K., Watts G. F., Oettgen H. C., Friend D. S., Pemberton A. D., Gurish M. F., and Lee D. M. (2008) Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of Trichinella spiralis infection. J. Immunol. 180, 4885–4891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McNeil H. P., Shin K., Campbell I. K., Wicks I. P., Adachi R., Lee D. M., and Stevens R. L. (2008) The mouse mast cell-restricted tetramer-forming tryptases mouse mast cell protease 6 and mouse mast cell protease 7 are critical mediators in inflammatory arthritis. Arthritis Rheum. 58, 2338–2346 [DOI] [PubMed] [Google Scholar]
- 48. Koolwijk P., Miltenburg A. M., van Erck M. G., Oudshoorn M., Niedbala M. J., Breedveld F. C., and van Hinsbergh V. W. (1995) Activated gelatinase-B (MMP-9) and urokinase-type plasminogen activator in synovial fluids of patients with arthritis: correlation with clinical and experimental variables of inflammation. J. Rheumatol. 22, 385–393 [PubMed] [Google Scholar]
- 49. Lees M., Taylor D. J., and Woolley D. E. (1994) Mast cell proteinases activate precursor forms of collagenase and stromelysin, but not of gelatinases A and B. Eur. J. Biochem. 223, 171–177 [DOI] [PubMed] [Google Scholar]
- 50. Magarinos N. J., Bryant K. J., Fosang A. J., Adachi R., Stevens R. L., and McNeil H. P. (2013) Mast cell-restricted, tetramer-forming tryptases induce aggrecanolysis in articular cartilage by activating matrix metalloproteinase-3 and -13 zymogens. J. Immunol. 191, 1404–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xie D. L., Meyers R., and Homandberg G. A. (1992) Fibronectin fragments in osteoarthritic synovial fluid. J. Rheumatol. 19, 1448–1452 [PubMed] [Google Scholar]
- 52. Razin E., Ihle J. N., Seldin D., Mencia-Huerta J. M., Katz H. R., LeBlanc P. A., Hein A., Caulfield J. P., Austen K. F., and Stevens R. L. (1984) Interleukin-3: a differentiation and growth factor for the mouse mast cell that contains chondroitin sulfate E proteoglycan. J. Immunol. 132, 1479–1486 [PubMed] [Google Scholar]
- 53. Levi-Schaffer F., Austen K. F., Gravallese P. M., and Stevens R. L. (1986) Coculture of interleukin-3-dependent mouse mast cells with fibroblasts results in a phenotypic change of the mast cells. Proc. Natl. Acad. Sci. U.S.A. 83, 6485–6488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Reynolds D. S., Gurley D. S., Austen K. F., and Serafin W. E. (1991) Cloning of the cDNA and gene of mouse mast cell protease 6: transcription by progenitor mast cells and mast cells of the connective tissue subclass. J. Biol. Chem. 266, 3847–3853 [PubMed] [Google Scholar]
- 55. Lazaar A. L., Plotnick M. I., Kucich U., Crichton I., Lotfi S., Das S. K., Kane S., Rosenbloom J., Panettieri R. A. Jr., Schechter N. M., and Puré E. (2002) Mast cell chymase modifies cell-matrix interactions and inhibits mitogen-induced proliferation of human airway smooth muscle cells. J. Immunol. 169, 1014–1020 [DOI] [PubMed] [Google Scholar]
- 56. Thakurdas S. M., Melicoff E., Sansores-Garcia L., Moreira D. C., Petrova Y., Stevens R. L., and Adachi R. (2007) The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J. Biol. Chem. 282, 20809–20815 [DOI] [PubMed] [Google Scholar]
- 57. Flanagan J. G., and Leder P. (1990) The kit ligand: a cell surface molecule altered in steel mutant fibroblasts. Cell 63, 185–194 [DOI] [PubMed] [Google Scholar]
- 58. Kaieda S., Shin K., Nigrovic P. A., Seki K., Lee R. T., Stevens R. L., and Lee D. M. (2010) Synovial fibroblasts promote the expression and granule accumulation of tryptase via interleukin-33 and its receptor ST-2 (IL1RL1). J. Biol. Chem. 285, 21478–21486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Katz H. R., and Austen K. F. (1986) Plasma membrane and intracellular expression of globotetraosylceramide (globoside) in mouse bone marrow-derived mast cells. J. Immunol. 136, 3819–3824 [PubMed] [Google Scholar]
- 60. Stevens R. L., and Austen K. F. (1982) Effect of p-nitrophenyl-β-d-xyloside on proteoglycan and glycosaminoglycan biosynthesis in rat serosal mast cell cultures. J. Biol. Chem. 257, 253–259 [PubMed] [Google Scholar]
- 61. Paton W. D. (1951) Compound 48/80: a potent histamine liberator. Br. J. Pharmacol. Chemother. 6, 499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fawcett D. W. (1954) Cytological and pharmacological observations on the release of histamine by mast cells. J. Exp. Med. 100, 217–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Abonia J. P., Friend D. S., Austen W. G. Jr., Moore F. D. Jr., Carroll M. C., Chan R., Afnan J., Humbles A., Gerard C., Knight P., Kanaoka Y., Yasuda S., Morokawa N., Austen K. F., Stevens R. L., et al. (2005) Mast cell protease 5 mediates ischemia-reperfusion injury of mouse skeletal muscle. J. Immunol. 174, 7285–7291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Younan G., Suber F., Xing W., Shi T., Kunori Y., Abrink M., Pejler G., Schlenner S. M., Rodewald H. R., Moore F. D. Jr., Stevens R. L., Adachi R., Austen K. F., and Gurish M. F. (2010) The inflammatory response after an epidermal burn depends on the activities of mouse mast cell proteases 4 and 5. J. Immunol. 185, 7681–7690 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.