Abstract
Pyoderma gangrenosum (PG) is an uncommon disorder characterized by the development of painful cutaneous ulceration, commonly precipitated by dermal injury at surgical sites. It is a diagnostic challenge as it manifests as necrotizing wounds which are commonly misdiagnosed as postoperative wound infection or ischemia. We discuss the clinical features and histopathological findings that allow for rapid identification of PG following autologous breast reconstruction and suggest an algorithm to aid diagnosis.
Keywords: Pyoderma gangrenosum (PG), breast reconstruction, mastectomy, pathergy
Introduction
Pyoderma gangrenosum (PG) is an uncommon dermatological disorder that poses a diagnostic challenge when it occurs in the postoperative period. It is an inflammatory neutrophilic dermatosis characterized by painful, sterile ulcerations with irregular, raised and violaceous borders (1) which expand at 1–2 cm/day (2). Although the aetiology is unclear, PG may involve dysfunctional innate immune responses (3): 70% are associated with underlying inflammatory bowel disease and inflammatory arthritides (4,5). It can occur spontaneously but is commonly precipitated by dermal injury in a process termed ‘pathergy’ (1,6).
PG may develop at recent surgical sites, where it is often misdiagnosed as wound infection or ischemia leading to delayed treatment and increased morbidity. Wound debridement is often performed which exacerbates the disease process by perpetuating it into surrounding, unaffected skin. This can produce devastating results in aesthetically sensitive areas such as the breasts (7).
The diagnosis of PG is hindered by a lack of diagnostic tests (6,8). Biopsy histopathology tends to show nonspecific neutrophilic inflammation indistinguishable from other ulcerative processes (1). A diagnosis of PG relies on recognition of subtle clinical features (1), some specific to PG after breast surgery (7).
Case presentation
A 48-year-old healthy female presented for immediate breast reconstruction with deep inferior epigastric perforator (DIEP) flaps following bilateral mastectomies for invasive breast carcinoma (Figure 1). Her past history was unremarkable apart from an immediate family history of multiple sclerosis and rheumatoid arthritis. She had no known allergies and was constitutionally well.
Figure 1.

Photograph of right breast of patient presenting with pyoderma gangrenosum (PG) 5 days after bilateral breast reconstruction using deep inferior epigastric perforator flaps. Irregularly shaped necrotizing lesions with raised, violaceous edges, and sterile pyogenic exudate at multiple surgical sites indicated PG.
On post-operative day four, a small circular ecchymotic lesion was noted centrally on her abdominal incision line which enlarged and ulcerated over the next two days. Similar lesions developed laterally on the abdominal incision line and on the breast incisions bilaterally but the flaps remained well perfused (Figure 1).
The patient recorded a temperature of 102° Fahrenheit. Blood tests revealed a white blood cell count of 17.0×109/L, and Vancomycin was started empirically. Wound swabs taken before initiation of antibiotics revealed a heavy sterile neutrophilia with negative wound and blood cultures. Autoimmune serology markers for antinuclear antibodies, extractable nuclear antigens, rheumatoid factor and anti-neutrophil cytoplasmic antibodies were also negative.
A diagnosis of PG was suspected based on the appearance of the ulcerations. The lesions were irregular with raised, violaceous borders and produced milky, sterile exudate (Figure 1). The distribution of wounds to all three distant surgical sites appeared atypical for infectious processes. On post-operative day seven wound biopsies were taken with care to minimize aggressive debridement. Histopathology revealed sharply demarcated ulcerations, a full thickness acute inflammatory exudate with a dense, sterile dermal neutrophilia, and necrosis extending into the subcutis. These findings were consistent with PG (Figure 2).
Figure 2.
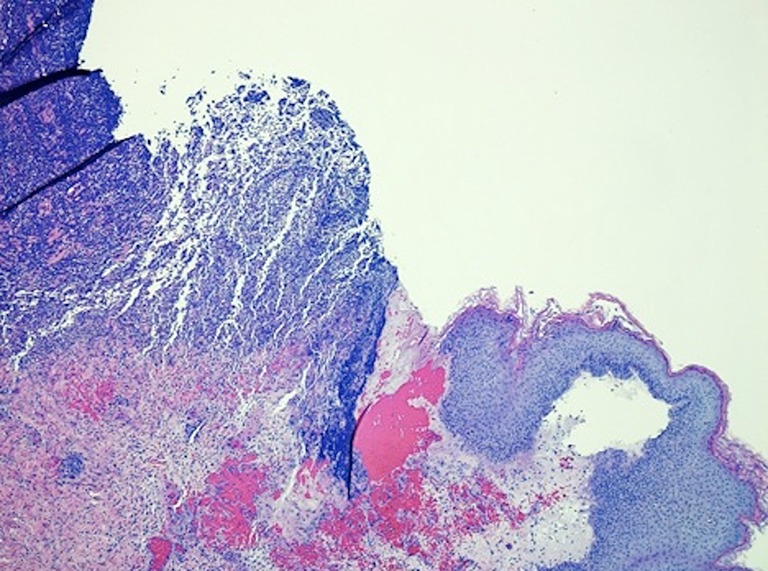
Photomicrograph illustrating a sharply demarcated area of epidermal ulceration with diffuse neutrophilic inflammatory infiltrate in the deep dermis associated with necrosis extending through the dermis to the subcutis (Haematoxylin & Eosin stain, 100×).
Tissue cultures grew Corynebacterium diphtheria, a common skin commensal. Based on these results, antibiotics were discontinued, a Dermatology review requested and intravenous steroid treatment initiated. Rapid improvement clinically confirmed the diagnosis and 3 days later the patient was transitioned to oral prednisone prior to discharge. Two months later her wounds had completely epithelialized, prednisone was tapered and she was offered nipple reconstruction under steroid cover.
Discussion
Diagnosis of PG is a process of exclusion with laboratory tests and histopathology serving only to exclude infection, ischemia, vasculitis and malignancy (1). Nevertheless, certain clinical features can raise suspicion of PG (1).
The necrotizing wounds of postoperative PG present within the first postsurgical week, often associated with fever and leukocytosis. Misdiagnosis as infection or ischemic necrosis can worsen morbidity by prolonging the disease course through debridement induced pathergy (1) and incorrect treatment. Recurrence of ulcerations may lead to suspicion of necrotizing fasciitis, however the timing of the progression of ulcerations and lack of a septic clinical picture are inconsistent with this diagnosis. Repeated, unnecessary debridements can leave the patients with massive wounds and permanent disfigurement. In some instances the diagnosis is never made and the true incidence of this condition is probably not known.
Laboratory tests and histopathology are nonspecific for PG (1) but clinical clues may increase suspicion. The unique characteristics of PG following breast surgery can facilitate diagnosis. The breasts are often affected symmetrically when bilateral surgery is performed, as is the abdomen when it is the tissue donor site. Lesions have a characteristic appearance with irregular, violaceous raised borders and often sterile micro-abscesses (Figure 1). When such necrotizing wounds affect multiple discrete surgical sites but spare the nipples, diagnosis of a systemic condition such as PG should be considered (7).
A thorough history may reveal previous episodes of cutaneous ulcerations suggestive of PG. A history of autoimmunity should also raise suspicion. If wound and blood cultures are negative, a wound biopsy should be taken. A dense neutrophilia with sterile pus is characteristic of PG. Discussion of the clinical presentation and suspicion with a Pathologist is helpful. A dermatology request is advisable and corticosteroids should be started with the presumptive diagnosis. A rapid response will confirm the diagnosis of PG. Adhering to our protocol may minimize misdiagnosis (Table 1).
Table 1. How to approach a patient with suspected pyoderma gangrenosum.
| Step | Feature |
|---|---|
| History | Necrotising, particularly painful well-demarcated ulcer; |
| Skin lesion prior to ulceration; macule, papule, pustule, bullae, vesicle; | |
| Minor trauma/ surgery to area of affected skin prior to ulceration (pathergy); | |
| Other autoimmune diseases in patient or family; | |
| Past medical history of ulcers/ similar lesions | |
| Clinical examination | Presence of necrotizing ulcers at multiple surgical sites; |
| Irregular, raised and violaceous borders; | |
| Tenderness and erythema around wound; | |
| Presence of microabscesses containing sterile pyogenic exudate; | |
| Patient constitutionally relatively well with no rapid deterioration; | |
| Lesions are nipple sparing; | |
| Fever may be present | |
| Laboratory investigations: not confirmatory for pyoderma gangrenosum (PG) but aid in ruling out differential diagnoses. Blood tests | Full blood count with white cell differential; |
| Inflammatory markers: C-reactive protein, erythrocyte sedimentation rate; | |
| Liver and renal function; | |
| Cultures; | |
| Serology for autoimmune markers: anti-neutrophil cytoplasmic antibodies (ANCA), antinuclear antibodies, extractable nuclear antigens, Rheumatoid factor, antiphospholipid antibody; | |
| Cryoglobulins and complement assay; | |
| Coagulation tests: platelets and clotting factors | |
| Imaging | Venous and arterial investigations, e.g., Doppler/contrast computed radiography/magnetic resonance imaging; |
| Chest roentgenogram | |
| Wound biopsy for histopathology: discuss PG differential with pathology | Ensure inflamed violaceous border is included in tissue sample and go deep enough to include subcutaneous fat; |
| Send for histological haematoxylin and eosin staining, and microbial staining, e.g., Gram staining, methenamine silver staining; | |
| Wound culture for microbial infection; | |
| Histopathology findings include well demarcated ulcers with a heavy sterile dermal neutrophilia, necrosis extending into the subcutis, lymphocytic vasculitis with reactive endothelial cells and mixed inflammation | |
| Treatment: discuss with dermatology | Minimal surgical debridement to remove already necrotic tissue; |
| Stop antibiotics if no superinfection; | |
| Start intravenous corticosteroids; | |
| For steroid resistant cases consider cyclosporine, azathioprine and cyclophosphamide; | |
| For rare, extremely resistant cases consider biologic agents like tumour necrosis factor alpha (TNFa) blockers | |
| Confirmation | Lack of response to antibiotics alone; |
| Rapidly successful response to corticosteroids with good wound healing; | |
| Monitor recurrence and side-effects of treatment |
Surgical debridement is contraindicated but deep wounds may benefit from vacuum assisted closure which improves granulation (9). Systemic treatment using corticosteroids is first line and a rapid response can be considered a diagnostic criterion (1). Cytotoxic drugs such as cyclosporine, azathioprine and cyclophosphamide are used in combination in patients with steroid resistant PG (10) and superimposed bacterial infections should be treated with appropriate antibiotics. Skin grafting can accelerate recovery but inevitably fails unless the inflammatory disease process is medically suppressed prior to grafting (9).
Conclusions
PG is a condition that mimics postoperative wound infection or ischemic necrosis, frequently resulting in misdiagnosis. The development of enlarging, necrotizing, ulcers with irregular, raised and violaceous borders at multiple surgical sites, presenting soon after surgery, unresponsiveness to antibiotics and/or recurrence of ulcerations after adequate initial debridement should raise suspicion for postoperative PG. Wound debridement should be minimal. Recognition of these features will enable rapid and accurate diagnosis and management with diminished morbidity.
Acknowledgements
None.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Su WP, Davis MD, Weenig RH, et al. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol 2004;43:790-800. 10.1111/j.1365-4632.2004.02128.x [DOI] [PubMed] [Google Scholar]
- 2.Brunsting LA, Goeckerman WH, O'Leary PA. Pyoderma (echthyma) gangrenosum: Clinical and experimental observations in five cases occurring in adults. Arch Derm Syphilol 1930;22:655-80. 10.1001/archderm.1930.01440160053009 [DOI] [Google Scholar]
- 3.Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol 2012;13:191-211. 10.2165/11595240-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 4.Greenstein AJ, Janowitz HD, Sachar DB. The extra-intestinal complications of Crohn's disease and ulcerative colitis: a study of 700 patients. Medicine (Baltimore) 1976;55:401-12. 10.1097/00005792-197609000-00004 [DOI] [PubMed] [Google Scholar]
- 5.Stolman LP, Rosenthal D, Yaworsky R, et al. Pyoderma Gangrenosum and rheumatoid arthritis. Arch Dermatol 1975;111:1020-3. 10.1001/archderm.1975.01630200080009 [DOI] [PubMed] [Google Scholar]
- 6.Powell FC, Schroeter AL, Su WP, et al. Pyoderma gangrenosum: a review of 86 patients. Q J Med 1985;55:173-86. [PubMed] [Google Scholar]
- 7.Tuffaha SH, Sarhane KA, Mundinger GS, et al. Pyoderma gangrenosum after breast surgery: diagnostic pearls and treatment recommendations based on a systematic literature review. Ann Plast Surg 2016;77:e39-44. 10.1097/SAP.0000000000000248 [DOI] [PubMed] [Google Scholar]
- 8.Crowson AN, Mihm MC, Jr, Magro C. Pyoderma gangrenosum: a review. J Cutan Pathol 2003;30:97-107. 10.1034/j.1600-0560.2003.00024.x [DOI] [PubMed] [Google Scholar]
- 9.Fulbright RK, Wolf JE, Tschen JA. Pyoderma gangrenosum at surgery sites. J Dermatol Surg Oncol 1985;11:883-6. 10.1111/j.1524-4725.1985.tb03559.x [DOI] [PubMed] [Google Scholar]
- 10.Wollina U. Clinical management of pyoderma gangrenosum. Am J Clin Dermatol 2002;3:149-58. 10.2165/00128071-200203030-00002 [DOI] [PubMed] [Google Scholar]


