Abstract
Pulmonary artery smooth muscle cell (PASMC) proliferation is one of the hallmark features of hypoxia-induced pulmonary hypertension. With only supportive treatment options available for this life-threatening disease, treating and preventing the proliferation of PASMCs is a viable therapeutic option. A key promoter of hypoxia-induced increases in the number of viable human PASMCs is arginase II, with attenuation of viable cell numbers following pharmacologic inhibition or siRNA knockdown of the enzyme. Additionally, increased levels of arginase have been demonstrated in the pulmonary vasculature of patients with pulmonary hypertension. The signaling pathways responsible for the hypoxic induction of arginase II in PASMCs, however, remain unknown. Hypoxia is a recognized activator of AMPK, which is known to be expressed in human PASMCs (hPASMCs). Activation of AMPK by hypoxia has been shown to promote cell survival in PASMCs. In addition, pharmacologic agents targeting AMPK have been shown to attenuate chronic hypoxia-induced pulmonary hypertension in animal models. The present studies tested the hypothesis that hypoxia-induced arginase II expression in hPASMCs is mediated through AMPK signaling. We found that pharmacologic inhibitors of AMPK, as well as siRNA knockdown of AMPKα1, prevented hypoxia-induced arginase II. The hypoxia-induced increase in viable hPASMC numbers was also prevented following both pharmacologic inhibition and siRNA knockdown of AMPK. Furthermore, we demonstrate that overexpression of AMPK induced arginase II protein expression and viable cells numbers in hPASMCs.
Keywords: pulmonary hypertension, pulmonary vasculature, l-arginine, vascular remodeling, vascular smooth muscle cells
pulmonary hypertension (PH) is a disease characterized by increased pulmonary vascular resistance leading to the elevation of right ventricular afterload, and eventual right-ventricle heart failure and death (34). Although the etiologies of PH are diverse, all of the etiologies have in common cellular proliferation and vascular remodeling as a response to various stimuli or lung injury (17, 30). Hypoxia is a well-established stimulus that induces pulmonary artery smooth muscle cell (PASMC) proliferation and PH (35). Currently, treatment options available for PH are vasodilators, which are only supportive, with no curative therapy available (14). Therefore, new therapeutic strategies are needed for the prevention and treatment of the pathogenic vascular remodeling and the vascular smooth muscle cell proliferation characteristic of PH.
Arginase, of which there are two described isoforms, has been shown to be important in proliferation of different cell types. Both isoforms are expressed in the lung and metabolize the conversion of l-arginine to l-ornithine and urea. This is the first step in polyamine and proline synthesis, which are critical for cell proliferation, differentiation, tissue repair, and growth (21, 26). We have previously demonstrated that hypoxia induced human PASMC (hPASMC) viability depends on the induction of arginase II (4); however, the signaling pathways responsible for the hypoxic induction of arginase II remain elusive.
AMPK is a ubiquitously expressed heterotrimer consisting of a catalytic α-subunit containing the kinase domain and regulatory β and γ subunits (10). There are two isoforms of the catalytic α-subunit that are expressed in the lung (11, 13). The basal activity of AMPK is low in unstressed cells, and metabolic stresses, including hypoxia, result in activation of AMPK (15, 19). AMPK regulates cell survival and proliferation. In systemic vascular smooth muscle cells, AMPK has been shown to inhibit cell proliferation (20), whereas in PASMCs, it has been reported that AMPK is required for cell survival following hypoxia exposure (19). In the present study, we hypothesized that hypoxia-induced arginase II expression and the increase in viable pulmonary artery smooth muscle cell numbers occurs via AMPK signaling.
MATERIALS AND METHODS
Cell culture.
Human PASMCs (cat. no. CL-2581, lot no. 7F3558, Lonza, Walkersville, MD; cat. no. 3110, lot no. 7449 and no. 4559, ScienCell Research Laboratories, Carlsbad, CA), between passages 5 and 8, were grown in 21% O2-5% CO2-balance N2 (normoxia) at 37°C in smooth muscle growth media (SmGM, cat. no. ml-3181, lot no. 389247, Lonza; cat. no. 1101, lot no. 19301, lot no. 18198), with no cell morphology changes noted. The cells were incubated in normoxia or 1% O2-5% CO2-balance N2 (hypoxia) for the indicated time points. We have previously used 1% O2 as our hypoxic stimulus because it provides consistent and reproducible changes in the hPASMCs (4–6). In some experiments, AMPK inhibitors 6-[4-(2-piperidin-1-yl-ethoxy)-phenyl)]-3-pyridin-4-yl-pyrrazolo[1,5-a]-pyrimidine (Compound C, 10 μM, cat. no. BML-EI369, lot no. 12171240, Enzo Life Sciences, Farmingdale, NY) or adenine 9-β-d-arabinofuranoside (Ara-A, 100 μM, cat. no. A5762, lot no. BCBL 6590V; Sigma Gel, Jandel Scientific, San Rafael, CA), the AMPK activator, N1-(β-d-ribofuranosyl)-5-aminoimidazole-4-carboxamide (AICAR; 150 μM, cat. no. sc-200659, lot no. A2413; Santa Cruz Biotechnology, Santa Cruz, CA), or vehicle (DMSO for the AMPK inhibitors or dH2O for AICAR) was added to the medium.
Protein isolation and Western blot analysis.
Protein was isolated from hPASMCs, as previously described (33), and concentration was determined by the Bradford method (2). Protein was assayed with evidence of specificity for arginase II (4) (1:500, cat. no. sc-20151, lot no. A3013; Santa Cruz), cleaved caspase 3 (28) (1:1,000, cat. no. 9664, lot no. 19, Cell Signaling, Danvers, MA), total caspase 3 (28) (1:1,000, cat. no. 9622, lot no. 18; Cell Signaling), AMPKα1 (40) (1:1,000, cat. no. 2795, lot no. 3; Cell Signaling), AMPKα2 (40) (1:1,000, cat. no. 2757, lot no. 2; Cell Signaling), p-AMPKα1/2 (Thr-172) (23) (1:500, cat. no. sc-33524, lot no. C0813; Santa Cruz), total AMPK (22) (1:500, cat. no. sc-25792; lot no. A2013, Santa Cruz), myc (27) (1:1,000, cat. no. TA150121, lot no. A002; OriGene, Rockville, MD), p-ACC (Ser-79) (29) (1:1,000, cat. no. 11818, lot no. 1; Cell Signaling), total ACC (1) (1:1,000, cat. no. 3676, lot no. 8; Cell Signaling), and β-actin (1:10,000, cat. no. 3700, lot no. 8, Cell Signaling) by Western blot analysis, as previously described (32, 33). Protein bands were visualized using enhanced chemiluminescence (Luminata Classico/Forte Western HRP Substrate; EMD Millipore Corporation, Billerica, MA) and were quantified using densitometry (UVP BioImaging Systems, Upland, CA).
AMPK overexpression.
Overexpression of AMPKα isoforms was performed using pCMV6-Entry vectors (OriGene) with in-frame MYC/DDK tags, allowing detection of the product with anti-myc antibody. Human PASMCs were seeded in six-well plates (2.5 × 105 cells/well) and were incubated in normoxia overnight. The cells were transfected with empty vector (vControl, cat. no. CW103174, lot no. 120457, OriGene; final concentration: 1 μg/ml), vector-AMPKα1 (cat. no. RC518572, lot no. 130457, OriGene), vector-AMPKα2 (cat. no. RC510226, lot no. SR013124, OriGene), or vector-AMPKα1+2 in transfection medium [Opti-MEM (200 μl, cat. no. 22600, lot no.460294, Thermo Fisher Scientific, Pittsburgh, PA)+DNA Turbofect (2 μl/μg, cat. no. R0531, lot no. 00197193; Thermo Fisher), serum/antibiotic-free media (800 μl)] for 6 h. Nontransfected hPASMCs were used as a control. Medium with FBS+antibiotics (500 μl) was added to each well and incubated. After 24 h, the hPASMCs were prepared for viable cell number assay, as described previously, or incubated in fresh medium in normoxia or hypoxia for 48 h and analyzed by Western blot.
siRNA-Transfection.
Human PASMCs were grown to ~70% confluence in six-well plates. Transient transfection with 50 nM of isoform-specific AMPKα-siRNA (50 nM, cat. no. AM51334, lot no. A5022YPK, Thermo Fisher), arginase II-siRNA (100 nM, cat. no. L-009454, lot no. 150119; GE Dharmacon, Lafayette, CO), or nontargeting scramble siRNA (cat. no. AM4635, lot no. AS021RMV; Thermo Fisher) was performed with DharmaFECT 1 transfection reagent (cat. no. T-2001, lot no. 15118T, Thermo Fisher), according to the manufacturer’s protocol. After 24 h, the hPASMCs were prepared for viable cell number assay, as described or incubated in fresh medium in normoxia or hypoxia for 1, 48, or 120 h, and analyzed by Western blot.
Viable cell number assay.
Viable cell numbers were determined, as previously described (4, 5). Briefly, hPASMCs were seeded on six-well plates (104 cells/well) in SmGM and incubated in hypoxia for 120 h. Adherent cells were trypsinized, and viable cells were counted using a hemocytometer and trypan blue exclusion. In a separate set of experiments, the viable cells were counted at each 24-h interval up to 120 h.
Lactate dehydrogenase cytotoxicity assay.
Lactate dehydrogenase (LDH) is a soluble cytosolic enzyme that is released into the culture medium following loss of membrane integrity, resulting from cytotoxicity. LDH activity is used as an indicator of cell membrane integrity and serves as a general means to assess cell viability by measuring membrane permeability. Human PASMCs were seeded in 48-well plates (4 × 104 cells/well) and incubated in normoxia overnight. The cells were transfected with isoform-specific AMPKα-siRNA or nontargeting scramble siRNA, as described previously, and incubated in normoxia or hypoxia for 48 h, and then LDH cytotoxicity was measured, according to the manufacturer’s protocol (cat. no. 601170, lot no. 0466208; Cayman Chemical, Ann Arbor, MI). Briefly, the assay measures cell death using a coupled two-step reaction. In the first step, LDH catalyzes the reduction of NAD+ to NADH and H+ by oxidation of lactate to pyruvate. In the second step, diaphorase uses the newly formed NADH and H+ to catalyze the reduction of a tetrazolium salt to highly colored formazan, which absorbs strongly at 490–520 nm.
Statistical Analysis.
Values are given as means ± SE. Unpaired Student's t-test, one-way or two-way ANOVA with post hoc Tukey’s or Dunnett’s for multiple comparisons were used to compare groups as appropriate (GraphPad Prism Software, La Jolla, CA). Differences were considered significant when P < 0.05.
RESULTS
Hypoxia activates AMPK.
AMPK has previously been shown to be activated by hypoxia (19). To confirm the effects of hypoxia on AMPK activation in our cell culture model, equally seeded hPASMCs were exposed to normoxia or hypoxia for 20 min, 30 min, 48 h, and 120 h, and p-AMPK and total AMPK expression was assessed by Western blot analysis and quantified by densitometry. Phosphorylated AMPKα1/2 (Thr-172) levels increased with hypoxic exposure at 20 and 30 min, levels of p-AMPKα1/2 (Thr-172) were ~3.5-fold greater than in normoxia (Fig. 1). There was no significant increase in p-AMPKα1/2 (Thr-172) after 48 or 120 h of hypoxia exposure (data not shown).
Fig. 1.

Hypoxia activates AMPK. Human pulmonary artery smooth muscle cells (PASMCs) were exposed to 21% O2 (normoxia) or 1% O2 (hypoxia) for 20 and 30 min. Protein was assayed for phosphorylated-AMPK (p-AMPK) by Western blot analysis and was quantified by densitometry. A: representative Western blots are shown for p-AMPK, total AMPK, and β-actin. B: data are shown as fold change ± SE after 20 and 30 min of hypoxia relative to normoxia control. *Significant difference, hypoxia vs. normoxia, P < 0.01; n = 3.
Pharmacologic inhibition of AMPK prevents hypoxia-induced arginase II protein expression, whereas AMPK activation increases arginase II protein expression.
To determine the effects of AMPK on arginase II protein expression, hPASMCs were treated with the AMPK inhibitor Compound C, the cell-permeable AMPK activator AICAR, or vehicle (dH2O), and incubated in either normoxia or hypoxia for 48 h. Protein was harvested for assessment of arginase II expression by Western blot analysis and quantified by densitometry. Consistent with previous results (4, 5, 7), hPASMCs exposed to hypoxia had substantially (P < 0.05) greater arginase II protein levels at 48 h than did hPASMCs exposed to normoxia (Fig. 2). The addition of Compound C for 48 h prevented hypoxia-induced arginase II protein expression (P < 0.0001; Fig. 2), such that there was no difference in arginase II expression in the Compound C-treated, hypoxia group compared with the vehicle-treated, normoxia controls. The addition of AICAR for 48 h resulted in increased arginase II levels in normoxia relative to vehicle-treated hPASMCs (P < 0.05); however, in hypoxia the addition of AICAR did not further increase arginase II levels compared with hypoxia alone (Fig. 2).
Fig. 2.

Pharmacologic inhibition of AMPK prevents hypoxia-induced arginase II protein expression, whereas AMPK activation increases arginase II protein expression. Human PASMCs were treated with the AMPK inhibitor Compound C, the cell-permeable AMPK activator AICAR, or vehicle (DMSO for the AMPK inhibitors or dH2O for AMPK activator, N1-(β-d-ribofuranosyl)-5-aminoimidazole-4-carboxamide (AICAR), and incubated in 21% O2 (normoxia) or 1% O2 (hypoxia) for 48 h. Protein was assayed for arginase II protein expression by Western blot analysis and was quantified by densitometry. A: representative Western blots are shown for arginase II and β-actin. B: data are shown as a fold change ± SE relative to vehicle-treated, normoxia-exposed human PASMC controls (n = 4). *Significant difference compared with vehicle-treated normoxia, P < 0.05. †Significant difference compared with vehicle-treated hypoxia, P < 0.0001.
AMPK overexpression induces arginase II expression and increases viable hPASMC numbers.
To determine the effect of AMPK overexpression on arginase II protein levels and viable cell numbers, hPASMCs were seeded on six-well plates and transfected with an empty pCMV6-Entry vector with in-frame MYC/DDK tags or pCMV6-Entry vector with in-frame MYC/DDK tags containing AMPKα1, AMPKα2, or AMPKα1/2. The transfected hPASMCs were placed in normoxia or hypoxia for 48 h and the protein was analyzed by Western blot. Protein was assayed for myc and arginase II expression by Western blot and was quantified by densitometry. We confirmed transfection of the vector by demonstrating that myc protein expression was present in the hPASMCs transfected with the empty vector (control), vAMPKα1, vAMPKα2, and vAMPKα1/2, and lack of myc expression in the nontransfected hPASMCs (Fig. 3A). We found that in normoxia, overexpression of either isoform of AMPKα significantly induced arginase II protein expression (P < 0.05, Fig. 3, B and C). Hypoxia induced arginase II protein expression in the empty vector control cells (P < 0.05, Fig. 3, B and C). vAMPKα1-transfected or vAMPKα2-transfected hPASMCs in hypoxia resulted in no further increase in arginase II protein expression than did hypoxia alone (Fig. 3, B and C). To determine the effect of AMPK overexpression on viable cell numbers, hPASMCs were seeded on six-well plates and transfected with an empty vector or vector containing AMPKα1, AMPKα2, or AMPKα1+2. The transfected hPASMCs were seeded onto six-well plates at 104 cells per well and incubated for 120 h in normoxia or hypoxia, and viable cell numbers were determined using trypan blue exclusion. In normoxia, overexpression of AMPK resulted in significantly greater viable cell numbers relative to empty vector controls (P < 0.01, Fig. 3D). Hypoxia significantly increased viable cell numbers (P < 0.0001, Fig. 3D). vAMPKα1-transfected hPASMCs in hypoxia resulted in no further increase in viable cell numbers than did hypoxia alone (Fig. 3D). Interestingly, vAMPKα2-transfected hPASMCs had a significant increase in viable cell numbers in hypoxia compared with hypoxia alone (P < 0.01, Fig. 3D).
Fig. 3.

AMPK overexpression induces arginase II expression and increases viable hPASMC numbers. Human PASMCs were seeded on six-well plates and transfected with an empty pCMV6-Entry vector with in-frame MYC/DDK tags or pCMV6-Entry vector with in-frame MYC/DDK tags containing AMPKα1, AMPKα2, or AMPKα1/2. The transfected hPASMCs were placed in 21% O2 (normoxia) or 1% O2 (hypoxia) for 48 h then protein was analyzed by Western blot analysis. Protein was assayed for myc and arginase II expression by Western blot analysis and was quantified by densitometry. Representative Western blots are shown for myc (A) and arginase II and β-actin (B). Data are shown as fold-change ± SE relative to empty vector control (vControl) (n = 4–6) (C). Human PASMCs were seeded on six-well plates and transfected with an empty pCMV6-Entry vector with in-frame MYC/DDK tags or pCMV6-Entry vector with in-frame MYC/DDK tags containing AMPKα1, AMPKα2, or AMPKα(1+2). The transfected hPASMCs were seeded onto six-well plates at 104 cells per well and incubated for 120 h in normoxia or hypoxia, and viable cell numbers were determined using trypan blue exclusion. D: data are shown as viable cell numbers as a percentage change from the number of seeded cells (n = 6). *Significant difference compared with vControl, P < 0.01. †Significant difference compared with hypoxia, vControl, P < 0.05.
AMPKα1 knockdown prevents hypoxia-induced arginase II protein expression.
To further test our hypothesis that AMPK regulates hypoxia-induced arginase II expression, we used RNA interference to decrease gene expression of AMPKα1, AMPKα2, or AMPKα1/2. Human PASMCs were treated with AMPKα isoform-specific siRNA or scramble siRNA for 24 h and then exposed to hypoxia for 48 or 120 h. In addition, a control group was added in which no transfection reagent was used. To verify knockdown, protein was harvested for assessment of AMPKα1, AMPKα2, and arginase II expression by Western blot analysis and quantified by densitometry. Appropriate knockdown of AMPKα1 and AMPKα2 was verified at the protein level in both normoxia and hypoxia at 48 h (Fig. 4, A and B) and 120 h (data not shown).
Fig. 4.
AMPKα1 knockdown prevents hypoxia-induced arginase II protein expression. Human PASMCs were transfected with AMPKα isoform-specific siRNA or scramble siRNA for 24 h, and then exposed to 21% O2 (normoxia) or 1% O2 (hypoxia) for 48 h. Protein was assayed for AMPKα1, AMPKα2, or arginase II protein expression by Western blot and was quantified by densitometry. Representative Western blots are shown for AMPKα1 (A), AMPKα2 (B), and arginase II and β-actin (C). Data are shown as means ± SE relative to normoxia, scramble siRNA-treated controls (n = 3–10). *Significant difference compared with normoxia, scramble siRNA-treated, P < 0.05. †Significant difference, arginase II vs. hypoxia, scramble siRNA-treated, P < 0.0001.
Arginase II was again significantly increased following hypoxia exposure in the scramble siRNA-transfected controls (P < 0.0001, Fig. 4C). As previously shown, arginase II protein expression was not different between the nontransfected control group, and the scramble siRNA-transfected controls in normoxia and hypoxia (data not shown) (4). Knockdown of AMPKα1 or AMPKα1/2 by siRNA transfection completely prevented the hypoxia-induced increase in arginase II (P < 0.0001), such that there was no difference in arginase II expression in the hypoxia, AMPKα1 or AMPKα1+2 siRNA-transfected group compared with the normoxia, scramble siRNA-transfected controls (Fig. 4C). Knockdown of AMPKα2 by siRNA transfection had no statistically significant effect on hypoxia-induced arginase II expression (Fig. 4C).
Knockdown of AMPKα1, but not AMPKα2, attenuates hypoxic activation of AMPK.
Given the difference in the effect of knockdown of AMPKα1 compared with AMPKα2 on hypoxia-induced arginase II protein expression, activation of AMPK following isoform-specific knockdown of AMPK was determined. Following transfection with siRNA against α1 or α2-specific AMPK, hPASMCs were exposed to hypoxia for 48 h. Protein was harvested for assessment of p-AMPK, total AMPK, phosphorylated acetyl-CoA carboxylase [ACC; an established marker for AMPK action (12)], and total ACC expression by Western blot analysis and quantified by densitometry. Knockdown of AMPKα1 resulted in significantly less p-AMPK and a trend toward decreased p-ACC than in the scramble siRNA-transfected cells (Fig. 5). Whereas both p-AMPK and p-ACC levels were significantly higher following AMPKα2 knockdown than in cells in which AMPKα1 was knocked down (Fig. 5).
Fig. 5.
Knockdown of AMPKα1, but not AMPKα2, attenuates hypoxic activation of AMPK. Human PASMCs were treated with AMPKα isoform-specific siRNA or scramble siRNA for 24 h, and then exposed to 1% O2 (hypoxia) for 48 h. Protein was assayed for p-AMPK, total AMPK, p-ACC, or total ACC protein expression by Western blot and was quantified by densitometry. Representative Western blots are shown for p-AMPK, total AMPK (A), p-ACC, total ACC, and β-actin (B). Data are shown as fold-change ± SE relative to scramble siRNA-treated controls (n = 3–7). *Significant difference, compared with scramble siRNA-treated, P < 0.01. †Significant difference, compared with siAMPKα1-treated, P < 0.05.
Pharmacologic inhibition of AMPK decreases the number of viable hPASMCs, whereas AMPK activation enhances hypoxia-induced increase in viable cell numbers.
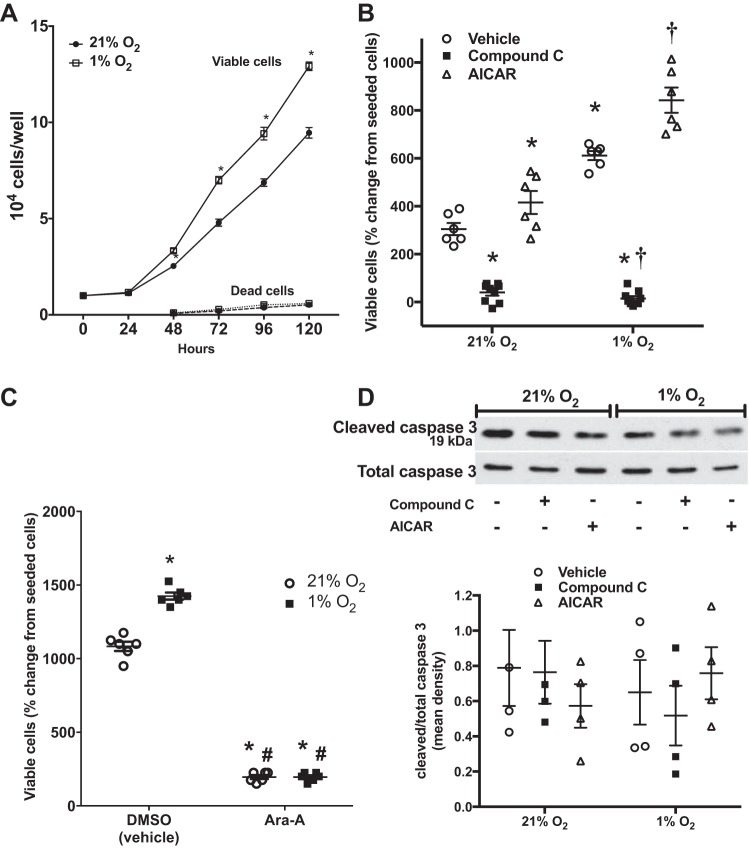
To determine the role of AMPK on hypoxia-induced viable cell numbers, hPASMCs were seeded on six-well plates at a density of 104 cells per well and Compound C, Ara-A, AICAR, or vehicle was added to the medium. The cells were then incubated for 120 h in normoxia or hypoxia and viable cell numbers were determined using trypan blue exclusion. Overall, as previously shown, hypoxia resulted in significantly greater viable cell numbers than in normoxia at 120 h (P < 0.0001, Fig. 6, A and B) (4, 5, 7). In addition, data for viable cell numbers at each 24-h time point were collected, and they are shown in Fig. 6A. Viable cell numbers were significantly greater in hypoxia starting at 48 h (P = 0.02) and continued to remain substantially different than in normoxia at 72, 96, and 120 h (P < 0.0001, Fig. 6A). The numbers of dead cells present in the media at 24-h intervals are also shown beginning at the 48-h time point, with no significant difference in cell death detected between normoxia and hypoxia at any of the evaluated time points (Fig. 6A). In both normoxia and hypoxia, treatment with Compound C resulted in fewer viable cells than in vehicle-treated hPASMCs (Fig. 6B). Given the nonspecific inhibitory action of Compound C on off-target molecules, particularly BMP signaling, which may also affect cell proliferation, a second AMPK inhibitor, Ara-A, was used to verify the results. Similar to compound C, viable cell numbers were significantly decreased in both normoxia and hypoxia following treatment with Ara-A (Fig. 6C). In contrast, the viable cell numbers were significantly enhanced in the normoxia, AICAR-treated group compared with those in the normoxia, vehicle-treated group (Fig. 6B, P < 0.05). In addition, the hypoxia, AICAR-treated group had greater viable cell numbers than after hypoxia exposure alone (P < 0.05, Fig. 6B). To determine whether apoptosis played a role in the decrease in viable cell numbers after Compound C treatment, hPASMCs were treated with the same treatment drugs and incubated in normoxia or hypoxia for 48 h. Protein was harvested for the assessment of cleaved caspase 3 expression by Western blot analysis and quantified by densitometry. There was no difference in cleaved caspase 3 expression in hypoxia exposure, Compound C, or AICAR treatment compared with vehicle-treated, normoxic cells (Fig. 6D).
Fig. 6.
Pharmacologic inhibition of AMPK decreases the number of viable hPASMCs, whereas AMPK activation enhances hypoxia-induced increase in viable cell numbers with no effect on apoptosis. Human PASMCs were seeded on six-well plates at a density of 104 cells per well, and the cells were incubated for 120 h in 21% O2 (normoxia) or 1% O2 (hypoxia), and viable cell numbers were determined at each 24-h interval using trypan blue exclusion. A: numbers of dead cells were counted beginning at the 48-h time point. Data are shown as viable or dead cell numbers per well (n = 3 for each time-point). Human PASMCs were treated with Compound C, AICAR, vehicle (B) or Ara-A or vehicle (C) (DMSO for the AMPK inhibitors or dH2O for AICAR), incubated in normoxia or hypoxia, and viable cell numbers were determined using trypan blue exclusion. Data are shown as viable cell numbers as a percent change from the number of seeded cells (n = 6–9). Human PASMCs were treated with Compound C, AICAR, or vehicle, and incubated in normoxia or hypoxia for 48 h. Protein was assayed for cleaved caspase 3 expression by Western blot analysis and quantified by densitometry. Representative Western blots are shown for cleaved caspase 3 and total caspase 3. Data are shown as fold change ± SE, relative to normoxia, vehicle-treated hPASMC controls (n = 4) (D). *Significant difference, compared with normoxia, vehicle-treated, P < 0.05. †Significant difference from hypoxia, vehicle-treated, P < 0.0001.
AMPK knockdown prevents hypoxia-induced increase in viable hPASMC numbers.
To determine the effect of siRNA-mediated AMPK knockdown on hypoxia-induced viable cell numbers, hPASMCs were transfected with siRNA against AMPKα1, AMPKα2, or AMPKα(1+2). The transfected hPASMCs were then seeded onto six-well plates at 104 cells per well and incubated for 120 h in normoxia or hypoxia and viable cell numbers determined using trypan blue exclusion. Overall, hypoxia resulted in significantly greater viable cell numbers in the scramble siRNA-transfected cells than in normoxia (P < 0.05, Fig. 7A). AMPKα1, AMPKα2, or AMPKα1/2 knockdown substantially attenuated viable cell numbers in both normoxia and hypoxia compared with scramble siRNA-transfected cells (P < 0.0001). The siRNA-mediated knockdown of AMPK had no appreciable effect on cell death as measured by cleaved caspase 3 (Fig. 7, B and C). Furthermore, to confirm these findings, LDH cytotoxicity assay was performed after 48 h of hypoxia exposure in hPASMCs transfected with siRNA against AMPKα1, AMPKα2, AMPKα1/2, or scramble siRNA. Overall, LDH cytotoxicity was very low at <5%, indicating little cell death. There were no differences in LDH cytotoxicity between hPASMCs exposed to normoxia compared with hypoxia (Fig. 7D). In normoxia, there was significantly less LDH cytotoxicity after siRNA transfection against each AMPKα isoform relative to scramble siRNA-transfected cells (Fig. 7D). In hypoxia, LDH cytotoxicity was significantly lower after siRNA transfection against each AMPKα isoform relative to scramble siRNA-transfected cells, however, with the least cytotoxic effects after siAMPKα1 (Fig. 7D). Interestingly, LDH cytotoxicity was substantially higher in the siAMPKα2 and siAMPKα1/2-transfected hypoxic cells compared with the siAMPKα2 and siAMPKα1/2-transfected normoxic cells (Fig. 7D).
Fig. 7.
siRNA knockdown of AMPK prevents hypoxia-induced increase in viable hPASMC numbers. Human PASMCs were transfected with AMPKα isoform-specific siRNA or scramble siRNA. The siRNA-transfected cells were then seeded on six-well plates at a density of 104 cells per well and incubated for 120 h in 21% O2 (normoxia) or 1% O2 (hypoxia), and viable cell numbers were determined using trypan blue exclusion. A: data are shown as viable cell numbers as a percentage change from number of seeded cells (n = 7). Human PASMCs were transfected with AMPKα isoform-specific siRNA or scramble siRNA and incubated in normoxia or hypoxia for 48 h. Protein was assayed for cleaved caspase 3 expression by Western blot analysis and was quantified by densitometry. B: representative Western blots are shown for cleaved caspase 3 and total caspase 3. Data are shown as fold-change ± SE relative to normoxia, scramble siRNA-treated hPASMC controls (n = 3–5) (C). D: alternatively, LDH cytotoxicity assay was performed. *Significant difference, compared with normoxia, scramble siRNA-treated, P < 0.05. †Significant difference from hypoxia, scramble siRNA-treated, P < 0.0001. ‡Significant difference, hypoxia siAMPKα2 or siAMPKα1/2 compared with normoxia siAMPKα2 or siAMPKα1/2, P < 0.0001.
Arginase II knockdown partially inhibits viable cell numbers in AMPK-activated, hypoxic hPASMCs.
To determine the effect of siRNA-mediated arginase II knockdown on AMPK-activated, hypoxia-induced viable cell numbers, hPASMCs were transfected with siRNA against arginase II. The transfected hPASMCs were then seeded onto six-well plates at 104 cells per well and incubated with AICAR or vehicle (dH2O) for 120 h in normoxia or hypoxia. Viable cell numbers were determined using trypan blue exclusion. Hypoxia resulted in significantly greater viable cell numbers in the scramble siRNA-transfected cells than in normoxia (P < 0.0001, Fig. 8). hPASMCs transfected with siArgII completely prevented the hypoxia-induced increase in viable cell numbers, such that there was no difference in viable cell numbers relative to normoxia, scramble siRNA-transfected cells. In hypoxia, viable cell numbers after treatment with siArgII+AICAR were less than scramble siRNA-transfected viable cell numbers, but greater than siArgII-transfected viable cell numbers (P < 0.002, Fig. 8).
Fig. 8.

siRNA knockdown of arginase II, in part, inhibits viable cell numbers in AMPK-activated, hypoxic hPASMCs. Human PASMCs were transfected with siRNA against arginase II. The transfected hPASMCs were then seeded onto six-well plates at a density of 104 cells per well and incubated with AICAR or vehicle (dH2O) for 120 h in 21% O2 (normoxia) or 1% O2 (hypoxia), and viable cell numbers were determined using trypan blue exclusion. Data are shown as fold change of viable cell numbers relative to normoxia, scramble siRNA-transfected hPASMCs. *Significant difference, compared with normoxia, scramble siRNA-treated, P < 0.05. †Significant difference from hypoxia, scramble siRNA-treated, P < 0.0005. ‡Significant difference, compared with siArgII.
DISCUSSION
The main objective of the present study was to determine whether hypoxia-induced arginase II expression and the subsequent increase in viable hPASMCs were dependent on AMPK. The major findings of this study were that in hPASMCs, 1) consistent with our previous findings (4, 5), hypoxia increased arginase II protein expression and viable cell numbers; 2) hypoxia activated AMPK; 3) overexpression of AMPK induced arginase II protein expression and viable hPASMC numbers in normoxia; 4) activation of AMPK by pharmacologic agents increased arginase II protein expression and viable hPASMC numbers; 5) inhibition of AMPK by pharmacologic agents and siRNA knockdown of AMPKα1 prevented the hypoxia-induced increase in arginase II protein expression, and 6) inhibition of AMPK by pharmacologic agents and siRNA knockdown of AMPKα1 prevented the hypoxia-induced increase in viable hPASMC numbers. Taken together, the results support our hypothesis that hypoxia induces arginase II protein expression and subsequent increase in viable hPASMCs at least partially via AMPK signaling.
AMPK is a ubiquitously expressed heterotrimer that is activated in response to metabolic stresses, such as hypoxia. AMPK comprises α1 and α2 catalytic subunits, as well as β and γ regulatory subunits, which also have multiple isoforms (10). Activation of AMPK has been reported to occur via several mechanisms. AMPK activation occurs when there is an increase in the AMP/ATP ratio, followed by phosphorylation at Thr-172 within the α-subunit by upstream kinases (10, 41). The tumor suppressor LKB1 is perhaps the most well-described AMPK kinase that phosphorylates the catalytic α-subunit of AMPK in an AMP-dependent manner (31). Another mechanism of AMPK activation, which occurs independently of cellular AMP levels, involves Ca2+/calmodulin-dependent protein kinase kinase (CaMKKβ) (18, 39). Additionally, hypoxia has been shown to trigger AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels, which occurs independently of both the AMP/ATP ratio and LKB1 (31). Thus, hypoxic activation of AMPK is well recognized. Figure 1 confirms the activation of AMPK by hypoxia in our established cell culture model. Inhibition of AMPK using Compound C completely prevented the hypoxia-induced increase in arginase II (Fig. 2B) and viable cell numbers (Fig. 6A). Furthermore, we found that in normoxia, overexpression of AMPK, either isoform or both, mimicked the hypoxia-induced increases in both arginase II protein expression levels and viable cell numbers (Fig. 3). In hypoxia, AMPKα1 overexpression did not further enhance arginase II induction or viable cell numbers than hypoxia exposure alone (Fig. 3, B–D). Although AMPKα2 overexpression in hypoxic conditions did not further increase arginase II than hypoxia exposure alone; interestingly, the viable cell numbers were significantly greater with AMPKα2 overexpression (Fig. 3, B–D). These data suggest that the enhanced viable cell numbers seen in hPASMCs that overexpress AMPKα2 is not due to an arginase II-dependent pathway. These studies demonstrate the importance of AMPK in hypoxic induction of arginase II and viable cell numbers.
Indeed, we found that siRNA-mediated knockdown of the α1 subunit attenuated phosphorylation of AMPK, as well as the downstream target ACC (a marker for AMPK action). Interestingly, siRNA-mediated knockdown of the α2 subunit resulted in higher levels of p-AMPK and p-ACC expression (Fig. 5). The concept that AMPK α1 and α2 have different functions has been reported by Ibe et al. (19), who demonstrated that AMPK α1 and α2 contribute to hPASMC survival via different mechanisms and that inhibition of AMPK α1 and α2 induced PASMC death. AMPKα1 was demonstrated to enhance autophagy to promote cell survival (19). Our data support these findings in that there were fewer viable cells after inhibition of AMPK (Figs. 6A and 7A). In contrast to Ibe et al. (19), we did not find an increase in apoptosis, as evidenced by cleaved caspase 3 expression following knockdown of either AMPKα isoform. However, we found that in our cell culture model, there was, overall, little cell death as measured by LDH cytotoxicity, despite a significant decrease in the viable cell numbers with AMPK isoform-specific knockdown. However, LDH cytotoxicity was significantly increased in hypoxia compared with normoxia in the siAMPKα2 and siAMPKα1/2-treated hPASMCs (Fig. 7D), suggesting that the AMPKα2 isoform is important in hPASMC survival. This supports the concept that there are functional differences in the AMPKα isoforms with hypoxia exposure. Furthermore, although there were differences in AMPKα isoform effects on arginase II and cell toxicity, both effects would contribute to lower viable cell numbers after siRNA-mediated knockdown, which would explain the similar effects of α1 and α2 subunit knockdown on viable cell numbers (Fig. 7A).
Arginase is critical for cell proliferation and growth, as it is the first step in the synthesis of polyamines and proline. We have previously shown that knockdown or pharmacologic inhibition of arginase II in pulmonary microvascular endothelial and pulmonary artery smooth muscle cells prevents hypoxia-induced viable cell numbers (4, 5, 38). An association has previously been shown between AMPK and arginase I expression in rat microglial cultures (24, 25). Interestingly, in this cell type, treatment with either the AMPK activator AICAR or the AMPK inhibitor Compound C, increased arginase I protein expression (24, 25), suggesting an indirect effect of AMPK on arginase. We demonstrate that the AMPK activator AICAR increased arginase II expression in normoxic hPASMCs (Fig. 2). We confirm a direct effect of AMPK on arginase II by overexpressing the AMPKα isoforms (Fig. 3). We found a significant increase in arginase II protein expression, as well as viable cell numbers following overexpression of AMPKα1, AMPKα2, or AMPKα1/2. Additionally, using siRNA transfection to knock down AMPK in hPASMCs, we demonstrated a direct link between AMPK and arginase, such that AMPKα1 isoform-specific knockdown prevented hypoxia-induced arginase II expression (Fig. 4C). More specifically, the induction of arginase II may be the result of hypoxic activation of AMPKα1, although we did not show differences in AMPKα1 protein expression. This is supported by the fact that cells transfected with siRNA against AMPKα2 had no effect on hypoxia-induced arginase II protein levels (Fig. 4C), which may be due to the enhanced activation of AMPK, as represented by greater p-AMPK and p-ACC levels, after knockdown of AMPKα2 than after knockdown of AMPKα1 (Fig. 5). We found that siRNA knockdown of arginase II attenuated the number of viable AICAR-treated hPASMCs in hypoxia (Fig. 8), supporting our hypothesis that AMPK regulates hypoxia-induced arginase-dependent enhancement of viable cell numbers. Arginase II knockdown did not completely prevent the hypoxia-induced increase in viable cell numbers in the AICAR-treated hPASMCs, which is likely due to the arginase-independent cell proliferative and/or cell survival mechanistic actions of AMPKα2. The different functions of the AMPKα isoforms have yet to be explored and may represent novel targets for potential therapeutic options in human disease.
It has yet to be determined the precise mechanism by which AMPKα1 regulates the expression of arginase II in hypoxia. AMPK has been known to regulate transcription factors, mitochondrial genes, and pathways that may be involved in pulmonary hypertensive diseases, including PGC1-α, NRF, PPARs, PI3K/AKT/mTOR, and cAMP response element binding protein (CREB) (3, 37). CREB is a cellular transcription factor that binds to cAMP-response elements, thereby regulating the transcription of downstream genes. We speculate that regulation of arginase II occurs via AMPK activation of CREB in our model. We have observed in unpublished data that siRNA knockdown of CREB results in prevention of hypoxia-induced arginase II protein expression and a link between AMPK activation and CREB has been previously reported (3, 37). Further studies evaluating this pathway, as well as isoform-specific activity, will be necessary to delineate the specific mechanism of AMPK regulation of arginase II.
Dysfunction of the l-arginine-nitric oxide (NO) pathway is an important contributor to the pathophysiology seen in pulmonary hypertension. Both arginase II and p-AMPK have independently been shown to play an important role in endothelial NO synthase (eNOS) expression and function in pulmonary vascular cells. Arginase and NOS compete for the same substrate, l-arginine, and when one l-arginine metabolic pathway is inhibited, the activity of the other is enhanced (8). Arginase II has been shown to be increased in pulmonary artery endothelial cells (PAECs) from patients with pulmonary hypertension, in association with a decrease in NO synthesis that may contribute to endothelial dysfunction (36, 42). Both a decrease in NO synthesis and hypoxia can further drive an increase in PASMC proliferation by both increasing substrate availability for arginase, as well as increase arginase expression. The role for AMPK in the pathogenesis of pulmonary hypertension remains unclear with conflicting data in the literature. In PAECs from an in utero sheep model of pulmonary hypertension induced by ductal ligation, Teng et al. (36) showed a decrease in p-AMPK relative to controls, although no differences were seen in the pulmonary arteries. AMPK activation was also decreased in PAECS from patients with idiopathic pulmonary hypertension, and pharmacologic activation of AMPK has been shown to increase eNOS expression, activity, and recoupling in those PAECs. On the contrary, in PASMCs isolated from patients with pulmonary arterial hypertension, Ibe et al. (19) found that p-AMPK was increased relative to controls. Additionally, the authors found that p-AMPK was increased in whole lung homogenates from mice with chronic hypoxia-induced pulmonary hypertension (19). Similarly, Huang et al. (16) showed an increase in p-AMPKα1 and AMPKα1 in pulmonary arterioles from a rat model of chronic hypoxia-induced pulmonary hypertension. Curiously, treatment with either an AMPK inhibitor or AMPK activator attenuated the chronic hypoxia-induced pulmonary hypertension in rodent models. For example, Ibe et al. (19) demonstrated that treatment with an AMPK inhibitor attenuated chronic hypoxia-induced pulmonary hypertension, as evidenced by right ventricular hypertrophy, right ventricular systolic pressure, and pulmonary arterial wall thickness. In contrast, Huang et al. (16) showed that treatment with an AMPK activator also attenuated pulmonary hypertension, as evidenced by improvement in right ventricular hypertrophy, mean pulmonary arterial pressures, and pulmonary vascular remodeling in rats. These intriguing results demonstrate the need for more in-depth inquiry into the mechanistic action of AMPK in pulmonary hypertensive diseases. We are uncertain as to the effects of hypoxia on AMPK and the l-arginine pathway in PAECs. Examining these pathways in PAECs and its effects on PASMCs using a coculture model or conditioned media will be necessary to gain a better understanding of the pulmonary vascular system as a whole. Through these studies, a more complex system recapitulating the pulmonary vasculature can be evaluated. It would be imperative to evaluate the effects of AMPK manipulation using more targeted tools, as it is known that pharmacologic inhibitors, such as Compound C may have other off-target effects (9).
There are several limitations to the present study that should be noted. We use commercially available hPASMCs that are isolated from main pulmonary artery segments. We chose these cells, in part, because they are human cells and are readily available. We acknowledge that these findings may need to be validated in resistance-level PASMCs. Second, the use of these cells in culture from later passages may potentially change the signaling pathways involved and may not translate directly in vivo. Finally, we use perhaps more severe levels of hypoxia to induce the number of viable PASMCs. On the basis of numerous trials in our laboratory using various conditions, including different oxygen levels, our cell culture model has consistently proven to be a reliable and reproducible model of hypoxia-induced increase in viable hPASMC numbers (4–6).
In conclusion, our data indicate that hypoxia activates AMPK, resulting in induction of arginase II protein expression and subsequent increase in viable hPASMCs. We show a direct effect of AMPKα1 on hypoxic induction of arginase II in hPASMCs. We also found functional differences between the α1 and α2 subunits in regard to activation of AMPK, knockdown-mediated effects on arginase II, and knockdown-mediated effects on cell toxicity. We postulate that further evaluation of the functional differences between the AMPK catalytic isoforms may provide evidence for development of specific targeted therapies in the prevention or treatment of the pathologic PASMC proliferation associated with hypoxic lung diseases.
GRANTS
This work was supported by Grant 5K08HL-105677 (B. Chen) from the National Heart Lung and Blood Institute of the National Institutes of Health and internal grant support from the Research Institute at Nationwide Children’s Hospital.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
J.X. performed experiments; J.X., L.D.N., and B.C. analyzed data; J.X., L.D.N., and B.C. interpreted results of experiments; J.X. and B.C. drafted manuscript; J.X., L.D.N., and B.C. approved final version of manuscript; L.D.N. and B.C. edited and revised manuscript; B.C. conceived and designed research; B.C. prepared figures.
REFERENCES
- 1.Ban HS, Xu X, Jang K, Kim I, Kim BK, Lee K, Won M. A novel malate dehydrogenase 2 inhibitor suppresses hypoxia-inducible factor-1 by regulating mitochondrial respiration. PLoS One 11: e0162568, 2016. doi: 10.1371/journal.pone.0162568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Cantó C, Auwerx J. AMP-activated protein kinase and its downstream transcriptional pathways. Cell Mol Life Sci 67: 3407–3423, 2010. doi: 10.1007/s00018-010-0454-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen B, Calvert AE, Cui H, Nelin LD. Hypoxia promotes human pulmonary artery smooth muscle cell proliferation through induction of arginase. Am J Physiol Lung Cell Mol Physiol 297: L1151–L1159, 2009. doi: 10.1152/ajplung.00183.2009. [DOI] [PubMed] [Google Scholar]
- 5.Chen B, Calvert AE, Meng X, Nelin LD. Pharmacologic agents elevating cAMP prevent arginase II expression and proliferation of pulmonary artery smooth muscle cells. Am J Respir Cell Mol Biol 47: 218–226, 2012. doi: 10.1165/rcmb.2011-0015OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen B, Nelin VE, Locy ML, Jin Y, Tipple TE. Thioredoxin-1 mediates hypoxia-induced pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol 305: L389–L395, 2013. doi: 10.1152/ajplung.00432.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen B, Xue J, Meng X, Slutzky JL, Calvert AE, Chicoine LG. Resveratrol prevents hypoxia-induced arginase II expression and proliferation of human pulmonary artery smooth muscle cells via Akt-dependent signaling. Am J Physiol Lung Cell Mol Physiol 307: L317–L325, 2014. doi: 10.1152/ajplung.00285.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chicoine LG, Paffett ML, Young TL, Nelin LD. Arginase inhibition increases nitric oxide production in bovine pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol 287: L60–L68, 2004. doi: 10.1152/ajplung.00194.2003. [DOI] [PubMed] [Google Scholar]
- 9.Emerling BM, Viollet B, Tormos KV, Chandel NS. Compound C inhibits hypoxic activation of HIF-1 independent of AMPK. FEBS Lett 581: 5727–5731, 2007. doi: 10.1016/j.febslet.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans AM, Hardie DG, Peers C, Mahmoud A. Hypoxic pulmonary vasoconstriction: mechanisms of oxygen-sensing. Curr Opin Anaesthesiol 24: 13–20, 2011. doi: 10.1097/ACO.0b013e3283421201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans AM, Hardie DG, Peers C, Wyatt CN, Viollet B, Kumar P, Dallas ML, Ross F, Ikematsu N, Jordan HL, Barr BL, Rafferty JN, Ogunbayo O. Ion channel regulation by AMPK: the route of hypoxia-response coupling in the carotid body and pulmonary artery. Ann N Y Acad Sci 1177: 89–100, 2009. doi: 10.1111/j.1749-6632.2009.05041.x. [DOI] [PubMed] [Google Scholar]
- 12.Evans AM, Lewis SA, Ogunbayo OA, Moral-Sanz J. Modulation of the LKB1-AMPK signalling pathway underpins hypoxic pulmonary vasoconstriction and pulmonary hypertension. Adv Exp Med Biol 860: 89–99, 2015. doi: 10.1007/978-3-319-18440-1_11. [DOI] [PubMed] [Google Scholar]
- 13.Evans AM, Mustard KJ, Wyatt CN, Dipp M, Kinnear NP, Hardie DG. Does AMP-activated protein kinase couple inhibition of mitochondrial oxidative phosphorylation by hypoxia to pulmonary artery constriction? Adv Exp Med Biol 580: 147–154, 2006. doi: 10.1007/0-387-31311-7_22. [DOI] [PubMed] [Google Scholar]
- 14.Ferns SJ, Wehrmacher WH, Serratto M. Pediatric pulmonary arterial hypertension—a review. Compr Ther 35: 81–90, 2009. [PubMed] [Google Scholar]
- 15.Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev 25: 1895–1908, 2011. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang X, Fan R, Lu Y, Yu C, Xu X, Zhang X, Liu P, Yan S, Chen C, Wang L. Regulatory effect of AMP-activated protein kinase on pulmonary hypertension induced by chronic hypoxia in rats: in vivo and in vitro studies. Mol Biol Rep 41: 4031–4041, 2014. doi: 10.1007/s11033-014-3272-9. [DOI] [PubMed] [Google Scholar]
- 17.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 43: Suppl S: S13–S24, 2004. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 18.Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem 280: 29060–29066, 2005. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 19.Ibe JC, Zhou Q, Chen T, Tang H, Yuan JX, Raj JU, Zhou G. Adenosine monophosphate-activated protein kinase is required for pulmonary artery smooth muscle cell survival and the development of hypoxic pulmonary hypertension. Am J Respir Cell Mol Biol 49: 609–618, 2013. doi: 10.1165/rcmb.2012-0446OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Igata M, Motoshima H, Tsuruzoe K, Kojima K, Matsumura T, Kondo T, Taguchi T, Nakamaru K, Yano M, Kukidome D, Matsumoto K, Toyonaga T, Asano T, Nishikawa T, Araki E. Adenosine monophosphate-activated protein kinase suppresses vascular smooth muscle cell proliferation through the inhibition of cell cycle progression. Circ Res 97: 837–844, 2005. doi: 10.1161/01.RES.0000185823.73556.06. [DOI] [PubMed] [Google Scholar]
- 21.Jänne J, Alhonen L, Leinonen P. Polyamines: from molecular biology to clinical applications. Ann Med 23: 241–259, 1991. doi: 10.3109/07853899109148056. [DOI] [PubMed] [Google Scholar]
- 22.Kim JS, Ro SH, Kim M, Park HW, Semple IA, Park H, Cho US, Wang W, Guan KL, Karin M, Lee JH. Sestrin2 inhibits mTORC1 through modulation of GATOR complexes. Sci Rep 5: 9502, 2015. doi: 10.1038/srep09502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitamura K, Shibata R, Tsuji Y, Shimano M, Inden Y, Murohara T. Eicosapentaenoic acid prevents atrial fibrillation associated with heart failure in a rabbit model. Am J Physiol Heart Circ Physiol 300: H1814–H1821, 2011. doi: 10.1152/ajpheart.00771.2010. [DOI] [PubMed] [Google Scholar]
- 24.Łabuzek K, Liber S, Gabryel B, Bułdak L, Okopień B. Ambivalent effects of compound C (dorsomorphin) on inflammatory response in LPS-stimulated rat primary microglial cultures. Naunyn Schmiedebergs Arch Pharmacol 381: 41–57, 2010. doi: 10.1007/s00210-009-0472-2. [DOI] [PubMed] [Google Scholar]
- 25.Łabuzek K, Liber S, Gabryel B, Okopień B. AICAR (5-aminoimidazole-4-carboxamide-1-β-4-ribofuranoside) increases the production of toxic molecules and affects the profile of cytokines release in LPS-stimulated rat primary microglial cultures. Neurotoxicology 31: 134–146, 2010. doi: 10.1016/j.neuro.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Meinginger CJ, Hawker JR, Haynes TE. Kepka-Lenhart D, Mistry SK, Morris SM, Wu G. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am J Physiol Endocrinol Metab 280: E75–E80, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Wang L, Zhao Q, Liu Y, He L, Xu Q, Sun X, Teng L, Cheng H, Ke Y. SHP2 positively regulates TGFβ1-induced epithelial-mesenchymal transition modulated by its novel interacting protein Hook1. J Biol Chem 289: 34152–34160, 2014. doi: 10.1074/jbc.M113.546077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Wu P, Wang Y, Du Y, A N, Liu S, Zhang Y, Zhou N, Xu Z, Yang Z. Ad-HGF improves the cardiac remodeling of rat following myocardial infarction by upregulating autophagy and necroptosis and inhibiting apoptosis. Am J Transl Res 8: 4605–4627, 2016. [PMC free article] [PubMed] [Google Scholar]
- 29.Madiraju AK, Alves T, Zhao X, Cline GW, Zhang D, Bhanot S, Samuel VT, Kibbey RG, Shulman GI. Argininosuccinate synthetase regulates hepatic AMPK linking protein catabolism and ureagenesis to hepatic lipid metabolism. Proc Natl Acad Sci USA 113: E3423–E3430, 2016. doi: 10.1073/pnas.1606022113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JX, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 54, Suppl: S20–S31, 2009. doi: 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mungai PT, Waypa GB, Jairaman A, Prakriya M, Dokic D, Ball MK, Schumacker PT. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol Cell Biol 31: 3531–3545, 2011. doi: 10.1128/MCB.05124-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelin LD, Krenz GS, Chicoine LG, Dawson CA, Schapira RM. l-Arginine uptake and metabolism following in vivo silica exposure in rat lungs. Am J Respir Cell Mol Biol 26: 348–355, 2002. doi: 10.1165/ajrcmb.26.3.4450. [DOI] [PubMed] [Google Scholar]
- 33.Nelin LD, Nash HE, Chicoine LG. Cytokine treatment increases arginine metabolism and uptake in bovine pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol 281: L1232–L1239, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakanishi N, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 54, Suppl: S43–S54, 2009. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99: 675–691, 2006. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 36.Teng RJ, Du J, Afolayan AJ, Eis A, Shi Y, Konduri GG. AMP kinase activation improves angiogenesis in pulmonary artery endothelial cells with in utero pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 304: L29–L42, 2013. doi: 10.1152/ajplung.00200.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomson DM, Herway ST, Fillmore N, Kim H, Brown JD, Barrow JR, Winder WW. AMP-activated protein kinase phosphorylates transcription factors of the CREB family. J Appl Physiol (1985) 104: 429–438, 2008. doi: 10.1152/japplphysiol.00900.2007. [DOI] [PubMed] [Google Scholar]
- 38.Toby IT, Chicoine LG, Cui H, Chen B, Nelin LD. Hypoxia-induced proliferation of human pulmonary microvascular endothelial cells depends on epidermal growth factor receptor tyrosine kinase activation. Am J Physiol Lung Cell Mol Physiol 298: L600–L606, 2010. doi: 10.1152/ajplung.00122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab 2: 21–33, 2005. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Wu Y, Song P, Zhang W, Liu J, Dai X, Liu Z, Lu Q, Ouyang C, Xie Z, Zhao Z, Zhuo X, Viollet B, Foretz M, Wu J, Yuan Z, Zou MH. Activation of AMPKα2 in adipocytes is essential for nicotine-induced insulin resistance in vivo. Nat Med 21: 373–382, 2015. doi: 10.1038/nm.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, Saiu P, Howell SA, Aasland R, Martin SR, Carling D, Gamblin SJ. Structure of mammalian AMPK and its regulation by ADP. Nature 472: 230–233, 2011. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu W, Kaneko FT, Zheng S, Comhair SAA, Janocha AJ, Goggans T, Thunnissen FB, Farver C, Hazen SL, Jennings C, Dweik RA, Arroliga AC, Erzurum SC. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J 18: 1746–1748, 2004. doi: 10.1096/fj.04-2317fje. [DOI] [PubMed] [Google Scholar]